Abstract
Poly(ethylene terephthalate) (polyester) fibers as solid-phase microextraction (SPME) adsorbent were directly filled in a poly(ether ether ketone) (PEEK) tube, for online analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental water samples, coupled with high-performance liquid chromatography. The facile, economic, and environmental polyester fibers-in-tube SPME device exhibited high extraction efficiency, good selectivity for PAHs, and satisfactory durability. Under optimum conditions, the polyester fibers provided satisfactory enhancement factors in the range of 307–1646, and low detection limits ranging from 0.01 to 0.03 μg L−1. The linearity was in the range of 0.03–80 μg L−1 with correlation coefficients (r) ranging from 0.9978 to 0.9997. Limit of quantification was defined as a concentration of the analytes with a ten-time signal-to-noise ratio (S/N = 10) and was in the range of 0.03–0.1 μg L−1. The intra-day and inter-day precisions for quantitative analysis were investigated and the relative standard deviation (RSD) was lower than 5.8 and 6.9 %, respectively. Extraction repeatability was also investigated and its RSD was in the range of 3.8–7.8 %. Finally, the fiber-in-tube SPME device was successfully applied to analyze PAHs in water samples.

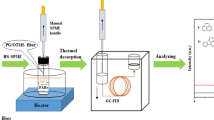
Schematic diagrams of polyester fibers-in-tube device and the automated in-tube SPME-HPLC system
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid-phase microextraction (SPME), a simple extraction method invented by Pawliszyn and co-workers in the 1990s, is a promising sample preparation technique [1, 2]. This method can overcome the problems of traditional methods by eliminating the use of organic solvents and by integrating sample extraction, concentration, and introduction into a single step [3, 4]. There are also many other merits such as rapidness, easy operation, environmental friendliness, and economical use [5]. SPME has become one of the most widely used sample preparation techniques in the past, due to its high sensitivity and selectivity. At early times, fiber-based SPME was more easily coupled to gas chromatography (GC), the extraction device was a fused silica or a stainless steel (SS) wire fiber externally coated with a polymeric sorbent at one of the ends [6]. Analytes were extracted by the coated sorbent and then the fiber was introduced into GC instrument for thermal desorption analysis. However, SPME-GC involves multi-steps which may lead to analytes losing before GC analysis and decrease determination precision [7]. In addition, thermally labile analytes and compounds with high boiling point are not achieved effective analysis using GC without any treatment. In contrast, high-performance liquid chromatography (HPLC) has a broader scope of analytes. It could be a wise choice to make SPME couple with HPLC to expand the area of analysis.
In order to couple with HPLC and accomplish online analysis, in-tube SPME was developed [8, 9]. In-tube SPME is suitable for automation, which can provide better accuracy, precision, and sensitivity than those offline methods [10, 11]. In-tube SPME is a mode of SPME which typically uses a GC capillary column with a proper coating to extract the analytes [12–15]. Its main processes are not complicated; simply speaking, an aqueous sample was flowed through an open capillary column and the extracted analytes on column coating can be analyzed by being desorbed into the HPLC. Although GC capillary columns are usually applied for in-tube SPME, many defects such as high cost, easy damage, and low extraction efficiency give a limit for further application. Many new style capillaries such as fiber-packed [16], sorbent-packed [17], and rod-type monolith capillaries [18, 19] were also developed to improve extraction efficiency and specificity. Sorbent-packed and rod-type monolith capillaries have excellent extraction efficiency for analytes, but complex preparation process and high requirements for equipment are necessary.
