Abstract
Multi-residue methods permitting the high-throughput and affordable simultaneous determination of an extended range of endocrine disrupting chemicals (EDCs) with reduced time and cost of analysis is of prime interest in order to characterize a whole set of bioactive compounds. Such a method based on UHPLC-MS/MS measurement and dedicated to 13 estrogenic EDCs was developed and applied to biological matrices. Two molecular recognition-based strategies, either molecular imprinted polymer (MIP) with phenolic template or estrogen receptors (ERα) immobilized on a sorbent, were assessed in terms of recovery and purification efficiency. Both approaches demonstrated their suitability to measure ultra-trace levels of estrogenic EDCs in aqueous samples. Applicability of the MIP procedure to urine and serum samples has also been demonstrated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
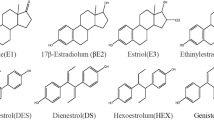
In recent decades, endocrine disrupting chemicals (EDCs) have attracted considerable interest from the scientific community as well as from public authorities and consumers because of their (1) ability to interfere with the function of the hormonal system and associated impact on human health, and (2) ubiquity. The role of endocrine disruptors is evocated with regard to a wide range of adverse health effects, such as reproductive abnormalities, cardiovascular diseases, obesity, diabetes, and cancers [1–9]. A specific concern is, however, related to the long-term, low-dose, and mixture effects of EDCs subsequently to early exposure during the perinatal period (pregnant women, fetus, new born). Among EDCs, estrogenic compounds are a matter of particular concern. These encompass natural estrogens (estrone, 17β-estradiol, estriol), synthetic estrogens (17α-ethinylestradiol, diethylstilbestrol), phytoestrogens (daidzein, genistein, equol), and other classes of environmental contaminants (phytosanitary products, plasticizers, alkylphenols).
Generally, the analysis of EDCs in biological samples has focused on a limited number of targeted compounds [10–13]. Conversely, multi-residue methods allowing the simultaneous determination of EDCs with a wide range of polarity using a single extraction and detection method are scarce. Hence, developing extended and rapid multi-residue methodologies to simultaneously determine human exposure to estrogenic EDCs mixtures would be of prime importance for accompanying not only academic research but also risk assessment.
Estrogenic endocrine disruptors are characterized by a wide range of chemical structures, but most of them are phenolic (Fig. 1). We established our extraction-purification strategy on the phenol recognition by either molecular imprinted polymer or nuclear receptor columns. In comparison with commonly used sorbents for which the adsorption of the compounds is driven by their polarity (e.g., C18-silica), molecularly imprinted polymers (MIPs) introduce a selectivity based both on the spatial conformation and a common chemical functionality of the target molecules [14, 15]. This last characteristic is then supposed to be an advantage in the scope of multi-class and multi-analyte analysis encompassing a range of substances from various polarities but with common phenolic sub-structure. Hence, the use of MIPs has been reported for efficient and selective extraction of estrogenic compounds (e.g., 17ß-estradiol, bisphenol A, and genistein) from biological matrices [16–18]. Another envisaged purification strategy may rely on the recognition of target analytes by a dedicated nuclear receptor, e.g., the estrogen receptors (ERs). Two main isoforms of ERs (ERα and ERβ) have been historically identified in humans with the same affinity for 17β-estradiol. Depending on their structure, EDCs exhibit high (i.e., estrone, 17β-estradiol, estriol, diethylstilbestrol), medium (i.e., genistein), or even low (i.e., bisphenol A) affinity for ERs [19].
