Abstract
Two new Standard Reference Materials (SRMs), SRM 2786 Fine Particulate Matter (<4 μm) and SRM 2787 Fine Particulate Matter (<10 μm) have been developed in support of the US Environmental Protection Agency’s National Ambient Air Quality Standards for particulate matter (PM). These materials have been characterized for the mass fractions of selected polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs, brominated diphenyl ether (BDE) congeners, hexabromocyclododecane (HBCD) isomers, sugars, polychlorinated dibenzo-p-dioxin (PCDD) and dibenzofuran (PCDF) congeners, and inorganic constituents, as well as particle-size characteristics. These materials are the first Certified Reference Materials available to support measurements of both organic and inorganic constituents in fine PM. In addition, values for PAHs are available for RM 8785 Air Particulate Matter on Filter Media. As such, these SRMs will be useful as quality control samples for ensuring compatibility of results among PM monitoring studies and will fill a void to assess the accuracy of analytical methods used in these studies.

Removal of PM from filter for the preparation of SRM 2786 Fine Particulate Matter
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Air pollution is composed of particulate matter (PM) often described in terms of the aerodynamic diameter of the particles with PM10 having an aerodynamic diameter <10 μm, PM2.5 having an aerodynamic diameter < 2.5 μm, and ultrafine particles having an aerodynamic diameter <0.1 μm. Sources of air pollution include traffic, fossil fuel combustion activities [1], harbor activities [2], wood processing, spray painting, car washing [3], agricultural activities [4], as well as many other sources. Fine particle pollution can be emitted directly or formed secondarily in the atmosphere [5]. The chemical composition of PM is very diverse including organic compounds, biological compounds, and metals [6].
Exposure to PM has been linked to a wide variety of diseases, including respiratory and cardiovascular problems [6, 7]. In response, the US Environmental Protection Agency (EPA) developed strict National Ambient Air Quality Standards for PM [8] along with establishing monitoring programs through its PM2.5 National Chemical Speciation Network and, more recently, the Interagency Monitoring of Protected Visual Environments (IMPROVE) [9].
In 2000, a working group of PM investigators from the US EPA’s research programs (PM2.5 Organic Speciation Working Group) was established to improve the quality and comparability of data on the organic composition of aerosols [10]. The goal of the working group was to improve the characterization and quantification of organic compounds associated with PM through participation in interlaboratory comparison exercises and to provide input for the development of appropriate Standard Reference Materials (SRMs). The National Institute of Standards and Technology (NIST) coordinated a series of three interlaboratory trials for the working group and conducted workshops to discuss the results of the trials and to assess the need for SRMs [10, 11].
The initial interlaboratory trial utilized PM from a bulk portion of SRM 1649a Urban Dust which had been sieved to < 63 μm and an extract of these particles. SRM 1649 was collected in Washington, DC and issued in 1982. The reissue, SRM 1649a, composed of the same bulk material as SRM 1649, was released in 2000. Both SRM 1649 and SRM 1649a were sieved to < 123 μm when prepared. The second interlaboratory trial used a PM2.5 collected in Baltimore, MD. Trial III included three samples: SRM 1648 Urban Particulate Matter, a second PM2.5 sample collected in Baltimore, MD, and Reference Material (RM) 8785 Air Particulate Matter on Filter Media, composed of a fine fraction (nominally < 2.5 μm) of SRM 1649a on quartz-fiber filters [12]. Participating laboratories were not constrained by a specific analytical method and reported data on those organic analytes that were typically measured in their laboratories, including alkanes, alkenes, aromatic and polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs, sterols, ketones, aldehydes, acids, phenols, and sugars. The results from these trials are summarized in two reports [10, 11].
An outcome of these studies and the associated workshops was the need for additional SRMs to support PM research. The proposed SRMs included calibration solutions for several classes of compounds, including nitrated PAHs, hopanes, steranes, and sugars, and additional PM samples, specifically having particles of smaller aerodynamic diameter. Several new calibration solutions were developed including SRM 2264 Nitrated Aromatic Hydrocarbons in Methylene Chloride I, SRM 2265 Nitrated PAHs in Methylene Chloride II, SRM 2266 Hopanes and Steranes in 2,2,4-Trimethylpentane, SRM 2267 Deuterated Levoglucosan in Ethyl Acetate, and SRM 2268 Carbon-13 Labeled Levoglucosan in Ethyl Acetate. In addition, two new PM SRMs were developed: SRM 2786 Fine Particulate Matter (<4 μm) and SRM 2787 Fine Particulate Matter (<10 μm).