Fiber-in-tube SPME not only enhances the extraction efficiency by placing SPME fibers into the tube but also improves the symmetry of chromatographic peaks via decreasing dead volume in tube [20–22]. Except for several kinds of fibers [23] only used to diminish the inner volume of the tube, more kinds of fibers including metallic materials [20, 24], fused silica [22, 25], and organic polymer [26], were employed as sorbent part to enhance extraction efficiency. However, they were often modified by a complicated chemical process. Poly(ethylene terephthalate) (polyester) is a chemically and physically stable, economic, and common polymer material. It is often used in clothing fabrics, modified asphalt [27, 28], and concrete materials [29, 30] without any special treatment. As shown in Fig. 1a, it is composed of aliphatic hydrocarbon, ester, benzene, and alcohol hydroxyl groups, which can provide multiple interactions including hydrophobic interaction, π-π stacking, and hydrogen bond with organic compounds.
In this work, a poly(ether ether ketone) (PEEK) tube was packed with a bundle of polyester fibers to obtain a new fibers-in-tube device (Fig. 1b) for in-tube SPME. Polyester fibers were characterized by a scanning electron microscope (SEM) and an energy-dispersive x-ray (EDX) spectrometer. By replacing the sample loop of HPLC equipment with a polyester fibers-in-tube device, online SPME-HPLC system was built. Polycyclic aromatic hydrocarbons (PAHs) are important environmental pollutants, which have been widely distributed in the environment. PAHs were usually selected as target to investigate novel SPE or SPME materials, and some methodologies were also developed to detect these analytes. While polyester fiber is not a kind of new material, as far as we know, it is firstly explored in SPME in currently published sources. Based on multiple extraction mechanism of the polyester fibers with PAHs, online SPME-HPLC system was evaluated with eight PAHs as listed in Table 1. Under the optimum extraction and desorption conditions, the online SPME-HPLC method was established and applied to detect model analytes in rain water and river water samples.
Experimental
Materials and reagents
Poly(ethylene terephthalate) fibers were obtained from Jinan Hongmei Thread Factory (Jinan, China). PEEK tube (0.16 cm o.d., 250 μm i.d.) was purchased from Haohai Chemical (Wuhan, China). Naphthalene (Nap), acenaphthylene (Any), acenaphthene (Ana), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Flt), pyrene (Pyr), bisphenol A, ethinylestradiol, estrone, diethylstilbestrol, hexestrol, dimethyl phthalate, diethyl phthalate, diallyl phthalate, benzyl butyl phthalate, di-n-butyl phthalate, di-n-pentyl phthalate, dicyclohexyl phthalate, phenol, o-Nitrophenol, 2,6-dimethyl phenol, 1-naphthol, p-aminophenol, aniline, 4-methyl aniline, 2,6-dimethylaniline, n-ethylaniline, diphenylamine, ethyl 4-hydroxybenzoate, propyl p-hydroxybenzoate, butyl paraben, methylbenzene, ethylbenzene, n-propylbenzene, and n-butylbenzene were purchased from Shanghai Jingchun Industry Co. (Shanghai, China). All above-mentioned reagents were analytical grade. Acetonitrile and methanol were HPLC grade and purchased from Tedia (USA) and Yuwang Chemical Reagent Co. (Dezhou, China), respectively.
Apparatus
All chromatographic analyses were performed on an Agilent 1260 HPLC system (USA) equipped with a 20-μL sample loop, a Zorbax C18 column (250 × 4.6 mm i.d., 5 μm), and a diode array detector (DAD). Nap was detected at 220 nm, Any and Flu were detected at 225 nm, Flt and Pyr were detected at 230 nm, Phe and Ant were detected at 250 nm, and Ana was detected at 260 nm. Bisphenol A, ethinylestradiol, estrone, diethylstilbestrol, and hexestrol were detected at 202 nm. Dimethyl phthalate, diethyl phthalate, diallyl phthalate, benzyl butyl phthalate, di-n-butyl phthalate, di-n-pentyl phthalate, dicyclohexyl phthalate, methylbenzene, ethylbenzene, n-propylbenzene, and n-butylbenzene were detected at 210 nm. Phenol, o-nitrophenol, 2,6-dimethyl phenol, 1-naphthol, p-aminophenol, aniline, 4-methyl aniline, 2,6-dimethylaniline, N-ethylaniline, diphenylamine, ethyl 4-hydroxybenzoate, propyl p-hydroxybenzoate and butyl paraben were detected at 254 nm. A P1201 HPLC pump was applied to move sample solution into extraction tube and purchased from Dalian Yilite analytical instruments Co., Ltd. (Dalian, China). Mobile phases were filtered with a 0.45-μm nylon membrane filter. All chromatographic tests used acetonitrile-water or methanol-water as the mobile phase at 25 °C and 1 mL min−1. Polyester fibers were characterized by a field-emission SEM (SUPRATM55, Carl Zeiss, AG, Germany) equipped with an energy-dispersive x-ray spectrometer (EDS, Oxford INCA X-Act, UK).
