Abstract
Microemulsion electrokinetic chromatography (MEEKC) is a powerful tool to separate neutral species based on differences in their hydrophobic and hydrophilic properties. However, as a major drawback the conventionally used SDS based microemulsions are not compatible with electrospray ionization mass spectrometry (ESI-MS). In this work, a surfactant-free microemulsion (SFME) consisting of water, ethanol, and 1-octanol is used for surfactant-free microemulsion electrokinetic chromatography (SF-MEEKC). Ammonium acetate was added to the SFME enabling electrophoretic separations. The stability of SFMEs containing ammonium acetate was investigated using small-angle X-ray scattering and dynamic light scattering. A method for the separation of a model system of hydrophobic and hydrophilic neutral vitamins, namely the vitamins B2 and D3, and the cationic vitamin B1 was developed using UV/VIS detection. The influence of the ammonium acetate concentration on the separation performance was studied in detail. The method was characterized concerning reproducibility of migration times and peak areas and concerning the linearity of the calibration data. Furthermore, SF-MEEKC was coupled to ESI-MS investigating the compatibility between SFMEs and the ESI process. The signal intensities of ESI-MS measurements of the model analytes were comparable for SFMEs and aqueous systems. Finally, the vitamin D3 content of a drug treating vitamin D3 deficiency was determined by SF-MEEKC coupled to ESI-MS using 25-hydroxycholecalciferol as an internal standard.

The concept of surfactant-free microemulsion electrokinetic chromatography coupled to electrospray ionization mass spectrometry
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capillary electrophoresis (CE) is a well-established analytical technique for the separation of charged analytes. There are special modes of CE that enable also the separation of neutral compounds using micellar solutions as background electrolytes (BGE). The corresponding technique is termed micellar electrokinetic chromatography (MEKC) [1]. In addition, microemulsion electrokinetic chromatography (MEEKC) is another CE separation method that typically uses a BGE consisting of an oil-in-water microemulsion to separate charged and neutral analytes based on electrophoresis and partition chromatography. Charged analytes migrate according to their size and charge whereas neutral analytes can be separated based on their hydrophobic and hydrophilic properties due to different interactions with microemulsion oil droplets [2, 3]. The thermodynamically stable and transparent microemulsions are typically composed of oil, water, surfactant, and co-surfactant forming nanometer-sized stable oil droplets in aqueous buffer [3]. Sodium dodecyl sulfate (SDS) is the most commonly used surfactant in MEEKC leading to negatively charged oil droplets, where the concentration of SDS determines the charge-to-size ratio of the droplet. This ratio affects the electrophoretic mobility and the migration time of the analytes that interact with the charged microemulsion droplets [3]. Apart from SDS, a microemulsion based on sodium bis(2-ethylhexyl) sulfosuccinate was used for MEEKC to determine natural and synthetic estrogens simultaneously by Lucangioli and co-workers [4]. MEEKC has been applied, furthermore, to several model mixtures such as steroids [5], preservatives in pharmaceutical products [6, 7] and foods [8], amino acid derivatives [9], as well as neutral products [10].
Furthermore, the comparison between micellar and microemulsion electrokinetic chromatography for the determination of water- and fat-soluble vitamins has been done by Terabe and Matsubara [11]. It was found that the separation efficiency is higher using MEEKC as the composition of the microemulsion droplets leads to a higher solubility of hydrophobic molecules compared to the micellar structures. MEEKC has also been used as a suitable tool for lipophilicity determination of acidic, neutral, and basic compounds [12].
MEEKC is most commonly coupled to UV/VIS detectors [13, 14]. As many organic compounds show UV absorbance, it is a useful technique for pharmaceutical applications. However, UV/VIS detection has a major drawback due to its relatively low sensitivity and selectivity [15] as a consequence of the short optical path length of the capillary detection window [13, 14]. Coupling to alternative detection techniques has been described such as laser-induced fluorescence detection [9], electrochemical detection [16], and inductively coupled plasma mass spectrometry [17].
MS detection has major advantages compared to conventional UV detection due to its high selectivity, low detection limits, and versatility [18, 19]. However, MEEKC with typically used SDS-based microemulsions has major limitations when it comes to the hyphenation to electrospray ionization mass spectrometry (ESI-MS), the most powerful detector in combination with separation techniques, due to the non-volatile properties of SDS [19].
