Abstract
The quantitative determination of the total free fatty acids (FFAs) is an important analytical task because FFAs exhibit important physiological effects and are also relevant in many other fields, for instance, in food research. Our aim was to investigate whether a commercially available enzymatic test kit developed for the determination of FFAs in human serum is also suitable to determine different physiological and nonphysiological FFAs and to which extent the impact on the sensitivities (i.e., the accuracy by which a given FFA can be determined) differ. It will be shown that the chain length as well as the double bond content has a significant impact on the sensitivity by which a given FFA can be determined. For instance, palmitic acid (16:0) is determined with an approximately 20 times higher sensitivity in comparison to docosahexaenoic acid (22:6n-3). All data were obtained by measuring the concentrations of the FFAs by gas chromatography, and selected FFAs were also determined in a complex matrix of human serum. It is concluded that this kit is not useful if major alterations of the FFA composition of a complex mixture are expected because the individual FFAs are not detected with the same sensitivities: the concentrations of polyunsaturated FFA determined by this kit are wrong.

The used enzymatic kit detects different free fatty acids with significantly different sensitivities: the number of carbon atoms and the number of double bonds massively contribute to these differences
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free fatty acids (FFAs) are of enormous physiological relevance. For instance, FFAs can be converted into triacylglycerols (TAG) which represent the physiological storage form of energy or FFAs can be oxidized to provide energy for the organism. Additionally, FFAs are necessary for the synthesis of phospholipids and represent the educts of important messenger molecules such as prostaglandins or leukotrienes. On the negative side, however, an excess of FFAs may also contribute to the development of different diseases, such as the nonalcoholic fatty liver disease (NAFLD) [1].
Therefore, the detailed (quantitative and qualitative) analysis of crude mixtures of FFAs is of considerable interest. This is valid as regards the clinical chemistry as well as the food chemistry: for instance, the FFA content is an important criterion for the “quality” of vegetable oils and fruit juices. Additionally, the differentiation between cis and trans FFAs (that are generated upon “fat hardening”) is very important in nutritional sciences but normally requires major efforts.
The available methods of FFA analysis range from spectroscopic techniques (such as infrared (IR) or nuclear magnetic resonance (NMR) spectroscopy [2]) to chromatographic methods such as silver ion thin-layer chromatography (Ag+–TLC) or silver ion high-performance liquid chromatography (Ag+–HPLC) [3]. Nowadays, gas chromatography (GC) and/or GC coupled with mass spectrometry (MS) are the most commonly used methods to analyze FFAs [4]. However, GC is time-consuming and tedious because derivatization (methylation or silylation) is required to enhance the volatilities of the FFAs [4].
To overcome these disadvantages, there were many efforts to use “soft” ionization MS techniques such as electrospray ionization (ESI) [5] or matrix-assisted laser desorption and ionization (MALDI) [6] which basically allow the direct analysis of FFA mixtures without the need of prior derivatization and/or separation. Recent studies have indicated that equally concentrated samples of saturated (18:0) and moderately unsaturated (18:1 and 18:2) FFAs result in comparable MS peak intensities of the individual FFAs [7]. In contrast, however, poor results are obtained if polyunsaturated FFAs (20:4n-6 and particularly 22:6n-3) are also present in the mixture because these FFAs are detected with reduced sensitivities in comparison to moderately unsaturated and saturated ones [7]. This effect is probably caused by the different volatilities of the individual FFAs at high vacuum conditions as they are normally used in commercially available MALDI mass spectrometers. Anyway, only the qualitative analysis of complex FFA mixtures is so far possible by soft ionization MS.
Established methods of quantitative FFA analysis are the classical techniques of the determination of the acid number, the iodine value, or the saponification number. However, the application of these techniques is hampered by their relatively low sensitivities [8].
Of course, there are also commercially available test kits allowing the overall FFA determination in biological samples. Although such kits are normally less accurate in comparison to GC or GC/MS as the standard methods, colorimetric assays have two significant advantages: (1) the required equipment is simple and the needed spectrophotometer is present in nearly all laboratories and (2) the FFA determination by a kit is simple and can be rapidly and conveniently performed. We have chosen a half-micro test kit from Roche Diagnostics that is widely used for the analysis of FFAs in human blood serum [9]. This kit relies on the colorimetric determination of in situ generated H2O2: in the presence of adenosine triphosphate (ATP), coenzyme A (CoA), and the enzyme acyl-CoA synthetase (acyl CS), FFAs are converted into acyl-coenzyme A (acyl-CoA) under the simultaneous generation of adenosine monophosphate (AMP) and pyrophosphate. Acyl-CoA subsequently reacts with oxygen in a reaction catalyzed by acyl-CoA oxidase (ACOD) under generation of 2,3-enoyl-CoA and H2O2. In the presence of the enzyme peroxidase (POD, presumably horseradish peroxidase [10]), H2O2 converts 2,4,6-tribromo-3-hydroxy-benzoic acid (TBHB) and 4-aminoantipyrine (4-AA) into a red dye [11]. This dye can be easily determined colorimetrically at 546 nm and the measured extinction can be used to calculate the FFA concentration. A survey of this complex reaction sequence is given in Fig. 1.
