Abstract
Dielectric-barrier-discharge ionization is an ambient-ionization technique. Since its first description in 2007, it has attracted much attention in such fields as biological analysis, food safety, mass-spectrometry imaging, forensic identification, and reaction monitoring for its advantages, e.g., low energy consumption, solvent-free method, and easy miniaturization. In this review a brief introduction to dielectric barrier discharge is provided, and then a detailed introduction to the dielectric-barrier-discharge-ionization technique is given, including instrumentation, applications, and mechanistic studies. Based on the summary of reported work, possible future uses of this type of ionization source are discussed at the end.

A dielectric barrier discharge ionization source to be used for ambient mass spectrometry (Reprinted from [33] with permission from Springer)
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studying ambient-ionization techniques has become one of the most productive subjects in mass spectrometry (MS) since the introduction of desorption-electrospray ionization (DESI) by Cooks’ group in 2004 [1]. Use of ambient mass spectrometry (AMS) is widespread in all application areas of mass spectrometry, including biological analysis [2, 3], food safety [4], mass-spectrometry imaging [5], forensic identification [6, 7], and reaction monitoring [8]. For example, probe-electrospray ionization (PESI) [9] has been used for the direct analysis of biological samples [10–12] and real-time reaction monitoring [13]. Compared with conventional electrospray ionization (ESI), PESI has the advantages of high tolerance to salts and direct ambient sampling. In addition, new AMS techniques are constantly emerging. According to incomplete statistics, more than 70 ambient-ionization techniques have been developed and described in the literature [14–29]. Of these AMS techniques, the direct analysis in real time (DART) proposed by Cody et al. in 2005 [30] has been developed into a commercialized instrument. In addition, DESI, laser-ablation-electrospray ionization (LAESI) [31], and liquid-extraction surface analysis (LESA) [32] are offered commercially.
Dielectric-barrier-discharge-ionization techniques [33, 34], as with DART, are a kind of plasma-based ambient-mass-spectrometry technique. This class of technique has become the most popular AMS technique except for DESI and DART. This review will give a comprehensive introduction to the technique and its application in biological and related subjects.
For a better understanding of this kind of technique, at the beginning of this review we give a brief introduction to dielectric barrier discharge (DBD). DBD is a typical non-equilibrium atmospheric-pressure alternating-current (AC) gas-discharge technology that was first reported by Siemens [35] in 1857, and it is widely used in plasma generation for its advantages of mild discharge, small size, stable operation at atmospheric pressure, and non-thermal characteristics. DBDs are characterized by the presence of one or more insulating layers (dielectric barriers) in the current path between metal electrodes in addition to the discharge space. The dielectric barriers usually use several common insulation materials, for example glass or silica glass, ceramic materials, mica, and thin enamel or polymer layers. There are two basic configurations for a DBD: planar and annular. Common DBD configurations are sketched in Fig. 1.
In planar arrangements (Fig. 1a–c) the two electrodes are parallel to each other, and either one dielectric barrier is located on one of the electrodes or between the two electrodes, or two dielectric barriers are located on each of the electrodes. In an annular, coaxial arrangement (Fig. 1d–f), one electrode is inside and the other is outside, with at least one or two dielectric barriers located between them. Figure 1 shows three common coaxial configurations:
-
1.
one dielectric barrier is placed on the outer side of the inner electrode;
-
2.
one dielectric barrier is placed on the inner side of the outer electrode; or
-
3.
two dielectric barriers are placed on both the electrodes facing each other.
In addition to these configurations, other variants of DBD are also used in a variety of applications. For example, the configuration in Fig. 1g is one variant of the DBD in which two annular electrodes are wrapped around a dielectric barrier: the capillary DBD or cylindrical DBD (C-DBD). DBDs can be realized with different gases in two major operating modes, namely the filamentary mode and the homogeneous mode. Filamentary DBD is the most common form of discharge, composed of many microdischarges that are randomly distributed over the electrode surface. Homogeneous discharge is similar to direct-current (DC) glow discharges, and its uniformity is better than that of a filamentary discharge.
DBD can generate a low-temperature plasma at atmospheric pressure, which provides a large amount of chemically active species at low temperatures ranging from room temperature (300 K) to approximately 2500 K. This is one reason DBD is widely used in many fields. DBD has a wide range of applications in analytical chemistry [36, 37], for example the DBD atomizer for analytical atomic spectrometry [38–41], DBD detector for gas chromatography [42, 43], DBD-induced and/or assisted chemiluminescence [44–46], and as the ionization source for ion-mobility spectrometry [47]. Generally speaking, the applications of DBD in analytical chemistry are beneficial to miniaturization of instruments because of the low power consumption and the simple and compact structure of DBD. On the basis of these applications and advantages, DBDs were adopted for use in ambient-ionization mass spectrometry.
Instrumentation
Since our group introduced the dielectric-barrier-discharge ion source (DBDI) into AMS [33], several innovative instruments based on diverse DBD configurations have emerged. All these instruments belong to the class of plasma-based ionization sources [29], and they all have one thing in common: plasma is induced by DBD. Hence, in this review, DBDI is given a broader meaning: all ambient-ionization sources based on DBD, including DBDI proposed by our group [33], the low-temperature plasma probe [34], and other similar ionization sources, are uniformly called DBDI. On the basis of the sampling method used in the ionization technique, DBDI techniques can be divided into three categories: plasma-jet ionization source, vapor-introduction ionization source, and multimode techniques.
Plasma-jet ionization source
In the plasma-jet ionization source, plasma is generated in the discharge region and ejected from a tubule or an orifice, carried by the airflow, to the surface of the analyte in an ambient environment. The plasma jet is used for desorption and ionization. This type of source includes the pin-plate DBDI, low-temperature-plasma (LTP) probe, dual-mode LTP source, adjustable LTP probe, LTP probe array, reactive-LTP, and portable LTP ionization source.
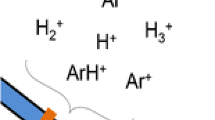
Pin-plate DBDI is a redefinition of the original DBDI setup proposed by our group in 2007 [33] to distinguish it from other DBDIs. The schematic of the pin-plate DBDI source is shown in Fig. 2. A low-temperature plasma jet in pin-plate configuration is used. An alternating voltage (3.5 kV to 4.5 kV p-p, 20.3 kHz) is applied between two electrodes, forming a stable plasma between the tip of the needle electrode and a glass slide. The analytes deposited on the surface of the glass slide are desorbed and ionized by impinging the sample surface with the reactive plasma species. We used DBDI coupled with MS to analyze 20 amino acids, and the test had good reproducibility [33]. Later, this setup was used for detecting trace amounts of explosives on different matrices, for example paper, cloth, chemical fiber, glass, paints, and soil [48].
Schematic of the pin-plate DBDI source (Reprinted from [33] with permission from Springer)
Based on pin-plate DBDI, the LTP probe was developed by a collaboration between our group and Cooks’ group in 2008 [34]. In the LTP probe, a glass tube (o.d. 6.35 mm and i.d. 3.75 mm) serves as the dielectric barrier and a stainless-steel needle (o.d. 1.57 mm) is used as an internal grounded electrode. The discharge gas can be either He, Ar, N2, or air. The plasma jet extending beyond the glass tube interacts directly with the sample. The LTP probe has some unique capabilities, including the use of air as the discharge gas, the ability to control fragmentation by a simple adjustment of the electrode configuration, the ability to directly analyze bulk aqueous solutions with no sample preparation, and the ability to analyze large surface areas. The LTP probe has good performance for the analysis of chemicals in a variety of situations (Fig. 3).
Schematic of the LTP probe (Reprinted from [34] with permission from American Chemical Society)
The dual-mode LTP source, in which a 2B pencil graphite is used as inner electrode, was also proposed by our group [49]. This source can work in two modes on the basis of its two different electrode arrangements: bar-plate and coaxial bar-cylinder shapes. The work mode can be altered at any time by using a simple double-stage electrical switch. The analysis of volatile organic compounds (VOCs) indicates there are several differences between the mass spectra obtained in the two modes. The differences can be very helpful for the identification of organic compounds and the study of gas-phase reactions in low-temperature plasmas. In addition to the feature mentioned above, the dual-mode LTP source with graphite electrode has another outstanding feature: it uses a disposable graphite electrode which is capable of acting as a sample adsorbent, providing a new sampling method for LTP-MS.
Sandra Martínez-Jarquín and Robert Winkler developed a novel LTP probe with adjustable output temperature and variable beam diameter [50], namely the adjustable LTP probe. As can be seen in Fig. 4, the plasma is guided through an internal second tube, and the plasma-beam diameter can be modified by changing the insert. The LTP temperature is controlled by the input voltage. It can achieve better spatial resolution for mass-spectrometry imaging (MSI) by varying the plasma-beam diameter, and achieve better sensitivity for detecting non-volatile samples using the high-temperature plasma.
Configuration of the adjustable LTP probe (Reprinted from [50] with permission from John Wiley and Sons)
The LTP probe array was proposed by Cooks’ group [51]. As shown in Fig. 5, there are two kinds of probe array: seven and 19 probes. For every probe, a quartz capillary (1.5 mm o.d., 0.75 mm i.d) served as the dielectric barrier, and steel wire (0.38 mm dia) was placed down the axis of each quartz tube to serve as the inner ground electrode and was positioned flush with the front of the epoxy-connected electrode array. The He flow rates were adjusted by use of rotameters. They used these probes for analysis of four “bath-salt” drugs, mephedrone, methylone, methylenedioxypyrovalerone (MDPV), and MDMA (ecstasy), and found that the limits of detection (LOD) improved as expected with increasing array size under the respective optimum conditions (0.1 L min−1 for one probe, 0.7 L min−1 for seven probes, and 1.9 L min−1 for 19 probes). They also used a modified seven-probe array, replacing the center capillary with a stainless-steel capillary to inject trifluoroacetic anhydride directly into the plasma stream for on-line derivatization. The result reveals increased selectivity towards mephedrone and methylone using trifluoracetylation under ambient-ionization conditions.