A new generation of surfactant-free microemulsions (SFMEs) has been recently studied by Klossek et al. [20]. Although such SFMEs were already observed and introduced by Barden and coworkers in the 1970s [21], it took a long time to characterize such systems and to understand the origin of their structuring. To get detailed insight into these structures, static and dynamic light scattering (DLS), as well as small-angle X-ray scattering (SAXS) and neutron scattering have been applied [20, 22, 23].
SFMEs basically consist of three components. Component A (usually water), which is completely mixable with a component B (usually a hydrotrope, like ethanol), but poorly miscible with the hydrophobic component C (e.g., 1-octanol), that in turn, is fully miscible with component B. In such systems, fine and remarkably time-stable emulsions can be formed in the two-phasic region, e.g., by adding a sufficient amount of water to a mixture of ethanol and 1-octanol. This phenomenon is known as the ouzo effect [24]. But what is more and of particular interest here, scattering experiments and molecular dynamics simulations revealed that in such systems well-defined aggregates on a nanometer scale can occur already in the monophasic region (near the phase separation border, but still far away from critical fluctuations) [22, 25]. This observation was called the “pre-ouzo” effect [20]. Ternary mixtures in this pre-ouzo region are thermodynamically stable, transparent, homogenous solutions, just as classical microemulsions. The aggregates inside are also similar to structures in conventional surfactant-based microemulsions and consist of a highly fluctuating oil-rich core in an outer aqueous pseudo-phase. The hydrotrope is distributed over both pseudo-phases, accumulating at the interface of the oil-rich domain. Furthermore, the thermodynamic stability of these aggregates could recently be explained and even predicted theoretically by an extended Derjaguin-Landau-Verwey-Overbeek (DLVO) theory [26]. Even bicontinuous or reverse aggregates can be formed, depending on the components and compositions. For these reasons, SFMEs provide a promising alternative to surfactant-based microemulsions for several applications [27–29].
In this work, the use of this new generation of SFMEs containing water, ethanol, and 1-octanol for SF-MEEKC is described for the first time. Ammonium acetate was added as electrolyte. The stability of the BGE was investigated using SAXS and DLS. A method for the separation of a model system consisting of hydrophobic and hydrophilic neutral vitamins with SF-MEEKC coupled to UV/VIS detection was developed and optimized. Furthermore, compatibility between the SF-MEEKC and the ESI process was investigated. Finally, the method was applied to a real sample, namely the quantification of vitamin D3 in a drug treating vitamin D3 deficiency using an internal standard.
Experimental
Chemicals and materials
Chemicals
Ethanol was purchased from Sigma-Aldrich (Steinheim, Germany, ≥99.8%). Ammonium acetate (≥96.0%), 1-octanol (≥99.0%), sodium hydroxide solution, formic acid, and propan-2-ol were purchased from Merck (Darmstadt, Germany). All chemicals were used without further purification. Water was purified using a Milli-Q System (Millipore, Bedford, MA, USA). The vitamins B1, B2, and D3 were obtained from Sigma-Aldrich (MO, USA) as well as 25-hydroxycholecalciferol, which was used as an internal standard in SF-MEEKC-MS experiments. The vitamins were stored at 4 °C and kept in the dark to avoid decomposition.
Buffer and sample preparation
SFMEs were prepared by weighing water, ethanol, and 1-octanol in the following proportions: 37.5% water, 43.75% ethanol, and 18.75% 1-octanol (weight fraction). Ammonium acetate was used to prepare the BGE in different concentrations (mmol kg−1 of SFME). The order of addition was found to be irrelevant for the formation of the SFME, as it could be expected for a thermodynamically stable system. All solutions were filtered through 0.45-μm PTFE Rotilabo® syringe filters (Carl Roth, Karlsruhe, Germany). The amount of ammonium acetate was varied (50, 100, 150, 200, and 250 mmol kg−1) to study its effect on the separation performance and is therefore specified further in the experiments.
Standard stock solutions of each vitamin were prepared daily by weighing certain amounts of each vitamin and dissolving them in the SFME. The final concentrations of standard stock solution for vitamin B1 and vitamin D3 were 10 and 0.085 mg mL−1 for vitamin B2. Samples were prepared daily by diluting the stock solutions appropriately with BGE and filtering them through 0.45-μm syringe filters before use.