In this study, we have developed a fast and sensitive analytical strategy for the simultaneous extraction and determination of a wide range of estrogenic endocrine disruptors in biological fluids. This method includes 13 analytes: estrone (E1), 17α-estradiol (α-E2), 17β-estradiol (β-E2), estriol (E3), 17α-ethinylestradiol (EE2), diethylstilbestrol (DES), bisphenol A (BPA), bisphenol S (BPS), 4-n-octylphenol (OP), 4-n-nonylphenol (NP), coumestrol (COU), genistein (GEN), and enterolactone (ENT) (Fig. 1). An external standard (diethylstilbestrol-d6, DES-d6) along with six internal standards [i.e., estradiol-d3 (E2-d3), bisphenol A 13C12 (BPA13C12), octylphenol 13C6 (OP13C6), nonylphenol 13C6 (NP13C6), daidzein 13C3 (DAID13C3), genistein 13C3 (GEN13C3)] were also included and monitored for accurate quantification according to the isotope dilution principle. A dansyl chloride derivatization was performed before liquid chromatography-tandem mass spectrometry (LC-MS/MS) measurement to enhance sensitivity as previously reported for the quantification of natural estrogens in biological fluids and drinking water [13, 20, 21]. We extended this methodology to the analysis of phytoestrogens, bisphenols, and alkylphenols. To characterize the interaction of targeted molecules with the sorbents tested (i.e., MIP phenolic and ERα-based extraction column), assays were first performed in aqueous medium. Extraction of biological matrices (serum, urine) was then conducted with the MIP sorbent, and the performances of the proposed method further evaluated. The developed method was further applied to quantify estrogenic endocrine disruptors in human maternal serum, cord serum, and urine samples.
Experimental
Chemicals and reagents
E1, α-E2, β-E2, EE2, DES, GEN, and OP13C6 were acquired from Sigma (St. Louis, MO, USA). BPA, BPS, OP, COU, ENT, and E2-d3 were from Sigma-Aldrich (St. Quentin Fallavier, France). E3 were purchased from Steraloids Inc. Ltd. (London, UK), whereas NP and NP13C6 were from from Cil-Cluzeau (Ste. Foy-la-Grande, France). BPA13C12 and DES-d6 were, respectively, from Cambridge Isotope Laboratories (Andover, MA, USA) and RIKILT (Wageningen, The Netherlands). GEN13C3 and DAID13C3 were obtained from KKJC2 (St. Andrews, Scotland, UK). Standard mixture solutions [estrogenic endocrine disruptors and internal standards (IS)] were prepared in ethanol at a concentration of 0.1 ng μL–1 and stored at –20 °C in the dark. Acetonitrile (ACN; LC-MS grade quality), anhydrous ethanol (EtOH; HPLC gradient-grade quality), and glacial acetic acid were purchased from Carlo-Erba Reagents (Rodano, Italy). Methanol (MeOH), dichloromethane (99.9 %), and hexane (analytical quality grade) were provided by Fisher Bioblock Scientific (Illkirch, France). Anhydrous carbonate sodium (Na2CO3) and ammonium hydroxide were from Merck (Darmstadt, Germany). Formic acid (Optigrade) was obtained from LGC-Standards (Molsheim, France). Ultrapurified water was dispensed by a Milli-Q-osmosis system from Millipore (Milford, MA, USA). The enzymatic preparation consisted of a purified extract from Patella vulgata and Helix pomatia (Sigma) dissolved in water (12 500 and 250 IU, respectively). Affinimip SPE phenolics (100 mg, 3 mL) were obtained from Polyintell (Val de Reuil, France), and C18-silica cartridges (500 mg, 6 mL) from Biotage (Uppsala, Sweden). The derivatization reagent was from Fisher Bioblock Scientific (Illkirch, France) and prepared as follows: 10 mg dansyl chloride was dissolved in 10 mL of ACN/Na2CO3 (10 mM) (50:50, v/v). Cell culture materials and luciferin were purchased, respectively, from Invitrogen (Cergy-Pontoise, France) and Promega (Charbonnieres, France).
Biological samples
Human samples were collected by the Gynaecology-Obstetric Unit of the Paule de Viguier Hospital, belonging to the Centre Hospitalier Universitaire (CHU) of Toulouse, France. Maternal serum, cord serum, and urine were obtained from volunteer women hospitalized between June 2010 and January 2013 for planned caesarean delivery with initial breastfeeding willingness. The experimental protocol was approved by a local ethical committee in accordance with French regulation, and the informed consent of all participating subjects was obtained. These samples were run for a first testing of the method, aiming at assessing analytical recoveries and matrix effects.