Basic principle of FFA determination by the commercially available test kit used in this study. Please note that the generation of the trans double bond between the second and the third (α and β) carbon atom of the FFA is most important. Abbreviations: 4-AA 4-aminoantipyrine, ACOD acyl-CoA oxidase, acyl CS, acyl-CoA synthetase, POD peroxidase, TBHB 2,4,6-tribromo-3-hydroxy-benzoic acid
Although this kit was originally developed for the determination of the FFA content of serum [9], we were interested in the question whether this assay is also useful for the determination of FFAs that do not occur in major amounts in biological samples. We will show here that different FFAs are determined by this kit with significantly different sensitivities. Therefore, using this kit for the determination of the concentration of different (in particular polyunsaturated) FFA samples should be regarded with caution.
Material and methods
Chemicals
All FFAs (myristic (14:0), palmitic (16:0), stearic (18:0), oleic (18:1cis-9), elaidic (18:1trans-9), cis-vaccenic acid (18:1trans-9), trans-vaccenic acid (18:1trans-11), linoleic (18:2n-6), nonadecanoic (19:0), arachidic (20:0), arachidonic (20:4n-6), and docosahexaenoic (22:6n-3) acid) were obtained from Sigma-Aldrich (Taufkirchen, Germany) in the highest commercially available quality and used without purification. Ethanol p.a. was also commercially available from Sigma-Aldrich, while the detergent Triton X-100 was purchased from Ferak (Berlin, Germany). Human serum was also purchased from Sigma-Aldrich (Taufkirchen, Germany).
FFA determination by the test kit
Stock solutions of the individual FFAs (300 μM) were prepared in ethanol and their concentrations were checked by means of GC (vide infra). The absence of any significant ethyl ester generation was verified by means of 1H NMR (600 MHz) in ethanol: there was no generation of the characteristic ethyl ester quadruplet at about 4.12 ppm.
Fifty microliters of the FFA solutions (or the FFA mixture of interest or an extract of the human serum sample) were subsequently evaporated under vacuum, redissolved in 5 μl ethanol and diluted to 50 μl with a 6 % aqueous solution of Triton X-100. Palmitic acid (350 μM) in the indicated Triton detergent served as reference. Using palmitic acid as the reference is also suggested in the “Roche” instruction protocol.
Afterwards, the concentration of each FFA (300 μM) was determined by the enzymatic test kit as indicated by the manufacturer (Roche, 11 383 175 001).
One selected human serum sample was also spiked with either palmitic or arachidonic acid to check whether the obtained results (in the presence of a more complex matrix) agree with the data obtained by using the isolated FFAs. For that purpose, the lipids of 3 ml of serum were extracted according to Bligh & Dyer [12] and the obtained organic phase equally distributed over nine vessels. Vessels 1–3 served as control, i.e., no FFA were added. Vessels 4–6 were spiked with arachidonic acid and 7–9 with palmitic acid, respectively. The final concentration was in both cases 300 μM. Afterwards, all samples were characterized regarding the total FFA content as described above and by GC (vide infra).