Capillary preparation
Fused silica capillaries were obtained from Polymicro Technologies (Phoenix, AZ, USA). The capillaries (outer diameter (OD) = 360 μm, inner diameter (ID) = 50 μm) were cut properly (total and effective lengths are specified further in the experiments) and the ends were polished with polishing paper (grid size 12 and 30 μm) to create a smooth surface. For CE-UV/VIS experiments a detection window (5–10 mm) was made by removing the polyimide coating with a microtorch. For CE-ESI-MS experiments, the polyimide coating was removed from the MS side end of the capillary. Before use, the capillaries were flushed for 15 min with 0.1 mol L−1 NaOH solution, 15 min with Milli-Q water, and 30 min with BGE. For CE-UV/VIS experiments, the capillary was additionally flushed with BGE for 5 min between the experiments to ensure reproducibility of migration times. After the experiments, the capillaries were flushed with water for storage. All solutions used for flushing were filtered through 0.45-μm filters before use.
Experiments
Dynamic light scattering
DLS experiments were performed using a temperature controlled CGS-3 goniometer system from ALV (Langen, Germany) equipped with an ALV-7004/FAST Multiple Tau digital correlator and a vertical-polarized 22-mW HeNe laser (wavelength λ = 632.8 nm). All samples were filtered into dust-free cylindrical light scattering cells (10 mm outer diameter) using a 0.2-μm PTFE membrane filter. The sealed measurement cells could be directly placed in the measurement apparatus. Measurements were performed at a scattering angle of 90° and a temperature of 25 ± 0.1 °C. Data points were collected for 300 s. It should be mentioned that for such systems calculations of droplet sizes is not evident. Possible aggregates, which might be formed in such solutions, are usually highly fluctuating systems. So, we used the DLS results only to have a rough, qualitative picture of the occurrence of aggregates.
Small-angle X-ray scattering
SAXS measurements were performed on a bench built by XENOCS. A sealed molybdenum tube with a 12:∞ multilayer Xenocs mirror (for Mo radiation) was used, coupled to two sets of scatter-less FORVIS slits, to generate a 0.8 × 0.8-mm2 X-ray beam (wavelength λ = 0.071 nm) at the sample position. Samples were placed in 2 mm quartz capillaries. Data were recorded using a large online scanner detector (MAR 345, MAR Research, diameter = 345 mm). A q-range from 0.24 to 30 nm−1 was covered applying off-center detection with an experimental resolution of ∆q/q = 0.05. Data integration of two-dimensional spectra was performed using FIT2D software. Corrections for background (empty cell and detector noise), as well as normalization with a high-density polyethylene film as a standard, were applied. Silver behenate in a sealed capillary was used as the scattering vector calibration standard. All measurements were performed at room temperature and the acquisition time was 3600 s per sample. In order to determine the correlation length and the radii of the aggregates, the Ornstein-Zernike equation was used for data fitting in the low-q-range (0.275–4 nm−1). The Ornstein-Zernike function can be linked to the radius of gyration R G of the aggregates by
where ξ is the correlation length and I 0 the intensity for q = 0 [30, 31]. The scattering vector q is given by
with ϑ being the scattering angle. The average spherical radius R S of the aggregates can be finally estimated as follows:
Hence, the following correlation between correlation length and the spherical radius of the aggregates is valid:
SF-MEEKC-UV/VIS
A laboratory built device consisting of a modified autosampler additionally equipped with a polished syringe needle electrode at the capillary inlet and a 30-kV high-voltage source (ISEG GmbH, Dresden, Germany) was used for all experiments. Control of the CE device was performed using a software developed by the Electronic workshop of the University of Regensburg.
For UV/VIS experiments, a second electrode made of a platinum wire was introduced to the system. A Bischoff Lambda 1010 UV/VIS system was used as detector. The wavelength of 272.0 nm was set for all experiments. Data acquisition was performed using a LabVIEW-based software written by a member of the working group. Injection was performed hydrodynamically by gravity flow. Ensuring equal injection conditions, the difference in height between inlet and outlet vial was kept constant at 10 cm for all experiments.
The performance of the SF-MEEKC-UV/VIS setup was investigated concerning selectivity, linearity, limit of detection (LOD), and reproducibility. If not stated differently, a sample containing 500 μg mL−1 of the vitamins B1 and D3 and 77 μg mL−1 of vitamin B2 was used. First, the resolution of the neutral species was optimized varying the concentration of ammonium acetate in the BGE (50, 100, 150, 200, and 250 mmol kg−1). The reproducibility (n = 9) of the migration times and the peak areas was further investigated using a BGE containing 150 mmol kg−1 ammonium acetate.