Preparation and characterization of polyester fibers-in-tube device
A bundle of polyester fibers including about 120 fibers (120 cm, 30 mg) was washed in ultra-pure water under ultrasonication for 0.5 h, and then washed in methanol for 1 h. A 30-cm PEEK tube was cleaned by ultra-pure water and methanol. The folded polyester fibers bundle was filled into the PEEK tube, and the polyester fibers-in-tube extraction device was successfully prepared. The polyester fibers were characterized by SEM. As shown in Fig. 2a, polyester fibers are twisted into a bundle, and they are relatively loose and avoid mutual obstacles. As shown in Figs. 2b–d, the single fiber with uniform size has many grooves on the surface, which is helpful to improve extraction efficiency and accelerate mass transfer. The elements of the polyester fiber composed of carbon and oxygen were obvious in the EDS spectrum (Fig. S1) in the Electronic Supplementary Material. The atom ratio of carbon and oxygen is 5.2:7.3, which is consistent with the theoretical component.
Sample preparation
Stock solutions of PAHs, estrogens, phthalates, alkyl benzenes, p-hydroxybenzoates, phenols, and anilines were prepared at concentrations of 10 mg L−1 in methanol solvent and stored at 4 °C, respectively. Working solutions were prepared daily by diluting the stock solution with ultra-pure water to 2 μg L−1 for all of these analytes. A serial of standard solution (0.312, 0.625, 1.25, 2.5, 5, 10 mg L−1) was directly injected into the HPLC system with a 20-μL sample loop to obtain the relationship between peak areas and analytes concentration (C SPME) for calculating enrichment factors. And 1 μg L−1 (C o) of aqueous solution was extracted to calculate the enrichment factors of analytes. Rain water and river water were collected locally as real samples. Before analysis, river water and rain water samples were filtered with a 0.45-μm nylon membrane filter.
Extraction conditions investigation
Several factors can influence the extraction efficiency, such as sampling rate, sample pH value, sample volume, extraction temperature, organic solvent content, desorption time, and so on. To achieve the best extraction efficiency of polyester fibers-in-tube extraction device for PAHs, main factors including sample pH value, sampling rate, sample volume, and methanol content were optimized.
Fibers-in-tube SPME-HPLC online system
The extraction tube device was directly mounted on the six-port valve of HPLC equipment via replacing the sample loop. A schematic diagram of the in-tube SPME-HPLC system is illustrated in Fig. 1c. Online SPME-HPLC method consisted of two steps, i.e., extraction and desorption processes. In extraction process, the six-port valve was set at load mode, sample solution was driven by sample pump (pump 2) with a certain flow rate through the polyester fibers-in-tube device. After extraction, the six-port valve was switched into injection mode for the desorption step. The mobile phase (acetonitrile-water or methanol-water) was flowed through the extraction PEEK tube with a flow rate of 1.0 mL min−1 (pump 1), eluting the analytes into the HPLC column for separation and further detection by DAD detector. After desorption, six-port valve was returned to load position, and the extraction process was carried out again. Gradient elution (A = acetonitrile, B = ultra-pure water; 0 min A = 70 %, B = 30 %; 10 min A = 70 %, B = 30 %; 20 min A = 100 %, B = 0 %; 17.5-min stop) was set for PAHs. Acetonitrile-water (50:50, v/v) was used for estrogens. Gradient elution (A = methanol, B = ultra-pure water; 0 min A = 80 %, B = 20 %; 5 min A = 80 %, B = 20 %; 15 min A = 100 %; 18-min stop) was used for phthalates. Gradient elution (A = acetonitrile, B = ultra-pure water; 0 min A = 70 %, B = 30 %; 5 min A = 70 %, B = 30 %; 15 min A = 100 %; 13 min stop) was used for alkyl benzenes and p-hydroxybenzoates. Methanol-water (70:30, v/v) and methanol-water (80:20, v/v) were used for phenols and anilines, respectively.
