Abstract
The epidermal growth factor receptor (EGFR) plays a key role in the pathogenesis of cancers of different types. It has been shown that EGFR and EGF-like peptides are often overexpressed in human carcinomas and that these proteins can cause cell transformation both in vivo and in vitro. In order to design a new apoptotic EGFR inhibitor, we used the essential pharmacophoric structural properties of EGFR inhibitors. We started with the natural alkaloid, theobromine, to get a new semisynthetic N-cyclohexyl acetamide derivative (T-1-NCA). T-1-NCA was extensively examined computationally for its potential against the EGFR protein. We initially performed deep density functional theory (DFT) computations to validate its 3D structure. The electrostatic potential, global reactive indices, and total density of states anticipating a high degree of reactivity were also indicated by the DFT analyses. Second, T-1-NCA's propensity to bind and inhibit the EGFR protein was investigated and verified using structure-based computational investigations such as molecular docking against EGFRWT, molecular dynamics (MD) over 100 ns, MM-GPSA, and PLIP experiments. T-1-NCA's computational ADME and toxicity profiles were examined before the synthesis, and its safety and general drug-likeness were anticipated. As a consequence, T-1-NCA was semi-synthesized to examine the proposed design and the in silico findings. In comparison with erlotinib, T-1-NCA suppressed EGFRWT in vitro with an IC50 value of 24.25 nM. (5.87 nM). Furthermore, T-1-NCA suppressed the proliferation of A549 and HCT-116 malignant cell lines with IC50 values of 40.20 and 34.05 µM, respectively, as compared to erlotinib, which had IC50 values of 17.13 and 17.32 µM. Interestingly, T-1-NCA’s selectivity indices were 3.29 and 3.89 against the two cancer cell lines indicating its general safety. Finally, the apoptotic effects of T-1-NCA were confirmed by flow cytometry and RT-PCR through the significant increase of the levels BAX, Casp3, and Casp9 in addition to the significant decrease of Bcl-2 level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer is defined as a condition in which the body is unable to regulate the spread of aberrant cell division and growth to neighboring tissues and other organs [1]. All over the world, it continues to be one of the major causes of death and morbidity despite decades of scientific and clinical research and trials of promising new medicines [2]. Therefore, cancer therapy is a significant challenge for medicinal chemists who want to create secure and efficient tailored chemotherapeutic drugs [3]. Apoptosis is a critical cellular process that eliminates damaged or potentially harmful cells in response to various oncogenic stresses, such as uncontrolled proliferation or DNA damage [4]. Through this process, apoptosis acts as a crucial safeguard against the development of cancer by removing cells that are at risk of transformation. Additionally, apoptosis is known to suppress tumorigenesis through various mechanisms, including the clearance of oncogenic proteins and the modulation of cellular signaling pathways [5]. The epidermal growth factor receptor (EGFR) is a particularly important factor that was linked to cancer cells’ apoptosis [6, 7]. It has been taken as a prime target for abnormal signaling-mediated cancer [8]. Additionally, EGFR has critical contributions in the development and progression of various carcinoma types [9,10,11]. Overexpression of EGFR promotes cell proliferation, differentiation, and survival [12]. A high level of EGFR expression has been associated with a lower survival rate across a variety of cancer types, and this expression serves as a powerful prognostic indicator [13].
Ligand and structure-based drug design are forefront of major anticancer drug design targeting EGFR [14, 15]. In addition to the discovery and repurposing of more potent drugs, the study of structure–activity relationships can also enhance the drug-likeness, pharmacokinetics, and pharmacodynamics of new drugs [16]. By using software employing a variety of techniques, computational chemistry can be used to study the interactions between potential drugs and biomolecules. Computational chemistry has been widely used in the pharmaceutical industry [17,18,19]. A range of computational chemistry applications has been developed over the years, including molecular and drug design [20, 21], docking simulations [22, 23], ligand-based approaches such as ADMET [24, 25], DFT [26], structure similarity [27, 28], and pharmacophore assessment [29].
As part of our team’s continuing efforts to search for compounds with potential anticancer activity targeting EGFR protein, including various derivatives of xanthines [30,31,32], thieno[2,3-d]pyrimidine [33,34,35], and theobromine [36,37,38], this paper describes a novel lead anticancer compound (T-1-NCA) that was synthesized for the first time and demonstrated promising in silico and in vitro anticancer properties.