A set of five standard solutions was measured three times, respectively, examining the linearity of the calibration curve. The concentrations of the samples were between 100 and 1000 μg mL−1 for vitamin B1 and between 250 and 1250 μg mL−1 for vitamin D3. For vitamin B2, four standard solutions with different concentrations ranging from 65 to 80 μg mL-1 were measured.
SF-MEEKC-ESI-MS
The ESI-MS system consisted of a Bruker micrOTOF (Bruker Daltonics, Bremen, Germany) time-of-flight mass spectrometer (TOF-MS), equipped with an orthogonal ESI source for coupling with capillary electrophoresis. In detail, a coaxial sheath liquid electrospray ionization interface (Agilent, Waldbronn, Germany) was used. The Bruker software micrOTOF control version 2.3 was used for data acquisition. For optimal ESI conditions, positioning of the tip of the capillary inside the interface was controlled by a microscope camera. Sheath liquid (water/isopropanol/formic acid, 49.9/49.9/0.2, v/v/v) was introduced by a syringe pump (KD Scientific, Holliston, MA, USA) with a flow rate of 0.48 mL h−1. Analysis was carried out in positive ion mode. The electrospray voltage was −4 kV (grounded sprayer tip), and other parameters were set as follows: end plate offset, −500 V; dry gas (N2) flow, 4.0 L/min; dry gas temperature, 190 °C; capillary exit, 75 V; skimmer 1, 25.3 V; hexapole 1, 23 V; hexapole RF, 65 Vpp; Skimmer 2, 23 V; lens 1 transfer, 49 μs; lens 1 prepulse storage, 5 μs; mass-to-charge (m/z) ratio, 22–550; spectra acquisition rate, 3 Hz. The mass traces of the analyte model mixture were selected as follows: vitamin B1, m/z 265.15; vitamin B2, m/z 377.19; vitamin D3, m/z 385.39, and 25-hydroxycholecalciferol, m/z 401.40.
The SF-MEEKC system was coupled to ESI-TOF-MS validating the compatibility of SFMEs with ESI by comparing the LOD of vitamins B1 and B2 using SF-MEEKC to a conventional aqueous CE method using 0.1 M acetic acid as BGE. Finally, SF-MEEKC-ESI-TOF-MS was applied for the quantification of vitamin D3 in a drug treating vitamin D3 deficiency using 25-hydroxycholecalciferol as an internal standard (I.S.).
The commercial drug formulation (Vitamin D3 Hevert 4000 IE, Hevert-Arzneimittel GmbH & Co. KG, Nussbaum) for treating vitamin D3 deficiency was bought in a local pharmacy in Regensburg (Germany). Samples were stored in their original packets at room temperature until the analysis was carried out. Drug tablets were finely powdered and one tablet corresponding to 0.1 mg of vitamin D3 was dissolved together with the internal standard in a total volume of 1 mL of the SFME. The solution was vortexed for 5 min, then centrifuged for 10 min at 3500 RPM × g. The supernatant was collected and filtered through a 0.45-μm syringe filter. 520 μL of supernatant was taken and filled up to the total volume of 1 mL for further analysis.
Results and discussion
Composition and structural investigations of the surfactant-free microemulsion
The SFME chosen for application in SF-MEEKC is composed of water, ethanol, and 1-octanol. Figure 1 shows the ternary phase diagram water/ethanol/1-octanol at room temperature, with α indicating the exact composition of the surfactant-free microemulsion in weight fractions used for SF-MEEKC. For sakes of clarity, the two-phasic region was redrawn from data given by Klossek et al. [20]. The mixture of 37.5% water, 43.75% ethanol, and 18.75% octanol (w/w/w) (solution α) was chosen, since recent publications revealed the presence of aggregates of well-characterized shape at this composition [20, 22, 23, 25].
