Abstract
Humic substances (HS) are complex and heterogeneous mixtures of organic compounds that occur everywhere in the environment. They represent most of the dissolved organic matter in soils, sediments (fossil), water, and landfills. The exact structure of HS macromolecules has not yet been determined because of their complexity and heterogeneity. Various descriptions of HS are used depending on specific environments of origin and research interests. In order to improve the understanding of the structure of HS extracted from landfill leachate (LHS) and commercial HS from leonardite (HHS), this study sought to compare the composition and characterization of the structure of LHS and HHS using elemental composition, chromatographic (high-performance liquid chromatography (HPLC)), and spectroscopic techniques (UV–vis, FTIR, NMR, and MALDI-TOF). The results showed that LHS molecules have a lower molecular weight and less aromatic structure than HHS molecules. The characteristics of functional groups of both LHS and HHS, however, were basically similar, but there was some differences in absorbance intensity. There were also less aliphatic and acidic functional groups and more aromatic and polyphenolic compounds in the humic acid (HA) fraction than in the fulvic acid (FA) and other molecules (OM) fractions of both origins. The differences between LHS and HHS might be due to the time course of humification. Combining the results obtained from these analytical techniques cold improve our understanding of the structure of HS of different origins and thus enhance their potential use.

Chemical and structural and characterization of landfill leachate and leonardite humic fractions
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Landfill is the most common way of storing waste around the world. This practice leads to the gaseous emissions and liquid leachates that are produced after waste decomposition and rainwater percolation [1]. The leachates usually contain a large spectrum of mineral (salt elements, heavy metals), microbiological, and organic components that could cause serious environmental pollutions of soils, surface and groundwater after migration from the landfills [2, 3]. This has encouraged scientists to look for ways of treating landfill leachates before their emission from landfill sites [3]. Their composition varies considerably, depending on the origin and nature of the disposed waste, the landfill geographic location and the climate conditions [4]. Humic substances (HS) and volatile fatty acids constitute the main organic matter present in landfill leachates.
HS are the most important pool of transient refractory organic carbon in the geosphere. They are highly complex mixtures of heterogeneous substances, naturally formed from the stochastic decomposition of diverse biogenic materials [5], and they represent most of the dissolved organic matter in soils, sediments (fossil), water and landfills. They can directly influence the transport and bioavailability of pollutants (e.g., organic micro-pollutants, toxic molecules and heavy metals) to plants and soil microorganisms [6–8]. HS are usually subdivided into three main fractions based on the polarity and water solubility of their components as a function of the pH: humic acids (HA), which are soluble at alkaline pH but insoluble at acidic pH (<2); fulvic acids (FA), which are soluble in water at any pH value and humins, which are a non-soluble fraction [9]. The boundaries that chemically separate these humic fractions, however, have not yet been clearly identified.
One of the characteristics of any HS is its heterogeneity in terms of elemental composition, chemical functionality and molecular size distribution [10]. Despite many years of research, there is still much debate about the exact chemical structure of HS macromolecules, their molecular weight, and their genesis because of their complexity. Various non-destructive spectroscopic methods, such as nuclear magnetic resonance (NMR) infrared (IR) and ultraviolet–visible (UV–vis), as well as destructive methods, such as elementary analysis, pyrolysis, gas chromatography–mass spectrometry (GC–MS) and high-performance size-exclusion chromatography (HPSEC), have been used to characterize HS [7, 11–13] and have provided qualitative and quantitative information on their molecular structure. A combination of these techniques, however, is required to overcome the limitations of each one and to understand the chemical structure of HS of different origins [14]. Determining the molecular size, weight and structural arrangement of the HS group of components is essential for an adequate understanding of their varied role in environmental processes [15]. This study was conducted, firstly, to characterize the structure and composition of humic fractions extracted from landfill leachate, compared with leonardite using chromatographic and spectroscopic analytical methods and, secondly, to infer possible links between their specific content and the biological effects presented in Tahiri et al. [16]. For the former, the method used was high-performance liquid chromatography (HPLC), whereas for the latter, the methods used were UV–vis, NMR, Fourier transform IR (FTIR) and matrix-assisted laser desorption/ionization time of flight (MALDI-TOF).
Materials and methods
Sources of humic substances
Two sources of HS were used in this study. The first was a commercial formulation extracted from leonardite (Humifirst®, Tradecorp, Belgium) and normalized to 12 % HA and 3 % FA. The second originated from the Cour-au-Bois (CB) landfill site (15 ha) in the Belgian municipality of Braine-le-Château. Operating since 1979, this site receives household and similar waste, non-hazardous and non-toxic industrial waste, inert waste, and bulky household waste. The samples were collected in January 2012 at the pumping station at the temporarily restored C3 cell (3.6 ha). They were driven to the laboratory and stored in the dark in a common house refrigerator until HS extraction and analysis.
Extraction and fractionation of humic substances
HS extraction from the landfill leachate was performed using an acid–base treatment method. The samples were adjusted to pH 10 with the addition of a 3 N KOH solution that solubilized the HA and centrifuged at 10,000g for 15 min (Beckman AvantiTM, J25I, Centrifuge) after 3 h of stirring.