Gas chromatography
All samples were independently investigated by GC analysis for total fatty acid composition, and this was performed as already described [13]. Briefly, dried samples were extracted in duplicate using chloroform/methanol (2:1, v/v) at room temperature. All solvents contained 0.005 % (w/v) of t-butylhydroxytoluene (BHT) to prevent the oxidation of PUFAs. The organic phase was dried with Na2SO4 and K2CO3 (10:1, w/w) and the solvent was subsequently evaporated under nitrogen at room temperature. The lipid extracts were redissolved in 300 μL of toluene, and an aliquot was used for methyl ester preparation. Next, 2 ml of 0.5 M sodium methoxide in methanol was added to the samples, which were shaken in a 60 °C water bath for 10 min. Subsequently, 1 ml of 14 % boron trifluoride (BF3) in methanol was added to the mixture, which was then shaken for additional 10 min at 60 °C. Saturated NaHCO3 (2 ml) was added, and the fatty acid methyl esters (FAMEs) were extracted three times in 2 ml of n-hexane. The solvent containing the FAMEs was reduced to dryness and the FAMEs were resuspended in 100 μl of n-hexane and stored at −18 °C until analysis. The detailed fatty acid analysis was performed using capillary GC with a CP-Sil 88 CB column (100 m × 0.25 mm, Chrompack-Varian, Lake Forest, CA, USA) that was installed in a Perkin Elmer gas chromatograph Autosys XL with a flame ionization detector and split injection (Perkin Elmer Instruments, Shelton, USA). The detailed GC conditions were the same as recently described [14]. The initial oven temperature was 150 °C, which was held for 5 min; subsequently, the temperature was increased to 175 °C and then to 200 °C at a rate of 2 °C min−1 and held constant for 10 min. Finally, the temperature was increased to 225 °C at a rate of 1.5 °C min−1 and held constant for 25 min. Hydrogen was used as the carrier gas at a flow rate of 1 ml min−1. The split ratio was 1:20, and the injector and detector were set at 260 and 280 °C, respectively. The proportions of individual fatty acids were calculated by internal standard method and 19:0 was used as internal standard. The fatty acid proportions were analyzed twice for each sample and expressed as g/100 g total fatty acids. The identification of fatty acids was verified by GC-MS/MS measurements. The analysis was performed using an Evolution GC-MS/MS system (Chromtech, Idstein, Germany). The injector temperature was set at 260 °C and the MS source at 250 °C, and the split ratio was 1:20. The oven temperature program was the same as with GC/FID conditions. The fatty acids were identified using NIST Library 2011.
Results and discussion
First, the concentrations of isolated FFA solutions of known composition and concentration were determined by the enzymatic test kit and the obtained data are shown in Fig. 2. There are obvious, significant differences between the prepared (theoretical) concentrations (300 μM) and the concentrations determined by the enzymatic test kit.
Determination of selected, isolated FFAs by using the commercially available test kit (n = 5). The FFA concentration was 300 μM in all cases. Significant differences between the experimental and the theoretical concentrations are obvious. In order to improve the clarity of presentation, the saturated FFAs are shown in (a) and the unsaturated ones in (b), respectively. Palmitic acid (16:0) was used to calibrate the assay: there is a linear dependence between the determined extinction and the FFA concentration up to (at least) 300 μM
Unequivocally, both, the chain lengths (panel a) and particularly the degree of unsaturation (double bond content, panel b) massively affect the determined FFA concentrations: the comparison of the different saturated FFAs (panel a) clearly indicates that an increasing chain length is accompanied by a decreased sensitivity and, thus, the concentrations of “longer” FFAs are underestimated in comparison to “shorter” ones and, thus, the determined concentrations of polyunsaturated FFAs are wrong. This effect is not seen if 14:0 and 16:0 are compared but is evident if 18:0 and particularly 19:0 and 20:0 are considered. Although a convincing explanation of this result is not yet available (presumably the enzymes used in this assay convert different FFAs with different efficiencies), it should be noted that all the determined concentrations are lower in comparison to the expected one (300 μM). Therefore, it is not reasonable to assume that the observed deviations are caused by the standard error of the method.
One potential reason for the observed differences might be coming from the ethanol/detergent solubility of the FFA whereby an increasing chain length correlates with an increase in the micellar size [15]. This is the solubility of FFAs decreases with an increasing chain length and, thus, a lower polarity of the FFA limits the solubility. However, it is not very likely that this effect plays a significant role because the used FFA concentrations (up to a maximum of 300 μM corresponding to 68.4 μg/ml (14:0) and 98.4 μg/ml (22:6n-3)) are relatively small: it is well known that even “longer” saturated FFAs are still soluble in ethanol in the concentrations which are here of interest [16]. For instance, the solubility of palmitic acid in ethanol (at 20 °C) is about 167 g/l and that of stearic acid about 34.2 g/l. This corresponds to about 0.65 and 0.12 M. Even if arachidic acid (20:0) is barely soluble in ethanol (about 0.1 g/l), this still corresponds to a concentration of 320 μM. Since the maximum concentration that we have used is 300 μM, it is not very likely that the solubility plays a major role. However, it was not the aim of this manuscript to clarify these aspects in more detail.