Results and discussion
Optimization of extraction conditions
Sample pH value
For in-tube SPME, changing the pH of the sample matrix could affect the extraction efficiency of a particular adsorbent. Various pH values in the range of 2–8 regulated with 10-mM phosphate buffers were investigated. As shown in Fig. 3a, the results demonstrated that pH values of solution have no obvious effect on the extraction performance of the polyester fibers. pH of working solution without regulation is about 7. So, the working solution was directly extracted without any pH regulation in the next investigations.
The effect of extraction conditions including effect of sample pH value (a), sampling rate (b), sample volume (c), methanol content (d), and desorption time (e) on extraction efficiency of the polyester fibers-in-tube device for PAHs. Conditions: sample pH value, 7; sampling rate, 1.50 mL min−1; sample volume, 40 mL; methanol content, 1 % (v/v); desorption time, 1.0 min; concentration of PAHs analytes, 2 μg L−1
Sampling rate
It is known that the required time to obtain extraction equilibrium is proportional to the length of the extraction tube and the volume of the coating, while it is inversely proportional to sampling rate [31, 32]. Sampling rate affects extraction efficiency and analysis time. Generally, high sampling rate is desirable for rapid analysis. However, in in-tube SPME, the sampling rate is limited by hindrance of sorbents inside the tube and mechanical strength of the tube. A relatively high sampling rate would lead to stripping of the sorbents, low extraction efficiency, and high pressure; but a relatively low sampling rate will prolong analysis time and reduce work efficiency. The high sampling pressure will bring damage to the instrument system as well.
In this experiment, sampling rate was investigated from 0.75 to 2.00 mL min−1 by extracting 40 mL of working solution. As shown in Fig. 3b, peak areas of all eight PAHs are almost unchanged with increasing sampling rate from 0.75 to 1.50 mL min−1, then peak areas of most analytes appear downward trend from 1.50 to 2.00 mL min−1. It seems that extraction efficiencies of PAHs are not affected much at low sampling rates, but cannot maintain with a high sampling rates. Sampling rate of 1.50 mL min−1 was selected for rapid analysis and satisfactory extraction efficiency.
Sample volume
For in-tube SPME, the extraction amount is closely associated with the volume of extracting phase and sample matrix. Extraction equilibrium volume is a minimum sample volume to obtain optimum extraction efficiency under the fixed sampling rate. The effect of sample volume on extraction efficiency was investigated from 10 to 50 mL with a sampling rate of 1.50 mL min−1. As can be seen in Fig. 3c, the peak areas of most PAHs increase rapidly along with the increase of the sample volume from 10 to 40 mL; there is no obvious change observed when sample volume increases more than 40 mL. The extraction equilibrium may be achieved at 40 mL for these analytes. Sample volume of 40 mL was selected for highest extraction efficiency.