1.1 Rational
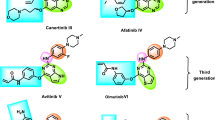
To counteract aberrant signal transduction through EGFR, the design of EGFR inhibitors using natural molecules as scaffolds has been potentiated [8]. Studies have demonstrated that some of the bioactive compounds in medicinal herbs overcome drug resistance to EGFR-TKIs and potentiate the therapeutic effects of EGFR-TKIs. These compounds include polyphenols, saponins, terpenoids, alkaloids, quinones, resins, and nucleosides [39]. Resveratrol I belongs to a class of polyphenolic compounds called stilbenes [40]. Resveratrol decreases CYP1A1 and ABCG2 expression and impairs gefitinib elimination from cells, increasing intracellular gefitinib concentration, which helps to overcome gefitinib resistance [41]. The accumulated gefitinib triggers apoptosis, autophagy, and senescence in gefitinib-resistant NSCLC cells [41]. Curcumin II potentiates the therapeutic effects of gefitinib. Curcumin induces apoptosis in TKI-resistant NSCLC cells by decreasing EGFR phosphorylation and increasing EGFR degradation, thus inhibiting cancer growth [42]. More importantly, the combination treatment of curcumin and EGFR-TKI markedly inhibits NSCLC growth by decreasing the expression of EGFR, c-MET, and cyclin D1 [43]. Shikonin III is a naphthoquinone compound that exhibits cytotoxicity, increases ROS production, and induces apoptosis in NSCLC cells. In addition, shikonin suppresses EGFR phosphorylation and increases EGFR proteasomal degradation in NSCLC [44]. Gambogenic acid IV is a polyprenylated xanthone that induces apoptosis in NSCLC cells by inhibiting the JAK/STAT3 signaling pathways. Furthermore, gambogenic acid abrogated the resistance to erlotinib in NSCLC, as demonstrated in a xenograft mouse model and patient-derived xenograft model by inhibiting c-Met activity and decreasing EGFR phosphorylation [45]. Cordycepin V induces cell cycle arrest at the G0/G1 phase and apoptosis in lung cancer cells by inhibiting the phosphorylation of EGFR, Akt, and ERK1/2 [46] and by interacting with and activating AMP-activated protein kinase [47, 48]. Cordycepin is also capable of inhibiting NSCLC cell cycle progression. NSCLC cells containing EGFR mutations are more sensitive to cordycepin treatment than those without EGFR mutations [49].
In this work, a new EGFR-based semisynthetic compound was designed and synthesized. Such compound has the same features of EGFR inhibitors [50, 51] (Fig. 1). The xanthine moiety was used as a heteroaromatic system to occupy the adenine binding pocket of the EGFR binding site. The acetamide moiety was used as a linker. The cyclohexyl moiety was used as a terminal hydrophobic head to occupy the hydrophobic region I. The two methyl groups at the 3- and 7-positions of xanthine moiety were used and proposed to occupy the hydrophobic region II.
2 Results and discussion
2.1 DFT
2.1.1 Geometry optimization and Mulliken charge
The geometry optimization of T-1-NCA was adopted at B3LYP/6-31G + + (d, p) utilizing the Gaussian 09 program. The important bond length (C14-N2) and bond angles at the two ends of the formed bond are given in the optimized structure, Fig. 2a. The ground energy or total energy (TE) was found to be -29,498.4 eV for the current chemical system of 44 atoms and 170 electrons, indicating a stable structure. The magnitude of the dipole moment was calculated to be 7.795 Debye, suggesting a polarizable structure with high reactivity.
The color scale of the Mulliken charge is represented in Fig. 2b, which shows the polarizability and distribution of charges along the chemical structure and hence predicts the charge transfer possibility in the designed molecule. The most electronegative and electropositive atoms are highlighted in Fig. 2b.
2.1.2 Frontier molecular orbital (FMO) analysis
HOMO and LUMO (FMO) orbitals can explain quantitative information regarding the affinity of T-1-NCA to bind with the target. According to Fig. 3, both HOMO and LUMO density lobes are mainly distributed over the structure except the cyclohexane ring. The HOMO energy (EHOMO), LUMO energy (ELUMO), and the energy gap between HOMO and LUMO, Egap are calculated and represented in Fig. 3. The Egap is a major stability metric that describes a molecule's reactivity and stability. Structures with a narrow Egap are more reactive and polar because electrons are readily offered to an acceptor. Theoretical Egap indicates high inhibition reactivity and feasible charge transfer [52]. The ionization potential (IP) and electron affinity (EA) amounts, Table 1, with the small Egap, suggesting strong binding with the target due to feasible electron donation from the prepared compound to the target molecule.