LC-MS/MS measurement
The chromatographic separation was performed using an Acquity ultraperformance LC system (Waters, Milford, USA) equipped with an Accucore phenyl-hexyl analytical column (100 × 2.1 mm; 2.6 μm particle size) supplied by Fisher Bioblock Scientific (Illkirch, France). The elution solvents were 0.1 % acetic acid in water (A) and 0.1 % acetic acid in ACN (B). Separation was performed at 50 °C using a flow rate of 0.5 mL min–1 with the following gradient: 30 % of eluent B (for 1 min), increasing to 60 % from 1 to 4 min (kept isocratic for 1 min), raising in 2 min to 100 % (kept for 1 min) and then back to initial conditions within 0.1 min. Total analysis time was 14 min. The injection volume was 5 μL.
A triple quadrupole mass spectrometer Xevo TQ-S (Waters) equipped with an electrospray interface Zspray operating in positive ionisation (ESI+) mode was used. The source and the desolvation temperatures were set at 150 and 500 °C, respectively. Nitrogen was used as nebulization and desolvation gas at flow rates of 150 and 650 L/h, respectively. After optimization, the capillary potential and cone voltage were set at 2.5 kV and 30 V, respectively. Argon was used as the collision gas at a flow rate of 0.1 mL min–1. Collision energies were optimized for each compound and each transition [see Electronic Supplementary Material (ESM) Table S1]. MassLynx ver. 4.1. and TargetLynx (Waters) softwares were used for data acquisition and processing, respectively. Acquisition was performed in the selected reaction monitoring mode (SRM). Identification of analytes was based on retention times and transition ratios as specified in the Decision 2002/657/EC [22].
Extraction on phenolic MIP sorbent
Deconjugation of phase II metabolites (glucuronide and sulfate forms) was done by adding 2 mL acetate buffer (0.2 M, pH 5.2) as well as 50 μL of homemade enzymatic mixture (Patella vulgata/Helix Pomatia/water, 1/1/0.5, v/v/v) to 1 mL sample. Incubation was performed 16 h at 37 °C; after centrifugation for 10 min at 7,164 g (4 °C), 1 mL ACN was added before loading the sample onto MIP cartridges.
For the recovery study, samples (1 mL) were spiked with a mixture of targeted compounds in EtOH to final concentration of 5 μg L–1. They were allowed to equilibrate for at least 30 min before extraction (without deconjugation). Then, 2 mL of H2O and 1 mL ACN were added.
The MIP cartridge was successively conditioned with 3 mL MeOH: formic acid (98:2, v/v), 3 mL ACN, and 3 mL H2O. After sample loading, the column was washed with 4 mL H2O followed by 5 mL H2O/ACN (80:20, v/v). Finally, the elution was performed by 10 mL MeOH followed by 10 mL MeOH: acetic acid (98:2, v/v).
All fractions were evaporated to dryness, reconstituted in 50 μL of dansyl chloride solution and held at 30 °C for 30 min. After further evaporation, a liquid/liquid extraction with 1.5 mL hexane and 1.5 mL H2O was performed. The organic solvent containing dansyl derivatives was collected and evaporated to dryness; the final residue was reconstituted in 50 μL ACN/H2O (50:50, v/v).
Extraction on ERα-based column
Experiments were performed at 4 °C to circumvent any protein denaturation. The recombinant ERα (25 nmol) was immobilised on Ni-NTA-agarose phase (Qiagen, France) previously applied in an empty column. After 2 h rolling, the immobilized receptor was washed twice with 500 μL of washing buffer (WB, i.e., 20 mM Tris-HCl, pH 7.5, 300 mM NaCl, 10 % glycerol, 0.1 mg mL–1 bovine serum albumin, and 10 mM imidazole) in order to remove impurities from the recombinant ERα production. Following this, a mixture of 13 estrogenic compounds diluted in WB (10 ng each, 500 μL) was loaded. The column was rinsed with 4 × 500 μL of WB and elution was performed with 4 × 500 μL of eluting buffer (EB, i.e., 20 mM Tris-HCl, pH 7.5, 300 mM NaCl, 10 % glycerol, 0.1 mg mL–1 bovine serum albumin, and 200 mM imidazole). To elute the remaining material, two supplementary fractions of EtOH were applied.