SRMs 2786 and 2787 were issued in 2011 as the first Certified Reference Materials (CRMs) for fine particulate matter. These two SRMs have been extensively characterized for PAHs, nitrated PAHs, brominated diphenyl ether (BDE) congeners, inorganic constituents, sugars, polychlorinated dibenzo-p-dioxin (PCDD) congeners, polychlorinated dibenzofuran (PCDF) congeners, and three hexabromocyclododecane (HBCD) isomers. Two additional CRMs for fine particulate matter have been developed by the Institute for Reference Materials and Measurements (IRMM) (Geel, Belgium): ERM CZ100 with values assigned for PAHs and ERM CZ120 with values assigned for toxic elements (As, Cd, Pb, and Ni). Both of these materials are titled “Fine Dust (PM10-Like)” and were produced from total suspended particulate matter (TSP). The preparation and certification measurements for SRMs 2786 and 2787 for selected organic and inorganic constituents are summarized in this paper.
Experimental
Sample collection and preparation
SRM 2786 and SRM 2787 were prepared from atmospheric PM collected in 2005 from an air intake filtration system of a major exhibition center in Prague, Czech Republic. While the sample is not intended to be representative of the area from which it was collected, it should typify atmospheric PM obtained from an urban area. The PM was removed from the reusable surface filters and sent to NIST. A particle suspension unit and ultra-high-volume sampler (UHVS) [13] were used to resuspend the total suspended particulate matter, and the face velocity of the cyclone in the UHVS was adjusted to control the particle size collected on Teflon membrane filters. The unit was adjusted such that larger particles (> PM10) were selectively removed from the cyclonic stream, leaving the PM < 4 μm fraction to be collected on filters. The size-fractionated PM was then brushed from the filters or collected from the bottom of the cyclone and stored in clean amber bottles with a Teflon-lined cap. The bottles containing the material were rolled for 1 h, after which the material was aliquoted into clean amber 4 mL bottles with Teflon-lined caps. Each bottle contains between 100 mg and 140 mg of material.
Conversion to dry-mass basis
The results for the constituents in SRM 2786 and SRM 2787 are reported on a dry-mass basis; the material “as received” contains residual moisture. The amount of moisture in the materials was determined by measuring the mass loss after freeze-drying test portions of 100 mg to 140 mg for 5 days at 1 Pa with a –20 °C shelf temperature and a –50 °C condenser temperature. The mass fraction of moisture in SRM 2786 at the time of certification analyses was 1.7 % ± 0.5 % (expanded uncertainty) at the 95 % confidence level, and the mass fraction of moisture in SRM 2787 at the time of certification analyses was 1.2 % ± 0.3 % (expanded uncertainty) at the 95 % confidence level.
PAHs, nitrated PAHs, and BDEs
The approach used for the value assignment of the PAHs, nitrated PAHs, and BDEs in SRM 2786 and SRM 2787 was similar to that reported for the recent certification of several environmental-matrix SRMs [14] and consisted of combining results from analyses using various combinations of different extraction techniques, cleanup/isolation procedures, and chromatographic separation and detection techniques. See Electronic Supplementary Material (ESM) Table S1 for details on the methods used for the PAHs, nitrated PAHs, and BDEs.
Five sets of gas chromatography/mass spectrometry (GC/MS) results were obtained at NIST. For the first GC/MS analyses, duplicate test portions of between 10 mg and 30 mg from six bottles of SRM 2786 or SRM 2787 were extracted using pressurized liquid extraction (PLE) at 150 °C with toluene. The extract was fractionated using an aminopropyl solid-phase extraction (SPE) column to isolate the fraction of interest. The processed extract was then analyzed by GC/MS using a 0.25 mm i.d. × 60 m fused silica capillary column with a 50 % phenyl methylpolysiloxane phase (0.25 μm film thickness; DB-17, Agilent Technologies, Wilmington, DE) and a 0.25 mm i.d. × 15 m fused silica capillary column with a 50 % liquid crystal polysiloxane phase (0.15 μm film thickness; LC-50, J&K Scientific, Milton, Ontario, Canada). The PAHs were determined on the DB-17 column using electron impact MS (EI-MS). The PAHs were also determined on the LC-50 column using EI-MS. The nitrated PAHs and BDEs were determined on the LC-50 column using negative chemical ionization MS (NCI-MS).
An additional method for the determination of the PAHs used one test portion (100 mg) from each of six bottles extracted using PLE at 100 °C with dichloromethane (DCM). Size exclusion chromatography (SEC) on a divinylbenzene-polystyrene column (10 μm particle size, 10 nm (100 angstrom) pore size, 7.5 mm i.d. × 300 mm, PL-Gel, Polymer Labs, Inc., Amherst, MA) was used. The fraction of interest was further isolated using an alumina (5 % deactivated) SPE column. The isolated fraction was then analyzed by GC/MS using a 0.25 mm i.d. × 60 m fused silica capillary column with a DB-17 MS phase (0.25 μm film thickness; Agilent Technologies).