2.1.2.1 Chemical reactivity descriptors and total density of state (TDOS)
Koopman's theorem was utilized to compute the electronic properties through various global reactivity parameters, including “ionization potential (IP), electron affinity (EA), chemical potential (μ), electrophilicity (ω), chemical hardness (η), maximal charge acceptance (Nmax), chemical electronegativity (χ), and global softness (δ)” as follows:
IP = –EHOMO.
EA = –ELUMO.
µ = (IP + EA)/2.
η = (IP−EA).
χ = −µ.
ω = µ2/(2 η).
σ = 1/η.
∆Nmax = − (μ/η).
∆E = −ω.
Egap = ELUMO–EHOMO.
The computed parameters listed in Table 1 show high (σ); softness, low (ω), and (η); electrophilicity and hardness, which indicate a high chemical reactivity. The magnitude of (μ), (ω), and (η) determines the efficiency of the compound to gain an extra charge ∆Nmax from an adjacent chemical system (target) [52]. In addition, the μ and χ control HOMO, LUMO, and MEP which are primarily assigned to a compound's reactivity and biological activity, as electronegativity (χ) is the negative of chemical potential (− μ) [53]. Hence, the easiness of gaining extra charge and the small value of Egap were the reason behind the high inhibition reactivity of the prepared drug.
In equilibrium, "the number of occupied states per unit volume" of a compound can be calculated by multiplying the probability function by the density of states and the results can be used to study various characteristics of the molecule [54]. The overall total density of states has been calculated and is depicted in Fig. 4. The TDOS spectrum indicated the highest density was found in the unoccupied orbitals.
2.1.3 Electrostatic potential maps (ESP)
Electrostatic attractions are one of the main variables that influence molecule–protein binding. The computational analysis of these attraction forces helps to determine the energy of the protein–drug complex. Electrostatic forces with steric force are the main driving forces in biomolecules.
Depending on the availability of electron density, the ESP surface has many colored spots. Atoms with a partial negative charge (highly electronegative) glow red and can act as hydrogen-bonding acceptors, while atoms with a low electronegativity (poor in electrons) and having a partial positive charge display blue and act as hydrogen-bonding donors. Neutral atoms (with zero charge) display green to yellow color and create p- and other kinds of staking attractions. These colored domains aid in anticipating their ability to participate in chemical bonding and understanding their reaction mechanism. T-1-NCA displayed blue and red patches, suggesting the probability of hydrogen bond formation with the target. The red patches at the carbonyl groups can create hydrogen bonds with polar amino acids, while the blue zones of hydrogen atoms in the methyl group of the imidazole ring could act as hydrogen donors. The high electron clouds with a green color primarily at cyclohexane moiety favor the π-staking bonding with aromatic amino acid residues (Fig. 5).
2.2 Molecular docking against wild and mutant EGFR
The molecular modeling tool was able to foresee interactions between molecules and their biological targets. Therefore, the binding mode of T-1-NCA was examined against EGFRWT (wild form) and EGFRT790M (mutant form) using MOE. As references, the co-crystallized ligands erlotinib and TAK-285 were utilized for wild and mutant types, respectively.
Before we go to how T-1-NCA binds wild and mutant types, a validation step was performed to verify the docking process Figs. 6 and 7.
With an affinity value of −21.44 kcal/mol, erlotinib binds the active site of EGFRWT. In details, the quinazoline moiety occupied the adenine pocket of EGFRWT forming one hydrogen bond with Met769. In addition, it was incorporated with many hydrophobic interactions with Leu820, Ala719, Leu694, Val702, and Thr830. The terminal ethynylphenyl moiety was oriented into the hydrophobic pocket I forming several hydrophobic interactions with Val702, Thr830, Lys721, Asp831, and Thr766. Additionally, the hydrophobic region II was occupied by the two 2-methoxyethoxy groups forming one hydrogen bonds with Lys692. Furthermore, it formed hydrophobic interactions with Gly695, Leu694, Gly772, Phe771, and Pro770. These findings were matched with the reported results [55] (Fig. 8).
