Abstract
We present the determination of the alkaloid hordenine and its forensic relevance as a qualitative and quantitative marker for beer consumption. A simple, rapid and sensitive ultra-performance liquid chromatography (UPLC)–tandem mass spectrometry (MS/MS) method for the determination of hordenine in human serum samples was developed and validated. The application was tested with serum samples after enzymatic cleavage. After addition of the synthesized internal standard hordenine-D 4, a liquid–liquid extraction with dichloromethane and diethyl ether was performed. Chromatographic separation was conducted with a Waters Acquity® UPLC system with gradient elution on an Agilent Eclipse XDB-C18 column (4.6 mm × 150 mm, 5-μm particle size). For quantification, a Waters Acquity® TQ detector (version SNC 627) with a positive electrospray ionization probe and multiple reaction monitoring mode was used. A flow rate of 0.4 ml/min was applied. The retention time for both the analyte and the internal standard was 3.67 min. Linearity was demonstrated from 0.2 to 16 ng/ml (R 2 > 0.999). The lower limit of quantification was 0.3 ng/ml in serum. Matrix effects and extraction recoveries for low and high concentrations were within acceptable limits of 75–125 % and 50 %, respectively. To the best of our knowledge there is no corresponding method for the determination of hordenine by UPLC–MS/MS in serum. By our drinking studies we demonstrate that beer consumption leads to detectable hordenine concentrations in serum and observed a linear elimination of total hordenine correlating to blood alcohol concentration, which shows that hordenine can be used as a reliable qualitative and quantitative marker for beer consumption. The validated method was successfully applied to serum from actual forensic cases.

Determination of hordenine in human serum samples by ESI+ UPLC-MS/MS
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The secondary plant metabolite hordenine was first isolated from Ariocarpus fissuratus by Heffter in 1894 [1] and since then has been detected in a number of plants, including cacti, grasses and bitter oranges. The most important source of hordenine is barley (Hordeum vulgare), in which it was detected by Léger in 1906 [1, 2]. It is formed in roots during germination of barley grains with increasing concentrations from day 3 to day 9. The hordenine content then decreases as the germs age. Starting from the amino acid tyrosine, hordenine is synthesized by decarboxylation and dimethylation by pyridoxal phosphate (PLP) dependent decarboxylase and N-methyltransferase [3–9].
The sympathomimetic effect of hordenine which results in hypertension (cats) or increasing respiratory and cardiac frequencies (horse) after application of 2 mg per kilogram of body weight is attributed to its structural similarity to neurotransmitters, including dopamine and adrenaline [10–14]. Hordenine is formed during malting of barley grains in beer brewing. The amount of hordenine in beer is dependent on temperature, germ duration and humidity, as well as carbon dioxide concentration [15]. In addition to our former investigations [16] we determined hordenine concentrations of 1.3–5.2 mg/l in 91 different types of beer by now. Consequently, beer consumption leads to detectable amounts of hordenine in blood and urine. Therefore, in addition to it being a congener alcohol, hordenine could be a suitable marker to prove the consumption of a specific alcoholic beverage in forensic toxicology.
Different extraction and analytical methods have been described for the determination of hordenine. Singh et al. [17] determined hordenine in horse blood and human urine samples (limits of detection, LOD, 25 ng/ml) using high-performance liquid chromatography (HPLC) and gas chromatography–mass spectrometry (MS). By HPLC, Hoult and Lovett [18] obtained an LOD of 2.5 μg/ml for the determination of hordenine in barley. Liquid chromatography (LC)–MS/MS was used for the determination of hordenine in bitter orange products; concentrations from 5.9 to 12.2 mg/kg were found [19]. Recently, hordenine was determined by solid-phase extraction and HPLC with tyramine as an internal standard with an LOD of 3 ng/ml [20]. Ma et al. [21] developed an LC–MS/MS method for the determination of hordenine in rat plasma with an LOD of 0.5 ng/ml. For our purposes it was necessary to develop and validate a specific and sensitive method for hordenine analysis in human serum samples after beer consumption. In this study we present a validated method with low LOD and low limits of quantification (LOQ) that uses hordenine-D 4 as an internal standard. Further, we performed drinking studies and observed a correlating elimination of hordenine and alcohol in serum after beer consumption. A hordenine concentration of 10 ng/ml in serum corresponded to blood alcohol concentrations of approximately 1 g/kg for example.Footnote 1
Materials and methods
Chemicals und reagents
Methanol and water were obtained from VWR International. Formic acid, ammonium formate and hordenine were obtained from Sigma-Aldrich. Ammonium acetate, dichloromethane, diethyl ether and sodium cyanoborohydride were obtained from Merck. Glucuronidase from Escherichia coli K12 was obtained from Roche Diagnostics (Mannheim, Germany). Deuterium-labelled formaldehyde was obtained from Cambridge Isotope Laboratories. Tyramine was obtained from J.T.Baker Chemicals.