In addition to the chain length, it is also evident that the saturation degree of the FFAs has also a significant impact on the determined concentrations: considering trace panel b, it is obvious that the detectability correlates reciprocally with the double bond content of the corresponding FFA. While there are nearly no differences in dependence on the position of the double bond and the occurrence of the cis or trans isomer, higher unsaturated FFAs such as 20:4n-6 and particularly 22:6n-3 are less efficiently detected by the used enzymatic assay. This confirms that the solubility of the FFA plays only a minor role: it is well known that the solubility of a given FFA increases with the number of double bonds [17]. Accordingly, 22:6n-3 should be most sensitively detectable if the solubility would play a significant role. However, this FFA is detected with the lowest sensitivity (panel b) and, thus, other reasons must be responsible for the observed differences.
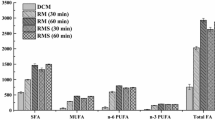
Of course, the applied kit was originally developed for the determination of the fatty acid content of human serum, but not the determination of isolated, arbitrarily selected FFAs. In order to check whether the same effects as so far described are also obtained if the FFA content of a human serum sample is analyzed, human serum was spiked with known concentrations of (1) 16:0 and (2) 20:4n-6. These FFAs were selected because they occur in major amounts in serum and are detected with marked sensitivity differences by the kit (cf. panels a and b). If both FFAs would be detectable with the same sensitivities, the addition of equal (molar) amounts of 16:0 and 20:4n-6 should result in the same absorption difference. However, the addition of 16:0 caused a more significant increase of the absorption (0.580 ± 0.018) in comparison to 20:4 (0.351 ± 0.049). Therefore, the same tendencies as in the case of the isolated FFAs are obvious, and the presence of a complex matrix (as in the case of human serum) does not influence the performance of the kit to a significant extent. In contrast, the simultaneously performed GC-FID analysis (Fig. 3) clearly indicates that the addition of both FFAs results in the expected increase of the corresponding relative amount, and there are no major differences between 16:0 and 20:4n-6.
Gas chromatographic determination of the total fatty acid composition (g/100 g total fatty acids) of human serum (white bar) and after spiking with either palmitic acid (light gray) or arachidonic acid (dark gray). All data are given in relative units. Please note that “18:1” is actually a mixture of different isomers which can be differentiated into 18:1trans-9 (0.64 %), 18:1trans-11 (1.13 %), 18:1cis-9 (90.0 %), and 18:1cis-11 (8.23 %). All these isomers were combined into “18:1” since a differentiation of the isomers was beyond the scope of this investigation. The shown data represent the average of three independent measurements together with the standard deviations. The used serum was purchased from Sigma-Aldrich to minimize deviations stemming from different blood donors
The more pronounced effect of 20:4n-6 addition in comparison to 16:0 is not surprising because the contribution of 16:0 is much higher; therefore, spiking with additional 16:0 leads to a smaller relative increase. The FFA composition shown in Fig. 3 agrees with previously performed studies on human serum [18]. However, it should be noted that the investigation of a larger set of samples (which would be necessary to minimize donor-to-donor deviations) was beyond the scope of this investigation. The serum was here exclusively used to check whether the same tendencies as in the case of isolated FFAs are also found in the case of serum, which represents a more complex sample matrix.
From these data, it is evident that the investigated test kit does not properly reflect changes of the FFA composition of complex mixtures. This particularly applies if there are major differences in the contents of highly unsaturated FFAs.
It should be noted that the data given in this study do not completely agree with the initial study by Shimizu and coworkers [11]. However, this discrepancy is presumably caused by the type of the used acyl-CoA synthetase which catalyzes the first step of the reaction sequence (cf. Fig. 1): for instance, the enzyme isolated from Pseudomonas aeruginosa converts FFAs with 6–18 carbon atoms [11], while acyl-CoA synthetase from Candida tropicalis converts FFAs with 4–20 carbon atoms [19]. Since details of the enzymes used in the commercially available test kit are not known, a more detailed evaluation of the molecular reasons would be very speculative. Nevertheless, it is suggested that the specificity of the applied acyl-CoA synthetase determines the sensitivity by which the concentration of a given fatty acid can be determined.
