Abstract
The size (hydrodynamic or Stokes radius, R H) of non-functionalized CdSeS/ZnS (core/shell) quantum dots (QDs) was characterized by size-exclusion chromatography with on-line quasi-elastic light scattering (SEC/QELS). Accurate determination of the size of QDs is important, because many of the optical properties of these materials are size dependent. A clear advantage of SEC/QELS over many batch techniques (e.g., QELS without separation) is the capability of the hyphenated technique to characterize the entire size range of a disperse sample, rather than merely providing a statistical average of the sizes present. Here, the SEC/QELS-determined R H values of CdSeS/ZnS QDs are compared to those determined by a traditional SEC experiment employing a calibration curve based on polystyrene standards, providing for the first reported study on SEC/QELS of non-functionalized QDs while also demonstrating the shortcomings of the widely-employed calibration curve approach. Furthermore, combining the R H of the QDs obtained by SEC/QELS with core size measurements derived from transmission electron microscopy allowed further calculation of the size of the QDs’ coronas. The latter result was found to be in close agreement to the previously measured dimension of the main corona constituent, as well as with the calculated size of this constituent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quantum dots (QDs) are semiconducting nanomaterials which exhibit quantum mechanical properties and which have applications in biological imaging and labeling, medicine, lasers, light-emitting diodes, and solar cells, among others [1, 2]. QDs are ideal for these applications because of their brightness and large extinction coefficients, which allow for easy detection and visualization. These unique optical properties of QDs stem from their small size; in fact, many of these properties are size dependent. In general, QDs have a diameter less than 10 nm, with emission wavelength and optical properties that can be tuned by modifying their size, a red shift in their electromagnetic spectrum being accompanied by an increase in QD size. QDs consist of materials such as cadmium selenide (CdSe), cadmium sulfide (CdS), zinc sulfide (ZnS), and indium phosphate (InP). So-called core/shell-structured QDs were developed to improve photoluminescence efficiency [2]. A common example of a core/shell QD is CdSeS/ZnS, in which the CdSeS core is capped with ZnS for enhanced quantum yield (Scheme 1).
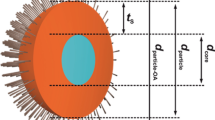
Schematic presentation of the CdSeS/ZnS quantum dot (as per QD naming custom, the CdS layer is considered implicit in the nomenclature) and experimental procedures employed in this study for determination of total radius of the QD (determined by SEC/QELS); radius of the metal core, composed of the CdSeS core plus the CdS and ZnS layers (determined by TEM); and thickness of the octadecylamine alkyl coating (determined by difference)
As discussed in the previous paragraph, the optical properties of QDs are size dependent. Thus, accurate determination of QD size is paramount in the characterization of these materials [3]. To date, the analytical methodologies employed for the size determination of QDs include off-line quasi-elastic light scattering (QELS, also known as dynamic light scattering), small-angle X-ray scattering and, most commonly, transmission electron microscopy (TEM) [4–6]. There are several shortcomings to the TEM approach, however. Firstly, in TEM only a limited area of a grid is examined, thus conveying information on only a small, perhaps nonrepresentative portion of the sample as a whole. Secondly, drying of the sample can cause physical and/or chemical changes to QDs, such as aggregation or oxidation. Finally, TEM underestimates the total size of QDs because it only “sees” the core, not the organic groups or passivating agents used for stabilizing the QD structure due to their destruction by the ionizing radiation of the TEM beam [4, 7].
Because QDs are usually synthesized in a liquid phase, high performance liquid chromatographic (HPLC) techniques coupled to various detection methods have been employed for separation and size characterization of QDs. For the separation of QDs (both in aqueous and organic solvents) reversed phase and size-exclusion chromatographic columns have been employed [4, 8, 9]. The detection methods coupled to HPLC include ultraviolet–visible (UV–vis) and fluorescence spectroscopy, and inductively coupled plasma mass spectrometry (ICP-MS). While these methods provide valuable information on QD properties, they are not able to give any direct information on their size. Size data become accessible when columns are calibrated for a size parameter (usually hydrodynamic radius, R H, or the equivalent hydrodynamic diameter 2R H) with well-characterized standards, such as polystyrenes [4]. The main disadvantage of this approach is that it is highly sensitive to abnormal elution behavior, as well as to normal chromatographic band broadening. For example, unwanted interactions between QDs and SEC stationary phases results in elution being affected by non-size-exclusion mechanisms which bias calculated QD sizes toward either artificially high values (for cases involving e.g., electrostatic ion-exclusion effects) or artificially low ones (when e.g., analyte sorption onto the stationary phase occurs) [10]. In the case of CdSe and CdSe/ZnS QDs it is the latter case, i.e., sorptive interactions between the analytes and the chromatographic column stationary phase, that has precluded accurate size characterization of these QDs by SEC using the traditional detection methods described earlier [6].