A third set of GC/MS analyses focused on the BDEs. Six test portions (between 100 mg and 140 mg) were extracted using PLE at 100 °C with DCM. The extracts were cleaned up using an alumina (5 % deactivated) SPE column. SEC on a divinylbenzene-polystyrene column (10 μm particle size, 10 nm (100 angstrom) pore size, 7.5 mm i.d. × 300 mm, PL-Gel, Polymer Labs, Inc.) was then used. This step was followed by an acidified silica SPE column step. Two fractions were collected from the acidified silica column. The BDEs were quantified using GC/EI-MS on a 0.18 mm i.d. × 30 m fused silica capillary column with a 5 % phenyl methylpolysiloxane phase (0.18 μm film thickness; DB-5MS, Agilent Technologies). The BDEs were also quantified using GC/NCI-MS on a 0.18 mm i.d. × 10 m fused silica capillary column with a DB-5 MS phase (0.18 μm film thickness; Agilent Technologies).
For the fourth GC/MS method, duplicate test portions of between 10 mg and 30 mg from three bottles of SRM 2786 and SRM 2787 were extracted using PLE at 100 °C (for one of the test portions from each bottle) or at 150 °C (for the other test portion from each bottle) with toluene. The extract was fractionated using an aminopropyl SPE column to isolate the fraction of interest. The processed extract was then analyzed by GC/MS using a 0.25 mm i.d. × 60 m fused silica capillary column with a proprietary non-polar phase (0.25 μm film thickness; DB-XLB, Agilent Technologies) and a 0.25 mm i.d. × 30 m fused silica capillary column with a DB-17MS phase (0.25 μm film thickness; Agilent Technologies). The PAHs were determined on the DB-XLB column using EI-MS. The nitrated PAHs were determined on the DB-17MS column using NCI-MS.
Duplicate test portions of 50 mg from three bottles of SRM 2786 and SRM 2787 were extracted using PLE at 100 °C with DCM for an additional GC/MS determination of the PAHs. The fraction of interest was further isolated using an alumina (5 % deactivated) SPE column. The isolated fraction was then analyzed by GC/MS using a 0.25 mm i.d. × 60 m fused silica capillary column with a DB-17MS phase (0.25 μm film thickness; Agilent Technologies).
For the methods described above, selected perdeuterated PAHs, perdeuterated nitrated PAHs, and fluorinated and 13C-labeled BDEs were added to the PM prior to solvent extraction for use as internal standards for quantification purposes. The reference mass fraction values for selected PAHs (biphenyl, dibenzothiophene, phenanthrene, anthracene, 1-methylphenanthrene, 2-methylphenanthrene, 3-methylphenanthrene, 9-methylphenanthrene, 1,7-dimethylphenanthrene, and retene) were significantly higher in the extracts obtained using PLE at 150 °C compared to those obtained using PLE at 100 °C.
HBCDs
The second fraction from the acidified silica SPE clean-up in the third GC/MS method above was analyzed by liquid chromatography with tandem mass spectrometry (LC/MS/MS) for the HBCDs using both electrospray ionization (ESI) and atmospheric pressurized photoionization (APPI). A C18 column (3.0 mm × 150 mm × 3.5 μm column, Eclipse Plus, Agilent Technologies) was used with a solvent gradient using 2.5 mmol/L ammonium acetate in 12.5 % water in methanol (volume fraction) and acetonitrile at a flow rate of 0.3 mL/min. 13C-labeled HBCDs were added to the PM prior to solvent extraction for use as internal standards for quantification purposes.
Inorganic constituents other than mercury
Value assignment of the mass fractions of Cd and Pb were performed using isotope dilution-inductively coupled plasma mass spectrometry (ID-ICP-MS) [15]. Two 50 mg samples taken from each of four bottles of SRM 2786 and SRM 2787 were spiked with enriched 111Cd and 206Pb and subjected to microwave digestion using 1 g of concentrated hydrofluoric acid and 9 g of concentrated nitric acid. For Pb, a portion of the digested samples was removed, heated to near-dryness, and redissolved in 2 % (volume fraction) nitric acid prior to ICP-MS analysis. For Cd, the remaining digested sample portions were subjected to anion exchange chromatography to separate Cd from the matrix prior to ICP-MS analysis.