All fractions were then purified on C18-silica cartridge (Isolute C18) previously conditioned with MeOH and H2O. Elution was performed with 6 mL MeOH and 6 mL dichloromethane. The fractions were analysed after derivatization as described above and eventually injected onto a LC-MS/MS system.
Luciferase assay
Assay was performed with HELN ERα cells. This stably transfected luciferase reporter cell line was obtained as described by [23]. Briefly, the estrogen-responsive reporter gene was first transfected into HeLa cells, generating the HELN cell line and, in a second step, these HELN cells were transfected with ERα expressing plasmid to obtain the HELN ERα cell line.
Reporter cells were seeded in 96-well plates for at least 8 h, and then cell lines were incubated with the compounds for 16 h at 37 °C. Experiments were performed in quadruplicate. At the end of incubation, the medium containing test compounds was removed and replaced by culture medium containing luciferin, which diffuses into the cell and produces a stable luminescent signal 10 min later. The luminescence was measured by a Wallac luminometer.
Evaluation of analytical performances
Linearity, repeatability, recovery determination, and detection limit were investigated for ultrapure water, serum, and urine. Linearity was evaluated by analysis of several concentration levels in the range 0.1–50 μg L–1 (1–500 μg L–1 for E3 in ultrapure water). Repeatability was checked by analyzing fortified samples at 1 and 5 μg L–1. Limit of detection was evaluated as the lowest concentration of the calibration curve that provides a signal to noise ratio of three.
Results and discussion
Choice of LC-MS/MS conditions
After a brief study on native estrogenic endocrine disruptors, derivatization with dansyl chloride and pyridine-3-sulfonyl previously reported to enhance the detection of estrogens in biological matrices was tested [13, 20, 24–26]. Higher intensities and lower noise were obtained (particularly for phytoestrogens) when using dansyl chloride as chemical derivative (data not shown). Therefore, derivatization by dansyl chloride was retained for the offered possibility to measure all targeted molecules (n = 13).
Optimizations of MS conditions were performed by direct introduction of each individual solution via the syringe pump at a 50 ng μL–1 concentration and 5 μL min–1 flow rates together with the introduction of mobile phase at 0.2 mL/min. For each compound, optimization of capillary and cone voltages was performed to reach the best sensitivity for the precursor ions corresponding to the protonated molecules [M + H]+ and [M + 2H]2+ (mono- and biphenolic compounds, respectively). For all the considered compounds, common diagnostic fragment ions were observed (e.g., m/z 171, 170, 156, and 115. This nonspecific fragmentation with dansyl chloride was previously reported for natural estrogens [13, 20, 21, 24]. Optimized collision energies and monitored transitions are displayed in ESM Table S1.
Derivatization (i.e., incubation time 5–35 min and temperature 30–80 °C) were also optimized through a factorial design using a mixed standard solution. The response surfaces were characterized using Statistica software. In most cases, optimal conditions were observed at 30 °C for 30 min (data not shown). Under these conditions, the repeatability (n = 5) was found satisfactory with relative standard deviation (RSD) values less than 10 % in most cases except for estriol (67 %).
Two stationary phases were first considered for efficient separation of dansyl derivatives (i.e., BEH C18 (100 × 2 mm, 1.7 μm) and Accucore phenyl-hexyl (100 × 2.1 mm, 2.6 μm), but better resolution was observed on the latter column because of π–π interactions with the phenolic functional groups characterizing the targeted analytes. A gradient based on H2O + 0.1 % acetic acid and ACN + 0.1 % acetic acid was optimized so that the more polar compound (estriol) was eluted around 4 min and the more apolar analyte (diethylstilbestrol) around 7 min (ESM Table S1).
