Abstract
Mass spectrometry imaging has become a popular tool for probing the chemical complexity of biological surfaces. This led to the development of a wide range of instrumentation and preparation protocols. It is thus desirable to evaluate and compare the data output from different methodologies and mass spectrometers. Here, we present an approach for the comparison of mass spectrometry imaging data from different laboratories (often referred to as multicenter studies). This is exemplified by the analysis of mouse brain sections in five laboratories in Europe and the USA. The instrumentation includes matrix-assisted laser desorption/ionization (MALDI)-time-of-flight (TOF), MALDI-QTOF, MALDI-Fourier transform ion cyclotron resonance (FTICR), atmospheric-pressure (AP)-MALDI-Orbitrap, and cluster TOF-secondary ion mass spectrometry (SIMS). Experimental parameters such as measurement speed, imaging bin width, and mass spectrometric parameters are discussed. All datasets were converted to the standard data format imzML and displayed in a common open-source software with identical parameters for visualization, which facilitates direct comparison of MS images. The imzML conversion also allowed exchange of fully functional MS imaging datasets between the different laboratories. The experiments ranged from overview measurements of the full mouse brain to detailed analysis of smaller features (depending on spatial resolution settings), but common histological features such as the corpus callosum were visible in all measurements. High spatial resolution measurements of AP-MALDI-Orbitrap and TOF-SIMS showed comparable structures in the low-micrometer range. We discuss general considerations for planning and performing multicenter studies in mass spectrometry imaging. This includes details on the selection, distribution, and preparation of tissue samples as well as on data handling. Such multicenter studies in combination with ongoing activities for reporting guidelines, a common data format (imzML) and a public data repository can contribute to more reliability and transparency of MS imaging studies.

Comparison of MS imaging platforms in international multicenter study
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mass spectrometry imaging has become a widely used analytical technique for the analysis of complex surfaces [1–3]. Applications range widely, from biomarker discovery (lipids, peptides, and proteins) to drug and metabolite distribution studies. Such wide applicability has drawn many new researchers to the field and lead to the development of a range of ionization methods, sample preparation protocols, and new instrumentation. It is thus desirable to compare the data output from different methodologies and mass spectrometers, both from an imaging perspective (i.e., Are the same spatial features observed?) and from a molecular perspective (i.e., What molecules are detected from each methodology and are they comparable?).
A comparison between mass spectrometry imaging laboratories could be broached in two ways: (1) a common sample could be distributed and complementary techniques that yield different, complementary molecular information could be compared, or (2) the same type of molecules could be targeted (e.g., lipids) and different sample preparation protocols and/or mass spectrometry instrumentation could be compared.
Here, we report on a multicenter study of mass spectrometry imaging that was conducted in five different laboratories, which were all partners in the E.U. project COMPUTIS for mass spectrometry imaging (www.computis.org). We have used a common benchmark sample (mouse brain) to compare results from a range of mass spectrometer types. General considerations for planning and performing multicenter studies in mass spectrometry imaging will be discussed.
Experimental
Sample
Tissue samples measured in this study originate from coronal mouse brain sections. Adjacent coronal sections of 10-μm thickness from the same mouse brain (female type 9 CFW-1, Harlan Laboratories, Boxmeer, The Netherlands) were sectioned on a cryo-microtome (Microm International, Walldorf, Germany) at −20 °C and were thaw mounted on indium-tin-oxide-coated glass slides (ITO; Delta Technologies, Stillwater, MN), stainless steel plates, or silicon wafers (see Table 1). As a reference, one section was stained with Hematoxylin/Eosin (H&E, Fisher Scientific, Landsmeer, NL) as shown in the Supplementary Material (ESM) Fig. S1. The samples were stored in polypropylene slide mailers (Fisher Scientific, Landsmeer, NL) at −80 °C before express shipment (on dry ice) to individual partners. Due to instrumental difficulties, some of these sections could not be measured or results were not satisfactory. For this reason, additional tissue sections (from different animals) were prepared. These sections were prepared from the same approximate coronal region of the mouse brain (for details, see Results section).