In this study, we demonstrate for the first time how SEC with on-line QELS detection provides a valuable tool for the size determination of QDs. The advantage of coupling SEC to QELS is that the hyphenated method proves to be less sensitive than SEC with column calibration to the aforementioned errors in size resultant from non-size-exclusion effects and chromatographic band broadening during elution. The SEC/QELS method also provides information on the size range of a sample, unlike off-line QELS which yields only the z-average of the R H distribution or which finds it necessary to invoke a number of assumptions to generate this distribution [11, 12]. In off-line QELS, for example, a distribution of diffusion rates from a monomodal size distribution is assumed, the correlation function is fit to a Gaussian distribution of decay times, and from this the distribution mean and width are calculated and the R H distribution and averages are obtained (this is the so-called “method of cumulants”). Alternatively in off-line QELS, the R H distribution is assumed to be smooth and a choice is made among various sets of exponentials that are used to fit the experimental data (the choice is generally made by the software based on the smoothness of the resultant distribution; this is the so-called “regularization method”). It should be noted that, for different species to be resolved from each other by the regularization method, their respective R H values should differ by a factor of 5 or greater. None of the assumptions on which either the cumulants or regularization method rely are employed when QELS is used on-line with SEC or related size-based separation methods, as the slices that elute from the chromatographic column are, in general, sufficiently narrow so as to generate single-exponential autocorrelation functions. An advantage of SEC/QELS over the more popular SEC with on-line multi-angle static light scattering (SEC/MALS) is that the latter, which has been employed (sometimes in conjunction with online QELS) in the size characterization of various colloids [13], nanoparticles [14] and colloidal assemblies [15], is generally limited to analytes of a size (radius) equal or greater than ≈1/40th of λ, the wavelength in the medium of the incoming radiation (λ ≡ λ 0/n 0 , where λ 0 is the vacuum wavelength of the radiation and n 0 is the refractive index of the medium) [16]. In general, most QDs fall below this size limit and their size is, thus, not accurately measureable by MALS. As discussed later, however, QELS measurements are based on diffusive transport properties, which makes this technique far more sensitive than MALS in the size range of interest for QDs.
A benefit of SEC/QELS over TEM is that the former is capable of giving information on the actual effective total particle radius (or diameter) of the QD, which includes the core, any surrounding metal layer(s), and any organic coating layer(s), while TEM can provide only information on the core and metal layers. Here, we take advantage of the complementarity of these two techniques in the characterization of CdSeS/ZnS core/shell QDs: TEM is employed to measure the combined size of the CdSeS core and the surrounding CdS and ZnS layers (for simplicity, the combination of all three of these will be referred to here as the “metallic core” of the QDs), with SEC/QELS being used to measure the total size of the QDs (see Scheme 1). The difference between these two measurements should provide the size of the organic coating layer of the QDs. To confirm the latter, our calculations of this octadecylamine coating layer size are compared to previous small-angle neutron scattering (SANS) measurements of the size of octadecyl chains, as well as to the calculated size of an all-trans octadecylamine molecule. Additionally, the SEC/QELS results are contrasted to those obtained by a traditional chromatographic approach, namely SEC employing a column calibration procedure, which serve to demonstrate the difference between the two approaches. We hope that the present study helps highlight the realized potential of SEC/QELS as a nanoparticle/quantum dot size characterization technique, as well as its complementarity with other characterization methods such as TEM.