Value assignment of the mass fractions of additional inorganic constituents is based on instrumental neutron activation analysis (INAA) and inductively coupled plasma optical emission spectrometry (ICP-OES). For INAA, direct analysis of pellets formed from duplicate test portions (median sample size of 1.3 mg) from each of 12 randomly selected bottles of SRM 2786 and SRM 2787 was achieved by the comparator technique with established multielement standards. Two sets of irradiations were carried out in the RT-1 pneumatic facility of the NIST Center for Neutron Research (NCNR). The irradiations for short-lived nuclides were done for 300 s. Irradiation time was (6 + 6) h with a 180 degree inversion of the irradiation capsule after the first 6 h for flux homogeneity. For the gamma-ray assay of short-lived nuclides, measurements were done 10 min after 8 min decay. Standards were counted either before or after the samples for 5 min or 10 min respectively. For the long irradiations, the first count was 2 h after 3 d decay, the second count was 12 h after at least 25 d decay with counts of the standards following the sample counts.
For ICP-OES, one 50 mg sample was taken from each of seven bottles of SRM 2786 and SRM 2787. The samples were microwave-digested using 1 mL of concentrated hydrofluoric acid and 9 mL of concentrated nitric acid. Following subsequent dilutions, the samples in 2 % (volume fraction) nitric acid were analyzed by ICP-OES using optimized parameters for each element.
Mercury
Value assignment of the mass fraction of Hg is based on isotope dilution cold-vapor ICP-MS [16–18]. Single subsamples (40 mg to 70 mg) were taken from each of four bottles and spiked with 201 Hg followed by microwave digestion.
Sugars
Value assignment of the mass fractions of sugars is based on the analysis of three samples (30 mg) of SRM 2786 and SRM 2787 at Texas A&M University [19]. The samples were extracted using PLE at 100 °C with DCM:methanol (9:1, volume fractions). The extracts were dried and redissolved in pyridine and then derivatized using N,O-bis-trifluoroacetamide (BSTFA) containing 1 % (volume fraction) trimethylchlorosilane (TMCS) at 75 °C for 1 h. The sugars were quantified using GC/EI-MS on a 0.25 mm i.d. × 30 m fused silica capillary column with a DB-5 phase (0.25 μm film thickness, Agilent Technologies).
Polychlorinated dibenzo-p-dioxins and dibenzofurans
Value assignment of the mass fractions of the 17 2,3,7,8-substituted polychlorinated dibenzo-p-dioxin and dibenzofuran congeners and the total tetra- through hepta-substituted polychlorinated dibenzo-p-dioxins and dibenzofurans is based on the analysis of four test portions (between 100 mg and 150 mg) of SRM 2786 and SRM 2787 by Environment Canada. Samples were Soxhlet-extracted overnight with toluene. Extracts were concentrated and solvent exchanged to hexane, then passed through a modified silica column followed by a basic alumina column. The dioxin/furan fraction was analyzed by using GC with high-resolution mass spectrometric detection (GC/HRMS) and a 0.25 mm i.d. × 60 m fused silica capillary column with a DB-5 phase (0.25 μm film thickness). The 2,3,7,8-tetrachlorodibenzofuran was quantified using a 50 % cyanopropylphenyl-substituted methylpolysiloxane (DB-225, Agilent Technologies) capillary column.
Particle-size information
Particle size distribution measurements for SRM 2786 and SRM 2787 were carried out using a laser diffraction instrument (Mastersizer 2000, Malvern Instruments, Southborough, MA) and the liquid suspension method with the instrument manufacturer’s small-volume sample dispersion unit (Hydro 2000 SM). A suspension of 0.1 % (mass fraction) of the PM in distilled water with 0.001 % Triton (volume fraction), was prepared by ultrasonication for 1 h. A measurement sequence of background and sample measurement was used. After the recording of the background, a portion of the suspension was added to the measurement cell to achieve an obscuration of 5 %. Three passes of the sample solution were recorded and averaged. A refractive index of 1.5 and absorption index of 0.1 were selected for the measurements. Results were calculated using the General Purpose Model provided by the instrument manufacturer.
Analysis of RM 8785 filters for PAHs
The individual filters of RM 8785 were placed directly in PLE cells that were then filled with hydromatrix and extracted using PLE at 100 °C with DCM as the solvent. The extracts were concentrated and fractionated using aminopropyl SPE columns to isolate the fractions of interest. The isolated fractions were then analyzed by GC/MS using a 0.25 mm i.d. × 60 m fused silica capillary column with a DB-17MS phase (0.25 μm film thickness; Agilent Technologies).