Ternary phase diagram in weight fractions of the system water/ethanol/1-octanol at 25 °C. The two phasic region (shaded) was redrawn by data of Klossek et al. [20]. The macroscopically homogeneous, one-phase region is given in white. The point α corresponds to the surfactant-free microemulsion used for SF-MEEKC consisting of 37.5% water, 43.75% ethanol, and 18.75% 1-octanol (w/w/w)
To be applicable in electrophoresis, ammonium acetate was added in order to establish a well-defined conductivity and in expectation of charging the interface between the two pseudo-phases by ion adsorption. In addition, ammonium acetate has the advantage to be compatible with ESI-MS detection. To exclude unwanted effects on the structuring within the SFME, the influence of the added salt on the structuring of the ternary mixture was investigated by DLS and SAXS measurements. The results of the light scattering experiments are shown in Fig. 2. Correlation functions for 0–250 mmol kg−1 are not significantly altered. However, correlation functions start to break down at 500 mmol kg−1 of added salt. For 1000 and 1500 mmol kg−1, there are already significant deviations compared to the correlation functions corresponding to samples with lower ammonium acetate concentrations. This means that structures, present in the initial ternary solution α, are maintained over a certain concentration range of ammonium acetate and progressively vanish after adding more than 500 mmol kg−1 of the salt. To make an independent check of the occurrence of pre-ouzo aggregates and in order to further analyze the influence of the added salt, SAXS measurements were performed (see Electronic Supplementary Material (ESM) Fig. S1). SAXS measurements for 0, 50, 100, 150, 200, and 250 mmol kg−1 showed a significant low-q scattering accompanied by successively enhanced I 0 values for increasing amounts of ammonium acetate. Radii calculated with the Ornstein-Zernike equation were in the range between 1.9 and 2.3 nm, increasing with increasing amount of ammonium acetate (see ESM Table S1). Addition of the analytes as described in “Buffer and sample preparation” section did not show a notable influence on the radius and on the structuring of the SFME. Further, the scattering results lead to the assumption that the polarizable ions, ammonium, and/or acetate are accumulating at the interface between the two pseudo-phases. The order of separation of the analytes (see “Chemical properties of the model analytes” section) leads to the conclusion that acetate has a higher affinity towards the interface between the water- and octanol-rich domains than the ammonium ions. Consequently, the hydrophobic octanol-rich aggregates can be assumed to be charged negatively overall, which allows successful application in SF-MEEKC. In addition, ammonium acetate could have salted out ethanol towards the interface or into the octanol-rich aggregates [23], which would also explain the increasing radii of these objects.
SF-MEEKC-UV/VIS
Chemical properties of the model analytes
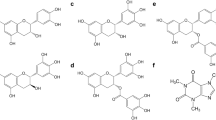
The structures of the analytes are depicted in Fig. 3.
The separation in SF-MEEKC is based on the electrophoretic and partitioning behavior of the analytes. The migration order of neutral compounds is therefore determined by their hydrophobicity, indicating that compounds which are more soluble in the oil phase will migrate differently than the ones that partition more into the aqueous phase [33, 34].
Optimization of SF-MEEKC-UV regarding electrolyte concentration
Figure 4 shows SF-MEEKC-UV separations of the model analytes using varying concentrations of ammonium acetate. Vitamin B1 migrates first as it is positively charged. Neutral fat-soluble species are mostly partitioning into the oil droplets, whereas the neutral hydrophilic species reside mainly in the aqueous phase [33]. The neutral and water-soluble vitamin B2 migrates before the neutral, fat-soluble vitamin D3. This indicates that the oil droplets are negatively charged and thus the migration time of the fat-soluble vitamin D3 is retained. The I.S. 25-hydroxycholecalciferol is comigrating with vitamin D3 as it has the same molecular structure apart from an additional hydroxyl group.
SF-MEEKC-UV separation of vitamin B1 (0.50 mg mL−1), B2 (0.077 mg mL−1), and D3 (0.50 mg mL−1) using the following concentrations of ammonium acetate in SFME. A 50 mmol kg−1, separation voltage 22.5 kV. B 100 mmol kg−1, separation voltage 22.5 kV. C 150 mmol kg−1, separation voltage 22.5 kV. D 200 mmol kg−1, separation voltage 20.5 kV. E 250 mmol kg−1, separation voltage 20.5 kV. Capillary: total length 31 cm × 50 μm I.D. (effective length 21 cm). Hydrodynamic injection with a height difference of 10 cm for 30 s. UV detection was set at 272 nm
The concentration of ammonium acetate in SFME was varied between 50 and 250 mmol kg−1. The conditioning of the capillary after changing the BGE was done by flushing 5 min with 0.1 M NaOH, 5 min with water, and 30 min with the new BGE. Some changes in the absolute migration times can occur depending on details of the conditioning protocol. However, the separation performance and the precision of repetitive measurements with the same BGE were quite good as specified later in this section.