Materials and methods
Materials
Non-functionalized, 1-octadecylamine-capped CdSeS/ZnS quantum dots (QD 510 and QD 620; the numbers denote the photoluminescence wavelength maxima, in nm) dispersed in toluene, were purchased from AC Diagnostics, Inc (Fayetteville, AR, USA). Polystyrene standards with nominal molar masses of 9000 g mol−1, 19,760 g mol-1, 31,420 g mol−1, 49,170 g mol−1, and 68,000 g mol−1 were from Agilent/Polymer Laboratories (Amherst, MA, USA). The polystyrene standard with nominal molar mass of 102,000 g mol−1 was acquired from Toyo Soda (Tokyo, Japan). The mass concentration of both quantum dots was 5 mg mL−1, according to the manufacturer. HPLC grade toluene was from Avantor Performance Materials Inc (Center Valley, PA, USA) and 1-octadecylamine was from Alfa Aesar (Ward Hill, MA, USA).
SEC/QELS analysis
The SEC instrument consisted of an Agilent 1260 isocratic HPLC pump (Agilent Technologies, Santa Clara, CA, USA), an autosampler (Agilent 1260), and a WyattQELS quasi-elastic light scattering detector (Wyatt Technology Co., Santa Barbara, CA, USA). QELS measurements were performed at a nominal angle of 108° relative to incident laser beam. A 0.22 μm inline Teflon filter was placed after the pump and before the injector to filter the mobile phase, prior to injection, of spurious particulate matter contaminants. QELS data were collected at a rate of 1 point per 2 s, and processed with ASTRA software (Wyatt Technology Co., version 6.1.1.17).
The SEC column employed for QD and polystyrene separations was either PLgel 500 Å nominal pore size (5 μm particle size) or PLgel 104 Å nominal pore size (10 μm particle size), both from Agilent/Polymer Laboratories and both packed with crosslinked styrene/divinyl benzene particles. The eluent was 2 mmol L−1 1-octadecylamine in toluene [4], at a flow rate of 0.5 mL min−1. Lower (or higher) flow rates were not investigated, as flow rate effects on on-line QELS results are essentially negligible at the size range examined in these experiment (it would require an order-of-magnitude or more increase in size for these effects to become manifest [15]). Addition of 1-octadecylamine to the organic solvent served to prevent QD sorption onto the column packing material and also as a prophylactic measure against QD aggregation in solution. Previous studies analyzing QDs, using the same type of SEC column packing material as employed here, both with and without the capping agent added to the mobile phase showed that addition of the capping agent is needed to help prevent or, at least, substantially reduce enthalpic non-size-exclusion interactions between the QDs and the column packing material [6]. The QDs were analyzed in triplicate without further sample dilution prior to analysis, i.e., at a concentration of 5 mg mL−1. According to preliminary SEC experiments with various injection volumes, a 50 μL injection volume was chosen for QD 510 and a 10 μL injection volume for QD 620. These injection volumes provided a QELS signal-to-noise ratio (S/N) sufficient for the determination of R H for each eluted QD fraction. Diluting the QD solutions by a factor of two while maintaining the same injection volumes yielded chromatograms with an S/N ratio too low for the reproducible determination of R H.
A calibration curve for R H was constructed employing narrow dispersity polystyrene standards of various molar masses. The peak apex molar masses M p provided by the manufacturer were converted into R H values employing the previously-published power law relationship between molar mass (M in g mol−1) and R H (in nm) of polystyrene in toluene at room temperature [17]:
The above relation was based on 38 polystyrene samples covering over two orders of magnitude in molar mass, with R H values determined mostly via QELS but also employing sedimentation techniques [17]. The concentration of polystyrene standards was 7 mg mL−1 (PS 9000), 5 mg mL−1 (PS 19,760, PS 31,420, PS 49,170), 4.5 mg mL−1 (PS 68,000), and 3.5 mg mL−1 (PS 102,000). The injection volume for all the polystyrenes was 100 μL, and each polystyrene was analyzed in duplicate. Both the column and the detector were temperature-regulated to 25 °C.
TEM imaging
Transmission electron microscopy measurements were performed using a JEM 3010 from JEOL (Waterford, VA, USA). A voltage of 300 kV was used for all samples. TEM sample preparation was performed by pipetting 6 μL of QD suspension onto a formvar-coated copper TEM grid (Electron Microscopy Sciences, Hatfield, PA, USA). Size distribution was determined by analysis of TEM images using ImageJ (National Institute of Health, Bethesda, MD, USA); at least 500 particles were counted in each population [18, 19].