The leachate HS (LHS) extract (supernatant: FA + HA) and the leonardite HS (HHS) were treated in order to isolate three different fractions. First, the extracts were acidified to pH 2 with H2SO4 (37 %) and stored in a cold room overnight for HA precipitation. The samples were recovered by centrifugation for 15 min at 10,000g. The resulting HA pellets was solubilized in a 0.1 N KOH solution and then in distilled water in order to produce HA solutions (fraction 1: HA). The supernatant was then introduced into a tangential ultrafiltration cell (Millipore Prep/ScaleTM TFF Cartridges, 1 kD) at a pressure of 4 bar with a pump variation frequency of 18–20 Hz. After 1 h of ultrafiltration, the retentate containing FA (fraction 2: FA) and the filtrate containing OM (fraction 3: OM <1 kD) were collected separately.
Measurement of humic fraction concentration
The HS concentrations were determined using a Hewlett-Parckard HPLC system (HP 1100 series) equipped with an automatic solvent degasser, a UV–vis spectrophotometric detector with scanning wavelengths ranging between 200 and 800 nm and Agilent PL aquagel-OH® columns (250 mm long, 7.7 mm Ø, 100 kD exclusion limit and poly-OH matrix). Then, 50 μl of filtered (nylon filter, 0.2 μm) humic fractions were injected and the chromatograms were detected at a wavelength of 280 nm. The composition of the mobile phase was water:acetonitrile:sodium hydroxide (2:10:88) and the flow was 1 ml/min. The HS content was quantified with a calibration curve using commercial HA (CHA, Flucka, 53680, Belgique) at different concentrations (100, 200, 400, 600, 800 and 1000 ppm).
Elemental analysis of humic fractions
Different physicochemical parameters of leachate samples were analyzed in the laboratory. Conductivity and pH were measured by a pH meter (WTW model pH 330i). Total nitrogen and NH4 + ware determined by the Kjeldahl and titration methods respectively and the chloride content was determined by the Volhard method. The heavy metal content was measured with an atomic absorption spectrophotometer.
Structural characterization of humic fractions
UV–visible analysis
HS samples were diluted in distilled water and the pH of solution was adjusted to 6–7. UV–vis spectra of the HS samples were obtained with a Shimadzu UV-1200 spectrophotometer within the wavelength range of 200–800 nm. The E2/E3 and E4/E6 ratios (parameters that are inversely related to molecular weight and aromaticity, and proportional to the O, C, and carboxyl group [COOH] content and total acidity) were determined by measuring the absorbance at 254 and 365 nm for E2/E3 ratio and at 465 and 665 nm for E4/E6 ratio.
1H NMR spectroscopy
NMR analysis was performed using a Varian Unity 600-MHz instrument at 298 K. About 50 mg of each sample was dissolved in 1 ml of deuterium oxide (D2O, Sigma, 99.9 % atom D). 1H-NMR spectra were recorded at 600 MHz under the following conditions: 1.7 s acquisition time, 1.0 s relaxation delay and pulse width of 45°.
FTIR spectroscopy
One milligram of freeze-dried humic fraction was mixed, ground with 100 mg of potassium bromide (KBr) and then mechanically pressed to form a pellet. These pellets were used to analyze the humic fractions structure using a Bruker IFS 48 spectrometer. FTIR spectra were recorded in the 4000–400 cm−1 range, with 16 scans performed for each acquisition at a resolution of 4 cm−1.
MALDI-TOF-MS spectrometry
Samples were prepared by dissolving 1 mg of freeze-dried humic fraction in 1 ml of 0.1 % TFA. The matrix 2,5-dihydroxybenzoic acid (DHBA) was dissolved in acetonitrile:water (50:50) with 0.1 % formic acid. All spots were prepared by mixing 1 μl of the sample with 1 μl of matrix (DHBA). MALDI-TOF spectra were recorded on a Bruker Ultraflex II spectrometer in positive and negative reflectron ion mode, using the following parameters:
Positive ion mode | Negative ion mode | |
Ion source voltage 1 | 25 kV | 20 kV |
Ion source voltage 2 | 21.85 kV | 17.55 kV |
Lens voltage | 9.5 kV | 6 kV |
Reflector voltage 1 | 25.96 kV | 21 kV |
Reflector voltage 2 | 13.8 kV | 11 kV |
Ion extraction delay | 30 ns | 30 ns |
Results and discussion
Chemical characteristics of leachate and leonardite humic fractions
HS are mixtures of a wide range of mineral and organic compounds. Leachate quality and composition are therefore site specific and variable, depending on the type of waste deposits, landfill age, geographic parameters, climate conditions and operational practices at the site [17, 18]. A variety of inorganic components, including heavy metals, dissolved organic matter and xenobiotic compounds, can bind together into an HS structure [19]. The chemical properties of CB leachate were presented in Tahiri et al. [20].