Serum samples for validation
Blank human serum was obtained from six outdated blood donation samples from the Institute of Haemostaseology and Transfusion Medicine (University Hospital of Düsseldorf).
Internal standard
Hordenin-D 4 was synthesized as an internal standard by dimethylation of tyramine and reductive amination with use of deuterium-labelled formaldehyde and sodium cyanoborohydride in accordance with the method of Guo et al. [22]. For this, 0.5 ml of 5 mM tyramine was mixed with 0.5 ml of 0.2 M ammonium acetate buffer pH 5.3 in a 2-ml sample tube. Then, 125-μl freshly prepared 1 M sodium cyanoborohydride was added and the tube contents were mixed thoroughly. Next, 100 μl of 4 % formaldehyde-D 2 was added. The tube contents were mixed in an incubator for 10 min at 37 °C. The reaction was stopped on ice. The solution was acidified by addition of 25 μl of formic acid. Hordenine-D 4 was identified by MS and HPLC–UV analysis, and the concentration of hordenine-D 4 was determined in comparison with a solution of hordenine by means of HPLC–UV analysis (diode-array detector).
Solutions and quality control samples
Stock solutions containing hordenine at 1 mg/ml and hordenine-D 4 at 3 mg/ml, respectively, in methanol, were stored at -20 °C. To obtain the working solutions, the hordenine stock solution was freshly diluted with methanol to obtain a different final concentration. The hordenine-D 4 stock solution was diluted with methanol to obtain the internal standard solution of approximately 0.15 μg/ml. We prepared homogeneous quality control (QC) pools by mixing blank serum samples with hordenine stock solution to obtain concentrations of 1, 5 and 10 ng/ml. QC samples were portioned and stored at -20 °C until analysis.
UPLC–MS/MS conditions
Analysis was performed with a Waters/Acquity® ultra-performance LC-MS system with use of MassLynx®. For quantification we used positive electrospray ionization and multiple reaction monitoring mode. The following transitions were used for quantification and identification: m/z 166 [M + H+] to m/z 121 (Q1) and m/z 103 (Q2) for hordenine and m/z 170 [M + H+] to m/z 121 (Q1) and m/z 103 (Q2) for hordenine-D 4. Collision energies and cone voltages were set as given in Table 1. The column used for chromatography was an Agilent Eclipse XDB-C18 column (5-μm particle size, 4.6 mm × 150 mm). The mobile phase consisted of 5 mM ammonium formate buffered water (mobile phase A) and methanol (mobile phase B), both containing 0.1 % formic acid. A total flow rate of 0.4 ml/min was applied, and the injection volume was 10 μl. The run time was set to 7 min. Gradient elution was performed starting at 35 % mobile phase B for 0.5 min and progressing to 79 % mobile phase B in 3.5 min. Within the next 2 min the gradient increased linearly to the initial conditions and was held for 7 min for reequilibration. The column temperature was adjusted to 50 ± 5 °C. The autosampler operated at 10 °C.
Sample preparation/extraction
Serum samples were prepared with and without enzymatic cleavage. For enzymatic cleavage, samples were incubated with β-glucuronidase for 1 h at 45 °C. Hordenine was extracted from 0.6 ml of serum after addition of 20 μl of internal standard solution. The pH was adjusted with 100 μl of carbonate buffer to 8.4. Samples were extracted with 1.2 ml of a mixture of diethyl ether and dichloromethane (30:70). Sample tubes were vortex-mixed for 10 min and then centrifuged for 10 min at 14,000 g at 10 °C. The organic phase was dried under a nitrogen stream at 60 °C. The residue was dissolved in 60 μl of mobile phase (65 % mobile phase A; 35 % mobile phase B), and 10 μl was injected into the LC–MS/MS system.