Schematic of the LTP-probe arrays (Reprinted from [51] with permission from John Wiley and Sons)
Reactive LTP is a completely new idea for MS. With the addition of extra chemical reagents into the discharge gas or the gaseous sample, ambient ion–molecule reactions can happen in the course of LTP ionization of analytes. These ambient ion–molecule reactions increase the specificity of LTP-based ambient analysis, improve the detection sensitivity, and reduce the detection limit. In this research field, Jacob T. Shelley et al. [52] investigated ionization-related matrix effects of three plasma-based AMS techniques: flowing atmospheric-pressure afterglow (FAPA), direct analysis in real time (DART), and the LTP probe. They introduced small amounts of vapor-phase matrices mixed with a continuous stream of gaseous analyte into each ionization source. Subsequently, ions generated in the reaction zone were detected. The results reveal that all sources had analyte-ion suppression when the matrix had a higher proton affinity than the analyte. Another study presented by Jonathan P. Wright et al. [53] discovered that the addition of a small amount of hydrogen to the He discharge gas can improve the analytical performance of LTP. Experimental data reveal that the addition of 0.9 % hydrogen to the helium increased signals for a range of test analytes, with enhancement factors of up to 68, without proportional increases in background levels. They believed the changes in signal levels resulted from a combination of changes to the desorption kinetics from the surface and increased ion production in the gas phase. Mario Benassi et al. [54] researched ambient ion–molecule reactions in LTP by adding some VOC reagents into He. When the reagents pass through the plasma region, reagent ions are generated. These ions are transported by the constant flow of gas and directed to the surface, where reaction with the neutral analyte takes place. The results reveal that an LTP source can be used to generate reagent ions that can undergo ion–molecule reactions in the ambient environment with an analyte at condensed phase on a surface. Cooks’ group performed a similar study using a modified LTP probe array [51]. They also performed a study on improving selectivity in the detection of selected explosives by adding trifluoracetic acid to the LTP discharge gas [55].
LTP is one of the AMS techniques meeting all of the requirements for portability in portable mass spectrometers. Cooks’ group and Ouyang’s group have done a lot of research on this subject [56–59]. They used a home-made LTP probe coupled with a portable mass spectrometer (Mini 10.5) [60] for the determination of melamine contamination in whole milk and related materials [56] and for detecting agrochemicals on the surface or in the tissue of fruit [57]. The results revealed that the LTP–Mini 10.5 combination is a robust method for in-situ analysis of complex samples, and therefore they subsequently proposed two portable devices, the handheld LTP [58] and Mini S [59]. The handheld LTP source consists of a battery-powered circuit, a glass tube for plasma formation, a miniature helium cylinder with a regulator, and a plastic housing (Fig. 6a). The overall weight of the source is 910 g, and it can work for 8–10 h before requiring a battery recharge or new helium cylinder. When coupled with a Mini 10.5 MS, LODs at the nanogram level can be achieved for a variety of agrochemicals. The Mini S (see Fig. 6b) is a backpack miniature mass spectrometer designed for maximum portability and flexibility for in-situ analysis. The overall weight of the Mini S is 12 kg. The instrument consumes an average of 65 W and can be operated autonomously under battery power for ca. 1.5 h. The geometry-independent LTP ionization source which makes direct surface sampling and analysis possible is an important part of Mini S. The mechanism of the geometry-independent LTP ionization source is as follows: helium supply generates a positive pressure inside the LTP source, and reactive ion species make contact with the sample surface; the discontinuous atmospheric-pressure interface (DAPI) valve is actuated for a short time; ions and neutrals are drawn into the mass analyzer through the ion-transfer capillaries. Detection of chemical warfare agent (CWA) simulants, illicit drugs, and explosives is achieved at nanogram levels directly from surfaces in near real time, including surfaces which have complex geometries, are heat-sensitive, and bear complex sample matrices.
Mohammad T. Jafari successfully realized the combination of LTP and ion-mobility spectrometry (IMS) [47]. In this work, the author analyzed some chemical compounds (gaseous, liquid, and solid) directly without their physical form being changed, and the result revealed that LTP-IMS is a promising technique.
Vapor-introduction ionization source
The vapor-introduction ionization source can only be used to ionize gaseous samples. Liquids and solids are ultimately introduced to the plasma as vapors. This type of source includes the double-cylindrical dielectric-barrier-discharge ion source, ambient-sampling chemi/chemical ionization, and the low-pressure dielectric-barrier-discharge ion source.
The double-cylindrical dielectric-barrier-discharge ion source is a non-ambient DBD ion source designed by Kenzo Hiraoka et al. [61]. The structure of the double-cylindrical DBDI is shown in Fig. 7. In this ion source, the sample gas is not exposed to the DBD He plasma and can only be ionized by the DBD-excited helium gas, because the sample inlet channel and plasma-generation area are separate. In addition, because ionization of sample vapor takes place inside the DBD ion source, this source is free from contaminants in ambient air, resulting in high reproducibility. An LOD of a few picograms for methamphetamine can be achieved using this source.
The structure of double-cylindrical DBDI (Reprinted from [61] with permission from Royal Society of Chemistry)
The ambient-sampling chemi/chemical ionization source was proposed by Lee Chuin Chen et al. [62, 63]. The schematic is shown in Fig. 8. In this source, metastable helium atoms (He*) generated from a DBD source are used as the primary ionizing agents. The gaseous sample in ambient air is sucked into an enclosed ionization chamber, which is electrically connected to the vacuum flange of the mass spectrometer, and reacts with He*. After this, the sample is ionized and detected. When detecting non-volatile samples, heated nitrogen or other desorption methods must be used. The authors used this source for rapid detection of gaseous hydrogen peroxide, and a detection limit of 0.8 ppbv was achieved [63]. For non-volatile compounds, an LOD of 1 ppb for methamphetamine in solution and of 5 pg for solid hexamethylene triperoxide diamine (HMTD) can be achieved [62].
Schematic of the ambient-sampling chemi/chemical ionization source (Reprinted from [62] with permission from John Wiley and Sons)
To achieve high ionization efficiency, Masuyuki Sugiyama et al. developed a low-pressure dielectric-barrier-discharge ion source (LP-DBDI), and integrated it into a portable mass spectrometer [64, 65]. As shown in Fig. 9a, because the outlet of LP-DBDI is connected with the vacuum region, high transmission efficiency from the ion source to the mass spectrometer is achieved. Meanwhile, gaseous samples pass through the discharge region, and therefore high ionization efficiency is expected. Mass spectra of methyl salicylate, 2-undecanone, and methamphetamine reveal that LP-DBDI is a soft ionization method characterized by only minor fragmentation, similar to atmospheric-pressure chemical ionization (APCI). In addition, it was found that the ionization efficiency and the specific ions generated by LP-DBDI depend on the pressure in the differential pumping region. Under optimum conditions, the sensitivity of the LP-DBDI source was revealed to be 40 times higher than that of a conventional APCI source.
Low-pressure dielectric-barrier-discharge ion source. (a) Configuration of the LP-DBDI source (Reprinted from [64] with permission from John Wiley and Sons), (b) schematic of a portable mass spectrometer with LP-DBDI, (c) configuration of the LP-DBDI source on a portable mass spectrometer, (d) control sequence of the portable mass spectrometer (Reprinted from [65] with permission from American Chemical Society)
Later, they developed a prototype of a portable mass spectrometer in which the LP-DBDI source and the vacuumed headspace method used for the sample introduction were combined with a homemade linear ion trap (LIT) (see Fig. 9). The prototype portable mass spectrometer is sensitive enough to detect 0.1 ppm methamphetamine, 1 ppm amphetamine, 1 ppm 3,4-methylenedioxymethamphetamine, and 10 ppm cocaine in liquid.
Multimode ionization techniques
Multimode ionization techniques are the ionization techniques in which two or more sampling and/or ionization methods are used, and can be classified into chromatography-based hyphenated techniques, sampling-based hyphenated techniques, and reactive multimode-ionization techniques.
Chromatography-based hyphenated techniques
Chromatography-based hyphenated techniques are those in which DBDI is combined with a chromatographic technique. There are two kinds of chromatography-based hyphenated technique: GC-LTP and HPLC-DBDI.
GC-LTP was proposed by Asger W. Nørgaard et al. [66]. As illustrated in Fig. 10a, an LTP probe and GC column are mounted in front of the inlet orifice. The LOD of GC-LTP coupled with time-of-flight (TOF) mass spectrometry is as low as ca. 0.5 ng (on column) for most compounds. The most commonly formed ion is [M + H]+ in the positive mode, and the M+• ions, [(M + O) + H]+, [(M + O2) + H]+, [M + NO]+, [M + NO2]+, and [M − H]+ can be observed in several cases.
Heiko Hayen used a DBD plasma jet as the DBDI source for liquid chromatography–mass spectrometry (LC–MS) [67]. A photograph of the HPLC-DBDI is presented in Fig. 10b. The setup was realized by modification of a commercial atmospheric-pressure-ionization (API) source. In this technique, the source must be coupled with a heated nebulizer in which nitrogen is used to nebulize the liquid eluent from LC. It is similar to APCI and atmospheric-pressure photoionization (APPI), and can be regarded as an API technique. What makes these three techniques different is the different ionization mechanism (corona discharge in APCI, gas-discharge lamp in APPI, and plasma jet in DBDI). Using the commercial API source hardware in which one can conveniently change between APCI, APPI, DBDI, and ESI, the performance of DBDI in comparison to APCI, APPI, and ESI was evaluated. The result reveals that DBDI can be regarded as a soft ionization technique characterized by only minor fragmentation, similar to APCI. HPLC-DBDI was also used for testing of multiclass organic contaminants in food and environment [68]. This technique is effective in detecting a wide array of organic compounds at concentration levels in the low ng L−1 to mg kg−1 range in wastewater and food matrices, respectively.