The elemental analysis performed in this study (Table 1) show that LHS sources typically contained a much higher concentration of nitrogen (mainly as ammonium) and chloride than the HHS sources and that they were all retrieved in leachate FA (LFA) and leachate OM (LOM) humic fractions, but not in HA, leonardite FA (HFA), or leonardite OM (HOM) fractions. The high amount of ammonia in the LHS could be released after the decomposition of proteins, where it can persist over time [19]. The high concentration of chloride, however, might have anthropogenic origins from the household wastes at the CB landfill. Fe concentration was higher in leonardite humic acids (HHA) and fulvic acids ( HFA) fractions than in leachate. The concentration of heavy metal in LHS and HHS was generally low apart from Pb, which varied between 0.1 and 0.9 mg/l in LHS and between 0.2 and 0.5 mg/l in HHS. Most heavy metals, especially in leachate, could stay trapped in waste mass [21]. François [22] estimated that 95 % of metals remain trapped in waste mass. The difference in heavy metal concentration in the humic fraction is probably due to chelation capacity of each fraction that is primary linked to pH and the content of oxygen containing functional groups, such as COOH and phenolic OH [23].
Structural characterization of leachate and leonardite humic fractions
HPLC
HS separation and quantification were performed using a gel permeation HPLC equipped with Agilent PL aquagel-OH column that separated HS in terms of their molecular weight. The chromatograms of standard HA and humic fractions extracted from leachate and leonardite are shown in Fig. 1. With regard to the standard HA chromatogram showing only one peak (Rt 5.7), two main regions could be distinguished in all humic fractions chromatograms. The first, between a retention time of 4 to 7.5 min, corresponded to high molecular weight molecules (HA) and the second, between 8 and 9 min, corresponded to low molecular weight compounds (FA and OM).
LHS presented two major peaks. The first one corresponded to leachate humic acids (LHA) molecules and the second to LFA and LOM molecules (Fig. 1a). HHS, however, revealed four incompletely resolved peaks. The first three dominant peaks corresponded to the HHA fraction (R t < 7.5) and the last one to the HFA and HOM fractions (Fig. 1b). As reported in the literature, the chromatograms of HS show broad, poorly resolved peaks because of the nature of the mixture of molecules in HS [24]. Given the complex nature of HS, it is possible that each region forms a near-continuum of chemical molecules with similar characteristics that are difficult to separate. Egeberg and Alberts [25] demonstrated that the reverse phase HPLC method separates natural organic matter (NOM) into two well-defined groups of molecules.
The LHA fraction chromatogram shows a distinct peak (R t = 5.6 min). The HHA fraction, however, shows three poorly resolved peaks (R t = 5.5, 6.1 and 6.8 min). The LFA fraction shows a major peak at R t = 8.6 min and a minor one at R t = 6.5 min. In contrast, the HFA fraction shows one peak at R t = 8.1 min and three more peaks that correspond to the rest of the HHA fraction (R t = 4.9, 5.9 and 6.2 min). The low molecular weight fraction (LOM < 1kD) presents a chromatogram similar to the LFA one, but differing in the intensity of peaks. This confirms the great difficulty of isolating individual compounds from the fractions in order to identify them, as noted by Sierra et al. [15].
As in previous studies that used size-exclusion or gel permeation chromatography, strong relationships between molecular size and physicochemical properties of HS were demonstrated. The first eluted peaks, corresponding to HA fractions, were more hydrophobic, with a high molecular weight and more aromatic compounds, than the last eluted peaks corresponding to FA fractions [15, 26–29].
HS quantification was performed using a calibration curve obtained with a standard HA (CHA, Fluka). There was a strong correlation (R 2 = 0.99, p < 0.001) between the total peak area of CHA chromatograms and CHA concentrations (Fig. 1c, d). The total area of each chromatogram was used to calculate HS concentration and distribution in both LHS and HHS without having to perform the extraction procedure.
The HS content measured by HPLC was 20 times higher in leonardite than in leachate (Table 1). In the leonardite humic extract, the HHA fraction was more dominant than HFA and HOM, with 97, 2, and 1 % of total HS, respectively. In the leachate extract, both LHA (43 %) and LFA (37 %) were more dominant than LOM (19 %). This difference between HS relative composition might be due to variation in the original organic matter and in the time course of their degradation. Landfill LHS come from the recent degradation of organic matter, whereas leonardite is the fossil form of HS.
UV–vis spectrometry
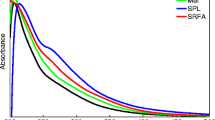
The UV–vis spectra of leonardite and leachate humic fractions recorded from 200 to 800 nm are shown in Fig. 2. In general, the UV–vis spectra of all fractions exhibited an exponential decline with increasing wavelength and strong UV absorption (at λ < 250 nm), which is characteristic of HS. The high absorbance at λ < 250 nm might result from the absorption of radiation by the double bonds, especially the aromatic C = C and ketonic C = O functional groups of aromatic chromophores and/or other organic compounds [30]. HHS spectra showed a higher absorbance than LHS spectra, especially those of HA fractions. There was no noticeable difference, however, in the spectra of the FA and OM fractions of both origins, and these were lower than HA fractions. These observations suggest that HHA has a higher amount of aromatic or polyphenolic organic compounds than LHA or other fractions. Kang et al. [10] also demonstrated that LHS generally had lower absorbance than purified HA and suggested that the aromaticity and molecular weight, known to increase as the humification process proceeds, increased in this HA source compared with HA of leachate origin.