Quantitative method validation
The LC–MS/MS method was validated according to the directives of the Society of Toxicological and Forensic Chemistry (http://www.gtfch.org) with use of Valistat 2.0.
Selectivity
For selectivity tests, blank serum samples were obtained from six different sources and screened without the internal standard. Two serum samples that tested negative were screened as a zero value after addition of the internal standard to test them for interferences.
Linearity of calibration
Linearity was proven by extraction of five calibrators different from zero. Blank serum samples with and without enzymatic cleavage were prepared by addition of hordenine stock solution to obtain seven different concentrations (0.2, 0.8, 2, 4, 8, 16 and 20 ng/ml). Each concentration was double-tested. Quantification was performed by linear regression of the peak-area ratios (analyte to internal standard) of seven calibrators.
Accuracy and precision
Inaccuracy and imprecision assays were performed on eight different days by extraction of two samples of each QC sample in an interday and an intraday assay. Inaccuracy was calculated as the percent deviation of the measured concentration from the original value. Imprecision was determined with Valistad 2.0 as the relative standard deviation (RSD).
Matrix effect (ion suppression) and extraction recoveries
For matrix effects and extraction recoveries, hordenine stock solution was added to five different serum samples to obtain concentrations of 1 and 10 ng/ml before and after extraction, respectively Appropriate concentrations were applied in the mobile phase. Each sample was double-tested. The peak-area ratios of extracted and mobile phase samples were used for matrix effect calculation. Extraction recoveries were calculated with the peak-area ratios of matrix and extraction samples.
Processed sample stability
Six already tested high-QC and low-QC samples (1 and 10 ng/ml) were merged, portioned and tested again within the duration of a regular batch run. Stability was calculated as the percent deviation of the measured concentration from the original value.
Freeze–thaw stability
Six high-QC and low-QC samples (1 and 10 ng/ml) were tested after three freeze–thaw cycles (20 h freezing, 1 h thawing and so on). The treated samples were tested against six freshly prepared samples. Stability was calculated as the percent deviation of the mean concentration of frozen–thawed samples from the mean concentration of untreated samples.
Long-term-stability
We performed stability tests by storing control samples at -20 °C. Approximately every month for 2.5 years the control samples were extracted and analysed against freshly prepared samples. Stability was calculated as the percent deviation of the mean concentration of stored samples from the mean concentration of untreated samples.
Limits
To determine the lower limit of quantification (LLOQ) and the LOD, hordenine stock solution was added to serum samples to obtain six different concentrations of 0.1, 0.2, 0.4, 0.6, 0.8 and 1 ng/ml. The samples were extracted and tested. The peak-area ratios of the qualifier were used for LLOQ calculation. The LOD was calculated with the quantifier peak-area ratios. Quantification was performed by linear regression of the peak-area ratios (analyte to internal standard).
Drinking studies
We performed two drinking studies. In the first one, 2 l beer (hordenine content 2.8 mg/l, alcohol content 4.8 vol %) was drunk by one of the authors within 2 h. Following this, blood samples were taken at different times (0.5, 1.25, 1.75, 2.25, 3.0, 3.75, 4.5 and 6 h) after drinking. In a second study, probands were allowed to chose different kinds of beverages, including different kinds of beer and liquors. Blood samples were taken after the resorption of blood alcohol was complete. All blood samples were centrifuged (10 min, 10 °C, 2000 rpm), and the serum phase was taken for the determination of hordenine. Blood alcohol concentrations (BAC) were determined by headspace gas chromatography according to the directions and instructions for blood alcohol measurements for forensic issues. BAC and total hordenine concentration were compared.
Results
MS data set
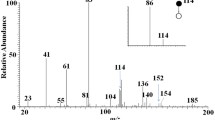
The mass spectra of hordenine and hordenine-D 4 are presented in Figs. S1 and S2. The parent ions m/z 166 [M + H]+ for hordenine and m/z 170 [M + H]+ for hordenine-D 4 were detected. Both the analyte and the internal standard show similar fragment ions with increasing cone voltage. The multiple reaction monitoring chromatograms of an extracted serum sample are shown in Fig. S3. The transitions m/z 166 [M + H]+ to m/z 121 and m/z 103 for hordenine and m/z 170 [M + H]+ to m/z 121 and m/z 103 for hordenine-D 4 were used for detection. The transition m/z 166 [M + H]+ to m/z 121 for hordenine and the transition m/z 170 [M + H]+ to m/z 121 for hordenine-D 4 were used for quantification. The retention time was 3.67 min for both hordenine and hordenine-D 4.