When the microemulsion containing 50 mmol kg−1 ammonium acetate was used as BGE in SF-MEEKC, the signals of the vitamins B2 and D3 were not completely separated. The resolution of the signals for the two neutral species improved with higher concentrations of ammonium acetate added to the mixture. Baseline separation of signals of vitamins B2 and D3 was first achieved using 150 mmol kg−1 ammonium acetate. The resolution of signals of vitamins B2 and D3 was in fact best when SFMEs with a content of 200 mmol and 250 mmol kg−1 ammonium acetate were used as BGE. However, due to higher concentrations of ammonium acetate in SFME, rather high electrophoretic currents (≥90 μA) were generated, when applying high voltages (larger than 20.5 kV) leading to a breakdown of electrophoretic current during separation. Therefore, a maximal separation voltage of 19 kV was applicable. However, when applying lower separation voltages, much longer migration times of analytes were observed. Therefore, a compromise between resolution and migration time was made and the SFME containing 150 mmol kg−1 and applying a separation voltage of 22.5 kV was chosen as the best compromise for separation of the vitamins B1, B2, and D3. Using this protocol, the reproducibility of the migration times and of the peak areas of the different vitamins was characterized by relative standard deviations ranging between 2.3 and 3.5% and between 8.6 and 12%, respectively (conditions as in Fig. 4c, n = 9). Further analytical characteristics are specified in Table 1.
Analytical characteristics in the concentration range from 100 to 1000 μg mL−1 for vitamin B1, from 65 to 80 μg mL−1 for vitamin B2, and from 25 to 1250 μg mL−1 for vitamin D3, with triplicate injection at each concentration level were determined by SF-MEEKC-UV. The relatively small range of concentrations of vitamin B2 was due to its low solubility. The linearity was evaluated by plotting peak areas versus concentrations of analytes. The corresponding calibration parameters of the three analytes are summarized in Table 1. Due to its low solubility, quantification of B2 is limited.
SF-MEEKC-ESI-TOF-MS
Investigation of the ESI compatibility of the SFMEs
Ensuring compatibility of the SFMEs with ESI, the LODs of the vitamins were compared using SF-MEEKC and a conventional aqueous CE method with 0.1 M acetic acid as BGE. The LODs of the SF-MEEKC-ESI-TOF-MS were determined to be 0.53 μg mL−1 for vitamin B1, 7.54 μg mL−1 for vitamin B2, and 2.89 μg mL−1 for vitamin D3 (Table 1). The LODs of vitamins B1 and B2 for aqueous CE were determined to be 0.63 and 12.5 μg mL−1, respectively. Vitamin D3 is not soluble in water and thus the LOD could not be determined with conventional aqueous CE. Comparing the LODs of CE and SF-MEEKC, it can be stated that the SFMEs are compatible with ESI as the LODs are in the same range. An exact comparison is limited as the injection conditions are different for both methods due to different viscosities of the media.
Application of the proposed SF-MEEKC-ESI-TOF-MS method
The potential of the developed method is demonstrated by quantifying vitamin D3 in a commercial drug formulation. The content of vitamin D3 in the commercial sample formulation was declared to be 100 μg. The UV/VIS detector was not suitable for the quantification of vitamin D3 due to problems with matrix effects and limited selectivity compared to MS. In real sample analysis, the noise level of SF-MEEKC-ESI-TOF-MS measurements was also higher than in case of standard solutions. Due to the more complex sample preparation protocol and to improve the precision of results, SF-MEEKC-ESI-TOF-MS measurements were performed using 25-hydroxycholecalciferol as an internal standard. Five different concentration levels (from 120 to 200 μM for 25-hydroxycholecalciferol and from 90 to 150 μM for vitamin D3) with triplicate injections were investigated. Different concentrations of standard solutions of vitamin D3 and I.S. were chosen to obtain similar MS intensities for both species and to investigate the response factor in this concentration range. Figure 5 shows exemplarily results for real sample analysis by SF-MEEKC-ESI-TOF-MS. The migration times of vitamin D3 and 25-hydroxycholecalciferol are equal indicating that the latter one is well suited as an I.S.. The response ratio (D3/I.S.) was found to be independent of the concentration of vitamin D3 and of I.S. in the studied range. The results of real sample analysis are summarized in Table 2. The contents of vitamin D3 determined in the individual drug samples ranged between 95 and 113 μg, which is in good agreement to the declared mass of 100 μg.