Results and discussion
The goal in preparing SRM 2786 and SRM 2787 was to provide more contemporary PM reference materials with smaller particle sizes. NIST attempted to perform a direct collection of PM2.5 from the Baltimore, MD airshed over multiple years with a yield of only gram-quantity samples. As an alternate approach to a direct collection of PM2.5, NIST began exploring the suspension of existing air particulate samples with the collection and processing of fractions of the particulate matter. The resuspension method was first successfully attempted using two SRMs that were available at the time, SRM 1648a Urban Particulate Matter and SRM 1649b Urban Dust, followed by the resuspension of the PM collected in Prague, Czech Republic for SRM 2786 and SRM 2787.
SRM 1648a and SRM 1649b were collected in the mid-1970s and sieved to < 53 μm and < 63 μm, respectively. Historically, SRM 1648a has been used primarily for the evaluation of methods used in inorganic analysis, and SRM 1649b has been used primarily for the evaluation of methods used in organic analysis although both materials have been characterized over the years for additional classes of analytes, organic and inorganic, respectively [14].
Comparing the particle size of the four materials, the shape parameters from the particle-size distributions indicating mean particle diameters are 5.85 μm for SRM 1648a, 10.5 μm for SRM 1649b, 2.8 μm for SRM 2786, and 8.3 μm for SRM 2787 and the particle sizes below which 90 % of the volume is present are 30.1 μm for SRM 1648a, 43.2 μm for SRM 1649b, 6.9 μm for SRM 2786, and 23.9 μm for SRM 2787. The mean particle size in SRM 1648a is smaller than that of SRM 2787; however, 90 % of the particles in SRM 2787 are below 23.9 μm compared to 30.1 μm for SRM 1648a. The particle size distributions for SRM 2786 and 2787 are shown in Fig. 1.
Since the development of the first natural-matrix SRMs for organic contaminants at NIST over 30 years ago, the assignment of certified mass fraction values has been based on the combination of results from two or more independent methods [20]. Currently, NIST SRMs for chemical composition are assigned mass fraction values designated as certified, reference, or information values based on the number and independence of the analytical methods used, source of the results, and degree of confidence in the accuracy of the assigned value [14, 20].
Tables 1, 2, 3, and 4 summarize the certified, reference, and information mass fraction values for selected PAHs, nitrated PAHs, BDE congeners, and inorganic constituents, respectively, in SRM 2786 and SRM 2787. In addition to the values listed in Tables 1 through 4, there are reference mass fractions for selected PCDD and PCDF congeners, for the total tetra-, penta-, hexa-, and hepta-substituted congeners of PCDD and PCDF, and for levoglucosan, mannosan, and glactosan [19] along with information values for three HBCD isomers included on the Certificates of Analysis for SRM 2786 and SRM 2787. The individual method data for all of the organic analytes listed on the Certificates of Analysis are summarized in ESM Tables S2 and S3 for SRM 2786 and SRM 2787, respectively, and the individual method data for the inorganic constituents characterized in the two SRMs are summarized in ESM Table S4.
As mentioned in the Experimental Section, the mass fractions of PAHs in these materials were determined using between one and six analytical methods all based on PLE with either DCM or toluene as the extraction solvent and extraction temperatures between 100 °C and 150 °C followed by analysis using GC/MS with one of three column phases, DB-17MS, DB-XLB, or LC-50. The mass fractions of nitrated PAHs (Table 2) were determined using between one and three analytical methods also based on PLE with toluene as the extraction solvent and extraction temperatures between 100 °C and 150 °C followed by analysis using GC/MS with either a DB-17MS or LC-50 column. Recent studies [24, 25] on solvent extraction of other air particulate matter SRMs have shown that using PLE at 200 °C removes higher quantities than using PLE at 100 °C or 150 °C for some PAHs and nitrated PAHs. As a result of these studies, SRM 2786 and SRM 2787 were reanalyzed for determination of PAHs using PLE at 200 °C, and no statistically significant differences were found.
The selection of the three GC columns, DB-17MS, DB-XLB, and LC-50, used for the analysis of the PAHs is based on the unique properties of each column to separate certain isomers of the PAHs. These characteristics have been discussed by Poster et al. [26]. Using the molecular mass 228 isomers as an example, chrysene and triphenylene are baseline separated on the DB-XLB column and the LC-50 column but not on the DB-17MS column. The DB-17MS and LC-50 columns are used for the nitrated PAHs due to their ability to separate 2-nitrofluroanthene from 3-nitrofluoranthene [27]. The use of two or more columns to separate isomers provides more confidence that there are no underlying interferences.