Quantitative method validation
Each blank sample was tested for interference. Selectivity is ensured at the LLOQ. Chromatograms for both the analyte and the internal standard are shown in Fig. 1. There was no chromatographic and mass-spectrometric interference at the retention time of hordenine and hordenine-D 4. Linear calibration curves were established for serum samples with and without enzymatic cleavage at concentrations from 0.2 to 20 ng/ml. The equation was y = 0.14x + 0.04 (R 2 = 0.9993) for the method without enzymatic cleavage and y = 0.14x + 0.04 (R 2 = 0.9996) for the samples treated with β-glucuronidase. The calibration curve of hordenine in serum after enzymatic cleavage can be applied for serum without preparation. Inaccuracy and imprecision were calculated for hordenine after extraction from serum. As shown in Table 2, inaccuracy was within the range 100.1–111.0 %. Interday precision was defined as the time-different intermediate precision, whereas intraday precision was defined as the repeatability. Imprecision values of 5.2-7.7 % for the time-different intermediate precision and 4.3–6.7 % for the repeatability were calculated. They were within the predefined interval of ±15 % (20 % for low concentrations). A 95 % β-tolerance interval is given within an acceptance interval of ±30 % (±40 % for low concentrations). The extraction recoveries were 64 ± 15.7 % and 76 ± 14.0 % for low (1 ng/ml) and high (10 ng/ml) concentrations. Recovery of 50 % or greater with a standard deviation 25 % or less is acceptable. Matrix effects were calculated for both low (100 ± 16.7 %) and high (116 ± 15.2 %) concentrations and were in the predefined interval of 75–125 % (Table 3). No significant concentration deficits were seen in processed sample stability tests. A maximum peak-area deficit of 25 % is acceptable. The mean assayed concentrations for low-QC and high-QC samples were within this limit (-2 % for low concentration and +4 % for high concentration; Table 4). No significant deficits were seen for freeze–thaw and long-term stability samples. The mean assayed concentrations were within 90–110 % of the mean QC concentration, as shown in Table 5. The 90 % confidence interval of the mean assayed concentrations is within 80–120 % of the mean QC concentrations as predefined. An LLOQ of 0.3 ng/ml and an LOD of 0.15 ng/ml in serum were calculated for the method presented (bias ± 20 %, RSD ≤ 20 %, inaccuracy 33 %, α = 0.01). Chromatograms obtained after extraction from serum spiked with hordenine concentrations according to the limits are shown in Fig. 2.
Drinking studies
In the first study we compared BAC and hordenine concentrations in serum samples after the consumption of 2 l beer within 2 h. The results are shown in Fig. 3. The highest concentration of hordenine after enzymatic cleavage in serum was 13.4 ng/ml (BAC 1.37 g/kg). Free hordenine was not detected after 2.5 h. After enzymatic cleavage of serum samples, hordenine was detected for up to 6 h after drinking. We observed a linear degradation of total hordenine (free and conjugated), which correlates to alcohol degradation. Hordenine concentrations (ng/ml) correlated to BAC (g/kg) multiplied by nearly 10.
Elimination of free hordenine (circles), total hordenine (triangles) and blood alcohol (diamonds) from blood after beer consumption (2 l beer within 2 h). Free hordenine is not detectable approximately 2 h after drinking. Total hordenine concentration and blood alcohol concentration (BAC) show correlating elimination up to 6 h after drinking
In the second study we compared BAC and hordenine concentrations in serum samples of probands, who chose to drink different kinds of beverages. Hordenine concentrations (ng/ml) correlated to BAC (g/kg) multiplied by values between 9.3 to 10.9 (mean 10.1) if only beer was drunk. Hordenine was not detectable in serum samples if probands drank wine. A mixed consumption of wine and beer led to a lower correlation value of 6.6 instead of 10 (see Table 6).