For a deeper understanding of these characteristics, the ratios between absorbance at 254 and 365 nm (E2/E3) and between 465 and 665 nm (E4/E6) were determined (Table 2). These ratios have been reported to be independent of the concentration of humic extract and are not the same as a function of the origin of the extracted HS [31]. The E2/E3 and E4/E6 values of LHS generally presented higher ratios than those of HHS. The HA fraction presented lower ratios in HHA (2.7 and 2.8, respectively) than in LHA (3.5 and 3.0, respectively). High ratios (>5) were observed, however, in the OM fractions of both origins, whereas the FA fractions had an intermediate ratio.
The value of E2/E3 and E4/E6 ratios is known to be inversely related to molecular weight and aromaticity, but proportional to O, C, and COOH content and total acidity [32, 33]. These ratios are often used to characterize HS from different sources. A low ratio (<5) indicates a high degree of condensation of aromatic humic components, high molecular weight and low acidity, which are characteristic of the HA fraction. A high ratio (>5), however, indicates a greater presence of aliphatic compounds, low molecular weight and high acidity, which are characteristic of FA. Our results also showed that LHS had a higher ratio than HHS. This means that the aromaticity, molecular weight and acidity of LHS was relatively lower than HHS, which accords with the results obtained by Kang et al. [10]. In addition, Tahiri et al. [34] reported that the difference between natural HS (e.g., from soil) and those from landfill leachate depends on the humification conditions. Leachate HS have low aromaticity and less oxygen but contain more aliphatic carbon, nitrogen, sulfur, and hydrogen than HS present in soil and aquatic environments [34, 35].
IR spectra spectrometry
FTIR spectroscopy is a method that provides information on molecular vibrations, which are related to specific molecular structure and functional groups [36]. This technique has been widely used for characterizing HS extracted from various sources [10, 14, 37, 38]. The FTIR spectra of LHS and HHS recorded in the 4000–400 cm−1 range are presented in Fig. 3. The recorded spectra indicate some differences between HS from leachate and leonardite sources. The spectra of FA and OM fractions of both origins are similar, with the main difference being the relative intensity of some absorption bands. Most of them are in the regions of 3300–3400 cm−1 (H-bonded OH groups and trace N-H stretching), 1400 cm−1 (O–H deformation, CH3 bending, C–O stretching of phenolic OH, and COO− anti-symmetric stretching), 1110 cm−1 (C–OH stretch of aliphatic alcohol) and 617 cm−1, which corresponds to the deformation of −COOH or the Si–O vibration of silicate impurities. The intensities of these peaks were higher for the FA and OM fractions than the HA fractions of both origins.
The LHA spectra had bands that were similar to those of the HHA fraction, with some differences at 2940, 880 and 700 cm−1, which can be attributed to aliphatic CH2 and CH3, aromatic C–H vibrations, and COOH deformation, respectively. In addition, both LHA and HHA spectra had similar bands around 3400–3300 cm−1 (O–H and N–H stretch), 1580 cm−1 (aromatic C = C, C = O, COO–), 1380 cm−1 (O–H deformation, CH3 bending, C–O stretching of phenolic OH and COO− anti-symmetric stretching), 1110 cm−1 (C–OH stretch of aliphatic alcohol) and 618 cm−1 (deformation of −COOH). These results indicate that the characteristics of the functional groups of both LHS and HHS were basically similar. There were some differences in absorbance intensity, indicating a potential difference in functional group distribution. These results accord with those reported by Kang et al. [10]. Both the leachate and leonardite FA and OM fractions presented a stronger band at around 1110 and 618 cm−1 than the HA fractions. This means that FA and OM contain more aliphatic and acidic groups (C–OH stretch of aliphatic alcohol and −COOH bound) than HA fractions. In contrast to the FA and OM fractions, the HA fractions presented a strong band at around 3400 and 1550–1360 cm−1 that could be attributed to aromatic and phenolic compounds. This indicates that HA contain more aromatic compounds than other fractions.
1H-NMR spectrometry
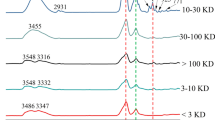
In order to obtain additional information on each sample, one-pulse proton experiments were performed. Based on an earlier study [10, 39–42], 1H-NMR spectra were subdivided into three main resonance regions (Fig. 4). The first was the aliphatic protons region (0.5–3.0 ppm); the second (3.0–4.5 ppm) was attributed to a broad resonance assigned to protons on carbons attached to O or N atoms (carbohydrate); and the third was the aromatic, phenol, and carboxylic proton region (6–9 ppm). LHS and HHS had similar chemical shift values, but they differed in intensity and linewidth. Comparing the different fractions, LFA and LOM fractions had very similar chemical shift values. The HFA and HOM fractions, however, had a slightly different spectrum, and the LHA and HHA spectra showed a range of different peaks. In general, the spectra of all fractions showed several highly resolved signals in the 0.8–4.5 ppm region and very weak resonances in the aromatic region (6.0–9.0 ppm).