Optimization and validation of extraction of 13 estrogenic endocrine disruptors with phenolic MIP-SPE
Development on ultrapure water
We studied the behavior of targeted EDCs on the sorbent by applying the manufacturer’s protocol that has been further optimized to improve extraction yields. The mixture of estrogenic endocrine disruptors [5 μg L–1 in 3 mL H2O/ACN (2:1, v/v)] was loaded onto the cartridge. A washing step was tested using 4 mL H2O followed by 5 mL H2O/ACN (60:40, v/v) as recommended by PolyIntell. Then, molecules were eluted with 2 × 3 mL MeOH. An additional elution fraction was recovered with either 2 mL MeOH/acetic acid (98:2, v/v) or 2 mL MeOH/ ammonium hydroxide (99:1, v/v). Using this protocol, losses during the second washing step were noticed, mainly for estriol (i.e., 50 %). Moreover, low recovery rates were observed for phytoestrogens (coumestrol and genistein), whereas some compounds showed different elution behaviour (i.e., eluted either in MeOH or in MeOH/acetic acid). Consequently, an adjustment of SPE conditions was required. To enhance solute retention on the sorbent, the ACN percentage was reduced both for the sample (to 25 %) and the second washing fraction (to 20 %). Also, acetic acid was chosen as modifier because of better recoveries in comparison with ammonium hydroxide (data not shown).
As shown in Table 1, satisfactory recoveries were achieved using elution with 10 mL MeOH followed by 10 mL MeOH/acetic acid (98:2, v/v). Natural estrogens (estrone, 17α-estradiol, 17β-estradiol, and estriol), synthetic estrogens (17α-ethinylestradiol, diethylstilbestrol), alkylphenols (octylphenol, nonylphenol), bisphenol A, and enterolactone were eluted in the MeOH fraction, while bisphenol S, coumestrol, and genistein were recovered in the MeOH/acetic acid fraction. The overestimated recoveries obtained for phytoestrogens can be partly attributed to an adsorption phenomenon of the targeted analytes on the glassware more important in standard solution than in biological extract (matrix protector effect). Recoveries estimated for nonylphenol and octylphenol appeared significantly lower than in the previous test (45 and 52 % against 92 and 120 %, respectively). During subsequent assays, a change in the behavior of these molecules on the MIP was observed (i.e., undetected or poorly detected in elutions and no losses during loading and washes). This observation confirms the presumed high interaction of these target compounds with the sorbent. Most probably, the two alkylphenols studied remain fixed on the MIP and the elution solvents previously used were not effective enough to break the interactions established with functional monomers. Unsatisfactory yields for this family of compounds were also reported by the manufacturer (i.e., Polyintell). Therefore, only part of the method performances was assessed for these two molecules.
With regard to the evaluation of the method performances, five standard calibration curves were constructed using seven levels of concentration in the range 0.1–50 μg L–1 (1–500 μg L–1 for E3) and average curves were defined for each compound. A good linearity was found for concentrations ranging from 0.1 to 10 μg L–1 (R2 higher than 0.99 in most cases) in comparison with concentrations up to 50 μg L–1 (Table 1). The intraday precisions evaluated at 1 μg L–1 in three independent series were less than 20 % for many of the compounds except estriol, bisphenol S, coumestrol, enterolactone, genistein, and diethylstilbestrol, which displayed values higher than 20 % in one series. The interday precisions were less than 20 % for 6 of the 13 compounds studied. However, values obtained for BPS and DES were between 30 and 40 % and higher than 45 % for genistein, estriol, and enterolactone. These results have been supposed to be associated to an inappropriate selection of IS in the case of these target compounds. The detection limits ranged from 0.01 to 0.80 μg L–1 and limits of quantification from 0.04 to 2.7 μg L–1.
Application to biological fluids
The method previously developed for ultrapure water was then applied to serum and urine samples.
As shown in Figs. 2 and 3, satisfactory results were achieved for both matrices, with recoveries above 70 % for a majority of compounds. Limited losses (below 4 %, except for BPA) during the percolation and washing steps clearly indicate efficient interaction of the targeted analytes with the phenolic imprint in the presence of the matrix. Lower recovery values were observed in the case of coumestrol and genistein from serum samples (45 and 61 %, respectively), while these two compounds were not recovered at all from urine samples. In the particular case of BPA, its ubiquitous presence in urine and blood samples, at levels higher than the one used in spiked samples, disturbed the recovery evaluation.