Discussion and application
In addition to congener alcohol analysis, the determination of hordenine may become an important analytical tool in forensic toxicological applications. After an offence has been committed, it is often necessary to prove or refute the consumption of specific alcoholic beverages. Although there are existing methods to give statements in evidence about this (congener alcohol, congener substances), new methods are necessary to verify exactly what kind of consumption occurred. The detection of substances in human matrices, which represent the consumption of ideally only one kind of beverage, is important in most forensic toxicological alcohol analytical cases.
We demonstrated that beer consumption leads to detectable hordenine concentrations in serum, so it is possible to use hordenine as a qualitative and quantitative marker of beer consumption. We observed higher concentrations of hordenine after enzymatic cleavage (“total hordenine”) of serum samples by β-glucuronidase, which shows that hordenine is metabolized to its glucuronide. Furthermore, we showed a linear elimination of total hordenine correlating to the BAC. A correlating factor of 10 was found after pure beer consumption; as an example, we found a total hordenine concentration of 5 ng/ml when the BAC was approximately 0.5 g/kg. In contrast, when beer and other alcoholic beverages such as wine were mixed, the hordenine concentration was lower in relation to the correspondent BAC.
Compared with other methods, the method presented here is sensitive, accurate and precise enough for the determination of hordenine in human serum samples after beer consumption. No ion suppressing or enhancing effect was observed.
We performed a brief validation for the quantification of hordenine in urine and other samples such as beverages with results as good as those in human serum samples. Further, we have successfully applied the determination of hordenine as a marker in addition to congener alcohol analysis in approximately 37 forensic cases so far. In the following, we present three examples.
In the first case the accused caused an accident. He stated he had drunk beer (five to ten glasses) during a dinner. After that he had amnesia (“blackout”). He suspected that a date rape drug (e.g. γ-hydroxybutyrate, flunitrazepam) had been administrated to him or that his beverages had been manipulated with the addition of higher alcoholic beverages (e.g. digestif). Our forensic toxicological analysis eliminated the suspicion of the administration of date rape drugs. We determined a BAC of 2.46 g/kg and a total hordenine concentration of 30 ng/ml, which results in a correlation factor of 12. From observations we concluded that the BAC was a result of beer consumption only. In the case of a manipulated drink (e.g. a mixture of beer and schnapps), the hordenine concentration in the blood of the accused would have been much lower than that determined.
In the second case we had to evaluate a statement of a person accused of driving under the influence of alcohol. He stated he had drunk 2.5 to three bottles of beer (approximately 0.5 l per bottle) as well as 100 ml of a digestif that contains no congener alcohol at home and not before he drove. The BAC was 1.18 g/kg. The consumption of the stated amount of beer would result in a BAC of 0.64–0.80 g/kg and a hordenine concentration between 6.4 and 8.0 ng/ml. We determined a total hordenine concentration of 7 ng/ml. The analysis for the other congener alcohol verified the statement of consumption of beer and a digestif.
In a third case a person was accused of driving under the influence of alcohol. Further, he was driving home. He was found at home by the police. He stated that he had consumed 600 ml of beer in a pub within 2 h (from 19:00 to 21:00). Then, he drove home and consumed 100–150 ml of a self-made beverage (94 % ethanol mixed with juice) from 22:25 to 22:50. We determined a BAC of 1.42 g/kg and a total hordenine concentration of 8 ng/ml at 00:16. If only 600 ml of beer had been drunk, we would not have found this high concentration of hordenine. Together with the results from other congener alcohol analyses, we could rule out his statement and proved that he had consumed more beer than he said he had.
Conclusion
The method presented here is suitable for pharmacokinetic studies and for defined intake of hordenine. It can be used to prove statements about the amount of beer that was consumed with or without other alcoholic beverages in forensic cases.
Notes
Data were partially presented as oral presentations at the International Traffic Medicine Association World Congress 2013, Hamburg.
References
Gaebel GO. Ueber das Hordenin. Vorläufige Mitteilung. Arch Pharm Pharm Med Chem. 1906;244(6-7):435–41.
Späth E. Über dieAnhalonium-Alkaloide. Monatsh Chem. 1919;40(2):129–54.
Frank AW, Marion L. The biogenesid of alkaloids: XVI. Hordenine metabolism in barley. Can J Chem. 1956;34(11):1641–6.