Extracted ion electropherogram for SF-MEEKC-ESI-TOF-MS determination of the vitamin D3 in a commercial sample (m/z = 385.39, black line) using 25-hydroxycholecalciferol (m/z = 401.40, red line) as an internal standard. Microemulsion composition: 37.5% of water, 43.75% of ethanol, 18.75% of 1-octanol (w/w/w), and 150 mmol kg−1 ammonium acetate. Capillary: total length 30 cm × 50 μm I.D. (effective length 21.5 cm); applied voltage 16 kV, hydrodynamic injection for 10 s
Conclusion
For the first time, we demonstrated the use of SFMEs for electrokinetic chromatography. In the so-called pre-ouzo region, aggregate formation in the water/ethanol/1-octanol microemulsion is occurring spontaneously with no additional surfactant present in the mixture. The DLS and SAXS measurements proved that the addition of ammonium acetate in the chosen concentration range was not altering aggregate formation in the SFME. SF-MEEKC allowed the separation of neutral species in the studied model mixture of vitamins. The compatibility of SF-MEEKC with ESI-MS could be demonstrated. Comparable sensitivity as in aqueous CE-ESI-MS was found. Finally, the practical applicability of SF-MEEKC-ESI-MS was demonstrated determining the content of vitamin D3 in a commercial drug.
References
Terabe S, Otsuka K, Ichikawa K, Tsuchiya A, Ando T. Electrokinetic separations with micellar solutions and open-tubular capillaries. Anal Chem. 1984;56:111–3.
Altria KD, Mahuzier PE, Clark BJ. Background and operating parameters in microemulsion electrokinetic chromatography. Electrophoresis. 2003;24(3):315–24.
Pyell U, ed. Electrokinetic chromatography: theory, instrumentation and applications. John Wiley & Sons; 2006.
Tripodi V, Flor S, Carlucci A, Lucangioli S. Simultaneous determination of natural and synthetic estrogens by EKC using a novel microemulsion. Electrophoresis. 2006;27:4431–8.
Vomastova L, Mikšik I, Deyl Z. Microemulsion and micellar electrokinetic chromatography of steroids. J Chromatogr B Biomed Sci Appl. 1996;681:107–13.
Mahuzier PE, Altria KD, Clark BJ. Selective and quantitative analysis of 4-hydroxybenzoate preservatives by microemulsion electrokinetic chromatography. J Chromatogr A. 2001;924(1–2):465–70.
Huang HY, Lai YC, Chiu CW, Yeh JM. Comparing micellar electrokinetic chromatography and microemulsion electrokinetic chromatography for the analysis of preservatives in pharmaceutical and cosmetic products. J Chromatogr A. 2003;993(1–2):153–64.
Hsi-Ya H, Chia-Ling C, Chen-Wen C, Jui-Ming Y. Application of microemulsion electrokinetic chromatography for the detection of preservatives in foods. Food Chem. 2005;89(2):315–22.
Xie JP, Zhang JY, Liu HX, et al. Microemulsion electrokinetic chromatography with laser-induced fluorescence detection: as tested with amino acid derivatives. Biomed Chromatogr. 2004;18(8):600–7.
Javor T, Buchberger W, Tanzcos I. Determination of low-molecular-mass phenolic and non-phenolic lignin degradation compounds in wood digestion solutions by capillary electrophoresis. Microchim Acta. 2000;135(1–2):45–53.
Terabe S, Matsubara N, Ishihama Y, Okada Y. Microemulsion electrokinetic chromatography: comparison with micellar electrokinetic chromatography. J Chromatogr A. 1992;608(1–2):23–9.
Subirats X, Yuan HP, Chaves V, Marzal N, Roses M. Micoemulsion electrokinetic chromatography as a suitable tool for lipophilicity determination of acidic, neutral, and basic compounds. Electrophoresis 2016;37(14):2010–2016.
Zemann AJ. Conductivity detection in capillary electrophoresis. Trends Anal Chem. 2001;20(6–7):346–54.
Gennaro LA, Salas-Solano O, Ma S. Capillary electrophoresis-mass spectrometry as a characterization tool for therapeutic proteins. Anal Biochem. 2006;355(2):249–58.
Olivares JA, Nguyen NT, Yonker CR, Smith RD. On-line mass spectrometric detection for capillary zone electrophoresis. Anal Chem. 1987;59(9):1230–2.
Yu LS, Xu XQ, Huang L, Ling JM, Chen GN. Separation and determination of flavonoids using microemulsion EKC with electrochemical detection. Electrophoresis. 2008;29(3):726–33.