Recovery profile for targeted estrogenic compounds in serum at 5 μg L–1 (n = 3). Load: serum/H2O/ACN (1:2:1, v/v/v) (total 4 mL); wash 1: 4 mL of H2O, wash 2: 5 mL H2O/ACN (80:20, v/v); elution 1: 6 mL methanol, elution 2: 3 mL methanol/acetic acid (98:2, v/v), elution 3: 3 mL methanol/acetic acid (98:2, v/v)
The linearity of the method was determined by spiking serum and urine samples at three levels of concentration ranging from 0.1 to 50 μg L–1, depending on the compound. As shown in Table 2, linearity was satisfactory in both matrices with R2 higher than 0.98.
The intraday precision of the method was evaluated at 1 μg L–1 (20 μg L–1 and 50 μg L–1 for estrone, 17β-estradiol, estriol, and enterolactone depending on matrices) and 5 μg L–1. At 1 μg L–1 RSD values ranged from 1.4 to 16.7 % for most compounds, demonstrating a good repeatability except for bisphenol S, coumestrol, genistein in serum bisphenol S, and enterolactone in urine. As discussed above, this can be attributed to inappropriate IS. In comparison with serum, a less satisfactory precision was achieved for urine at 5 μg L–1 with values overall higher than 20 %. The detection limits were between 0.01 and 1 μg L–1 and were similar for both matrices. The limits of quantification ranged from 0.04 to 3.4 μg L-1 in serum and from 0.04 to 1.8 μg L-1 in urine.
Finally, natural estrogens and phytoestrogens were determined in a set of samples both by a validated official method used in the laboratory and the MIP-SPE method developed with a view of demonstrating the suitability of our MIP-SPE method for the analysis of natural estrogens and phytoestrogens. Results are presented in Table 3. Overall, similar results were obtained with both methods, except in the case of phytoestrogens from urine.
ERα-based extraction
The first extraction assay was performed using 5 nmol of receptor (ERα) immobilized on column. Estrogenic activities of all collected fractions (percolation, washing, and elution) were determined by HELN ERα cell luciferase assay after dilution in cell culture medium. A transactivation signal was observed in percolation and washing fractions indicating losses of targeted analytes during these stages (data not shown). So, the assay was renewed using 25 nmol of receptor for enhancing its interaction with the target compounds. Under the latter conditions, losses were reduced. Furthermore, a strong transactivation signal was observed in the first and the second elution fractions (Fig. 4). The estrogenic activity of these collected fractions was evaluated, and the dose-response curve drawn. According to E2 equivalent, the estrogenic activity of the elution fractions was estimated at 84.1 % in comparison with the mixture of estrogenic compounds not extracted (i.e., initial).
HELN ERα cell luciferase assay for the different fractions collected from ERα-based extraction column (25 nmol of receptor). Results are expressed as a percentage of luciferase activity measured per well (mean ± RSD, n = 4). The value obtained in the presence of estradiol 10–8 M was used as reference (i.e., 100 %). The dashed line represents the basal response of cells in presence of EtOH
All fractions collected were further extracted on C18-silica column and analyzed by LC-MS/MS. Recoveries were higher than 50 % except for diethylstilbestrol and octylphenol, which displayed 25 and 43 %, respectively (Fig. 5). Moreover, losses in the loading and washing fractions were less than 10 % (except for BPA: 14.8 %) showing a good interaction of targeted EDCs with the receptor. In fact, the affinity of targeted estrogenic compounds for ERα has already been reported [27–29] and mechanisms of interactions have been also elucidated by crystallography [19, 30–32]. The resulting diagnostic ion chromatograms obtained for the first fraction of elution are presented in Fig. 6. In comparison with the similar diagnostic ion chromatograms obtained for ultrapure water after extraction on MIP phenolic (data not shown), the purification on ERα-based extraction column was estimated to be less efficient. At this stage, these results indicate the suitability of the ERα-based method for the extraction of a mixture of estrogenic compounds at least from an aqueous medium.