Leete E, Kirkwood S, Marion L. The formation of hordenine and N-methyltyramine from tyramine in barley. Can J Chem. 1952;30(10):749–60.
Leete E, Marion L. The biogenesis of alkaloids: VII. The formation of hordenine and N-methyltyramine from tyrosine in barley. Can J Chem. 1953;31(2):126–8.
Liu DL, Lovett JV. Biologically active secondary metabolites of barley. II. Phytotoxicity of barley allelochemicals. J Chem Ecol. 1993;19(10):2231–44.
Lovett JV, Hoult AHC, Christen O. Biologically active secondary metabolites of barley. IV. Hordenine production by different barley lines. J Chem Ecol. 1994;20(8):1945–54.
Lovett JV, Ryuntyu MY, Liu DL. Allelopathy, chemical communication, and plant defense. J Chem Ecol. 1989;15(4):1193–202.
Meyer E. Separation of two distinct S-adenosylmethionine dependent N-methyltransferases involved in hordenine biosynthesis in hordeum vulgare. Plant Cell Rep. 1982;1(6):236–9.
Russo CA, Burton G, Gros EG. Metabolism of [methyl-13C2]hordenine in homogenates from Hordeum vulgare roots. Phytochemistry. 1983;22(1):71–3.
Frank M, Weckmann TJ, Wood T, Woods WE, Tai CL, Chank S, et al. Hordenine: pharmacology, pharmacokinetics and behavioural effects in the horse. Equine Vet J. 1990;22(6):437–41. doi:10.1111/j.2042-3306.1990.tb04312.x.
Barwell CJ, Basma AN, Lafi MAK, Leake LD. Deamination of hordenine by monoamine oxidase and its action on vasa deferentia of the rat. J Pharm Pharmacol. 1989;41(6):421–3.
Hapke HJ, Strathmann W. Pharmakologische Wirkungen des Hordenin. DTW Dtsch Tierarztl Wochenschr. 1995;102(6):228–32.
Rietschel HG. Über die Pharmakologie des Hordenins. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1937;16(20):714.
Narziß L, Back W. Die Bierbrauerei Band I: die Technologie der Malzbereitung. Weinheim: Wiley-VCH; 2009.
Brauers G, Steiner I, Daldrup T. Quantification of the biogenic phenethylamine alkaloid hordenine by LC-MS/MS in beer. Toxichem Krimtech. 2013;80:323.
Singh AK, Granley K, Misrha U, Naeem K, White T, Jiang Y. Screening and confirmation of drugs in urine: interference of hordenine with the immunoassays and thin layer chromatography methods. Forensic Sci Int. 1992;54(1):9–22.
Hoult AHC, Lovett JV. Biologically active secondary metabolites of barley. III. A method for identification and quantification of hordenine and gramine in barley by high-performance liquid chromatography. J Chem Ecol. 1993;19(10):2245–54.
Nelson B, Putzbach K, Sharpless K, Sander L. Mass spectrometric determination of the predominant adrenergic protoalkaloids in bitter orange (Citrus aurantium). J Agric Food Chem. 2007;55(24):9769–75.
Chen Y, Meng J, Zou J, An J. Selective extraction based on poly(MAA-VB-EGMDA) monolith followed by HPLC for determination of hordenine in plasma and urine samples. Biomed Chromatogr. 2015;29(6):869–75.
Ma J, Wang S, Huang X, Geng P, Wen C, Zhou Y, et al. Validated UPLC-MS/MS method for the determination of hordenine in rat plasma and its application to pharmacokinetic study. J Pharm Biomed Anal. 2015;111:131–7.
Guo K, Ji C, Li L. Stable-isotope dimethylation labeling combined with LC − ESI MS for quantification of amine-containing metabolites in biological samples. Anal Chem. 2007;79(22):8631–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments in this study comply with current German laws.
Conflict of interest
The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 93 kb)
Rights and permissions
About this article
Cite this article
Steiner, I., Brauers, G., Temme, O. et al. A sensitive method for the determination of hordenine in human serum by ESI+ UPLC-MS/MS for forensic toxicological applications. Anal Bioanal Chem 408, 2285–2292 (2016). https://doi.org/10.1007/s00216-016-9324-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9324-3