A comparable affinity value was obtained by T-1-NCA (−18.71 kcal/mol). It interacts with the EGFRWT active site similarly to erlotinib. The purine moiety formed one hydrogen bond with Met769 in the adenine pocket. In addition, it formed many hydrophobic interactions with Lue694, Leu820, Val702, Thr830, and Ala719. On the other side, the cyclohexyl group was oriented into the hydrophobic pocket I forming several hydrophobic interactions with Lys721, Leu764, Leu820, Thr830, Lys721, Asp831, Ala719, and Thr766. Furthermore, the two methyl group formed a close contact with Gly695, Leu694, Val702, Gly772, and Phe771 (Fig. 9).
To support the aforementioned findings of the wild type, the docking outcomes of the mutant type of EGFR (EGFRT790M) were examined. The binding pattern TAK-285 to the EGFRT790M active site revealed an important hydrogen bond with Met793 [56] as presented in Fig. 10.
As displayed in Fig. 11, T-1-NCA was stacked onto the EGFRT790M catalytic site in a way like to that of TAK-285. The purine arm was fixed in the adenine pocket to form one hydrogen bond with Met793 and four pi-pi bonds with Leu844, Leu718, and Cys797. Additionally, the cyclohexyl moiety interacted with the hydrophobic pocket forming three hydrophobic bonds with Lys745, Met790, and Val726.
2.3 Molecular dynamics (MD) simulations
According to the analyses performed on the production run, the protein shows a stable behavior, while the T-1-NCA shows large fluctuations which result from the unbinding and rebinding to the active site. The RMSD plot of the protein (Fig. 12a (blue curve)) shows a stable average during the whole trajectory with a value of 1.79 Å. On the other hand, the RMSD values of the complex (green curve) show two states. For the first 20 ns, the RMSD of the complex has a stable average of, approximately, 2 Å. After that, it shows a slightly large average of around 2.65 Å except for the spike at 29 ns, reaching 4.3 Å, which corresponds to the transient unbinding of the T-1-NCA. On the other hand, the RMSD of the T-1-NCA shows very large values with the maximum value reaching 27 Å corresponding to the unbinding of the compound. After rebinding, the RMSD shows a slightly large fluctuation before stabilizing at an average of 13.3 Å for the last 20 ns. The RoG (Fig. 12c) and SASA (Fig. 12d) show a similar trend. The first 80 ns show an average of 19.42 Å and 15,088 Å2, respectively. On the other hand, the last 20 ns show a decreasing trend due to the motion of the loop Glu841:Lys851 as this loop has a large RMSF value as can be seen in Fig. 12f. H-bonds (Fig. 12e) show a stable fluctuation with an average of 61 bonds. The fluctuation of the amino acids depicted in the RMSF plot (Fig. 12f) shows very low fluctuation (less than 2 Å) except for the Glu841:Lys851 loop, the Asp892:Ile899 loop, and the free C-terminal reaching 5, 2.7, and 8 Å, respectively. During the simulation time, the ligand transiently dissociated from the protein before rebinding again for the rest of the MD duration (Fig. 12g), with an average of 14.4 Å. This confirms that the large RMSD values are due to the free movement of the ligand inside the binding pocket.
The RMSD values from the trajectory. A the RMSD for the EGFR protein and EGFR & T-1-NCA complex. EGFR is the blue curve, and complex is the green curve, B the RMSD for the T-1-NCA compound, C the radius of gyration of the EGFR protein, D the solvent-accessible surface area (SASA) of the EGFR protein, E the change in the number of H-bonds during the trajectory, F the RMSF values of the amino acids, G the center of mass distance between the T-1-NCA compound and the EGFR protein, H the different energetic components of MM-GBSA and their values. Bars represent the standard deviations, I the binding free energy decomposition of the EGFR-T-1-NCA complex
The binding free energy analysis using MM-GBSA (Fig. 12i) shows the different components that contribute to the binding. T-1-NCA shows a total binding with an average value of −11.63 kcal/Mol. The largest favorable contribution is the van der Waals energy with an average value of −22.73 kcal/Mol followed by the electrostatic interaction energy with an average value of −9.65 kcal/Mol. Moreover, we did a decomposition analysis (Fig. 12i) to know which amino acids within 1 nm of T-1-NCA have a contribution to the interaction with a value less than −0.5 kcal/mol. Leu694 (−1.01 kcal/Mol), Gly695 (−0.86 kcal/Mol), Ser696 (−1.29 kcal/Mol), Gly697 (−0.73 kcal/Mol), Val702 (−0.81 kcal/Mol), and Arg817 (−0.64 kcal/Mol) are the amino acids that have a contribution with a value better (less) than −0.5 kcal/Mol. In addition, one amino acid Asp776 shows a positive contribution to the binding with an average value of + 0.48 kcal/Mol.