Application to the analysis of real samples
To evaluate the applicability of the MIP-SPE method, maternal serum, cord serum, and urine of newborn (4, 3, and 10 samples respectively) were analyzed as described above. Among EDCs studied, the presence of β-E2 was observed only in serum, whereas E1, E3, BPA, and ENT were all detected in maternal serum, cord serum, and urine (Tables 4 and 5). Specially, E3 was found with the highest concentrations in comparison with other natural estrogens (>100 μg L–1 in serums and >20 μg L–1 in urine). In all cases, concentrations of E1 in serum were higher than β-E2 (9.2 to 108.2 μg L–1 versus 3.6 to 24.3 μg/L) and ranged from below the limit of quantification 0.2 to 6 μg/L in urine. The levels of BPA were from below 0.04 (limit of quantification) to 0.1 μg L–1 in serum, and 0.1 to 5.8 μg L–1 in urine. For enterolactone, concentrations ranged from 0.7 to 3.5 μg L–1 in serum and 0.2 to 27 μg L–1 in urine. The detection of other compounds was observed only one or two times with values under quantification limit except for α-E2, which was quantified in one urine sample at 0.5 μg/L. The results presented in ESM Fig. S1 illustrate an example of serum and urine treated with the developed method. These results are in agreement with previous tendencies reported for the levels of these estrogenic compounds in such biological samples [10, 33–35].
Conclusion
A multi-residue isolation and measurement method dedicated to estrogenic endocrine disrupting chemicals in biological fluids has been proposed, with a final view of rapidly assessing the levels of such compounds simultaneously as this could be of precious help for assessing human internal dose levels and biomonitoring studies. It is based on selective sorbent extraction followed by UHPLC-MS/MS after dansyl chloride derivatization, enabling simultaneous measurement of a range of 13 structurally varied EDCs. Two sample pretreatment methods based on molecular recognition of the target compounds were evaluated: MIP phenolic and ERα-based extraction column. Application of these two strategies to aqueous medium shows that molecules of interest are well retained on both types of sorbents, so that both strategies are suitable as sample preparation method for the isolation of estrogenic compounds. The procedure developed with ERα can be coupled to in vitro assay allowing the analysis of specific estrogenic fractions; this is probably the main advantage of this strategy, since estrogenic molecules not identified before can thus be characterized. However, the specificity of this biological material and its associated sensitivity in terms of production, stability, and handling, still represent some limitations for its democratized use in routine analysis. As the MIP-based strategy is more convenient to put in practice, it was further applied to serum and urine samples. Performances of the developed method appear acceptable for most of the targeted estrogenic compounds in both matrices, with level ranges assessed comparable to previously reported levels for similar biological samples. Yet the proposed method was found not appropriate in the particular case of phytoestrogens from urine; we do believe that the introduction of two identified levels of improvement (internal calibration system and additional purification) would permit overcoming these limitations in a near future, thereby extending the applicability of the developed method.
References
Skakkebaek N, Rajpert-De Meyts E, Main KM (2001) Hum Reprod 16:972–978
Zhou F, Zhang L, Liu A, Shen Y, Yuan J, Yu X, Feng X, Xu Q, Cheng C (2013) J Chromatogr B 938:80–85
Shen Y, Ren M-L, Feng X, Gao Y-X, Xu Q, Cai Y-L (2014) Eur J Obstet Gynecol Reprod Biol 178:137–137