As mentioned in the introduction, RM 8785 Air Particulate Matter on Filter Media was used in the third interlaboratory study for the PM2.5 Organic Speciation Working Group. This RM, which consists of a fine fraction, particle sizes nominally < 2.5 μm, of SRM 1649a deposited on quartz-fiber filters (nominally 1 mg of particulate matter on each filter), was developed for the evaluation of analytical methods used to characterize the carbon composition of atmospheric fine PM. The preparation and characterization of RM 8785 is described by Klouda et al. [12]. Prior to using it in the third interlaboratory study, the mass fractions of selected PAHs and nitrated PAHs were determined on whole (n = 16) and partial (n = 16) filters to evaluate the homogeneity of the RM for PAH measurements. The overall relative standard deviations (RSDs) for measurements of the PAHs ranged from 3 % to 11 % with no significant differences in the RSDs for analysis of the whole filters and the partial filters (see ESM Fig. S1). Comparing the mass fractions determined for selected PAHs and nitrated PAHs for the whole filters to the mass fractions of those compounds in SRM 1649a (Table 5), the mass fractions are generally higher (between 3 % and 85 % with the majority < 25 %) in the fine fraction deposited on the filters (RM 8785). The two exceptions are benz[a]anthracene and benzo[b]fluoranthene which have lower mass fractions, 13 % and 10 %, respectively, in RM 8785 than in SRM 1649a. The data in Table 5 will be useful for investigators who need a filter material for use as a control material for PAH analyses in PM.
The BDE values (Table 3) were based on one to three sets of data using PLE with DCM or toluene as the extraction solvent and extraction temperatures between 100 °C and 150 °C and analysis using GC/MS with either a 10 m DB-5 column, 30 m DB-5 column, or an LC-50 column. The use of the shorter 10 m DB-5 column and on-column injection results in the elution of the fully brominated congener (BDE 209) without degradation on-column [28].
The inorganic data (Table 4) were based on results from a definitive analytical method (ID-ICP-MS) for total Hg, Cd, and Pb, or data from either INAA, ICP-OES, or combining data from INAA and ICP-OES. The inorganic data from the individual methods are listed in ESM Table S4 for both SRMs.
For the certified values, the majority of the relative expanded uncertainties are < 8 %. The heterogeneity of SRM 2786 and SRM 2787 was assessed for the PAHs, nitrated PAHs, and BDEs by analyzing duplicate 10 mg to 30 mg test portions from six bottles selected by stratified random sampling. No statistically significant differences among bottles were observed for the three classes of compounds at the 30 mg sample size. The assessment of the heterogeneity of SRM 2786 and SRM 2787 for inorganic constituents was based on the INAA results for the test portions from 12 bottles (median sample size of 1.3 mg). Calculated elemental homogeneity factors (He) [29] range from 1.3 for Al to 10 for Ti, which indicates that SRM 2786 and SRM 2787 are suitable for milligram-size sampling for inorganic constituents.
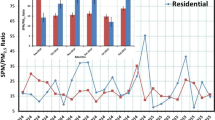
The total suspended PM used to prepare SRM 2786 and SRM 2787 was collected in Prague, Czech Republic during 2005, approximately 30 years later than the particulate matter used for SRM 1648a which was collected in St. Louis, MO and for SRM 1649b which was collected in Washington, DC. Figure 2 shows a comparison of the mass fractions of selected compounds in the four materials; note, however, that the BDEs, PCDDs, and PCDFs were not characterized in SRM 1648a. The mass fractions of the PAHs and nitrated PAHs are not significantly different among the materials with the exception of some of the methylated phenanthrenes. The mass fraction of 1-methylphenanthrene is approximately 2.5 times higher in SRM 1648a than in SRM 2787 which is almost 3 times higher than SRM 1649b. The other three methylphenanthrenes, 2-, 3-, and 9-, have similar mass fractions in the four materials. The only dimethylphenanthrene quantified in the materials is 1,7-dimethylphenanthrene; its mass fraction is highest in the two contemporary materials. Retene which is indicative of wood burning is found at higher mass fractions in the two contemporary materials. This difference most likely indicates the tendency of US city dwellers, particularly in the 1970s, not to use wood stoves. The mass fractions of the BDEs also tend to be higher in the 2005 collection. This may be due to the increased use of brominated flame retardants in the late 1990s. The total mass fraction of PCDDs and PCDFs were higher in SRM 1649b than in the more contemporary materials.