Results and discussion
SEC/QELS of individual QDs and of QD blend
The SEC/QELS chromatograms of the QDs are presented in Fig. 1. For comparison, two different SEC columns were employed for the separations, a PLgel 104 Å (10 μm particle size) and a PLgel 500 Å (5 μm particle size) column. Chromatographic resolution R s was calculated using Eq. (2) [10]:
where V R is the peak retention volume (retention volume of peak maxima; W b is the chromatogram peak width, in retention volume units, formed by intersection of the baseline with the tangents to the curve inflexion points; and the subscripts 1 and 2 refer to each of the two peaks (or to each peak centroid). As can be seen in Fig. 1 A and B, the larger particle/pore size column provided better resolution between the two QDs than did the column with smaller particle/pore size. The R s between QD 620 and QD 510 was 0.16 with the 500 Å column, doubling to 0.32 when the 104 Å pore size column was used. The fact that worse resolution was achieved with the smaller particle size and smaller pore size column than with its larger particle size and pore size counterpart appears, at first, counterintuitive. This result is explained, however, by the fact that QD 620 appears to elute at the exclusion volume of the smaller particle/pore size column, as determined by comparing the retention volume of this QD to those of a series of narrow dispersity PS standards (data not shown). By analyzing these standards at the same solvent/temperature conditions as the QDs, it was determined that the size exclusion limit of the smaller column was similar to, and perhaps even slightly smaller than, the size of QD 620, meaning that some, perhaps all, of this QD eluted at the exclusion limit of the smaller column. Hence, the separation space was reduced in the smaller pore size column as compared to the larger pore size one, effectively negating any resolution advantage that might have been derived from differences in packing particle size between these columns.
A, B SEC/QELS chromatograms of QD 620 (red line) and QD 510 (blue line) and corresponding R H values across the chromatographic peaks (solid red squares for QD 620 and open blue circles for QD 510). Data in A and C were obtained using a PLgel 10 μm particle size, 104 Å pore size column and, in panel B, employing a PLgel 5 μm particle size, 500 Å pore size column. C is the SEC/QELS chromatogram (purple line) of a blend of the two QDs (5:1 blend of QD 620 and QD 510, respectively), with R H values across the chromatogram shown as open purple triangles
Regardless of which column was used, QD 620 was observed to elute earlier than did QD 510. This elution order is in accordance with the size-exclusion principle of larger analytes sampling a smaller pore volume, and thus being less retained, than smaller analytes. Employing the on-line QELS detector, the size (hydrodynamic radius R H, also known as the Stokes radius) of the QDs was measured across their individual chromatograms. (It should be noted that QELS actually measures the translational diffusion coefficient D T of the analytes, from which the hydrodynamic radius is calculated via the Stokes-Einstein equation R H ≡ k B T/(6πη 0 D T), where k B is Boltzmann’s constant, T is the absolute temperature in Kelvin, and η 0 is the solvent viscosity at the experimental temperature). As seen in Table 1 and in Fig. 1A, B, the R H of QD 620 is indeed larger than that of QD 510, 6.6 nm versus 4.8 nm, respectively. Furthermore, the very small change in R H as a function of QD elution volume, for both analytes, attests to the near-monodispersity of these materials.