Methanol content
Methanol was usually added into the sample solutions as a cosolvent, to increase the solubility of PAHs in aqueous solution. In SPME, excessive organic solvent will reduce extraction efficiency due to its influence on the adsorption of analytes on extraction materials. Under fixed sample volume (40 mL) and sampling rate (1.50 mL min−1), PAHs sample solutions containing different content methanol in the range of 0.5–5 % (v/v) were loaded into polyester fibers-in-tube extraction device for SPME. As can be seen in Fig. 3d, peak areas of most PAHs are found to increase with increasing methanol content from 0.5 to 1 %, and decrease when methanol content is further increased. In order to obtain satisfactory extraction efficiency and repeatability, methanol content in samples was selected as 1 % (v/v).
Desorption time
In SPME, desorption process is very important for extraction efficiency, since it must be sufficient to release all the analytes without damaging the extraction coating. The desorption profiles were investigated ranging from 0.2 to 2.0 min. As shown in Fig. 3e, the effect of desorption time on extraction efficiency is slightly improved in the range of 0.2–1.0 min. The desorption was almost complete when desorption time of 1.0 min was used. To protect extraction coating and avoid any possible peak broadening, desorption time was set as 1.0 min for all tests.
Method evaluation
Based on the above-mentioned optimization process, a sampling rate of 1.50 mL min−1, sample volume of 40 mL, methanol content of 1 % (v/v) and desorption time of 1.0 min were chosen as the optimum conditions. The polyester fibers-in-tube SPME-HPLC method, including linear range, correlation coefficients (r), intra-day and inter-day precision, limits of detection (LODs), and limits of quantification (LOQs) were investigated. As outlined in Table 1, linear ranges are 0.03–80 μg L−1 for Nap, Any, Flu, Phe, Ant, and Pyr; 0.1–80 μg L−1 for Ana and Flt with correlation coefficients (r) ranging from 0.9978 to 0.9997. LODs were defined as a concentration of the analytes with a signal-to-noise ratio (S/N) of 3 and are 0.01–0.03 μg L−1. LOQs were defined as a concentration of the analytes with a signal-to-noise ratio (S/N) of 10 and are 0.03–0.1 μg L−1. Method precision was assessed by reduplicate extraction and analysis (n = 5) of standard solutions (2 μg L−1) on the same and different days. The intra-day and inter-day relative standard deviation (RSD) for quantitative analysis were lower than 5.8 and 6.9 %, respectively. Extraction repeatability was also investigated by extracting standard PAHs aqueous solution spiked at 2 μg L−1 three times, and its RSD is in the range of 3.8–7.8 %. Good precision, as well as low LODs and LOQs indicated that the extraction device can provide good extraction repeatability and ensure the accuracy of the experiment.
In order to further evaluate extraction efficiency of the polyester fibers-in-tube, the enrichment factors (F E) for PAHs were investigated. The enrichment factors of model analytes were calculated by the ratio of analytes concentrations after and before extraction (F E = C SPME/C o, C o = 1 μg L−1). As shown in Table 1, the polyester fibers-in-tube extraction device exhibits high extraction capability for PAHs with enrichment factors ranging from 307 to 1646.
Sensitivity and enrichment factors of this method for PAHs analytes were compared with that of other reported methods. As can be seen from Table 2, the sensitivity of this method is obviously superior to the in-tube SPME-HPLC methods using stainless steel sample loop [31] and zeolitic imidazolate framework-8 polydopamine PEEK tube [33]. The better sensitivity of this method is attributed to higher enrichment capacity of more fiber sorbent of fiber-in-tube SPME than in-tube SPME. This method also provides lower or comparable LODs for PAHs than other methods, based on some sorbents including CP-Sil 19CB capillary column [34], SE-54 capillary column [35], sol-gel-based copper tube device [36], and metal-organic hybrid gels capillary [37]. It results from good extraction performance of the polyester fibers to PAHs.