In contrast to HHS, LHS showed a poorly resolved aromatic carbohydrate proton region and an intense and broad region attributed to the aliphatic region. The spectra of the HA fraction of both origins showed a highly resolved signal at 8.4 ppm arising from formate (HCO2 −) [42], which is absent in the FA and OM fractions of both origins. A relatively high amount of aromatic proton, between 6.5 and 8.0 ppm, was observed only in the HHS, especially in the HHA fraction and less in HFA, indicating that HHS contain more aromatic structures than LHS. In the second region (3.0–4.5 ppm, carbohydrates, proteins), there was proportionally less resonance intensity in LHS than in HHS. A strong signal, however, with some narrow components attributed to sugar-like components [42], was present in HHS. The resonance signals at 3.5–3.8 ppm could be attributed to CHOH and CH2OH functional groups, which typically occur in lignin and polysaccharides moieties, as reported by Adani et al. [43]. These signals are stronger in HHS than LHS, indicating that HHS have a higher molecular weight than LHS. Aminomethine groups [−CH(NH−)] and methylene groups bonded to amide functional groups [−CH2(NHCO−)], which had resonance signals between 3.1–3.3 ppm, were present in the HA fractions of both origins and absent in other fractions.
In the aliphatic region, characteristic peaks between 2.5 and 1.2 ppm (especially at 2.1, 1.9, and 1.2 ppm) were observed in all spectra. These peaks are believed to be attached to aliphatic carbons (methyl or methylene groups), which are themselves attached to functional groups (carboxyl group or aromatic ring) [43]. A peak at 2.8 ppm assigned to protons in αCH3 of acetoacetate bound to the HS macrostructure was recorded only in the HA fraction of both origins [44, 45]. A peak at 0.8–2.0 ppm (assigned mainly to CH2 groups) was recorded only in LHS.
In general, LHS contain more protons of aliphatic components than HHS. They also contain fewer aromatic and carbohydrate protons. These results are consistent with those obtained previously from the UV–vis and FTIR spectra and reported by Kang et al. [10].
The intensity of the signals for the aliphatic, aromatic, and carboxyl groups tended to vary, depending on the origin and type of humic matter. The FA fractions and low molecular weight molecules (OM) exhibited spectra dominated by strong signals in the aliphatic region and sometime with signals for aromatic protons that were often manifested as very weak peaks. The HA fractions, however, showed spectra with weaker signals in the aliphatic regions and very strong signals in the aromatic regions [46].
MALDI-TOF-MS
In order to gain more information about the structure and composition of LHS and HHS, a MALDI-TOF-MS spectrometry study was performed. This spectrometric technique does not have limitations with regard to the molecular weight of the analytes [47]. It is also an appropriate technique because it provides minimum noise and the highest resolution and degree of ionization, but it cannot identify individual molecules [48]. The choice of matrix for the HS analysis was based on the literature [48, 49]. Mugo and Bottaro [49] demonstrated that DHBA was the most suitable matrix for HS characterization.
The MALDI spectra of LHS and HHS obtained with the DHBA matrix were acquired in both positive and negative ion mode (Figs. 5 and 6). The sample spectra recorded with DHBA produced low spectral background arising from the matrix and the matrix peaks were almost completely suppressed, causing no interference with analyte peaks, as demonstrated by Lyubomirova and Djingova [48].
In positive ion mode (Fig. 5), most of the peaks were in the m/z region between 200 and 1000 m/z, the same as that observed in Antarctic soil HA samples by Gajdǒsová et al. [50]. Significant differences were observed between HS samples. This could be linked to differences in the functional groups (e.g., carboxylic, phenolic) or multiple alkyl groups (CH2). The relative abundance of each structure depended on the HS source (leachate or leonardite). In addition, a number of dominant common peaks were observed in all the humic fractions, regardless of origin, at 360.9, 392.7, 422.7 and 476.9 m/z, with some differences in the intensity of these peaks. The presence of common peaks in all fractions accorded with the hypothesis that common structural components are shared by all organic matter, irrespective of origin [49].
HHS showed more peaks than LHS, especially in the HA and FA fractions. It is important to note that the spectra of humic fractions of the same origin were similar, suggesting the presence of the same compounds but in different amounts. The highest masses observed in LHS were at 392.8 m/z for both LFA and LOM and at 422.7 m/z for LHA. In HHS, however, the highest masses were at 318.1, 338.9 and 360.9 m/z for HOM, HFA, and HHA, respectively.
A prominent repeating pattern of peaks separated by 14, 18, 28 and 58 Da mass spacing was clearly observed in all fractions, suggesting the quasi-polymeric or oligomeric structure of HS. These repeating patterns can be attributed to the loss of −CH2, H2O, −CO and −C3H6O− units, respectively, as described in the literature for FA and HA [49, 50]. The 28- and 58-Da mass spacing was more abundant in LHS, whereas the 14 and 18 Da mass spacing was more abundant in HHS.
In negative ion mode (Fig. 6), most peaks were observed in the m/z region between 250 and 500 m/z. The spectra of all fractions of both origins showed similar specific peaks at 270.2, 288.3, 314.4, 344.3, 360.4 and 387.6 m/z, with differences in the intensity. The most intensive peaks were observed at 314.3 for LFA and LOM and at 344.4 m/z for LHA. For HHS, the most intensive peaks were observed at 288.3 m/z for HHA, 344.3 m/z for HFA, and 360.4 m/z for HOM.
The comparison between the spectra of negative and positive ionization modes indicated that the spectra in negative ion mode were less intensive and contained fewer peaks than in positive ion mode. These results are in contrast to those reported by Lyubomirova and Djingova [48], who found that positive ion mode presented less intensive and fewer peaks than negative ion mode because of a low concentration of total N2 (0.5 %) and the small number of NH2 groups in the HA composition. In addition, peaks at 328.3 and 360.4 m/z were present in both ionization modes, indicating that these peaks presented double-charged fragments.