Comparison of the mass fractions of selected compounds in the four PM SRMs available from NIST. Note that the BDEs, PCDDs, and PCDFs were not characterized in SRM 1648a. Values shown are the certified or reference values and associated expanded uncertainties from the respective Certificates of Analysis
Pb and Cd show interesting differences in the four materials but are not shown in Fig. 2. The mass fractions of Pb and Cd in SRM 2786 and SRM 2787 are similar (Table 4). In SRM 1649b, however, the mass fraction of lead is (12,860 ± 60) mg/kg approximately 50 times higher than in the fine PMs. It is even higher in SRM 1648a at (65,500 ± 3300) mg/kg. The mass fraction values of Cd in SRM 1649b and SRM 1648a are (26.10 ± 0.28) mg/kg and (73.7 ± 2.3) mg/kg, respectively, which are approximately 6 and 18 times higher than in the fine PM SRMs. SRM 1648 and SRM 1649 were collected in the mid-1970s when the use of Pb-containing gasoline was still prevalent. Clements et al. [4] noted that the concentrations for Cd and Pb in their study (collections in 2010 and 2011) were more than an order of magnitude lower than values reported in January 1982. These differences illustrate why particulate materials from contemporary collections are important for quality control in contemporary studies.
SRMs 2786 and 2787 were both obtained from the same total suspended PM; however, there are differences in the mass fractions of the individual analytes. For the PAHs, the mass fractions in SRM 2787 range from 46 % lower to 163 % higher (with the majority between 15 % lower and 20 % higher) than those in SRM 2786. The differences are not as large for the BDEs with all of the values in SRM 2787 being lower than those in SRM 2786 (from 6 to 30 % lower). For the inorganic constituents, the largest difference is for total Hg with the value in SRM 2787 being 87 % lower than that in SRM 2786. This difference illustrates the need for having the two SRMs with differing particle sizes.
SRM 2786 and 2787 are the first “natural” or field-collected fine particulate matter CRMs with values assigned for both organic and trace element. The two fine particulate matter CRMs issued by IRMM (ERM CZ100 and ERM CZ120), both issued in 2010, were prepared from TSP collected from a road tunnel in Warsaw, Poland. The TSP was sieved to less than 0.5 mm and then ground using a jet-mill to produce a fine dust with 50 volume percent below 7.59 μm and 90 volume percent below 20 μm. ERM CZ100 is characterized for content of seven PAHs and the mass fractions of the individual PAHs are approximately a factor of 4 less than the content of SRM 2786 and SRM 2787. For the trace element content, SRM 2786 and SRM 2787 have mass fractions a factor of 3 to 5 higher than the content in ERM-CZ120.
SRM 2786 and SRM 2787 have been used to evaluate new methods and determine additional analytes as illustrated for the sugars in the paper by Louchouarn et al. [19] and more recently to evaluate a relatively new extraction method (Quick Easy Cheap Effective Rugged and Safe, QuEChERS) for the analysis of nitrated and oxygenated PAHs [30]. Such studies are important for adding information on additional compounds and parameters to the materials. This need for additional characterization of available reference materials was recently reiterated in a review article by Parshintsev and Hyötyläinen [31].
Conclusion
SRM 2786 Fine Particulate Matter (<4 μm) and SRM 2787 Fine Particulate Matter (<10 μm) are the most characterized fine PM reference materials currently available, having been characterized for a wide range of organic and inorganic constituents. The mass fractions of the constituents represent those in a contemporary urban environment and complement the mass fractions found in existing SRM PMs collected in the mid-1970s which have a wider particle size range. These SRMs will be useful in method development and quality control for measurements related to constituents in fine PM.
References
Wang M, Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Hoffmann B, et al. Environ Int. 2014;66:97–106.
Donateo A, Gregoris E, Gambaro A, Merico E, Giua R, Nocioni A, et al. Environ Sci Pollut Res. 2014;21:9415–29.
Traumann A, Reinhold K, Tint P. Environ Eng Manag J. 2014;13:2233–41.
Clements N, Eav J, Mingjie X, Hannigan MP, Miller SL, Navidi W, et al. Atmos Environ. 2014;89:273–381.
Fine Particulate (PM2.5) Designations. US Environmental Protection Agency, Washington, DC. 2013. http://www.epa.gov/airquality/particlepollution/designations/basicinfo.htm. Accessed 13 April 2015.
Kim K-H, Kabir E, Kabir S. Environ Int. 2015;74:136–43.
Karottki DG, Bekö G, Clausen G, Madsen AM, Andersen ZJ, Massling A, et al. Environ Int. 2014;73:372–81.
Lippmann M, Frampton M, Schwartz J, Dockery D, Schlesinger R, Koutrakis P, et al. Environ Health Perspect. 2003;111:1074–92.
Solomon PA, Crumpler D, Flanagan JB, Jayanity RKM, Rickman EE, McDade CE. J Air Waste Manage Assoc. 2014;64:1410–38.