Application to real samples
PAHs can effect on organisms with the potential carcinogenic, which is widely distributed in environment including water, soil, and air. In this work, the polyester fibers-in-tube SPME-HPLC method was used to the determination of PAHs analytes in rain water and river water samples. Chromatograms of two samples are shown in Fig. 4, and simultaneous analysis of eight PAHs could be performed in a single run through the polyester fibers-in-tube SPME-HPLC method. The peaks were identified according to the retention time of each standard compound. The analysis results are listed in Tables 3 and 4. As can be seen from Fig. 4a, Any, Ana, Phe, and Ant are detected in rain water, their concentration ranges from 0.15 to 0.32 μg L−1. The relative recoveries spiked at 2 and 10 μg L−1 in river water are in the range of 95.5–118.3 and 93.8–107.4 %, respectively. As shown in Fig. 4b, no analytes are detected in river water. The relative recoveries spiked at 2 and 10 μg L−1 in river water samples are in the range of 93.1–113.4 and 91.3–105.7 %, respectively. These results indicated the polyester fibers-in-tube SPME-HPLC method was an efficient online analysis method to PAHs analytes in samples.
HPLC chromatograms of PAHs in rain water sample (a) and river water sample (b) extracted using the polyester fibers-in-tube device and detected at 230 nm. Peaks: 1 Nap, 2 Any, 3 Ana, 4 Flu, 5 Phe, 6 Ant, 7 Flt, and 8 Pyr. Extraction conditions are the same as in Fig. 4
Extraction selectivity
Extraction selectivity of the polyester fibers-in-tube device was investigated by several different types of compounds including PAHs, estrogens, phthalates, alkyl benzenes, p-hydroxybenzoates, anilines, and phenols. The enrichment factors of PAHs have been listed out in Table 1, enrichment factors of the rest of the six types of compounds are shown in Table 3. Enrichment factors are in the range of 33–484 for five estrogens, enrichment factors are in the range of 0–509 for seven phthalates, enrichment factors are 0 for all five phenols, enrichment factors are in the range of 0–378.6 for five anilines, enrichment factors are in the range of 195.4–1065.3 for alkyl benzenes, and enrichment factors are in the range of 0–208.3 for p-hydroxybenzoates.
Chromatograms of different types of compounds before and after extraction on the polyester fibers-in-tube are shown in Fig. 5. As can be seen from Fig. 5a, the PAHs analytes cannot be detected for direct injection of a sample spiked at 2 μg L−1, but after the extraction, chromatographic peaks become very obvious. It indicated that the polyester fibers-in-tube possessed excellent extraction ability for PAHs. As shown in Fig. 5b, estrogens including bisphenol A, ethinylestradiol, estrone, diethylstilbestrol, and hexestrol were all extracted on the extraction device. Compared with PAHs, the extraction capability of polyester fibers for estrogens was significantly decreased. It may result from their hydrophobic property weaker than PAHs. As shown in Fig. 5c, more hydrophobic phthalates including benzyl butyl, di-n-butyl, di-n-pentyl, and dicyclohexyl phthalates are extracted on the extraction tube, but less hydrophobic phthalates including dimethyl, diethyl, and diallyl phthalates almost not be extracted. As shown in Figs. 5d, e, except for the weak extraction for N-ethylaniline and diphenylamine, other anilines and phenols cannot be enriched. As shown in Fig. 5f, the polyester fibers exhibit stronger extraction ability for hydrophobic alkyl benzene than hydrophilic p-hydroxybenzoates. It is further explained that the polyester fibers have a greater ability to extract hydrophobic analytes.