A 1:5 (QD 510:QD 620) blend by volume of the two QDs was also analyzed, employing the 10 μm particle size, 104 Å pore size column. The results of this analysis are shown in Fig. 1C. There are a few things to note from this figure. First, this particular SEC column was not able to separate the two QDs from each other in a manner that allowed differentiation of the two peak apexes. This was to be expected, however, given that the R s between the individual QDs on this column was only 0.32 (see above). Second, the retention volume of the blend peak is 7.01 mL, in between the retention volumes of the component QDs, which were 6.96 mL and 7.41 mL. While this result was also expected, the fact that the retention volume of the blend is substantially closer to that of QD 620 than to the retention volume of QD 510 may not, at first, seem obvious. The reason for this bias is that the chromatograms in Fig. 1C (and, indeed, all chromatograms in this paper) are based on the signal from the QELS photometer. Being a light scattering detector, the QELS response is larger for analytes with a larger molar mass as, all other factors being equal, light scattering photometers are molar-mass-sensitive detectors. All factors also being equal, a larger QD size corresponds to a larger molar mass. Consequently, the peak apex of the QD blend is expected to be closer to the peak apex of the larger QD, QD 620, which is, indeed, what is observed. Lastly, we note that, even though the SEC column lacked the necessary chromatographic resolution to distinguish between the two components of the blend, the on-line QELS detector provides affirmative evidence to their presence, as observed from the distribution of R H values across the chromatogram: From smallest to largest retention volume we observe, in turn, first a near-plateau in R H around 6.4 nm, then a decrease in size from 6.4 nm to 5.1 nm and, lastly, another near-plateau around 5.1 nm. These correspond, respectively, to almost exclusively QD 620 (as seen in Fig. 1A, fronting of the QD 510 peak is minimal, meaning that there is little “contamination” of the QD 620 peak by QD 510); then to a combination of the two QDs, where the QELS detector measures the average size of the analytes eluting from the column at each retention slice; and, lastly, the portion of the peak which is, mostly, QD 510, but which also contains some of QD 620, as can be expected from the tailing of the peak of the latter QD as seen in Fig. 1A. The QELS detector thus provides evidence of the two blend components present, even though the chromatographic peak is monomodal.
QD size by SEC with polystyrene-relative calibration
In the SEC analysis of macromolecules, a common practice is to construct peak-position calibration curves using a set of well-characterized narrow molar mass dispersity standards. From these curves, the molar mass averages and distributions of the sample(s) being analyzed are obtained. The results obtained from this approach are highly suspect if either the chemistry and/or architecture of the sample(s) differ from those of the calibrants. Unfortunately, an analogous method for obtaining the size averages and distributions of QDs appears to have gained favor in the literature. For QDs, this approach has been used by e.g., Krueger and colleagues to determine the size of QDs dispersed in toluene [4]. Given the increasing popularity of this approach, we decided to apply it here and to compare the calibrant-relative results to the “absolute” results obtained above by SEC/QELS. To this effect, a series of narrow dispersity polystyrene standards, of nominal molar mass between 9000 g mol−1 and 102,000 g mol−1, were analyzed by SEC. The molar masses were converted into hydrodynamic radii via Eq. 1, previously derived by Fetters et al. [17]. As can be seen in Fig. 2, the resultant R H versus retention volume calibration curve covers the range of ≈2 nm to ≈8 nm, bracketing the expected size of the QDs based on the SEC/QELS experiments described above. From this calibration curve, R H for peak apex of 4.13 ± 0.04 nm and 5.83 ± 0.01 nm were obtained for QD 510 and QD 620, respectively. As can be seen from Table 1, these values are lower than those obtained from SEC/QELS, likely as a result of sorptive non-size-exclusion interactions between the QDs and the column packing material at the given solvent/temperature conditions of the experiment.
While differences in the average R H obtained by SEC/QELS and SEC with PS-relative calibration are relatively small (between 0.7 and 0.8 nm; see Table 1), larger differences are observed in the R H range determined by each method. Because of the “absolute” nature of QELS detection, results obtained by SEC/QELS are less sensitive to chromatographic band broadening than are those derived using the calibration curve approach. As can be seen when comparing the R H results in Fig. 1A to the PS-relative size range covered by the chromatograms is Fig. 2, the ranges derived from the calibration curve approach are several nm larger than those obtained by SEC/QELS: For QD 510, the peak (retention volume between 7 and 8 mL) covers the R H range from ≈4.3 to ≈5.2 nm according to SEC/QELS and from ≈2.6 to ≈5.6 nm when the calibration curve is employed. The corresponding ranges (retention volume between 6.5 and 7.75 mL) for QD 620 were from ≈6.1 to ≈8.3 nm by SEC/QELS, and from ≈3.2 to ≈8.3 nm when applying the calibration curve. The fact that larger differences are observed at the lower end of the size range in both cases is likely due to the predominant effect of longitudinal diffusion (van Deemter B term) being responsible for the band broadening of these very small-sized analytes, in contradistinction to SEC of large-sized polymers, where band broadening is chiefly governed by mass transfer effects (van Deemter C terms).