Instrumentation
A variety of mass spectrometers were used within the COMPUTIS consortium. Table 1 gives an overview of the instrumentation used for this study. Details of each instrument and associated experimental parameters are given in the Supplementary Material. The best combination of sample preparation, ionization type, and mass analyzer is highly dependent on the analyte of interest and sample properties. Thus, the correct combination must be chosen carefully. In our study, the samples were prepared according to the established protocols at each partner laboratory in order to obtain optimum results. Thus, this study is not a pure comparison of mass spectrometry (MS) imaging instruments, but also takes into account different preparation protocols. The laboratories involved were the FOM Institute for Atomic and Molecular Physics, Amsterdam, Netherlands (AMOLF); Institut de Chimie des Substances Naturelles, Gif-sur-Yvette, France (CNRS); Justus Liebig University, Giessen, Germany (JLU); and Novartis Institutes for BioMedical Research, Basel, Switzerland (Novartis).
Data processing
All datasets were converted to the common mass spectrometry imaging data standard imzML [4]. Procedures for each data type are described in the Supplementary Material. In addition to proprietary software, the open-source software MSiReader (version 0.04) was used to navigate and display mass spectral images of the imzML datasets [5]. All data was exchanged through a central sever location (ftp server of CEA-LIST, Saclay, France).
Results and discussion
Phospholipids and proteins were chosen as examples to display and discuss mass spectrometric images. Experimental details and exemplary results for each method (including mass spectra) are shown in the ESM (Fig. S2–S12). There are several approaches on how to compare different datasets in MS imaging. It is important to keep in mind that mass spectral parameters and spatial/histological parameters need to be considered in combination for MS imaging experiments. Here, we focus on the evaluation of detected spatial features and discussion of practical aspects of the experiment.
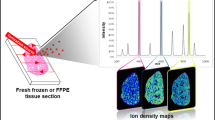
The MS images of all measurements are shown in Fig. 1. Phospholipid data is shown for matrix-assisted laser desorption/ionization (MALDI)-QStar (B), MALDI-Fourier transform ion cyclotron resonance (FTICR) (C), and atmospheric-pressure (AP)-MALDI-Orbitrap (D). Proteins are shown for MALDI-time-of-flight (TOF) (A) in order to demonstrate the higher mass range of this instrument type. Cholesterol is shown for cluster TOF-secondary ion mass spectrometry (SIMS) (E). Due to the different areas analyzed with the techniques, the MS images shown in Fig. 1 do not directly match each other. The areas analyzed by AP-MALDI-Orbitrap (Fig. 1d) and TOF-SIMS (Fig. 1e) are indicated in the H&E-stained section in Fig. 1f. As discussed above, not all measured sections originate from the initial set of adjacent mouse brain sections. MALDI-TOF, MALDI-QStar, and MALDI-FTICR data was acquired from different sections. The corpus callosum is, however, visible in all measurements as a common structural feature. These datasets are also suitable to discuss technical and practical aspects of this comparison study. First of all, datasets were converted to imzML (www.imzml.org), and MS images in Fig. 1 were displayed in the open-source software “MSiReader” with identical visualization parameters (“jet” color map, linear interpolation of first order, TIC normalization). These settings might not be the default choice for all measurements, but the described workflow enables visualization parameters to be uniformly adjusted for all datasets. This is an important step towards objective evaluation and comparison of different MS imaging techniques. The different software tools normally used for these experiments resulted in diverse images due to use of different color maps and varying options for interpolation and normalization (see Supplementary Material). Alternative options to display imzML data are Datacube Explorer [6] and “OmniSpect” [7], which are freely available, or the commercial tools “Quantinetix” [8] and “MALDIVision” [9]. An updated list of available tools is available at www.imzml.org.
Spatial distribution of selected ion images as acquired with different MS imaging platforms: a MALDI-TOF (AMOLF), m/z 14,114; b MALDI-QStar (Novartis), m/z 810.6; c MALDI-FTICR (PNNL), m/z 810.5983; d AP-MALDI-Orbitrap, m/z 848.638; and e cluster TOF-SIMS (CNRS), m/z 369.35. All datasets were converted to imzML and displayed in the open-source software (MSiReader) with identical settings for color map, spatial interpolation, and intensity normalization. Details on pixel size, dimensions, image bin width and mass spectral parameters for each measurement are given in Table 2. f H&E staining of coronal mouse brain section. Colored rectangles indicate analyzed area of measurements shown in d and e
The reporting of experimental parameters and display of data is a crucial issue in mass spectrometry imaging. Experimental parameters such as laser frequency, sample preparation procedures, and pixel size are usually described. However, parameters such as acquisition time, (statistical) mass accuracy, and mass resolution are often missing. No or little information is often given about the processing of data, such as mass bin size used to generate the image, details of image interpolation, and mass spectral normalization. These details are, however, essential to evaluate the quality and information content of an MS imaging experiment. Therefore, a summary of mass spectrometry imaging measurement parameters of this study is shown in Table 2. This data includes image dimensions, pixel size, and acquisition time, as well as mass accuracy and mass resolving power and bin size for image generation. This data is based on actual measurements and corresponds directly to the MS images shown in Fig. 1 and in the ESM. Some of the mass spectrometers used here have been superseded by newer generation instruments with better performance. The results presented here are therefore not meant to be representative for a given technique, but it still shows general trends for different ionization and mass spectrometer types.