HPLC chromatograms of extraction selectivity investigation of the polyester fibers-in-tube device. a PAHs sample detected at 230 nm, peaks: 1 Nap, 2 Acy, 3 Ana, 4 Flu, 5 Phe, 6 Ant, 7 Flt, and 8 Pyr. b Estrogens sample detected at 202 nm, peaks: 1 bisphenol A, 2 ethinylestradiol, 3 estrone, 4 diethylstilbestrol, and 5 hexestrol. c Phthalates sample detected at 210 nm, peaks: 1 dimethyl phthalate, 2 diethyl phthalate, 3 diallyl phthalate, 4 benzyl butyl phthalate, 5 di-n-butyl phthalate, 6 di-n-pentyl phthalate, and 7 dicyclohexyl phthalate. d Phenols sample detected at 254 nm, peaks: 1 p-aminophenol, 2 phenol, 3 o-nitrophenol, 4 2,6-dimethyl phenol, and 5 1-naphthol. e Anilines sample detected at 254 nm, peaks: 1 aniline, 2 4-methyl aniline, 3 2,6-dimethylaniline, 4 N-ethylaniline, and 5 diphenylamine. f Alkyl benzenes and p-hydroxybenzoates sample detected at 210 nm, peaks: 1 ethyl 4-hydroxybenzoate, 2 propyl p-hydroxybenzoate, 3 butyl paraben, 4 methylbenzene, 5 ethylbenzene, 6 N-propylbenzene, and 7 N-butylbenzene
On the basis of the above-mentioned results, the polyester fibers-in-tube SPME device showed strongest extraction selectivity for PAHs due to the hydrophobic interaction, π-π stacking, and hydrogen bond between polyester molecule and electron-rich PAHs [31]. The extraction device presented weak extraction ability for estrogens, phthalates, alkyl benzenes, and p-hydroxybenzoates, and it has almost no enrichment ability for hydrophilic phenols and anilines. The results indicated its extraction selectivity for electron-rich or strong hydrophobic analytes.
Durability
Durability is essential for practical application of a SPME material. Damage of the adsorbent of in-tube SPME was mainly caused by its exposure to high temperature, high pressure, organic solvent, and acidic or basic solution during consecutive extractions [38]. PEEK tube is an ideal substrate of in-tube SPME with good resistance to high pressure. Polyester fibers in PEEK tube show excellent stability, and the extraction capacity is still maintained after more than 100 times of extraction and desorption process. The extraction efficiency of the polyester fibers-in-tube was investigated at different extraction times. A 3D bar diagrams can be seen in Fig. 6; the peak areas of eight PAHs had no obvious change within 180 times according to the results at 1st, 60th, 120th, and 180th. It is obvious that the polyester fibers-in-PEEK tube was a durable SPME device.
Conclusions
In this work, a facile and efficient poly(ethylene terephthalate) fibers-in-tube for online solid-phase microextraction towards polycyclic aromatic hydrocarbons was developed. The form of fiber-in-tube extraction device not only overcomes the defect of larger dead volume of open tube, but also increases the sorbent to improve extraction efficiency. The polyester fibers-in-tube device possesses the advantages of easy preparation, low-cost, high extraction efficiency, high selectivity, durability, and environmental friendliness. The method was established under the optimized conditions, and it provided low LODs and LOQs, good extraction repeatability and wide linear ranges, and good intra-day and inter-day precisions. So, the poly(ethylene terephthalate) fibers-in-tube SPME extraction device has potential application to determination of polycyclic aromatic hydrocarbons.
References
Belardi RP, Pawliszyn J. Water Pollut Res J Can. 1989;24:179–91.
Arthur CL, Pawliszyn J. Anal Chem. 1990;62:2145–8.
Wu J, Mester Z, Pawliszyn J. Anal Chim Acta. 2000;424:211–22.
Ahmadi SH, Manbohi A, Heydar KT. Anal Chim Acta. 2015;853:335–41.
Mei M, Yu J, Huang X, Li H, Lin L, Yuan D. J Chromatogr A. 2015;1385:12–9.
Kima TY, Alhooshania K, Kabira A, Friesb D-P, Malik A. J Chromatogr A. 2004;1047:165–74.
Fan Y, Feng Y-Q, Zhang J-T, Da S-L, Zhang M. J Chromatogr A. 2005;1074:9–16.
Pawliszyn J. Wiley-VCH, New York, 1997.
Eisert R, Pawliszyn J. Anal Chem. 1997;69:3140–7.
Wen Y, Zhou B-S, Xu Y, Jin S-W, Feng Y-Q. J Chromatogr A. 2006;1133:21–8.