TEM of QDs
As indicated in the Introduction, TEM is the most frequently used technique for size characterization of QDs. One of the disadvantages of TEM is its inability to detect the organic layer(s) that stabilize the inorganic core. Here, TEM was employed to determine the size of the metallic core, composed of the CdSeS core and concentrically surrounding CdS and ZnS layers (see Scheme 1), of the QDs studied. In this manner, TEM provides information complementary to that obtained from SEC/QELS (or from calibrant-relative SEC), which yields the size (R H) of the QDs as a whole, i.e., as metallic core plus organic coating layer.
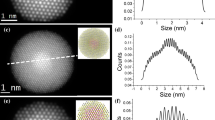
The TEM images of the QD 510 and QD 620, shown in Fig. 3, indicate that both QDs have spherical shape and a narrow size dispersity. The radius obtained for the core of QD 510 was 2.4 ± 0.3 nm, and for that of QD 620 3.5 ± 0.6 nm (Table 1). The difference between these data and the R H values obtained from SEC/QELS provides the thickness of the octadecylamine (C18NH2) coating layer, i.e.:
From the above relation, the thickness of the octadecylamine layer was calculated as 2.4 ± 0.3 nm for QD 510 and 3.1 ± 0.6 nm for QD 620. Thus, according to our calculations the alkyl layer of QD 510 was 0.7 nm thinner than that of QD 620. One explanation for this difference in organic layer thickness between the two QDs examined could, possibly, be due to a difference in “grafting density” of octadecylamine, with a lower density in QD 510 than in QD 620. High packing density is known to restrict the movement of polymer chains, leading to more extended conformation than when the packing density is lower [20, 21]. It should be noted, however, that both these values for the size of the alkyl layer appear to be quite reasonable. This conclusion stems from the fact that Sander et al., employing small-angle neutron scattering (SANS), found the length of an octadecyl (C18) chain, in methanol and bonded to silica, to be 2.1 ± 0.3 nm [22]. Our values for the size of the organic layer of the QDs, calculated from the difference between the SEC/QELS and TEM measurements (Eq. (3)), are also found to bracket the theory-predicted value of ≈2.7 nm for the length of an all-trans C18NH2 chain [23]. The similarity between our values and those found either via SANS or theory provides strong support as to the robustness of the present method for determining the thickness of the various portions of organically-coated QDs by employing the combined SEC/QELS + TEM method.
Conclusions
We have demonstrated here the successful coupling of size-exclusion chromatography to quasi-elastic light scattering detection for the purposes of determining the average size, and the size range, of quantum dots. In the present case, the SEC/QELS technique was applied to the characterization of two CdSeS/ZnS QDs with an average size of 4.8 and 6.6 nm and covering a size range of ≈4 to ≈8 nm. The method was also applied to the study of a blend of these QDs. The use of on-line QELS detection allows for determination of the hydrodynamic radius R H of the QDs at each slice eluting from the columns. Results from SEC/QELS are compared to those obtained by a widely applied calibrant-relative approach. The latter is found to underestimate the average size of the individual QDs, though only slightly. Larger errors from applying a polystyrene-relative calibration curve are seen when trying to determine the size range as a whole, especially as this technique tends to exaggerate the lower end of the size range, likely as a result of longitudinal diffusion band broadening effects in the chromatography of these small-sized analytes.
The SEC/QELS method is complemented here by TEM analysis of the QDs. While the former measures the R H of the QDs as a whole, metal core and alkyl coating combined, the latter measures the geometric size of the metal core alone. The difference between these two measurements provides the size of the alkyl layer, octadecylamine in the present case. The size of this layer, as determined by the difference between SEC/QELS and TEM measurements, was found to be in good agreement with previous measurements of the size of a C18 group and in even better agreement with the calculated size of an all-trans C18NH2 molecule. We believe the method presented here should be of interest to those attempting to determine the size of QDs as, in addition to chemistry, size is a fundamental parameter governing the optical properties of these materials.
References
Huang H, Zhu J. The electrochemical applications of quantum dots. Analyst. 2013;138:5855–65.
Vasudevan D, Gaddam RR, Trinchi A, et al. Core-shell quantum dots: properties and applications. J Alloys Compd. 2015;636:395–404.