The trajectory was clustered to get a representative frame for each cluster produced. The number of clusters was selected automatically using the elbow method, and this produced five clusters. For each cluster representative, the PLIP webserver was utilized to know the number and types of interactions between T-1-NCA and the EGFR protein. Table 2 shows the number and types of interactions obtained from the PLIP webserver. There are two types of interactions detected with nearly the same numbers (5 hydrophobic interactions and 4 H-bonds). Val702 and Ser696 are the most common amino acids forming hydrophobic interactions and H-bonds, respectively. In addition to producing the interaction types and numbers from PLIP, it also generates a.pse file to see the 3D conformation of the ligand and its interaction with the protein (Fig. 13).
2.4 ADMET profiling study
In medicinal chemistry, the field of predictive ADMET (absorption, distribution, metabolism, excretion, and toxicity) has grown in importance since Lipinski's rule of five was introduced [57]. Nowadays, it consists of a wide range of techniques including high-throughput assay development, machine learning, data mining, visualization, and structure-based modeling [58]. During the early stages of drug development, it is urgent to evaluate the ADMET properties of the new compounds to avoid dragging them off the market later [59]. Herein, the ADMET properties for T-1-NCA were computationally predicted using Discovery Studio® based on its reference molecule, erlotinib. The ADMET results of T-1-NCA versus erlotinib (Fig. 14) demonstrated an acceptable degree of drug-likeness because it was predicted to have a very low ability to cross the blood–brain barrier, BBB, and to be non-hepatotoxic and non-inhibitor of the cytochrome P-450, CYP2D6. T-1-NCA also had good aqueous solubility and medium intestinal absorption levels, as shown in Table 3.
2.5 In silico toxicity studies
The use of in silico methods has proven to be crucial in drug development because they reduce the need for in vitro and in vivo experiments and reduce the associated time delay [60]. As part of in silico toxicity prediction, the software compares the basic chemical structural descriptors of the studied molecule/s with those of thousands of molecules that have been described as safe or toxic [61], using the structure–activity relationship (SAR)-predictive toxicity method. Through Discovery Studio software, toxicity models have been created, which are used to estimate toxicity parameters: FDA Rodent Carcinogenicity in Mouse-male (FDA-C-MM), Ames Mutagenicity (A-M), a mouse tumor cell potency (TD50-R), a rat maximum tolerable dose, a rat oral LD50 (R-O-LD50), a rat chronic LOAEL (R–C-LOAEL). Based on the computed models, the designed theobromine derivative T-1-NCA revealed general safety levels, as shown in Table 4.
2.6 Chemistry
In the current study, theobromine 1 was treated with alcoholic KOH while being continuously stirred to produce potassium salt 2 [51]. In order to generate the corresponding target product T-1-NCA (5), the formed salt 2 was then reacted with 2-chloro-N-cyclohexylacetamide 4 in DMF (Scheme 1).
Compound 5 was obtained as off-white crystals (yield, 84%); m. p. = 257–259 °C. The molecular formula C15H21N5O3 was deduced based on the basis of the elemental analysis and EI-MS. Besides, the 1H NMR spectrum in (DMSO-d6) (Table 5) displayed signals for amidic proton at δH 8.05 ppm and singlet signal for the proton of theobromine moiety at δH 7.97 ppm. On the other hand, the aliphatic region contains two protons that appeared as a singlet signal at δH 4.41 ppm corresponding to the methylene group, the α-amino-methine proton at 3.52 (m), and two methyl signals, at δH 3.89 (s, 3H) and 3.42 (s, 3H). Finally, the 1H NMR spectrum displayed the multiple protons of the cyclohexyl ring at δH (1.69–1.03) ppm. The 13C NMR spectrum afforded 13 carbon signals that were assigned as two methyls, one methylene, six sp3 methines, one sp2 methine, two quaternary sp2, and three amide carbonyl carbons. All the above-mentioned data confirmed the validity of the proposed structure.