Kataoka H, Lord HL, Pawliszyn J. J Chromatogr A. 2000;880:35–62.
Campíns-Falc P, Verdú-Andrés J, Sevillano-Cabeza A, Herráez-Hernández R, Molins-Legua C, Moliner-Martinez Y. J Chromatogr A. 2010;1217:2695–702.
Ahmadi F, Assadi Y, Milani Hosseini SMR, Rezaee M. J Chromatogr A. 2006;1101:307–12.
Chafer-Pericás C, Herraez-Hernández R, Campíns-Falcó P. J Chromatogr A. 2006;1125:159–71.
Fan Y, Feng YQ, Shi ZG, Wang JB. Anal Chim Acta. 2005;543:1–8.
Kataoka H, Ishizaki A, Nonaka Y, Saito K. Anal Chim Acta. 2009;655:8–29.
Kataoka H. Anal Bioanal Chem. 2002;373:31–45.
Lord HL. J Chromatogr A. 2007;1152:2–13.
Kataoka H. Curr Pharm Anal. 2005;1:65–84.
Sun M, Feng J, Bu Y, Luo C. J Chromatogr A. 2015;1408:41–8.
Saito Y, Kawazoe M, Hayashida M, Jinno K. Analyst. 2000;125:807–9.
Liu X-Y, Ji Y-S, Zhang H-X, Liu M-C. J Chromatogr A. 2008;1212:10–5.
Li J, Su Q, Li K-Y, Sun C-F, Zhang W-B. Food Chem. 2013;141:3714–20.
Sun M, Feng J, Bu Y, Luo C. J Sep Sci. 2014;37:3691–8.
Yan X, Wu D, Peng H, Ding K, Duan C, Guan Y. J Chromatogr A. 2012;1244:69–76.
Chen B, Hu B, He M, Mao X, Zu W. J Chromatogr A. 2012;1227:19–28.
Wu S, Ye Q, Li N. Constr Build Mater. 2008;22:2111–5.
Tayfura S, Ozenb H, Aksoy A. Constr Build Mater. 2007;21(2):328–37.
Tang WC, Lo Y, Nadeem A. Cement Concrete Comp. 2008;30:403–9.
Xiao J, Falkner H. Fire Safety J. 2006;41:115–21.
Bagheri H, Piri-Moghadam H, Es’haghi A. J Chromatogr A. 2011;1218:3952–7.
Lord H, Pawliszyn J. J Chromatogr A. 2000;885:153–93.
Zhang J, Zhang W, Bao T, Chen Z. J Chromatogr A. 2015;1388:9–16.
Ishizaki A, Saito K, Hanioka N, Narimatsu S, Kataoka H. J Chromatogr A. 2010;1217:5555–63.
Campíns-Falcó P, Verdú-Andrés J, Sevillano-Cabeza A, Molins-Legua C, Herráez-Hernández R. J Chromatogr A. 2008;1211:13–21.
Hu Y, Fan Y, Huang Z, Song C, Li G. Chem Commun. 2012;48:3966–8.
Zhang W, Zhang Z, Meng J, Zhou W, Chen Z. J Chromatogr A. 2014;1365:19–28.
Sun M, Feng J, Qiu H, Fan L, Li L, Luo C. J Chromatogr A. 2013;1300:173–9.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC, nos. 21205048 and 21405061), the Shandong Provincial Natural Science Foundation of China (no. ZR2014BQ019) and the Graduate Innovation Foundation of University of Jinan (GIFUJN, no. YCXS15007).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 110 kb)
Rights and permissions
About this article
Cite this article
Bu, Y., Feng, J., Sun, M. et al. Facile and efficient poly(ethylene terephthalate) fibers-in-tube for online solid-phase microextraction towards polycyclic aromatic hydrocarbons. Anal Bioanal Chem 408, 4871–4882 (2016). https://doi.org/10.1007/s00216-016-9567-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9567-z