Pitkänen L, Striegel AM. Size-exclusion chromatography of metal nanoparticles and quantum dots. Trends Anal Chem. 2015;in press.
Krueger KM, Al-Somali AM, Falkner JC, Colvin VL. Characterization of nanocrystalline CdSe by size exclusion chromatography. Anal Chem. 2005;77:3511–5.
Lee M, Chung WJ, Park SK, Kim M, Seo HS, Ju JJ. Structural and optical characterizations of multi-layered and multi-stacked PbSe quantum dots. Nanotechnology. 2005;16:1148–52.
Wilcoxon JP, Provencio PP. Chemical and optical properties of CdSe and CdSe/ZnS nanocrystals investigated using high-performance liquid chromatography. J Phys Chem B. 2005;109:13461–71.
Wilcoxon JP, Martin JE, Provencio P. Size distributions of gold nanoclusters studied by liquid chromatography. Langmuir. 2000;16:9912–20.
Fischer CH, Weller H, Katsikas L, Henglein A. Photochemistry of colloidal semiconductors. 30. HPLC investigation of small CdS particles. Langmuir. 1989;5:429–32.
Trapiella-Alfonso L, Bustos ARM, Encinar JR, Costa-Fernandez JM, Pereiro R, Sanz-Medel A. New integrated elemental and molecular strategies as a diagnostic tool for the quality of water soluble quantum dots and their bioconjugates. Nanoscale. 2011;3:954–7.
Striegel AM, Yau WW, Kirkland JJ, Bly DD. Modern size-exclusion liquid chromatography. 2nd ed. Hoboken: Wiley; 2009.
Berne BJ, Pecora R. Dynamic light scattering. Mineola: Dover; 2000.
Johnson Jr CS, Gabriel DA. Laser light scattering. Mineola: Dover; 1981.
Brewer AK, Striegel AM. Characterizing the size, shape, and compactness of a polydisperse prolate ellipsoidal particle via quadruple-detector hydrodynamic chromatography. Analyst. 2011;136:515–9.
Brewer AK, Striegel AM. Characterizing a spheroidal nanocage drug delivery vesicle using multi-detector hydrodynamic chromatography. Anal Bioanal Chem. 2011;399:1507–14.
Brewer AK, Striegel AM. Characterizing string-of-pearls colloidal silica by multidetector hydrodynamic chromatography and comparison to multidetector size-exclusion chromatography, off-line multiangle static light scattering, and transmission electron microscopy. Anal Chem. 2011;83:3068–74.
Wyatt PJ. Light scattering and the absolute characterization of macromolecules. Anal Chim Acta. 1993;272:1–40.
Fetters LJ, Hadjichristidis N, Lindner JS, Mays JW. Molecular weight dependence of hydrodynamic and thermodynamic properties for well-defined linear polymers in solution. J Phys Chem Ref Data. 1994;23:619–40.
Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophoton Int. 2004;11:36–41.
Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Burchard W. Solution properties of branched macromolecules. Adv Polym Sci. 1999;143:113–94.
Krueger KM, Al-Somali AM, Mejia M, Colvin VL. The hydrodynamic size of polymer stabilized nanocrystals. Nanotechnology. 2007;18:475709/1–7.
Sander LC, Glinka CJ, Wise SA. Determination of bonded phase thickness in liquid chromatography by small angle neutron scattering. Anal Chem. 1990;62:1099–101.
Hiemenz P, Lodge T. Polymer chemistry. 2nd ed. Boca Raton: CRC Press; 2007.
Harris DC. Quantitative chemical analysis. 8th ed. New York: WH Freeman; 2010.
Acknowledgments
The authors are grateful to Liz Nguyen for her help with the TEM measurement.
Disclaimer
Commercial products are identified to specify adequately the experimental procedure. Such identification does not imply endorsement or recommendation by the National Institute of Standards and Technology, nor does it imply that the materials identified are necessarily the best available for the purpose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Rights and permissions
About this article
Cite this article
Pitkänen, L., Striegel, A.M. Determining the core, corona, and total size of CdSeS/ZnS quantum dots by SEC/QELS and TEM. Anal Bioanal Chem 408, 4003–4010 (2016). https://doi.org/10.1007/s00216-016-9487-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9487-y