Sampling-based hyphenated techniques
As well as chromatography-based hyphenated techniques, there are other hyphenated techniques in which DBDI, as an ionization method, is combined with sampling methods including laser desorption, thermal desorption, or nebulization, or, sometimes, as a sampling method, is combined other ionization methods. Here we call these techniques sampling-based hyphenated techniques (analyte molecules are first formed through laser irradiation, shockwave treatment, thermal energy, or nebulization and then transferred to the ionization source for post-ionization). Sampling-based hyphenated techniques can be classified into nebulization-DBDI (Fig. 11), desorption-DBDI (Fig. 12), and DBD-inductively-coupled-plasma mass spectrometry (DBD-ICP-MS).
Desorption-DBDI techniques. (A) Active-capillary plasma source for ambient mass spectrometry. (a) Active capillary based on the wire-electrode configuration, (b) active capillary based on the cap-electrode configuration, (c) schematic diagram of the laser-ablated active-capillary plasma-ionization source (Reprinted from [71] with permission from John Wiley and Sons), (d) real-time breath analysis with active-capillary plasma-ionization ambient mass spectrometry (Reprinted from [72] with permission from IOP Publishing Ltd). (B) Schematic diagram of non-contact halogen-lamp-heating-assisted LTP ionization and water-assisted low-temperature-plasma (WALTP) torch. (a) Schematic diagram of non-contact halogen-lamp-heating-assisted LTP ionization (Reprinted from [73] with permission from Royal Society of Chemistry), (b) schematic diagram of water-assisted low-temperature-plasma (WALTP) torch (Reprinted from [74] with permission from Royal Society of Chemistry). (C) Schematic diagram of flash-desorption LTP ionization source (Reprinted from [76] with permission from Springer). (D) Diagram of experimental setup for LPTD ambient-ionization mass spectrometry (Reprinted from [78] with permission from Springer). (E) Schematic diagram of diode-laser-desorption dielectric-barrier-discharge ambient-ionization mass spectrometry (LD-DBDI-MS) (Reprinted from [79] with permission from American Chemical Society). (F) Schematic diagram of DBDI-based source, with desorption using an ultrasonic cutter and ionization by DBDI (Reprinted from [80] with permission from Springer)
Nebulization-DBDI techniques, including neutral desorption sampling in conjunction with dielectric-barrier-discharge ionization (ND-DBDI) and atmospheric-pressure chemi/chemical ionization (APC/CI), are illustrated in Fig. 11a. ND-DBDI in which the DBDI and neutral desorption are integrated was proposed by Zhou et al. [69]. In contrast with neutral-desorption extractive-electrospray ionization (ND-EESI), ND-DBDI uses ionized gas rather than charged droplets to ionize the sample. This technique was used to qualify a variety of hogwash-oil (HHO) and edible-oil samples, and the result revealed that ND-DBDI-MS is a rapid tool for fast and accurate identification of different HHO.
A schematic of the APC/CI is depicted in Fig. 11b. This source was proposed by Lee Chuin Chen et al. [70]. With this source, the gaseous sample is introduced through a nitrogen-flowing glass pipette, DBD produces He* to ionize sample molecules via chemi/chemical ionization, and a needle held at 100–500 V is used to guide the analyte ions into the mass spectrometer. Based on this source, they developed a rapid and sensitive method for the high-throughput analysis of methamphetamine, amphetamine, 3,4-methylenedioxymethamphetamine (MDMA), ketamine, and valproic acid directly from pH-adjusted biofluid samples (e.g. urine and serum). The detection limit for methamphetamine in urine is as low as 5 ng mL−1. Compared with (LC)–APCI-MS, this method is very simple.
In desorption DBDI many desorption methods, including laser desorption and thermal desorption, are used. A desorption-DBDI technique named active-capillary plasma source was proposed by Robert Winkler et al. for MSI [71], as shown in Fig. 12A. The active-sampling capillary based on DBD serves as the ionization source and atmospheric interface of the mass spectrometer. For solid or liquid samples, the active-capillary plasma source must couple with other desorption methods (laser-ablated in Fig. 12A (c)). The authors studied the relationship between the geometry of the electrodes (Fig. 12A (a), (b)) and ionization efficiency using vapor samples, and found that the taper cap-tape active capillary is ten times more sensitive than the wire-tape capillary, achieving an LOD of 0.1 ppb for decylamine. Subsequently, they used the active-capillary plasma source to analyze exhaled human breath in real time (see Fig. 12A (d)) and revealed that the technique is sensitive enough to detect over 100 relevant VOCs including fatty acids [72].
The technique shown in Fig. 12B uses halogen-lamp heating for assisted desorption. This technique has two outstanding features: high sensitivity for explosives with low volatility, and rapid analysis of explosives on large surface areas. Chen et al. used the technique in Fig. 12B (a) coupled with a miniature rectilinear-ion-trap mass spectrometer (RIT-MS) to analyze organic and inorganic explosives on solid surfaces [73]. LODs down to the picogram level can be achieved (e.g., 10 pg and 20 pg for TNT (2,4,6-trinitrotoluene) and RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine), respectively). This technique was also used to analyze wastewater from an explosives factory, and the feasibility of environmental analysis using this technique was proved. Based on the technique in Fig. 12B (a), Chen et al. developed a water-assisted low-temperature-plasma (WALTP) ionization source (see Fig. 12B (b)) in which wet air was introduced into the plasma jet [74]. Compared with the former technique, the LODs of explosives detection increased by 3–5 times (at the low picogram level, e.g., 5 pg for RDX) and the mass spectra were simplified.
Dilshadbek T. Usmanov et al. developed a technique in which a novel preheated-filament-probe method proposed by Ovchinnikova et al. [75] and DBDI were combined [76] (Fig. 12C). The preheated-filament-probe method is a flash-desorption method by which rapid heating followed by rapid cooling is achieved, enabling suppression of the successive thermal decomposition for the primary desorbed molecules. The mechanism of flash-desorption DBDI is as follows: the preheated stainless-steel filament (100–760 °C) is moved down and up automatically to achieve a slight and transient contact with the sample surface (an invasion depth of 0.1 mm and a contact time of 50 ms). With this level of contact, flash heating (at a rate of ~104 °C s−1) and fast cooling occurs. The desorbed gaseous molecules were then ionized by use of a dielectric-barrier-discharge ion source. This technique achieves high-throughput qualitative analysis with minimal sample consumption, and is very suitable for analyzing less and nonvolatile samples including pharmaceuticals, narcotics, explosives, and synthetic polymers.
Another desorption-DBDI technique composed of thermal desorption and DBDI is illustrated in Fig. 12D [77, 78]. This technique was proposed by Subhrakanti Saha et al. and uses the Leidenfrost phenomenon for spontaneous sample enrichment. The Leidenfrost phenomenon is common: when a liquid is exposed to an extremely hot substance, it produces an insulating vapor layer keeping that liquid from boiling rapidly, and the Leidenfrost temperature is defined as the substance temperature above which the solvent droplet starts levitating instead of splashing. In Leidenfrost-phenomenon-assisted thermal desorption (LPTD), the sample-solution droplet is dropped on the sample heater, the temperature of which is set above the Leidenfrost temperature. During the desorption process slow and gentle solvent evaporation takes place, but the less volatile and nonvolatile sample compounds remain in the liquid droplets, which leads to the pre-concentration of sample molecules. At the last moment of droplet evaporation, the explosive molecules are released and ionized by DBDI. This technique is very sensitive for rapid detection of less volatile and nonvolatile compounds at atmospheric pressure. The LODs for a variety of small organic molecules and peptides are at the level of ppt and 10−9 mol L−1, respectively [78]. The LOD for explosives is in the range 1–10 ppb, or absolute sample amount 90–630 fmol [77].
Figure 12E shows laser-desorption dielectric-barrier-discharge-ionization mass spectrometry (LD-DBDI-MS) [79]. A continuous-wave near-infrared diode laser is used for desorption and/or sampling. This source can be used for detection of nonvolatile chemicals on surfaces. The glass substrate is coated on the back with a black point to achieve an efficient desorption, and on this glass substrate a wide variety of multiclass species with low vapor pressure were tested, including pesticides, pharmaceuticals, and explosives. A comparative experiment using DBDI with a heated substrate was also performed, and the result revealed LD-DBDI to be better.
Figure 12F shows a very interesting technique, in which an ultrasonic cutter is used for desorption [80]. The ultrasonic cutter is a tool based on mechanical friction, for moving and smashing the matter at the interface. The experiment revealed that there is only a little thermal decomposition in the desorption process, and the ultrasonic cutter coupled with DBD-MS is a sensitive and quick analysis method for contaminants including narcotics, insecticides, and explosives. LODs of 1 ng for drugs, pharmaceuticals, amino acids, and explosives are obtained with this technique.
There is another sampling-based hyphenated technique usually used for inorganic mass spectrometry, in which DBD serves as a tool for sampling. Our group reported a depth-profile analysis method for thin layer coatings, combining an LTP probe with inductively-coupled-plasma mass spectrometry (ICP-MS) [81]. In this method, the sample material is ablated by the LTP probe and converted into an aerosol, and then transported by a carrier-gas flow to the ICP-MS, ionized, and analyzed. This technique is characterized by high spatial resolution, fast analysis, and ease of implementation. It should be a complementary technique to existing depth-profiling methods including glow discharge (GD)-MS, GD-optical emission spectrometry (OES), auger-electron spectroscopy (AES), and secondary-ion mass spectrometry (SIMS).
Reactive multimode-ionization techniques
Reactive multimode ionization is a multimode-ionization technique in which the function of DBD is neither desorption nor ionization. It serves as a source providing reactive molecules used for surface treatment of the nanoESI emitter [82] or for reaction with the ions from another ionization source [83, 84].
The setup in Fig. 13a was proposed by Cooks’ group. There are two examples of applications using this setup. In the first [82], LTP was used for treating the surface of the nanoESI emitter. The working sequence of this source is: first, turn on the LTP for 2 min with the nanoESI power off; then turn off the LTP and turn on the nanoESI. The study reveals that peptide fragmentation in nanoESI with and without pretreatment is different. For phosphopeptides, rich backbone fragmentation was usually observed and, most noticeably, labile phosphate groups are preserved. As the author believed, there are two root causes for this phenomenon. One is that electrolytes are released from the glass emitter by the plasma. Consequently they can be taken up into the spray solution, causing a high local electrolyte concentration. The other is the relatively low spray voltage used in nanoESI.