The results obtained for both ionization modes indicated that, the presence of specific peaks, the way in which the MALDI peaks were grouped and the global shape of spectra, reflected the global structure of HS. In addition, the molecular weight of all fractions was below 1000 Da. This could suggest that HS are supramolecular structures formed by variable molecules bound together by numerous forces, including weak Vander Waals forces, to form a macromolecule, as already hypothesized [9, 51, 52].
Conclusions
In this study, we extracted HS from CB landfill leachate. The LHS, as well as the HHS derived from Humifirst, a commercial product, were fractionated into HA, FA, and OM as a function of their solubility and molecular weight. Chromatographic and spectroscopic characterizations of these fractions were performed. The results indicated that the structural characteristics of functional groups of both LHS and HHS were basically similar, with some differences in absorbance intensity. Elemental analysis showed that LHS (apart from the HA fraction) typically had a much higher nitrogen (mainly as ammonium) and chloride concentration than HHS and that heavy metal concentration was low in both cases. HPLC analysis indicated that the HA fractions were more hydrophobic and had a higher molecular weight and more aromatic compounds than the FA and OM fractions. Spectral features obtained from UV/Vis, FTIR, 1H NMR spectroscopy and MALDI-TOF spectrometry showed that LHS had low molecular weight molecules and fewer aromatic structures than HHS. This might be due to the difference in the time course of organic matter degradation between the two HS origins. We also found that there was a higher degree of condensation of the aromatic components, higher molecular weight, and lower acidity in the HA fractions than in the FA and OM fractions of both origins. This suggests that HA fractions have fewer aliphatic and carboxylic compounds, but more aromatic and phenolic compounds.
HS of different origins (especially leonardite) are being used commercially as organic fertilizers. The results of this study show that there are some similarities between LHS and HHS, suggesting that LHS could be used as a bio-fertilizer to improve soil structure or as a biostimulant to improve plant growth. While, the differences observed between humic fractions could be used to explain the difference in their biological activity. For these purposes, further studies will be needed to investigate and compare the biological activity of leachate and leonardite HS on plant growth and development.
Abbreviations
- FA:
-
Fulvic acids
- HA:
-
Humic acids
- HFA:
-
Leonardite fulvic acids
- HHA:
-
Leonardite humic acids
- HHS:
-
Leonardite HS
- HOM:
-
Leonardite other molecules
- HS:
-
Humic substances
- LFA:
-
Leachate fulvic acids
- LHA:
-
Leachate humic acids
- LHS:
-
Leachate HS
- LOM:
-
Leachate other molecules
References
Vilar V, Rocha E, Mota F, Fonseca A, Saraiva I, Boaventura R. Treatment of a sanitary landfill leachate using combined solar photo-Fenton and biological immobilized biomass reactor at a pilot scale. Water Res. 2011;45(8):2647–58. doi:10.1016/j.watres.2011.02.019.
Sang N, Han M, Li GK, Huang M. Landfill leachate affects metabolic responses of Zea mays L. seedlings. Waste Manage. 2010;30:856–62. doi:10.1016/j.wasman.2010.01.023.
Zhao R, Novak JT, Goldsmith CD. Evaluation of on-site biological treatment for landfill leachates and its impact: a size distribution study. Water Res. 2012;46:3837–48. doi:10.1016/j.watres.2012.04.022.
Renou S, Givaudan JG, Poulaina S, Dirassouyan F, Moulin P. Landfill leachate treatment: review and opportunity. J Hazard Mater. 2008;150:468–93. doi:10.1016/j.jhazmat.2007.09.077.
Hou D, He J, Lü C, Wang W, Zhang F. Spatial distributions of humic substances and evaluation of sediment organic index on Lake Dalinouer, China. J Geophys Res. 2014;2014:1–13. doi:10.1155/2014/502597.
Xiaoli C, Shimaoka T, Qiang G, Youcai Z. Characterization of humic and fulvic acids extracted from landfill by elemental composition, 13C CP/MAS NMR and TMAH-Py-GC/MS. Waste Manage. 2008;28:896–903. doi:10.1016/j.wasman.2007.02.004.
Canellas LP, Zandonodi DB, Busato JG, Baldotto MA, Simoes ML, Martin-Neto L, et al. Bioactivity and chemical characteristics of humic acids from tropical soils sequence. Soil Sci. 2008;173(9):24–637. doi:10.1097/SS.0b013e3181847ebf.
Badis A, Ferradji FZ, Boucherit A, Fodil D, Boutoumi H. Characterization and biodegradation of soil humic acids and preliminary identification of decolorizing actinomycetes at Mitidja plain soils (Algeria). Afri J Microbiol Res. 2009;3(13):997–1007.
Piccolo A. The supramolecular structure of humic substances. A novel understanding of humus chemistry and implications in soil science. Adv Agron. 2002;75:57–134. doi:10.1016/S0065-2113(02)75003-7.
Kang KH, Shin HS, Park H. Characterization of humic substancs present in landfill leachates with different ages and implications. Water Res. 2002;36(16):4023–32. doi:10.1016/S0043-1354(02)00114-8.