This is also exemplified in more detail with a comparison of MS imaging datasets from cluster TOF-SIMS and MALDI-Orbitrap. Typically, TOF-SIMS is used for high spatial resolution and MALDI for analysis of intact molecular ions, accurate mass measurements, and MS/MS experiments. Until recently, larger molecules could only be detected by MALDI, typically at 100-μm pixel size. Recent developments have allowed MALDI analyses of peptides and lipids at 5 μm [10] and proteins at 10 μm [11] spatial resolution in biological samples. On the other hand, the mass range of TOF-SIMS has been significantly increased by employing cluster ion sources, thus improving its applicability to biomolecules such as lipids. Consequently, the capabilities of SIMS and MALDI are converging. This is illustrated in Fig. 2, showing the distribution of phospholipids in the mouse brain striatum as acquired by two instruments in our study, an AP-MALDI-Orbitrap instrument at 5-μm pixel size and a cluster TOF-SIMS instrument at 1-μm pixel size (the corresponding spectra are shown in Figs. S3c and S11, respectively). The two measurements (which were acquired from neighboring tissue sections) show good correlation of spatial features and therefore demonstrate that comparable results can be obtained with very different MS imaging techniques. Similar structures are obtained in the low-micrometer range, regardless that the samples were shipped across Europe, prepared in different laboratories, and measured with different instrumental platforms. Such quality and comparability have only recently become possible due to advancements in MSI technology for both MALDI and SIMS. Nevertheless, these results also highlight the complementarity of different MS imaging techniques, in this case by the highest spatial resolution of TOF-SIMS and molecular specificity of the MALDI data. Each of the discussed MS imaging techniques has specific characteristics which make them suitable for certain applications, e.g., MALDI-TOF covers a wide mass range and MALDI-FTICR offers the highest mass resolving power. The complementarity of different MS imaging methods can be utilized in a “multimodal” approach in order to obtain a wealth of spatially correlated chemical information.
Comparison of phospholipid distribution. a TOF-SIMS measurement at 1-μm pixel size: Overlay of selected ion images of C16 fatty acids (red), cholesterol (green), and ST(42:2) sulfatide (blue). b AP-MALDI-Orbitrap measurement at 5-μm pixel size: Overlay of selected ion images of PC(32:0)+K+ (m/z = 772.525, red) and PS(40:0)+K+ (m/z = 848.638, green) without normalization or interpolation
In general, instrumental advantages of MS imaging methods are well known, but the actual performance depends on the exact application (targeted compound class and sample type) as well as experience and instrument status in the respective laboratory. These practical parameters and real-life performance can be evaluated in a multicenter study as discussed in this Note.
Here, we discuss general considerations and practical aspects for planning and performing multicenter studies in mass spectrometry imaging. The first step is to decide on a common sample. This choice obviously depends on the scope and purpose of the planned comparison study. Mouse brain tissue was chosen for our comparison study for several reasons: It is a widely used model tissue for MS imaging studies and thus results can easily be compared to measurements of rodent brain samples obtained by other groups/methods. Its histological structure and molecular composition are well known and thus obtained results can be readily evaluated, e.g., by comparison to online resources such as the “High Resolution Mouse Brain Atlas” [12]. A critical factor for comparison studies (and quality control efforts) is the fact that mouse brain tissue is readily available and comparable tissue can be obtained in the future. In addition, mouse brain tissue offers histological features of different spatial dimensions including individual cells (e.g., Purkinje cells). Its symmetric structure can provide a first indication of measurement reproducibility over the tissue (if the section is measured completely). These factors make mouse brain sections an ideal sample for our study, and similar activities where the focus is on comparison of methodology (and data analysis) rather than biological interpretation. If more complex (or more diverse) samples are used for comparison studies, they should be as similar as possible, i.e., adjacent sections cut from the same tissue sample. We originally distributed adjacent sections of one mouse brain, but not all of them could be used for the measurements of this comparison. Sections which were prepared subsequently do not exactly match the histological structure of the original sections. This problem could be avoided by two approaches: (a) distribute more than one section per laboratory and (b) provide a better definition of the targeted region (e.g., by referring to the “Allen Mouse Brain Atlas” [13]) to obtain comparable tissue sections.