We conclude that the used test kit provides the highest sensitivity regarding fatty acids with chain lengths up to C16. Using “longer” FFA, the achievable sensitivity decreases rapidly and already stearic acid (18:0) is significantly less sensitively detected, i.e., the determined concentration is wrong. In contrast, if there is only a single double bond, for instance in oleic acid (18:1cis-9), the sensitivity loss is less pronounced and oleic acid is detected with a sensitivity that is very similar to palmitic acid. In a nutshell, this kit can be readily used (as indicated by the manufacturer) for the determination of the FFA concentration in serum samples where saturated and weakly unsaturated FFAs represent the overwhelming majority of the FFAs [19]. However, great caution is needed if either (1) significant changes of the FFA composition are expected or (2) the concentrations of different isolated FFAs are to be determined.
References
Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ (2005) Sources of fatty acids stored in liver and secreted via lipoproteins in patients with non-alcoholic fatty liver disease. J Clin Investig 115:1343–1351
Eibisch M, Riemer T, Fuchs B, Schiller J (2013) Differently saturated fatty acids can be differentiated by 31P NMR subsequent to derivatization with 2-chloro-4,4,5,5-tetramethyldioxaphospholane: a cautionary note. J Agric Food Chem 61:2696–2700
Nikolova-Damyanova B, Momchilova S (2001) Silver ion thin-layer chromatography of fatty acids. A survey. J Liq Chromatogr Rel Technol 24:1447–1466
Griffiths WJ (2003) Tandem mass spectrometry in the study of fatty acids, bile acids, and steroids. Mass Spectrom Rev 22:81–152
Yang WC, Adamec F, Regnier FE (2007) Enhancement of the LC/MS analysis of fatty acids through derivatization and stable isotope coding. Anal Chem 79:5150–5157
Shroff R, Svatoš A (2009) 1,8-Bis(dimethylamino)naphthalene: a novel superbasic matrix for matrix-assisted laser desorption/ionization time-of-flight mass spectrometric analysis of fatty acids. Rapid Commun Mass Spectrom 23:2380–2382
Eibisch M, Süss R, Schiller J (2012) Time-dependent intensity changes of free fatty acids detected by matrix-assisted laser desorption and ionization time-of-flight mass spectrometry in the presence of 1,8-bis(dimethylamino)naphthalene—a cautionary note. Rapid Commun Mass Spectrom 26:1573–1576
Blank EW (1962) Errors in the acid and saponification values of fatty acids. J Am Oil Chem Soc 39:122–123
Omar F, van der Watt G, November V, Pillay TS (2010) Plasma free fatty acid reference interval in South African neonates in the first week of life. Ann Clin Biochem 47:381–382
McGowan MW, Artiss JD, Strandbergh DR, Zak B (1983) A peroxidase-coupled method for the colorimetric deterrmination of serum triglycerides. Clin Chem 29:538–542
Shimizu S, Tani Y, Yamada H, Tabata M, Murachi T (1980) Enzymatic determination of serum-free fatty acids: a colorimetric method. Anal Biochem 107:193–198
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Dannenberger D, Nuernberg K, Nuernberg G, Priepke A (2012) Different dietary protein and PUFA interventions alter the fatty acid concentrations, but not the meat quality, of porcine muscle. Nutrients 4:1237–1246
Shen X, Dannenberger D, Nuernberg K, Nuernberg G, Zhao R (2011) Trans-18:1 and CLA isomers in rumen and duodenal digesta of bulls fed n-3 and n-6 PUFA-based diets. Lipids 46:831–841
Holmberg K, Jönsson B, Kronberg B, Lindman B (2003) Surfactants and polymers in aqueous solution. Wiley, Chichester
Ralston AW, Hoerr CW (1942) The solubilities of the normal saturated fatty acids. J Org Chem 7:546–555
Ralston AW, Hoerr CW (1952) The solubilities of oleic and linoleic acids in common organic solvents. J Org Chem 56:1068–1073
Wang DC, Sun CH, Liu LY, Sun XH, Jin XW, Song WL, Liu XQ, Wan XL (2012) Serum fatty acid profiles using GC-MS and multivariate statistical analysis: potential biomarkers of Alzheimer's disease. Neurobiol Aging 33:1057–1066
Shimizu S, Yasui K, Tani Y, Yamada H (1979) Acyl-CoA oxidase from Candida tropicalis. Biochem Biophys Res Commun 91:108–113
Acknowledgments
This work was supported by the Federal Ministry of Education and Research (BMBF Grant 0315735, Virtual Liver Network) and by the German Research Council (SFB 1052/B6).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eibisch, M., Popkova, Y., Süß, R. et al. Evaluation of a commercial enzymatic test kit regarding the quantitative analysis of different free fatty acids. Anal Bioanal Chem 406, 7401–7405 (2014). https://doi.org/10.1007/s00216-014-8162-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8162-4