Abbt-Braun G, Lankes U, Frimmel FH. Structural characterization of aquatic humic substances—the need for a multiple method approach. Aquat Sci. 2004;66:151–70. doi:10.1007/s00027-004-0711-z.
Chung TL, Chen JS, Chiu CY, Tian G. 13C-NMR spectroscopy studies of humic substances in subtropical perhumid montane forest soil. J For Res. 2011;17:1–10. doi:10.1007/s10310-011-0319-9.
Nasir S, Sarfaraz TB, Verheyen TV, Chaffee AL. Structural elucidation of humic acids extracted from Pakistani lignite using spectroscopic and thermal degradative techniques. Fuel Process Technol. 2011;92(5):983–91. doi:10.1016/j.fuproc.2010.12.020.
Fernández-Gómez MJ, Nogales R, Plante A, Plaza C, Fernández JM. Application of a set of complementary techniques to understand how varying the proportion of two wastes affects humic acids produced by vermicomposting. Waste Manage. 2015;35:81–8. doi:10.1016/j.wasman.2014.09.022.
Sierra MD, Giovanela M, Parlanti E, Soriano-Sierra EJ. 3D-Fluorescence spectroscopic analysis of HPLC fractionated estuarine fulvic and humic acids. J Braz Chem Soc. 2006;17(1):113–24. doi:10.1590/S0103-50532006000100017.
Tahiri A, Destain J, Thonart P, Druart P. In vitro model to study the biological properties of humic fractions from landfill leachate and leonardite during root elongation of Alnus glutinosa L. Gaertn and Betula pendula Roth. Plant Cell Tiss Organ Cult. 2015;122(3):739–49. doi:10.1007/s11240-015-0807-2.
Thonart P, Steyer E, Drion R, Hiligsmann S. La gestion biologique d’une décharge. Tribune de l’Eau. 1998;590(591):3–12.
Sang N, Li GK, Xin XY. Municipal landfill leachate induces cytogenetic damage in root tips of Hordeum vulgare. Ecotoxicol Environ Saf. 2006;63:469–73. doi:10.1016/j.ecoenv.2005.02.009.
Kjeldsen P, Barlaz MA, Rooker AP, Baum A, Ledin A, Christensen TH. Present and long-term composition of MSW landfill leachate. A review. Crit Rev Environ Sci Technol. 2002;32(4):297–336. doi:10.1080/10643380290813462.
Tahiri A, Destain J, Thonart P, Druart P. Valorization and properties of landfill leachates humic substances. J Mater Environ Sci. 2014;5(S2):2495–8.
Berthe C. Etude de la Matière Organique contenue dans des lixiviats issus de différentes filières de traitement des déchets ménagers et assimilés. France: Thèse de doctorat, Université de Limoges; 2006.
François V. Détermination d’indicateurs d’accélération et de stabilisation de déchets ménagers enfouis. Etude de la recirculation de lixiviats sur colonnes de déchets. Thèse de doctorat, Université de Limoges. 2004.
Rose MT, Patti AF, Little KR, Brown AL, Jackson WR, Cavagnaro TR. A meta-analysis and review of plant-growth response to humic substances: practical implications for agriculture. Adv Agron. 2014;124:37–89. doi:10.1016/B978-0-12-800138-7.00002-4.
Ryan D, Zhu B. Humic substances: high performance liquid chromatography. Ref Mod Chem Mol Sci Chem Eng. 2013. doi:10.1016/B978-0-12-409547-2.04781-8.
Egeberg PK, Alberts JJ. Determination of hydrophobicity of NOM by RP-HPLC, and the effect of pH and ionic strength. Water Res. 2002;36:4997–5004. doi:10.1016/S0043-1354(02)00228-2.
Her N, Amy G, Foss D, Cho J. Variations in molecular weight estimation by HP-size exclusion chromatography with UVA versus online DOC detection. Environ Sci Technol. 2002;36:3393–9. doi:10.1021/es015649y.
Wu FC, Evans RD, Dillon PJ. High-performance liquid chromatographic fractionation and characterization of fulvic acid. Anal Chim Acta. 2002;464:47–55. doi:10.1016/S0003-2670(02)00476-2.
Wu FC, Evans RD, Dillon PJ, Cai YR. Rapid quantification of humic and fulvic acids by HPLC in natural waters. Appl Geochem. 2007;22:1598–605. doi:10.1016/j.apgeochem.2007.03.043.
Hutta M, Gora R, Halko R, Chalanyova M. Some theoretical and practical aspects in the separation of humic substances by combined liquid chromatography methods. J Chromatog A. 2011;1218:8946–57. doi:10.1016/j.chroma.2011.06.107.
Chen J, Gu B, Le Boeuf EJ, Pan H, Dai S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere. 2002;48:59–68. doi:10.1016/S0045-6535(02)00041-3.
Pansu M, Gautheyrou J. Handbook of soil analysis: mineralogical, organic and inorganic methods. Berlin Heidelberg: Springer; 2006. p. 399–451.
Chin YP, Aiken G, O’Loughlin E. Molecular weight, polydispersity, and spectroscopic properties of aquatic humic substances. Environ Sci Technol. 1994;28(11):1853–8. doi:10.1021/es00060a015.