2.7 Biology
2.7.1 In vitro EGFR inhibition
The potentiality of T-1-NCA against EGFRWT protein was investigated in vitro to examine the performed design as well as the great computational results. Compare the strongly inhibited EGFR protein with an IC50 value of 24.25 nM (Table 6). These results were in agreement with those acquired in silico, demonstrating the strong suppression potential of the compound.
2.7.2 Cytotoxicity
Considering the notably inhibitory potentials (in silico and in vitro) of T-1-NCA against EGFRWT, it is expected to exert promising anticancer effects. Therefore, the cytotoxicity of T-1-NCA was examined in vitro against the lung carcinoma epithelial A549 and the human colon cancer HCT-116 malignant cell lines using erlotinib as a reference drug (Table 7). Interestingly, T-1-NCA exhibited potent anticancer against the aforementioned cancer cells with IC50 values of 40.20 and 34.05 µM, respectively, compared to erlotinib, which had IC50 values of 17.32 and 17.13 µM, respectively.
To verify the safety profile of T-1-NCA and investigate its specificity, experiments were conducted using a Vero cell line. Results indicated that T-1-NCA exhibited a significant IC50 value of 132.4 μM and remarkably high selectivity index values (SI) of 3.89 and 3.29 against the two cancer cell lines.
2.7.3 Flow cytometric analysis and apoptosis assay
Apoptosis (programmed cell death) is a regular sequential process of cell death that maintains a homeostatic equilibrium between the rate of cell production and cell death. A misalignment of this balancing function, on the other hand, can contribute to aberrant cell development/proliferation, autoimmune diseases, and so on. Apoptosis is therefore believed to be critical from the development of an embryo through the growth of an organism, contributing to tissue regeneration and the elimination of inflammatory cells [62]. Since the discovery of DNA breakage in thymocytes following exposure to glucocorticoids in the 1980s, the induction of apoptosis became a logical and viable therapeutic strategy [5, 63].
To confirm the apoptotic properties of T-1-NCA, the apoptosis percentage in the A549 cells was checked by Annexin V and PI double stains [64, 65]. The results revealed that T-1-NCA induced an increase of the apoptotic cells percentage in the early stage of apoptosis (from 0.71 to 4.39%), late stage of apoptosis (from 0.13 to 21.02%), as well as the total stage (from 3.06 to 33.52%). Furthermore, the necrosis percentage was promoted to be 8.11, compared to 2.22% in the control cells (Fig. 15 and Table 8). Briefly, T-1-NCA successfully arrested the HCT-116 cell cycle at the G2/M phase resulting in cytotoxic potentialities that may be related to apoptosis.
2.7.4 Apoptotic proteins assay
The effects of compound T-1-NCA on HCT-116 were investigated in this study, with a focus on the expression levels of BAX, Bcl-2, caspase-3, and caspase-9. T-1-NCA was administered at a concentration of 34.05 µM, which is the IC50 value. As shown in Table 9, T-1-NCA had a significant impact on the expression levels of these proteins compared to the control group. The results showed that the proapoptotic protein BAX was increased by 3.8 times when compared to the control. BAX is a protein that promotes cell death or apoptosis. The anti-apoptotic protein Bcl-2 was also significantly reduced by 5.8-fold compared to the control. Bcl-2 is a protein that inhibits apoptosis and promotes cell survival. Therefore, the increase in BAX and decrease in Bcl-2 indicate that T-1-NCA is promoting apoptosis in HCT-116 cells.
The study also found that T-1-NCA significantly increased the levels of caspase-3 and caspase-9. Caspases are a family of enzymes that play a critical role in apoptosis. Caspase-3 is an initiator caspase, which means it is activated at the start of the apoptotic pathway, while caspase-9 is an executioner caspase, which means it is activated downstream in the pathway. The increase in caspase-3 levels by 4.6-fold and caspase-9 levels by threefold compared to the control indicates that T-1-NCA is an apoptosis promotor. These findings provide a better understanding of the mechanism of action of T-1-NCA and its potential as an anti-cancer agent (Fig. 16).