One important aspect for preparation and distribution of sample is the sample support. Some instrument types require specific properties such as conductive surface and/or physical dimensions. Sample supports used in this study include glass slides (uncoated), ITO-coated glass slides, stainless steel, and silicon wafers (see Table 1). Sample thickness is another aspect that can be relevant in some cases. These parameters should be discussed and agreed on between all participating groups before starting a multicenter study. As a practical aspect, samples which are not chemically fixed should be shipped on dry ice with a short shipping duration (overnight is best). Based on our experience, extra care should be taken with scheduling and documentation when shipping samples on dry ice to non-Schengen countries such as the United Kingdom and Switzerland.
As a general note, it is difficult to use a standardized sample preparation protocol for a comparison study, especially for instruments as diverse as the ones used in our study. A common sample preparation protocol could favor one of the instrumental setups and would thus lead to biased results. We therefore decided to use the sample preparation protocols which are routinely used in each of the laboratories. Another possibility would have been the analysis of two samples per laboratory with a standardized protocol and an in-house protocol, respectively. The choice of sample preparation protocol must obviously meet the demands of the experimental study.
The conversion to imzML is an important step for comparing and evaluating MS imaging data from different instrument platforms. It has two main advantages: (1) All partners can view and analyze data from other laboratories. They can inspect raw mass spectrometric data and vary display parameters for MS images rather than evaluating the graphic representation of selected mass spectra and predefined MS images. This proved to be very useful in the course of the discussion of technical and practical details of our study. (2) All datasets can be displayed in a common software with identical options for visualization, e.g., normalization and interpolation (as shown in Fig. 1). If the measured area and pixel size match, data from different instruments could even be overlaid directly.
Conclusions
Our study could be used as a guideline for future multicenter studies in MS imaging. The concepts described here can also be applied for more extensive collaboration studies, e.g., to compare protocols and to establish best-practice guidelines, an activity that is currently being pursued in the EU-funded “COST action (European Cooperation in Science and Technology) Mass Spectrometry Imaging: New Tools for Healthcare Research” (BM1104) [14].
There are different goals for multicenter comparison studies. “Technical” studies (using “standard” tissue, e.g., mouse brain) focus on practical aspects of data acquisition, sample, and data handling. The goal of such a study could be to ensure comparable performance of an analysis workflow in different laboratories. This kind of comparison could be the basis for a study that focuses on a biological/biomedical application, e.g., the validation of biomarker discovery in different laboratories. An example for a bilateral study as a first step in this direction was recently reported [15].
Such multicenter studies in combination with ongoing activities for reporting guidelines [16], a common data format (imzML) [4], and a public data repository [17] will provide for more reliability and transparency of MS imaging studies in the future.