The second application was studying peptides containing disulfide bonds [83]. In this application, LTP functions as a source providing reagents for radical-induced reactions, whereas the T-shaped glass tube serves as a flow reactor in which peptide ions formed from nanoESI react with radical species generated from the LTP source. The authors observed the cleavage of the disulfide bond within a polypeptide, and believed that hydroxyl radicals initiated from the plasma may cause this by dissociative addition of a hydroxyl radical to the disulfide bond, forming RSH and R′SO• at the cleavage site.
The technique in Fig. 13b should be named reactive paper spray [84]. As in the second application of the setup in Fig. 13a, LTP in this technique also serves as a source of reagents. Based on reactive paper spray, the author proposed a method for determining the position of unsaturation in lipids in both simple and complex matrices. For determination of a double-bond position, oxygen is used as the discharge gas instead of helium, producing ozone. Ozone acts as a reagent and reacts with the spray of ions produced by paper-spray ionization, giving rise to cleavage of double bonds. The resulting ozone-cleavage product ions are used to determine the position of unsaturation through characterization of these ions in the precursor-ion-scan mass spectra.
Applications
DBDI has become very popular for applications in analytical science because of its outstanding features, including flexibility and simplicity of setup, good portability, and high chemical activity. In addition, the versatility of DBDI has been thoroughly revealed for a series of applications. Application fields of DBDI analysis include environmental analysis, ambient-mass-spectrometry imaging, high-throughput analysis, real-time monitoring of chemical reactions, food safety, public safety, clinical and pharmaceutical applications, industrial analysis, and many others. It is difficult to make a full summary of these diverse applications. In this review, we only briefly summarize some typical applications.
Real-time monitoring of chemical reactions
The study of reaction mechanisms has a critical function in synthetic chemistry, and the development of methods used for reaction monitoring constitutes an important part of analytical chemistry. Because of their low matrix effect and variety of sample-introduction approaches and ionization mechanisms, AMS techniques are beginning to be used to monitor reaction progress and intercept crucial reaction intermediates.
Our group developed an online-reaction-monitoring method based on LTP-MS [85]. We first proved the feasibility of using the LTP probe for liquid-sample analysis by letting the plasma directly interact with the liquid sample. Based on this and the in-situ analysis capacity of LTP, we used the LTP probe for the online monitoring of such simple organic reactions as esterification, acetylation, and Schiff’s-base formation. During the reaction the intermediates were detected, and the concentrations of reactants and reaction products were monitored. There are three main advantages of the LTP probe for reaction monitoring: low power consumption, no use of matrix, and little pollution to the MS. The result revealed that the LTP probe is suitable for probing even stepwise reactions. MS integrated with an LTP probe may be suitable for monitoring a large variety of organic reactions.
Ambient-mass-spectrometry imaging
Mass-spectrometry imaging (MSI, also known as imaging mass spectrometry, IMS) is a technique used in mass spectrometry to visualize the spatial distribution of, e.g., compounds, biomarkers, metabolites, peptides, or proteins by their molecular masses. As a promising technique in many fields, MSI is currently receiving a substantial amount of attention [5, 86–89]. Ambient MSI is an MSI technique that enables ambient sampling and ionization. In contrast with MALDI imaging and SIMS imaging, ambient MSI:
-
1.
enables large-area imaging analysis;
-
2.
enables direct analysis of the sample with little or no sample pretreatment; and
-
3.
some ambient MSI techniques can achieve in-situ imaging analysis with no damage to the sample.
LTP-MSI is one kind of ambient MSI technique. Its main advantage over other spray-related and laser-assisted techniques is that it enables MSI of artworks because of its spray-free and nondestructive characteristics. However, one inherent disadvantage of this technique is that it has poor spatial resolution. Figure 14 shows the applications of LTP-MSI.
LTP-MSI applications. (A) LTP-MSI of works of art. (a) Analysis of the calligraphy patterns using LTP-MSI, (b) expanded view of the LTP probe scanning the pattern, (c) imaging of the inkpads of seals on rice paper by use of the LTP probe (Reprinted from [90] with permission from John Wiley and Sons). (B) LTP-MSI of a cross-section of a Capsicum annuum fruit. (a) Photograph of the fresh sample, (b–d) ion traces reveal either the uniform distribution or the tissue-specific localization of compounds, (e) components which need to be integrated for a LTP-MSI system (Reprinted from [91] with permission from Elsevier). (C) LTP-MSI of volatile and semi-volatile compounds. (a) Diagram of one plasma source, (b) photograph of imaging setup, (c) images for 2 ng γ-decalactone (m/z 171, M + H]+) spotted on a 3 × 3 mm piece of Whatman #1 filter paper in the center of a 2.5 × 2.5 cm surface, and analyzed using LTP probes with a variety of i.d. (Reprinted from [92] with permission from John Wiley and Sons)
In 2010 our group presented a novel MSI method based on an LTP probe for analysis of paintings and calligraphy [90], as shown in Fig. 14A. The structure of the LTP probe was specially designed for high-resolution MSI. The inner diameter of the inner capillary is the most important factor controlling the dimension of the plasma plume. A very slender plasma plume was obtained by discharging in a quartz capillary with a diameter of 100 μm, and the spatial resolution of the images was approximately 250 μm. The results revealed that discrimination between the genuine and counterfeit seals could be achieved by mapping the ion of m/z 116 present only in genuine seals. More importantly, no damage was made to the surface, as confirmed by scanning electron microscopy (SEM) after MSI analysis. It is believed that LTP can contribute to the identification, conservation, and restoration of valuable artworks.
Another study of LTP-MSI was presented by Mauricio Maldonado-Torres et al. [91]. They constructed an LTP-MSI system using an adjustable LTP probe [50] with a plasma-beam diameter of approximately 300 μm. The constitution of the LTP-MSI system is detailed in Fig. 14B. The system has a practical resolution of 1 mm and a sampling area of 100 × 100 mm2, resulting in up to 10,000 sampling points. Imaging of the distribution of metabolites in the longitudinal cross-section of a chili fruit (Capsicum annuum, “Jalapeño pepper”) was performed to prove the suitability of the LTP-MSI system for macroscopic samples. The result reveals that LTP-MSI can detect and localize small organic compounds in biological tissue without prior sample work-up. This study is important regarding broadening the application of MSI in biology.
An LTP probe was used for chemical imaging of volatile and semi-volatile compounds by using a Peltier cooling stage (Fig. 14C) [92]. In this study, authors investigated the effect reducing the dimensions of the probe had on the chemical image quality, and found that an LTP probe of i.d. 0.5 mm is the best choice for all probes (2.0, 0.75, 0.50, and 0.30 mm, and probe array).
Biological, clinical, and pharmaceutical applications
Biology studies, clinical research, and pharmacology studies have an important function in the promotion of human health. In these subjects, mass spectrometry is an important tool, and DBDI-MS, with its high sensitivity and simple operation, is effective in these fields.
The first application in these fields was by our group in 2009 [93]: we developed a high-throughput method for rapid screening of active ingredients in drugs by use of LTP-MS. This method was proved to be effective by analyzing 18 identical pharmaceutical tablets within 1.9 min. The potential of LTP-MS in clinical and pharmaceutical analysis is proved by the following examples.
Lee Chuin Chen et al. used APC/CI-MS for direct and rapid detection of volatile drugs in raw biological fluids including urine and serum (see “Instrumentation” section) [70]. Jackson et al. used LTP for the analysis of 14 drugs of abuse, including amphetamine, caffeine, cocaine, diazepam, and heroin, in saliva, urine, and hair extracts, achieving detection limits as low as 1 ng mL−1 (0.5 ng mL−1 with heating) [94]. M. Jeanette Stein et al. used plasma-pencil atmospheric mass spectrometry (PPAMS) for rapid, simultaneous detection of the crucial micronutrients zinc, iron, folate, vitamin A, and iodine in porcine plasma, and then classified the nutrients by type and quantity into lower and upper physiological blood nutrient levels by applying the multivariate-statistical-modeling method of principal component analysis (PCA) to the spectra [95]. Lukas Bregy et al. used an active-capillary plasma source to analyze exhaled human breath in real-time (see “Instrumentation” section) [72].
In biology, as well as the three applications of reactive multimode techniques mentioned in the “Multimode ionization techniques” section, there are two other applications. One is determination of the double-bond position in unsaturated fatty acids and esters by LTP-MS [96]. Unsaturated fatty acids and esters can be oxidized by in-situ-generated ozone during ionization using LTP. Fragmentation patterns of the resulting oxidation products can be used to assign the double-bond positions. The other is rapid bacterial differentiation by detecting fatty-acid ethyl esters (FAEE) using LTP-MS [97]. Gram-positive and gram-negative bacteria and 11 out of 13 Salmonella strains can be distinguished by the mass spectra of FAEE, which have highly reproducible and characteristic patterns.
Food safety, public safety, and environmental analysis
In today’s globalized world, food safety is an international concern. Food safety has an important function in maintaining people’s health and in the prevention of food-borne diseases. The introduction of DBDI provides another important tool for food quality control. There are already some applications in food safety.
Huang et al. used an LTP coupled with tandem mass spectrometry to detect and quantitate melamine in milk powder, with an LOD down to low ppb level [98]. A similar application was performed by Huang et al. using LTP combined with a miniature ion-trap mass spectrometer, in which the analysis rate was two samples per minute and the detection limit of melamine was as low as 250 ng mL−1 in whole milk [56]. As well as detecting illegal additives in food, screening of agrochemicals in foodstuffs is another important application. Agrichemicals analysis using LTP-MS has passed through several stages. At first, the analysis was performed with a homemade LTP probe and a Thermo LTQ linear-ion-trap mass spectrometer. A total of 13 agricultural chemicals were directly detected on fruit peel and in fruit and vegetable extracts, with a detection limit in the picogram range [99]. Later, a homemade LTP probe coupled with a handheld mass spectrometer was used to detect diphenylamine (DPA) directly from the skin of apples [57]. This is much closer to in-situ analysis. Recently, Cooks’ group took this work one stage further by developing a completely field-portable instrument composed of a handheld LTP probe and a miniature mass spectrometer (see “Instrumentation” section) [58]. They evaluated this instrument by direct detection of pesticides. When using He as the discharge gas, the LOD of atrazine is 0.1 ng. In food safety, there are some attempts to use LTP in food-quality monitoring. Juan F. Garcia-Reyes et al. used LTP-MS–MS to detect both minor and trace components (free fatty acids, phenolics, and volatiles) in raw untreated olive oil. They expect data from mass spectra will be used for authentication of olive oil [100]. A similar study was done by Zhou et al., in which ND-DBDI-MS (see “Instrumentation” section) was established for the fast and accurate identification of a variety of HHO and edible-oil samples under ambient conditions [69].