Schantz MM, Wise SA, Lewtas J. NIST Internal Report (NISTIR) 7229, Gaithersburg, MD. 2005. http://www.nist.gov/manuscript-publication-search.cfm?pub_id=904512. Accessed 13 April 2015.
Schantz MM, Poster DL, Kucklick JR, Wise SA, McDow S, Lewtas J et al. NIST Internal Report (NISTIR) 7303, Gaithersburg, MD. 2006. http://www.nist.gov/manuscript-publication-search.cfm?pub_id=904424. Accessed 13 April 2015.
Klouda GA, Filliben JJ, Parish HJ, Chow JC, Watson JG, Cary RA. Aerosol Sci Technol. 2005;39:173–83.
Heller-Zeisler S, Ondov JM, Zeisler R. Biol Trace Elem Res. 1999;71–72:195–202.
Wise SA, Poster DL, Kucklick JR, Keller JM, VanderPol SS, Sander LC, et al. Anal Bioanal Chem. 2006;386:1153–90.
Murphy KE, Beary ES, Rearick MS, Vocke RD. Fres J Anal Chem. 2000;368:362–70.
Christopher SJ, Long SE, Rearick MS, Fassett JD. Anal Chem. 2001;73:2190–9.
Long SE, Kelly WR. Anal Chem. 2002;74:1477–83.
Kelly WR, Long SE, Mann JL. Anal Bioanal Chem. 2003;376:753–8.
Louchouarn P, Kuo L-J, Wade TL, Schantz MM. Atmos Environ. 2009;43:5630–6.
May W, Parris R, Beck C, Fassett J, Greenberg R, Guenther F et al. NIST Special Publication 260-136, Gaithersburg, MD. 2000. http://www.nist.gov/manuscript-publication-search.cfm?pub_id=200172. Accessed 13 April 2015.
Horn RA, Horn SA, Duncan DB. J Am Stat Assoc. 1975;70:380–5.
JCGM 100:2008; Evaluation of Measurement Data — Guide to the Expression of Uncertainty in Measurement (ISO GUM 1995 with Minor Corrections); Joint Committee for Guides in Metrology. 2008. available at http://www.bipm.org/utils/common/documents/jcgm/JCGM_100_2008_E.pdf (accessed 9 September 2015).
JCGM 101:2008; Evaluation of Measurement Data – Supplement 1 to the Guide to Expression of Uncertainty in Measurement; Propagation of Distributions Using a Monte Carlo Method; Joint Committee for Guides in Metrology. 2008. available at http://www.bipm.org/utils/common/documents/jcgm/JCGM_101_2008_E.pdf (accessed 9 September 2015).
Schantz MM, McGaw E, Wise SA. Anal Chem. 2012;84:8222–31.
Bergvall C, Westerholm R. Anal Bioanal Chem. 2008;391:2235–48.
Poster DL, Schantz MM, Sander LC, Wise SA. Anal Bioanal Chem. 2006;386:859–81.
Bamford HA, Bezabeh DZ, Schantz MM, Wise SA, Baker JE. Chemosphere. 2003;50:575–87.
Stapleton HM, Dodder NG, Offenberg JH, Schantz MM, Wise SA. Environ Sci Technol. 2005;39:925–31.
Stoeppler M, Kurfürst U, Grobecker KH. Fresenius’ Z Anal Chem. 1985;322:687–91.
Albinet A, Nalin F, Tomaz S, Beaumont J, Lestremau F. Anal Bioanal Chem. 2014;406:3131–48.
Parshintsev J, Hyötyläinen T. Anal Bioanal Chem. 2015;407:5877–97.
Acknowledgments
Partial funding support for the preparation and certification of this SRM was provided by the U.S. Environmental Protection Agency, Office of Research and Development, Human Exposure and Atmospheric Science Division, National Exposure Research Laboratory, Research Triangle Park, N.C. The particulate matter was collected by J. Kucera and staff from the Nuclear Physics Institute, Prague, Czech Republic. The sugar measurements were performed by P. Louchouarn of the Department of Marine Science and Department of Oceanography, Texas A&M University, Galveston, TX and College Station, TX, and the PCDD and PCDF measurements were performed by G. Poole of Environment Canada, Environmental Technology Centre, Analysis and Air Quality Division, Ottawa, Ontario, Canada.
Certain commercial equipment, instruments, or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 369 kb)
Rights and permissions
About this article
Cite this article
Schantz, M.M., Cleveland, D., Heckert, N.A. et al. Development of two fine particulate matter standard reference materials (<4 μm and <10 μm) for the determination of organic and inorganic constituents. Anal Bioanal Chem 408, 4257–4266 (2016). https://doi.org/10.1007/s00216-016-9519-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9519-7