Uyguner CS, Bekbolet M. Evaluation of humic acid photocatalytic degradation by UV–Vis and fluorescence spectroscopy. Catal Today. 2005;101(3):267–74. doi:10.1016/j.cattod.2005.03.011.
Tahiri A, Destain J, Druart P, Thonart P. Propriétés physico-chimiques et biologiques des substances humiques en relation avec le développement végétal. Biotechnol Agron Soc Environ. 2014;18(3):336–45.
Zhang L, Li A, Lu Y, Yan L, Zhong S, Deng C. Characterization and removal of dissolved organic matter (DOM) from landfill leachate rejected by nanofiltration. Waste Manage. 2009;29:1035–40. doi:10.1016/j.wasman.2008.08.020.
Atkins P, De Paula J. Physical chemistry. 9th ed. New York: Oxford University Press; 2009.
Baigorri R, Fuentes M, González-Gaitano G, García-Mina JM, Almendros G, González-Vila FJ. Complementary multianalytical approach to study the distinctive structural features of the main humic fractions in solution: gray humic acid, brown humic acid, and fulvic acid. J Agric Food Chem. 2009;57(8):3266–72. doi:10.1021/jf8035353.
Baglieri A, Vindrola D, Gennari M, Negre M. Chemical and spectroscopic characterization of insoluble and soluble humic acid fractions at different pH values. Chem Bioll Technol Agr. 2014;1:9. doi:10.1186/s40538-014-0009-x.
Russell, Stokes AR, Macdonald H, Muscolo A, Nardi S. Stomatal responses to humic substances and auxin are sensitive to inhibitors of phospholipase A2. Plant Soil. 2006;283:175–85. doi:10.1007/s11104-006-0011-6.
Schiavon M, Pizzeghello D, Muscolo A, Vaccaro S, Francioso O, Nardi S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J Chem Ecol. 2010;36:662–9. doi:10.1007/s10886-010-9790-6.
Trubetskoj O, Hatcher PG, Trubetskaya OE. 1H-NMR and 13C-NMR spectroscopy of chernozem soil humic acid fractionated by combined size-exclusion chromatography and electrophoresis. Chem Ecol. 2010;26(4):315–25. doi:10.1080/02757541003785825.
Ertani A, Francioso O, Tugnoli V, Righi V, Nardi S. Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J Agric Food Chem. 2011;59:11940–8. doi:10.1021/jf202473e.
Adani F, Genevini P, Tambone F, Montoneri E. Compost effect on soil humic acid: a NMR study. Chemosphere. 2006;65:1414–8. doi:10.1016/j.chemosphere.2006.03.070.
Fan TWM. Metabolite profiling by one- and two-dimensional NMR analysis of complex mixtures. Progr Nucl Magn Reson Spectrosc. 1996;28:161–219. doi:10.1016/0079-6565(95)01017-3.
Muscolo A, Sidari M, Attinà E, Francioso O, Tugnoli V, Nardi S. Biological activity of humic substances is related to their chemical structure. Soil Sci Soc Am J. 2007;71:75–85. doi:10.2136/sssaj2006.0055.
Kim HT. Humic matter in soil and the environment. Principles and controversies. CRC Press 294p. 2014.
Hillenkamp F, Peter-Katalinic J. MALDI MS: a practical guide to instrumentation, methods and applications. Hardcover. Wiley-Blackwell, 480p. 2007.
Lyubomirova V, Djingova R. Mass spectrometric techniques for characterisation of platinum–humic substance complexes in soil and street dust samples. Chem Spec Bioavailab. 2013;25(4):223–34. doi:10.3184/095422913X13844520283032.
Mugo SM, Bottaro CS. Characterization of humic substances by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2375–82. doi:10.1002/rcm.1635.
Gajdǒsová D, Pokorná L, Láska K, Prosěk P, Havel J. Are there humic acids in Antarctica? In: Davies G, Ghabbour EA, editors. Humic substances structures, models and functions. Cambridge: Royal Society of Chemistry; 2001. p. 121–31.
Peña-Méndez EM, Gajdošová D, Novotná K, Prošek P, Havel J. Mass spectrometry of humic substances of different origin including those from Antarctica. A comparative study. Talanta. 2005;67:880–90. doi:10.1016/j.talanta.2005.03.032.
Gajdǒsová D, Novotná K, Prosěkb P, Havela J. Separation and characterization of humic acids from Antarctica by capillary electrophoresis and matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Inclusion complexes of humic acids with cyclodextrins. J Chromato A. 2003;1014:117–27. doi:10.1016/S0021-9673(03)01040-9.
Acknowledgments
The authors would like to thank the TRADECORP Company for supplying its “Humifirst” formulation and the Van Gansewinkel Groep (Cour-au-Bois landfill) for providing the leachate needed to conduct our study. We also thank Maesen Ph. (GxABT-ULg) for the chemical analysis; De Pauw E. and Smargiasso N. (GIGA-Ulg) for the MALDI-TOF analysis; and Salvé A., Chemotti C., and Byttebier V. for their help in the chemical analysis and their technical assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Tahiri, A., Richel, A., Destain, J. et al. Comprehensive comparison of the chemical and structural characterization of landfill leachate and leonardite humic fractions. Anal Bioanal Chem 408, 1917–1928 (2016). https://doi.org/10.1007/s00216-016-9305-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9305-6