Applications of DBDI in public safety focus on the detection of explosives. The main difference between these studies is experimental setup. The setups used for detecting explosives include pin-plate DBDI [48], LTP [101], reactive LTP [55], LPTD [77], non-contact halogen-lamp-heating-assisted LTP [73], and WALTP [74]. Descriptions of these setups are provided in “Multimode ionization techniques” section. These studies were performed not only on benchtop mass spectrometers [48, 55, 77], but also on miniature mass spectrometers [58, 73, 74, 102]. The LOD is down to the low femtogram range on benchtop mass spectrometers and picogram range on miniature mass spectrometers. Another application related to public safety is detecting hydrogen peroxide in ambient air using an ambient-sampling chemi/chemical ionization source (see “Instrumentation” section) [63].
Environmental analysis using DBDI-MS includes analysis of PM 2.5 filters [103] and the online detection of indoor volatile organic compounds [104]. In PM 2.5-filters analysis, the fingerprinting of the relative abundance of dominating species is achieved. Air is used as the discharge gas for detection of indoor VOCs including alcohols, ketones, aldehydes, and aromatics. This source is characterized by minimal fragmentation and domination of protonated ions.
Other applications
Other applications concentrate on industrial analysis and materials analysis. We list some examples below.
In industrial analysis, Dahlia I. Campbell et al. [92] developed a semi-quantitative method based on LTP-MS for volatile compounds in commercial cleaning products, in which a Peltier cooling stage was used to improve the retention time of VOCs on a surface. Mario Benassi et al. used LTP-MS for rapid screening of low-molecular-weight and relatively volatile components in petroleum crude oil [105]. The spectral data were used for characterization and differentiation of crude-oil samples using principal components analysis (PCA).
In materials analysis, Zhang et al. [81] reported the use of LTP coupled to inductively coupled plasma MS for depth profiling of thin-layer coatings of electronics (see “Instrumentation” section). Ma et al. used LTP-MS for direct analysis of self-assembled monolayers (SAMs) on copper surfaces [106]. In their group’s later work, LTP-MS was used for detection of layer-by-layer self-assembly multilayer films [107]. Using high-resolution mass spectrometry (HR-MS) and an LTP probe, Britta Vortmann et al. [108] presented a rapid method for in-situ analysis and reaction monitoring in lithium-ion-battery (LIB) electrolytes. Major thermal-decomposition products in the battery were detected and identified. In the report of Asger W. Nørgaardet al. [109], LTP, two other AMS techniques (transmission-mode desorption-electrospray ionization and nano-assisted laser-desorption ionization), and two API techniques (ESI and APCI) were used for the analysis of two nanofilm products (NFPs) for surface coating.
There are some other researches worth mentioning. In the study of Asger W. Nørgaard et al. [110], Limonene and its ozone-initiated reaction products were investigated in situ by an LTP probe coupled with a quadrupole time-of-flight (QTOF) mass spectrometer. Yang et al. [111] investigated the distribution of water clusters under ambient conditions using a triple-quadrupole mass spectrometer coupled with a DBDI source.
Mechanistic studies
Studies on the mechanism of an AMS technique can be divided into two subjects: desorption mechanism and ionization mechanism. The desorption mechanism of DBDI is often claimed to be essentially thermal, which is a helpful auxiliary for ionization in the direct analysis of solid analytes. In most cases, signal enhancement is achieved at a modestly elevated temperature. It is believed that elevated temperature of the sample plate or discharge gas serves to volatilize organic molecules from surfaces. This assertion is supported by Refs. [34, 50, 55, 98, 99]. Although thermal effects can certainly affect the desorption process, other factors, including the etching reaction and sputtering reaction [112], could also contribute; ablation of nanometer coatings in [81] and etching of polymer films in [113] are examples of this.
Ionization mechanisms of DBDI have received more study, and we can summarize them in three categories by their research focus. The first kind focuses on how ionization-source settings affect ionization. Generally speaking, the settings of DBDI, including the type and flow rate of discharge gas and the settings of the power supply, have some effect on MS spectra [33, 34]. For example, Mohammad Reza Almasian et al. [49] studied the effect of electrode-arrangement mode, electrode length, and electrode material on in-source fragmentation patterns. Jacob T. Shelley et al. [114] found that the frequency and waveform shape of the power supply have a significant effect on the number of reagent ions generated by LTP probe. Although these studies did not reveal the mechanism of the ionization source in depth, they revealed the complexity of the mechanism and could serve as inspiration for future research. Cordula Meyer et al. [115] went further than the studies mentioned above. In their study, the basic discharge mode of DBD was classified by electrical measurements and its effect on ionization efficiency was taken into account. The study indicated that filamentary DBDI has poor ionization efficiency (absolute MS intensity and minor fragmentation) compared with homogeneous DBDI. In subsequent work, they measured the emission spectra of atomic He, N2 +, and N2 molecules in DBD plasma under two modes, searching for optimum conditions under which the source is expected to be most effective for soft ionization of molecules [116].
The second type of research is characterization of DBDI by comparing it with other ionization sources. Heiko Hayen et al. [67] compared DBDI with three commercial API sources, ESI, APCI, and APPI, for the analysis of a heterogeneous compound library using LC–MS. The result revealed that DBDI can be regarded as a soft ionization technique characterized by only minor fragmentation, similar to APCI, and serves as an attractive ionization source for the ionization of low-molecular-weight compounds over a relatively wide polarity range. A similar study was performed by Anastasia Albert and Carsten Engelhard [117], in which LTP was compared with ESI and APCI via analysis of several model analytes from different compound families, and the same conclusion was reached. Bienvenida Gilbert-López et al. [118] revealed minor ionization matrix effects in DBDI compared with APCI. Jacob T. Shelley and Gary M. Hieftje [52] investigated ionization matrix effects of FAPA, DART, and LTP, and revealed that all three sources had analyte-ion suppression when the matrix had a higher proton affinity than the analyte. Jan Kratzer et al. [119] compared the three ambient-ionization sources based on plasma. They indicated that BDBI, atmospheric-pressure radiofrequency-driven glow discharge, and DART have the same mechanism for their desorption and ionization processes. The intensity of the MS signal relates to the density of ions and activated atoms in plasma. Excessive positively charged particles and negatively charged particles are both bad for the ionization process, meaning the effect of removing the charged particles from the plasma has to be determined. They also believed that temperature had a strong effect on the response signal for the plasma-based ionization source. The desorption efficiency increased with increased temperature, but excessive temperature will lead to decomposition of the analytes.
The last category is elucidation of the ionization mechanism by means of plasma-spectroscopy diagnostics. Plasma-spectroscopy diagnostics is a good method for identifying atomic and diatomic species created within the discharge. J. Franzke’s group has done much work on this. They investigated the mechanism of N2 + production in the plasma jet of a capillary DBD used as a soft ionization source in ion-mobility spectrometry by means of optical emission spectroscopy (OES) [120–122], because N2 + has an important function in the ionization process. They also proposed a method for the determination of the populations of the lowest excited helium states 2s3S1, 2s1S, 2p3P0 J, and 2p1P0, created in an atmospheric-helium capillary DBD [123]. This work is helpful in understanding the generation process for N2 +. Matthew S. Heywood et al. used collision-assisted laser-induced fluorescence (LIF) to image density distributions of helium metastable atoms [124]. George C.-Y. Chan et al. performed a spatially resolved optical characterization of the DBD and afterglow of the LTP probe to identify atomic and diatomic species present in the plasma under a variety of operating conditions [125]. Subsequently, they used a similar measurement to reveal the reaction mechanisms (Fig. 15) ultimately responsible for analyte ionization. They believed that there is a set of reactions for the generation of reagent ions [126].
Schematic diagram showing identified spatially dependent reactions for the afterglow and reagent-ion formation in the LTP-probe ambient-ionization source (Reprinted from [126] with permission from American Chemical Society)
There are also some studies on characterization of mass spectra of specific compounds. For example, Na et al. [127] observed dehydrogenation of benzene and other arenes when a helium-supported LTP probe was used for ionization of these compounds. Zhang et al. [128] studied the plasma reaction of benzene in an atmospheric-pressure low-temperature plasma of air using a planar DBDI coupled to a high-resolution Exactive Orbitrap mass spectrometer, and observed a reaction pathway for the replacement of one carbon atom in benzene with atomic nitrogen, which leads to the generation of nitrogen-heterocyclic compounds. There are few studies of this aspect and the plasma chemical reaction makes the MS spectrum complicated, so that the mass spectra of some compounds are hard to understand. More studies are expected with the development of DBDI-MS.
Summary and perspective
DBDI, as one of the AMS techniques, has received much attention for its outstanding features, including the flexibility and simplicity of setup, good portability, and high chemical activity. Researchers have done a lot of studies on the instrument innovation, application, and ionization mechanism of this ionization source. This review gives a detailed introduction to these studies, which it is hoped will provide clarification. Based on the studies discussed in this review, the following list details some possible prospects for DBDI research.
-
1.
Miniaturization and portability. From previous studies of its applications, DBDI has special advantages regarding detection of explosives and analysis of agrochemical residues. It is also easily miniaturized because of its simple structure. In consequence, miniaturization is one development possibility for DBDI. A portable mass spectrometer with a DBDI source would have an important function in public safety and food safety.
-
2.