References
Neubert P, Walch A (2013) Current frontiers in clinical research application of MALDI imaging mass spectrometry. Expert Rev Proteomics 10(3):259–273. doi:10.1586/epr.13.19
Römpp A, Spengler B (2013) Mass spectrometry imaging with high resolution in mass and space. Histochem Cell Biol 139(6):759–783. doi:10.1007/s00418-013-1097-6
Chughtai K, Heeren RMA (2010) Mass spectrometric imaging for biomedical tissue analysis. Chem Rev 110(5):3237–3277. doi:10.1021/cr100012c
Schramm T, Hester A, Klinkert I, Both J-P, Heeren RMA, Brunelle A, Laprévote O, Desbenoit N, Robbe M-F, Stoeckli M, Spengler B, Römpp A (2012) imzML—a common data format for the flexible exchange and processing of mass spectrometry imaging data. J Proteomics 75(16):5106–5110. doi:10.1016/j.jprot.2012.07.026
Robichaud G, Garrard K, Barry J, Muddiman D (2013) MSiReader: an open-source interface to view and analyze high resolving power MS imaging files on Matlab platform. J Am Soc Mass Spectrom 24(5):718–721. doi:10.1007/s13361-013-0607-z
Klinkert I, Chughtai K, Ellis SR, Heeren RMA (2014) Methods for full resolution data exploration and visualization for large 2D and 3D mass spectrometry imaging datasets. Int J Mass Spectrom 362:40–47. doi:10.1016/j.ijms.2013.12.012
Parry RM, Galhena A, Gamage C, Bennett R, Wang M, Fernández F (2013) OmniSpect: an open MATLAB-based tool for visualization and analysis of matrix-assisted laser desorption/ionization and desorption electrospray ionization mass spectrometry images. J Am Soc Mass Spectrom 24(4):646–649. doi:10.1007/s13361-012-0572-y
Imabiotech (2012) Quantinetix MALDI Imaging Software. http://www.imabiotech.com/Quantinetix-TM-Maldi-Imaging.html?lang=en. Accessed 8 Jul 2012
PREMIERBiosoft (2012) MALDIVision. http://www.premierbiosoft.com/maldi-tissue-imaging/index.html. Accessed 3 Jul 2012
Römpp A, Guenther S, Schober Y, Schulz O, Takats Z, Kummer W, Spengler B (2010) Histology by mass spectrometry: label-free tissue characterization obtained from high-accuracy bioanalytical imaging. Angew Chem Int Ed 49(22):3834–3838. doi:10.1002/anie.200905559
Yang J, Caprioli RM (2011) Matrix sublimation/recrystallization for imaging proteins by mass spectrometry at high spatial resolution. Anal Chem 83(14):5728–5734. doi:10.1021/ac200998a
Sidman RL (2011) High resolution mouse brain atlas. http://www.hms.harvard.edu/research/brain/intro.html
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen T-M, Chi Chin M, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong H-W, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf K-R, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Feng Yuan X, Zhang B, Zwingman TA, Jones AR (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445(7124):168–176, http://www.nature.com/nature/journal/v445/n7124/suppinfo/nature05453_S1.html
McDonnell LA, Heeren RMA, Andrén PE, Stoeckli M, Corthals GL (2012) Going forward: increasing the accessibility of imaging mass spectrometry. J Proteomics 75(16):5113–5121
Dekker TJA, Balluff BD, Jones EA, Schöne CD, Schmitt M, Aubele M, Kroep JR, Smit VTHBM, Tollenaar RAEM, Mesker WE, Walch A, McDonnell LA (2014) Multicenter matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI) identifies proteomic differences in breast-cancer-associated stroma. J Proteome Res 13(11):4730–4738
McDonnell LA, Römpp A, Balluff B, Heeren RMA, Albar JP, Andren P, Corthals G, Walch A, Stoeckli M (2014) Discussion point: reporting guidelines for mass spectrometry imaging. Anal Bioanal Chem. doi:10.1007/s00216-014-8322-6
Römpp A, Wang R, Albar JP, Urbani A, Spengler B, Hermjakob H, Vizcaino JA (2014) A public data repository for mass spectrometry imaging data. Anal Bioanal Chem. doi:10.1007/s00216-014-8357-8
Acknowledgments
This work was supported by the European Union (Contract LSHG-CT-2005-518194 COMPUTIS). JLU acknowledges support by the Hessian Ministry of Science and Art (LOEWE focus Ambiprobe) and the European Research Council Starting Grant of Zoltan Takats. Alexandre Seyer is indebted to the Institut de Chimie des Substances Naturelles for a Ph.D. research fellowship. A portion of the research was performed using EMSL, a national scientific user facility sponsored by the U.S. Department of Energy’s Office of Biological and Environmental Research and located at Pacific Northwest National Laboratory.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in the topical collection Mass Spectrometry Imaging with guest editors Andreas Römpp and Uwe Karst.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 2.80 mb)
Rights and permissions
About this article
Cite this article
Römpp, A., Both, JP., Brunelle, A. et al. Mass spectrometry imaging of biological tissue: an approach for multicenter studies. Anal Bioanal Chem 407, 2329–2335 (2015). https://doi.org/10.1007/s00216-014-8410-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8410-7