Tang R, Chen M-J, Ding G-D, Chen X-J, Han X-M, Zhou K, Chen L-M, Xia Y-K, Tian Y, Wang X-R (2013) Environ Pollut 178:115–120
Casals-Casas C, Desvergne B (2011) Annu Rev Physiol 73:135–162
Virtanen HE, Adamsson A (2012) Mol Cell Endocr 355:208–220
Grün F, Blumberg B (2009) Mol Cell Endocr 304:19–29
Ziegler RG, Faupel-Badger JM, Sue LY, Fuhrman BJ, Falk RT, Boyd-Morin J, Henderson MK, Hoover RN, Veenstra TD, Keefer LK, Xu X (2010) J Steroid Biochem Mol Biol 121:538–545
Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC (2009) Endocr Rev 30:293–342
Aris A (2014) Reprod Toxicol 45:8–13
Prasain JK, Arabshahi A, Moore RD II, Greendale GA, Wyss JM, Barnes S (2010) J Chromatogr B 878:994–1002
Ferrara F, Ademollo N, Orrù MA, Silvestroni L, Funari E (2011) Chemosphere 82:1044–1049
Kushnir MM, Rockwood AL, Bergquist J, Varshavsky M, Roberts WL, Yue B, Bunker AM, Meikle AW (2008) Am J Clin Pathol 129:530–539
Pichon V, Chapuis-Hugon F (2008) Anal Chim Acta 622:48–61
Hu Y, Pan J, Zhang K, Lian H, Li G (2013) Trends Anal Chem 43:37–52
Zhang J-H, Jiang M, Zou L, Shi D, Mei S-R, Zhu Y-X, Shi Y, Dai K, Lu B (2006) Anal Bioanal Chem 385:780–786
Gadzała-Kopciuch R, Ričanyová J, Buszewski B (2009) J Chromatogr B 877:1177–1184
Chrzanowska AM, Poliwoda A, Wieczorek PP (2015) J Chromatogr A 1392:1–9
Le Maire A, Bourguet W, Balaguer P (2010) Cell Mol Life Sci 67:1219–1237
Nguyen HP, Li L, Gatson JW, Maass D, Wigginton JG, Simpkins JW, Schug KA (2011) J Pharma Biomed Anal 54(4):830–837
Lin Y-H, Chen C-Y, Wang G-S (2007) Rapid Commun Mass Spectrom 21:1973–1983
Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (Text with EEA relevance) (notified under document number C(2002) 3044). Off J Eur Commun 2002:L221, 8
Balaguer P, François F, Comunale F, Fenet H, Boussioux A-M, Pons M, Nicolas J-C, Casellas C (1999) Sci Total Environ 233:47–56
Li W, Li Y-H, Li AC, Zhou S, Naidong W (2005) J Chromatogr B 825:223–232
Blonder J, Johann DJ, Veenstra TD, Xiao Z, Emmert-Buck MR, Ziegler RG, Rodriguez-Canales J, Hanson JA, Xu X (2008) Anal Chem 80:8845–8852
Xu L, Spink DC (2008) Anal Biochem 375:105–114
Pillon A, Boussioux A-M, Escande A, Aït-Aïssa S, Gomez E, Fenet H, Ruff M, Moras D, Vignon F, Duchesne M-J, Casellas C, Nicolas J-C, Balaguer P (2005) Environ Health Perspect 113(3):278–284
Liu Z-H, Kanjo Y, Mizutani S (2010) Water Res 44:567–577
Molina-Molina J-M, Amaya E, Grimaldi M, Sáenz J-M, Real M, Fernández MF, Balaguer P, Olea N (2013) Toxicol Appl Pharmacol 272:127–136
Chandsawangbhuwana C, Baker ME (2014) Steroids 80:37–43
Delfosse V, Grimaldi M, Cavaillès V, Balaguer P, Bourguet W (2014) Environ Health Perspect 122(12):1306–1313
Delfosse V, Grimaldi M, Pons J-L, Boulahtouf A, Le Maire A, Cavailles V, Labesse G, Bourguet W, Balaguer P (2012) Proc Natl Acad Sci U S A 109:14930–14935
Adlercreutz H, Yamada T, Wähälä K, Watanabe S (1999) Am J Obstet Gynecol 180(3):737–743
Kuijper E, Ket JCF, Caanen MR, Lambalk CB (2013) Reprod Biomed Online 27:33–63
Pirard C, Sagot C, Deville M, Dubois N, Charlier C (2012) Environ Int 48:78–83
Acknowledgments
The authors want to express their special thanks to all the volunteers who have permitted the collection of biological samples exploited in this work. They also thank the Centre Hospitalier Universitaire de Toulouse as the promoter of the corresponding clinical protocol.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 71 kb)
Rights and permissions
About this article
Cite this article
Bousoumah, R., Antignac, J.P., Camel, V. et al. Development of a molecular recognition based approach for multi-residue extraction of estrogenic endocrine disruptors from biological fluids coupled to liquid chromatography-tandem mass spectrometry measurement. Anal Bioanal Chem 407, 8713–8723 (2015). https://doi.org/10.1007/s00216-015-9024-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-9024-4