Reactive DBDI. Low-temperature plasma contains many reactive molecules, providing a favorable environment for chemical reactions. More research into reactive DBDI is desired to take full advantage of this feature. It will be very helpful regarding reaction dynamics. In addition, reactive DBDI can increase the specificity of analyte identification and improve sensitivity for the targeted species.
-
3.
New tool for healthcare research. There are many studies on use of the low-temperature plasma jet in healthcare, including plasma treatment of cancer cells [129], living human cells [130], prevention of nosocomial infections [131], and therapy for infected wounds [132]. From the features of low-temperature plasma, we believe that DBDI-MS could be a promising tool for clinical analysis, real-time, in-situ, and in-vivo analysis of organ tissue, and identification of molecular biomarkers.
-
4.
Studying the ionization mechanism. The ionization mechanism of DBDI is still unclear. It will not be until the ionization mechanism is fully understood that the specific range of compounds DBDI is adapted for can be discovered and the performance can be improved (e.g. reducing the background noise).
-
5.
In-situ real-time reaction monitoring. Previous study has proved the LTP probe to be a simple approach for reaction monitoring, with the fewest requirements regarding experimental devices and procedures [85]. Also, DBDI is an in-situ real-time chemical-analysis tool. Therefore, it is expected that a miniaturized mass spectrometer coupled with DBDI may be suitable for the in-situ real-time monitoring of a large variety of organic reactions.
References
Takats Z, Wiseman JM, Gologan B, Cooks RG (2004) Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science 306(5695):471–473
Nemes P, Vertes A (2012) Ambient mass spectrometry for invivo local analysis and insitu molecular tissue imaging. TrAC Trends Anal Chem 34:22–34
Chingin K, Liang J, Chen H (2014) Direct analysis of in vitro grown microorganisms and mammalian cells by ambient mass spectrometry. RSC Adv 4(11):5768–5781
Hajslova J, Cajka T, Vaclavik L (2011) Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. TrAC Trends Anal Chem 30(2):204–218
Wu C, Dill AL, Eberlin LS, Cooks RG, Ifa DR (2013) Mass spectrometry imaging under ambient conditions. Mass Spectrom Rev 32(3):218–243
Ifa DR, Jackson AU, Paglia G, Cooks RG (2009) Forensic applications of ambient ionization mass spectrometry. Anal Bioanal Chem 394(8):1995–2008
Green FM, Salter TL, Stokes P, Gilmore IS, O’Connor G (2010) Ambient mass spectrometry: advances and applications in forensics. Surf Interface Anal 42(5):347–357
Ma X, Zhang S, Zhang X (2012) An instrumentation perspective on reaction monitoring by ambient mass spectrometry. TrAC Trends Anal Chem 35:50–66
Hiraoka K, Nishidate K, Mori K, Asakawa D, Suzuki S (2007) Development of probe electrospray using a solid needle. Rapid Commun Mass Spectrom 21(18):3139–3144
Mandal MK, Chen LC, Hashimoto Y, Yu Z, Hiraoka K (2010) Detection of biomolecules from solutions with high concentration of salts using probe electrospray and nano-electrospray ionization mass spectrometry. Anal Methods 2(12):1905–1912
Yoshimura K, Chen LC, Asakawa D, Hiraoka K, Takeda S (2009) Physical properties of the probe electrospray ionization (PESI) needle applied to the biological samples. J Mass Spectrom 44(6):978–985
Yu Z, Chen LC, Suzuki H, Ariyada O, Erra-Balsells R, Nonami H, Hiraoka K (2009) Direct profiling of phytochemicals in tulip tissues and in vivo monitoring of the change of carbohydrate content in tulip bulbs by probe electrospray ionization mass spectrometry. J Am Soc Mass Spectrom 20(12):2304–2311
Yu Z, Chen LC, Erra Balsells R, Nonami H, Hiraoka K (2010) Real‐time reaction monitoring by probe electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom 24(11):1507–1513
Cooks RG, Ouyang Z, Takats Z, Wiseman JM (2006) Ambient mass spectrometry. Science 311(5767):1566–1570
Venter A, Nefliu M, Graham Cooks R (2008) Ambient desorption ionization mass spectrometry. TrAC Trends Anal Chem 27(4):284–290
Harris GA, Nyadong L, Fernandez FM (2008) Recent developments in ambient ionization techniques for analytical mass spectrometry. Analyst 133(10):1297–1301
Van Berkel GJ, Pasilis SP, Ovchinnikova O (2008) Established and emerging atmospheric pressure surface sampling/ionization techniques for mass spectrometry. J Mass Spectrom 43(9):1161–1180
Chen H, Gamez G, Zenobi R (2009) What can we learn from ambient ionization techniques? J Am Soc Mass Spectrom 20(11):1947–1963
Chen H, Hu B, Zhang X (2010) Principle and application of ambient mass spectrometry for direct analysis of complex samples. Chin J Anal Chem 38(8):1069–1088
Ifa DR, Wu C, Ouyang Z, Cooks RG (2010) Desorption electrospray ionization and other ambient ionization methods: current progress and preview. Analyst 135(4):669–681
Weston DJ (2010) Ambient ionization mass spectrometry: current understanding of mechanistic theory; analytical performance and application areas. Analyst 135(4):661–668
Huang M, Yuan C, Cheng S, Cho Y, Shiea J (2010) Ambient ionization mass spectrometry. Annu Rev Anal Chem 3:43–65
Alberici RM, Simas RC, Sanvido GB, Romão W, Lalli PM, Benassi M, Cunha IB, Eberlin MN (2010) Ambient mass spectrometry: bringing MS into the “real world”. Anal Bioanal Chem 398(1):265–294
Huang M, Cheng S, Cho Y, Shiea J (2011) Ambient ionization mass spectrometry: a tutorial. Anal Chim Acta 702(1):1–15
Harris GA, Galhena AS, Fernandez FM (2011) Ambient sampling/ionization mass spectrometry: applications and current trends. Anal Chem 83(12):4508–4538
Monge ME, Harris GA, Dwivedi P, Fernández FM (2013) Mass spectrometry: recent advances in direct open air surface sampling/ionization. Chem Rev 113(4):2269–2308
Badu-Tawiah AK, Eberlin LS, Ouyang Z, Cooks RG (2013) Chemical aspects of the extractive methods of ambient ionization mass spectrometry. Annu Rev Phys Chem 64:481–505
Li L, Feng B, Yang J, Chang C, Bai Y, Liu H (2013) Applications of ambient mass spectrometry in high-throughput screening. Analyst 138(11):3097–3103
Ding X, Duan Y (2014) Plasma‐based ambient mass spectrometry techniques: the current status and future prospective. Mass Spectrom Rev
Cody RB, Laramée JA, Durst HD (2005) Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem 77(8):2297–2302
Nemes P, Vertes A (2007) Laser ablation electrospray ionization for atmospheric pressure, in vivo, and imaging mass spectrometry. Anal Chem 79(21):8098–8106
Kertesz V, Van Berkel GJ (2010) Fully automated liquid extraction‐based surface sampling and ionization using a chip‐based robotic nanoelectrospray platform. J Mass Spectrom 45(3):252–260
Na N, Zhao M, Zhang S, Yang C, Zhang X (2007) Development of a dielectric barrier discharge ion source for ambient mass spectrometry. J Am Soc Mass Spectrom 18(10):1859–1862
Harper JD, Charipar NA, Mulligan CC, Zhang X, Cooks RG, Ouyang Z (2008) Low-temperature plasma probe for ambient desorption ionization. Anal Chem 80(23):9097–9104
Siemens WV (1857) Ueber die elektrostatische Induction und die Verzögerung des Stroms in Flaschendrähten. Ann Phys Berlin 178(9):66–122
Meyer C, Müller S, Gurevich EL, Franzke J (2011) Dielectric barrier discharges in analytical chemistry. Analyst 136(12):2427–2440
Hu J, Li W, Zheng C, Hou X (2011) Dielectric barrier discharge in analytical spectrometry. Appl Spectrosc Rev 46(5):368–387
Miclea M, Kunze K, Musa G, Franzke J, Niemax K (2001) The dielectric barrier discharge—a powerful microchip plasma for diode laser spectrometry. Spectrochim Acta B At Spectrosc 56(1):37–43
Zhu Z, Liu J, Zhang S, Na X, Zhang X (2008) Determination of Se, Pb, and Sb by atomic fluorescence spectrometry using a new flameless, dielectric barrier discharge atomizer. Spectrochim Acta B At Spectrosc 63(3):431–436
Yu Y, Du Z, Chen M, Wang J (2008) Atmospheric‐pressure dielectric‐barrier discharge as a radiation source for optical emission spectrometry. Angew Chem Int Ed 47(41):7909–7912
Tombrink S, Müller S, Heming R, Michels A, Lampen P, Franzke J (2010) Liquid analysis dielectric capillary barrier discharge. Anal Bioanal Chem 397(7):2917–2922
Monagle M (1999) Trace constituent detection in inert gases. Google Patents
Gras R, Luong J, Monagle M, Winniford B (2006) Gas chromatographic applications with the dielectric barrier discharge detector. J Chromatogr Sci 44(2):101–107
He Y, Lv Y, Li Y, Tang H, Li L, Wu X, Hou X (2007) Dielectric barrier discharge-induced chemiluminescence: potential application as GC detector. Anal Chem 79(12):4674–4680
Almasian MR, Na N, Wen F, Zhang S, Zhang X (2010) Development of a plasma-assisted cataluminescence system for benzene, toluene, ethylbenzene, and xylenes analysis. Anal Chem 82(9):3457–3459
Han J, Han F, Ouyang J, He L, Zhang Y, Na N (2014) Low temperature CO sensor based on cataluminescence from plasma-assisted catalytic oxidation on Ag doped alkaline-earth nanomaterials. Nanoscale 6(6):3069–3072