Bytzek AK, Reithofer MR, Galanski M, Groessl M, Keppler BK, Hartinger CG. The first example of MEEKC-ICP-MS coupling and its application for the analysis of anticancer platinum complexes. Electrophoresis. 2010;31(7):1144–50.
Hoffmann E, Stroobant V. Mass spectrometry. Principles and applications. John Wiley & Sons; 2007.
Matysik F-M, Neusüss C, Pelzing M. Fast capillary electrophoresis coupled with time-of-flight mass spectrometry under separation conditions of high electrical field strengths. Analyst. 2008;133(12):1764–6.
Klossek ML, Touraud D, Zemb T, Kunz W. Structure and solubility in surfactant-free microemulsions. ChemPhysChem. 2012;13(18):4116–9.
Smith GD, Donelan CE, Barden RE. Oil-continuous microemulsions composed of hexane, water, and 2-propanol. J Colloid Interface Sci. 1977;60(3):488–96.
Diat O, Klossek ML, Touraud D, et al. Octanol-rich and water-rich domains in dynamic equilibrium in the pre-ouzo region of ternary systems containing a hydrotrope. J Appl Crystallogr. 2013;46(6):1665–9.
Marcus J, Touraud D, Prévost S, Diat O, Zemb T, Kunz W. Influence of additives on the structure of surfactant-free microemulsions. Phys Chem Chem Phys. 2015;17(48):32528–38.
Vitale SA, Katz JL. Liquid droplet dispersions formed by homogeneous liquid - liquid nucleation: “the ouzo effect”. Langmuir. 2003;19:4105–10.
Schöttl S, Marcus J, Diat O, et al. Emergence of surfactant-free micelles from ternary solutions. Chem Sci. 2014;5(8):2909–3340.
Zemb T, Klossek ML, Lopian T, et al. How to explain the occurrence of microemulsions formed by solvent mixtures without surfactants. Proc Natl Acad Sci U S A. 2015;113(16):4260–5.
Fischer V, Marcus J, Touraud D, Diat O, Kunz W. Toward surfactant-free and water-free microemulsions. J Colloid Interface Sci. 2015;453:186–93.
Hankel RF, Rojas PE, Cano-Sarabia M, et al. Surfactant-free CO2 -based microemulsion-like systems. Chem Commun. 2014;50(60):8215–8.
Zoumpanioti M, Karali M, Xenakis A, Stamatis H. Lipase biocatalytic processes in surfactant free microemulsion-like ternary systems and related organogels. Enzym Microb Technol. 2006;39(4):531–9.
Gawrys KL, Blankenship GA, Kilpatrick PK. Solvent entrainment in and flocculation of asphaltenic aggregates probed by small-angle neutron scattering. Langmuir. 2006;22(10):4487–97.
Lopian T, Schöttl S, Prévost S, Pellet-Rostaing S, Horinek D, Kunz W, et al. Morphologies observed in unltraflexible microemulsions with and without the presence of a strong acid. ACS Cent Sci. 2016;2:467–75.
Callmer K, Davies L. Separation and determination of vitamin B1, B2, B6 and nicotinamide in commercial vitamin preparations using high performance cation exchange chromatography. Chromatographia. 1974;7:644–50.
Boso RL, Bellini MS, Miksik I, Deyl Z. Microemulsion electrokinetic chromatography with different organic modifiers: separation of water- and lipid-soluble vitamins. J Chromatogr A. 1995;709(1):11–9.
Altria KD. Background theory and applications of microemulsion electrokinetic chromatography. J Chromatogr A. 2000;892(1–2):171–86.
Acknowledgments
The authors thank Tobias Lopian from the Institut de Chimie Séparative Marcoule (ICSM) for SAXS measurements. S.K. thanks the Fonds der chemischen Industrie (FCI) for a scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Fundamental Aspects of Electromigrative Separation Techniques with guest editors Carolin Huhn and Pablo A. Kler.
Andrea Beutner and Sebastian Krickl contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1.72 mb)
Rights and permissions
About this article
Cite this article
Mohorič, U., Beutner, A., Krickl, S. et al. Surfactant-free microemulsion electrokinetic chromatography (SF-MEEKC) with UV and MS detection - a novel approach for the separation and ESI-MS detection of neutral compounds. Anal Bioanal Chem 408, 8681–8689 (2016). https://doi.org/10.1007/s00216-016-0057-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-0057-0