Jafari MT (2011) Low-temperature plasma ionization ion mobility spectrometry. Anal Chem 83(3):797–803
Na N, Zhang C, Zhao MX, Zhang SC, Yang CD, Fang X, Zhang XR (2007) Direct detection of explosives on solid surfaces by mass spectrometry with an ambient ion source based on dielectric barrier discharge. J Mass Spectrom 42(8):1079–1085
Almasian MR, Yang CD, Xing Z, Zhang SC, Zhang XR (2010) Development of a graphite low-temperature plasma source with dual-mode in-source fragmentation for ambient mass spectrometry. Rapid Commun Mass Spectrom 24(6):742–748
Martinez-Jarquin S, Winkler R (2013) Design of a low-temperature plasma (LTP) probe with adjustable output temperature and variable beam diameter for the direct detection of organic molecules. Rapid Commun Mass Spectrom 27(5):629–634
Dalgleish JK, Wleklinski M, Shelley JT, Mulligan CC, Ouyang Z, Cooks RG (2013) Arrays of low-temperature plasma probes for ambient ionization mass spectrometry. Rapid Commun Mass Spectrom 27(1):135–142
Shelley JT, Hieftje GM (2010) Ionization matrix effects in plasma-based ambient mass spectrometry sources. J Anal Atom Spectrom 25(3):345–350
Wright JP, Heywood MS, Thurston GK, Farnsworth PB (2013) The effects of added hydrogen on a helium atmospheric-pressure plasma jet ambient desorption/ionization source. J Am Soc Mass Spectrom 24(3):335–340
Benassi M, Garcia-Reyes JF, Spengler B (2013) Ambient ion/molecule reactions in low-temperature plasmas (LTP): reactive LTP mass spectrometry. Rapid Commun Mass Spectrom 27(7):795–804
Garcia-Reyes JF, Harper JD, Salazar GA, Charipar NA, Ouyang Z, Cooks RG (2011) Detection of explosives and related compounds by low-temperature plasma ambient ionization mass spectrometry. Anal Chem 83(3):1084–1092
Huang G, Xu W, Visbal-Onufrak MA, Ouyang Z, Cooks RG (2010) Direct analysis of melamine in complex matrices using a handheld mass spectrometer. Analyst 135(4):705–711
Soparawalla S, Tadjimukhamedov FK, Wiley JS, Ouyang Z, Cooks RG (2011) In situ analysis of agrochemical residues on fruit using ambient ionization on a handheld mass spectrometer. Analyst 136(21):4392–4396
Wiley JS, Shelley JT, Cooks RG (2013) Handheld low-temperature plasma probe for portable “point-and-shoot” ambient ionization mass spectrometry. Anal Chem 85(14):6545–6552
Hendricks PI, Dalgleish JK, Shelley JT, Kirleis MA, McNicholas MT, Li L, Chen T, Chen C, Duncan JS, Boudreau F, Noll RJ, Denton JP, Roach TA, Ouyang Z, Cooks RG (2014) Autonomous in situ analysis and real-time chemical detection using a backpack miniature mass spectrometer: concept, instrumentation development, and performance. Anal Chem 86(6):2900–2908
Gao L, Song Q, Patterson GE, Cooks RG, Ouyang Z (2006) Handheld rectilinear ion trap mass spectrometer. Anal Chem 78(17):5994–6002
Hiraoka K, Ninomiya S, Chen LC, Iwama T, Mandal MK, Suzuki H, Ariyada O, Furuya H, Takekawa K (2011) Development of double cylindrical dielectric barrier discharge ion source. Analyst 136(6):1210–1215
Chen LC, Yu Z, Furuya H, Hashimoto Y, Takekawa K, Suzuki H, Ariyada O, Hiraoka K (2010) Development of ambient sampling chemi/chemical ion source with dielectric barrier discharge. J Mass Spectrom 45(8):861–869
Chen LC, Yu Z, Hiraoka K (2010) Vapor phase detection of hydrogen peroxide with ambient sampling chemi/chemical ionization mass spectrometry. Anal Methods 2(7):897–900
Sugiyama M, Kumano S, Nishimura K, Hasegawa H, Hashimoto Y (2013) Sensitive low-pressure dielectric barrier discharge ion source. Rapid Commun Mass Spectrom 27(9):1005–1010
Kumano S, Sugiyama M, Yamada M, Nishimura K, Hasegawa H, Morokuma H, Inoue H, Hashimoto Y (2013) Development of a portable mass spectrometer characterized by discontinuous sample gas introduction, a low-pressure dielectric barrier discharge ionization source, and a vacuumed headspace technique. Anal Chem 85(10):5033–5039
Norgaard AW, Kofoed-Sorensen V, Svensmark B, Wolkoff P, Clausen PA (2013) Gas chromatography interfaced with atmospheric pressure ionization-quadrupole time-of-flight-mass spectrometry by low-temperature plasma ionization. Anal Chem 85(1):28–32
Hayen H, Michels A, Franzke J (2009) Dielectric barrier discharge ionization for liquid chromatography/mass spectrometry. Anal Chem 81(24):10239–10245
Gilbert-Lopez B, Garcia-Reyes JF, Meyer C, Michels A, Franzke J, Molina-Diaz A, Hayen H (2012) Simultaneous testing of multiclass organic contaminants in food and environment by liquid chromatography/dielectric barrier discharge ionization-mass spectrometry. Analyst 137(22):5403–5410
Zhou Y, Wu Z, Li C, Wang N, Zhang X, Chen H, Xiao S (2014) Coupling neutral desorption sampling to dielectric barrier discharge ionization mass spectrometry for direct oil analysis. Anal Methods 6(5):1538–1544
Chen LC, Hashimoto Y, Furuya H, Takekawa K, Kubota T, Hiraoka K (2009) Rapid detection of drugs in biofluids using atmospheric pressure chemi/chemical ionization mass spectrometry. Rapid Commun Mass Spectrom 23(3):333–339
Nudnova MM, Zhu L, Zenobi R (2012) Active capillary plasma source for ambient mass spectrometry. Rapid Commun Mass Spectrom 26(12):1447–1452
Bregy L, Sinues PML, Nudnova MM, Zenobi R (2014) Real-time breath analysis with active capillary plasma ionization-ambient mass spectrometry. J Breath Res 8(2):27102–27108
Chen W, Hou K, Xiong X, Jiang Y, Zhao W, Hua L, Chen P, Xie Y, Wang Z, Li H (2013) Non-contact halogen lamp heating assisted LTP ionization miniature rectilinear ion trap: a platform for rapid, on-site explosives analysis. Analyst 138(17):5068–5073
Chen W, Hou K, Hua L, Xiong X, Li H (2014) Water-assisted low temperature plasma ionization source for sensitive detection of explosives. RSC Adv 4(28):14791–14794
Ovchinnikova OS, Van Berkel GJ (2010) Thin‐layer chromatography and mass spectrometry coupled using proximal probe thermal desorption with electrospray or atmospheric pressure chemical ionization. Rapid Commun Mass Spectrom 24(12):1721–1729
Usmanov DT, Ninomiya S, Hiraoka K (2013) Flash desorption/mass spectrometry for the analysis of less-and nonvolatile samples using a linearly driven heated metal filament. J Am Soc Mass Spectrom 24(11):1727–1735
Saha S, Mandal MK, Chen LC, Ninomiya S, Shida Y, Hiraoka K (2013) Trace level detection of explosives in solution using leidenfrost phenomenon assisted thermal desorption ambient mass spectrometry. Mass Spectrom (Tokyo, Japan) 2(Spec Iss):S8
Saha S, Chen LC, Mandal MK, Hiraoka K (2013) Leidenfrost phenomenon-assisted thermal desorption (LPTD) and its application to open ion sources at atmospheric pressure mass spectrometry. J Am Soc Mass Spectrom 24(3):341–347
Gilbert-Lopez B, Schilling M, Ahlmann N, Michels A, Hayen H, Molina-Diaz A, Garcia-Reyes JF, Franzke J (2013) Ambient diode laser desorption dielectric barrier discharge ionization mass spectrometry of nonvolatile chemicals. Anal Chem 85(6):3174–3182
Habib A, Ninomiya S, Chen LC, Usmanov DT, Hiraoka K (2014) Desorption mass spectrometry for nonvolatile compounds using an ultrasonic cutter. J Am Soc Mass Spectrum 1–4
Xing Z, Wang JA, Han GJ, Kuermaiti B, Zhang SC, Zhang XR (2010) Depth profiling of nanometer coatings by low temperature plasma probe combined with inductively coupled plasma mass spectrometry. Anal Chem 82(13):5872–5877
Yu X, Zheng O, Cooks RG (2008) Peptide fragmentation assisted by surfaces treated with a low-temperature plasma in NanoESI. Angew Chem Int Ed 47(45):8646–8649
Xia Y, Cooks RG (2010) Plasma induced oxidative cleavage of disulfide bonds in polypeptides during nanoelectrospray ionization. Anal Chem 82(7):2856–2864
Oradu SA, Cooks RG (2012) Multistep mass spectrometry methodology for direct characterization of polar lipids in green microalgae using paper spray ionization. Anal Chem 84(24):10576–10585
Ma XX, Zhang SC, Lin ZQ, Liu YY, Xing Z, Yang CD, Zhang XR (2009) Real-time monitoring of chemical reactions by mass spectrometry utilizing a low-temperature plasma probe. Analyst 134(9):1863–1867
Amstalden Van Hove ER, Smith DF, Heeren R (2010) A concise review of mass spectrometry imaging. J Chromatogr A 1217(25):3946–3954
McDonnell LA, Heeren R (2007) Imaging mass spectrometry. Mass Spectrom Rev 26(4):606–643
Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM (2001) Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med 7(4):493–496
Chaurand P, Schwartz SA, Billheimer D, Xu BJ, Crecelius A, Caprioli RM (2004) Integrating histology and imaging mass spectrometry. Anal Chem 76(4):1145–1155
Yueying L, Xiaoxiao M, Ziqing L, Mingjia H, Guojun H, Chengdui Y, Zhi X, Sichun Z, Xinrong Z (2010) Imaging mass spectrometry with a low-temperature plasma probe for the analysis of works of art. Angew Chem Int Ed 49(26):4435–4437
Maldonado-Torres M, López-Hernández JF, Jiménez-Sandoval P, Winkler R (2014) ‘Plug and Play’ assembly of a low-temperature plasma ionization mass spectrometry imaging (LTP-MSI) system. J Proteome 102:60–65
Campbell DI, Dalgleish JK, Cotte-Rodriguez I, Maeno S, Cooks RG (2013) Chemical analysis and chemical imaging of fragrances and volatile compounds by low-temperature plasma ionization mass spectrometry. Rapid Commun Mass Spectrom 27(16):1828–1836
Liu YY, Lin ZQ, Zhang SC, Yang CD, Zhang XR (2009) Rapid screening of active ingredients in drugs by mass spectrometry with low-temperature plasma probe. Anal Bioanal Chem 395(3):591–599
Jackson AU, Garcia-Reyes JF, Harper JD, Wiley JS, Molina-Diaz A, Ouyang Z, Cooks RG (2010) Analysis of drugs of abuse in biofluids by low temperature plasma (LTP) ionization mass spectrometry. Analyst 135(5):927–933
Stein MJ, Lo E, Castner DG, Ratner BD (2012) Plasma pencil atmospheric mass spectrometry detection of positive ions from micronutrients emitted from surfaces. Anal Chem 84(3):1572–1578
Zhang JI, Tao WA, Cooks RG (2011) Facile determination of double bond position in unsaturated fatty acids and esters by low temperature plasma ionization mass spectrometry. Anal Chem 83(12):4738–4744
Zhang JI, Costa AB, Tao WA, Cooks RG (2011) Direct detection of fatty acid ethyl esters using low temperature plasma (LTP) ambient ionization mass spectrometry for rapid bacterial differentiation. Analyst 136(15):3091–3097
Huang GM, Zheng OY, Cooks RG (2009) High-throughput trace melamine analysis in complex mixtures. Chem Commun 5:556–558
Wiley JS, Garcia-Reyes JF, Harper JD, Charipar NA, Ouyang Z, Cooks RG (2010) Screening of agrochemicals in foodstuffs using low-temperature plasma (LTP) ambient ionization mass spectrometry. Analyst 135(5):971–979
Garcia-Reyes JF, Mazzoti F, Harper JD, Charipar NA, Oradu S, Ouyang Z, Sindona G, Cooks RG (2009) Direct olive oil analysis by low-temperature plasma (LTP) ambient ionization mass spectrometry. Rapid Commun Mass Spectrom 23(19):3057–3062
Zhang Y, Ma XX, Zhang SC, Yang CD, Ouyang Z, Zhang XR (2009) Direct detection of explosives on solid surfaces by low temperature plasma desorption mass spectrometry. Analyst 134(1):176–181
Dalgleish JK, Hou K, Ouyang Z, Cooks RG (2012) In situ explosive detection using a miniature plasma ion source and a portable mass spectrometer. Anal Lett 45(11):1440–1446
Vibenholt A, Nørgaard A, Wolkoff P (2011) LTP-MS of PM2. 5 filters sampled in dwellings—a new and simple fingerprinting method
Gong XY, Xiong XC, Peng YE, Yang CD, Zhang SC, Fang X, Zhang XR (2011) Low-temperature plasma ionization source for the online detection of indoor volatile organic compounds. Talanta 85(5):2458–2462
Benassi M, Berisha A, Romao W, Babayev E, Roempp A, Spengler B (2013) Petroleum crude oil analysis using low-temperature plasma mass spectrometry. Rapid Commun Mass Spectrom 27(7):825–834
Xu XY, Na N, Wen JY, Ouyang J (2013) Detection of layer-by-layer self-assembly multilayer films by low-temperature plasma mass spectrometry. J Mass Spectrom 48(2):172–178
Ma L, Jia MZ, Hu JB, Ouyang J, Na N (2012) The characterization of self-assembled monolayers on copper surfaces by low-temperature plasma mass spectrometry. J Am Soc Mass Spectrom 23(7):1271–1278
Vortmann B, Nowak S, Engelhard C (2013) Rapid characterization of lithium ion battery electrolytes and thermal aging products by low-temperature plasma ambient ionization high-resolution mass spectrometry. Anal Chem 85(6):3433–3438
Norgaard AW, Janfelt C, Benassi M, Wolkoff P, Lauritsen FR (2011) Nebulization ionization and desorption ionization analysis of reactive organo-functionalized silanes in nanofilm products. J Mass Spectrom 46(4):402–410
Norgaard AW, Vibenholt A, Benassi M, Clausen PA, Wolkoff P (2013) Study of ozone-initiated limonene reaction products by low temperature plasma ionization mass spectrometry. J Am Soc Mass Spectrom 24(7):1090–1096
Yang P, Ye ZL, Jiang GY, Li Z, Ding CF, Hou HQ (2009) Water cluster distribution under ambient conditions and dissociation of H+(H2O)(n) (n = 4 similar to 16) using mass spectrometry in atmosphere. Acta Chim Sin 67(17):2031–2037
Braithwaite NSJ (2000) Introduction to gas discharges. Plasma Sources Sci Technol 9(4):517–527
Muramoto S, Staymates ME, Brewer TM, Gillen G (2012) Ambient low temperature plasma etching of polymer films for secondary ion mass spectrometry molecular depth profiling. Anal Chem 84(24):10763–10767
Shelley JT, Stindt A, Riedel J, Engelhard C (2014) Time-resolved mass-spectral characterization of ion formation from a low-frequency, low-temperature plasma probe ambient ionization source. J Anal Atom Spectrom 29(2):359–366
Meyer C, Mueller S, Gilbert-Lopez B, Franzke J (2013) Impact of homogeneous and filamentary discharge modes on the efficiency of dielectric barrier discharge ionization mass spectrometry. Anal Bioanal Chem 405(14):4729–4735
Mueller S, Kraehling T, Veza D, Horvatic V, Vadla C, Franzke J (2013) Operation modes of the helium dielectric barrier discharge for soft ionization. Spectrochim Acta B 85:104–111
Albert A, Engelhard C (2012) Characteristics of low-temperature plasma ionization for ambient mass spectrometry compared to electrospray ionization and atmospheric pressure chemical ionization. Anal Chem 84(24):10657–10664
Gilbert-Lopez B, Geltenpoth H, Meyer C, Michels A, Hayen H, Molina-Diaz A, Garcia-Reyes JF, Franzke J (2013) Performance of dielectric barrier discharge ionization mass spectrometry for pesticide testing: a comparison with atmospheric pressure chemical ionization and electrospray ionization. Rapid Commun Mass Spectrom 27(3):419–429
Kratzer J, Mester Z, Sturgeon RE (2011) Comparison of dielectric barrier discharge, atmospheric pressure radiofrequency-driven glow discharge and direct analysis in real time sources for ambient mass spectrometry of acetaminophen. Spectrochim Acta B 66(8):594–603
Olenici-Craciunescu SB, Michels A, Meyer C, Heming R, Tombrink S, Vautz W, Franzke J (2009) Characterization of a capillary dielectric barrier plasma jet for use as a soft ionization source by optical emission and ion mobility spectrometry. Spectrochim Acta B 64(11–12):1253–1258
Michels A, Tombrink S, Vautz W, Miclea M, Franzke J (2007) Spectroscopic characterization of a microplasma used as ionization source for ion mobility spectrometry. Spectrochim Acta B 62(11):1208–1215
Olenici-Craciunescu SB, Mueller S, Michels A, Horvatic V, Vadla C, Franzke J (2011) Spatially resolved spectroscopic measurements of a dielectric barrier discharge plasma jet applicable for soft ionization. Spectrochim Acta B 66(3–4):268–273
Horvatic V, Mueller S, Veza D, Vadla C, Franzke J (2014) Atmospheric helium capillary dielectric barrier discharge for soft ionization: determination of atom number densities in the lowest excited and metastable states. Anal Chem 86(1):857–864
Heywood MS, Taylor N, Farnsworth PB (2011) Measurement of helium metastable atom densities in a plasma-based ambient ionization source. Anal Chem 83(17):6493–6499
Chan GCY, Shelley JT, Jackson AU, Wiley JS, Engelhard C, Cooks RG, Hieftje GM (2011) Spectroscopic plasma diagnostics on a low-temperature plasma probe for ambient mass spectrometry. J Anal Atom Spectrom 26(7):1434–1444
Chan GCY, Shelley JT, Wiley JS, Engelhard C, Jackson AU, Cooks RG, Hieftje GM (2011) Elucidation of reaction mechanisms responsible for afterglow and reagent-ion formation in the low-temperature plasma probe ambient ionization source. Anal Chem 83(10):3675–3686
Na N, Xia Y, Zhu Z, Zhang X, Cooks RG (2009) Birch reduction of benzene in a low-temperature plasma. Angew Chem Int Ed 48(11):2017–2019
Zhang Z, Gong X, Zhang S, Yang H, Shi Y, Yang C, Zhang X, Xiong X, Fang X, Ouyang Z (2013) Observation of replacement of carbon in benzene with nitrogen in a low-temperature plasma. Sci Rep 3
Kim GC, Kim GJ, Park SR, Jeon SM, Seo HJ, Iza F, Lee JK (2009) Air plasma coupled with antibody-conjugated nanoparticles: a new weapon against cancer. J Phys D Appl Phys 42(3):032005
Tümmel S, Mertens N, Wang J, Viöl W (2007) Low temperature plasma treatment of living human cells. Plasma Process Polym 4(S1):S465–S469
Morfill GE, Shimizu T, Steffes B, Schmidt H-U (2009) Nosocomial infections—a new approach towards preventive medicine using plasmas. New J Phys 11:115019
Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A (2008) Applied plasma medicine. Plasma Process Polym 5(6):503–533
Acknowledgments
This research is supported by the Ministry of Science and Technology of China (2011YQ090005; 2012IM030400).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in the topical collection Spectrochemical Plasmas for Clinical and Biochemical Analysis with guest editors Alfredo Sanz-Medel and María Montes Bayón.
Rights and permissions
About this article
Cite this article
Guo, C., Tang, F., Chen, J. et al. Development of dielectric-barrier-discharge ionization. Anal Bioanal Chem 407, 2345–2364 (2015). https://doi.org/10.1007/s00216-014-8281-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8281-y