3 Experimental
3.1 Docking studies
It was operated for T-1-NCA against EGFRWT (wild form) and EGFRT790M (mutant form) by MOE2014 software [66]. The supplementary section provides additional illustrations and details.
3.2 M D simulations
It was operated for EGFR-T-1-NCA complex by the CHARMM-GUI web server [67] and GROMACS 2021. The supplementary section provides additional illustrations and details. The supplementary section provides additional illustrations and details [68, 69].
3.3 MM-GBSA
It was operated for EGFR-T-1-NCA complex by the Gmx_MMPBSA package [70, 71]. The supplementary section provides additional illustrations and details.
3.4 ED analysis
Principal component analysis (PCA) was employed for EGFR-T-1-NCA complex to investigate the dynamic motion of alpha carbons located in the amino acid sequence spanning from Glu826 to Leu1161 [72]. Supplementary section provides additional illustrations and details.
3.5 Bi-dimensional assays
To compare frames within the reduced subspace, we merged, aligned, created a new C matrix, and plotted the projections [73]. The supplementary section provides additional illustrations and details.
3.6 DFT
It was operated for T-1-NCA by Gaussian 09 and GaussSum3.0 programs. The supplementary section provides additional illustrations and details.
3.7 ADMET studies
It was operated for T-1-NCA by Discovery Studio 4.0 [74]. The supplementary section provides additional illustrations and details.
3.8 Toxicity studies
It was operated for T-1-NCA by Discovery Studio 4.0 [75]. The supplementary section provides additional illustrations and details.
3.9 In vitro EGFR inhibition
It was operated for T-1-NCA by Human EGFR ELISA kit. The supplementary materials show a comprehensive explanation.
3.9.1 In vitro antiproliferative activity
It was operated for T-1-NCA by MTT procedure [76, 77]. The supplementary materials show a comprehensive explanation.
3.9.2 Safety assay
It was operated for T-1-NCA by the calculation of selectivity index after MTT assay utilizing Vero cell lines [78]. The supplementary section provides additional illustrations and details.
3.9.3 Flow cytometry of apoptosis
It was operated for T-1-NCA flow cytometry analysis technique. The supplementary section provides additional illustrations and details.
3.9.4 Apoptotic proteins assay
It was operated by RT-PCR using the kit (Qiagen RNA extraction/BioRad SYBR green PCR MMX). The supplementary section provides additional illustrations and details.
4 Conclusion
In conclusion, this study has shown that the newly designed semisynthetic N-cyclohexyl acetamide derivative, T-1-NCA, has potent anti-EGFR apoptotic anti-proliferative activities in vitro. The computational investigations, including molecular docking, molecular dynamics simulations, and PLIP experiments, suggested that T-1-NCA can effectively bind and inhibit the EGFR protein. The in vitro assays demonstrated that T-1-NCA had a significantly strong IC50 value for EGFRWT inhibition. Moreover, T-1-NCA demonstrated significant and safe anti-proliferative effects against A549 and HCT-116 cell lines compared to erlotinib, indicating its potential as a therapeutic agent for EGFR-dependent cancers. The apoptotic effects of T-1-NCA were also confirmed through the significant increase in BAX, Casp3, and Casp9 levels and the significant decrease in Bcl-2 levels. These results suggest that T-1-NCA is a promising candidate for further development as an anti-cancer drug targeting EGFR.
Data availability
Data are available with corresponding authors upon request. Sample availability: T-1-NCA is available from the authors.
References
Nandi S, Bagchi MC (2022) Exploring CDKs, Ras-ERK, and PI3K-Akt in abnormal signaling and cancer. J Cancer Res Updates 11:63–69
Siegel RL et al (2022) Cancer statistics 72(1): 7–33
Khan F, Akhtar S, Kamal MA (2023) Nanoinformatics and personalized medicine: an advanced cumulative approach for cancer management. Curr Med Chem 30(3):271–285
Voss AK, Strasser A (2020) The essentials of developmental apoptosis. F1000Research, 9
Carneiro BA, El-Deiry WS (2020) Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol 17(7):395–417
Goel S, Hidalgo M, Perez-Soler R (2007) EGFR inhibitor-mediated apoptosis in solid tumors. J Experiment Therapeutics Oncol 6(4)
Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, Pao W (2007) Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 4(10):e294
Nandi S et al (2022) Natural Sourced inhibitors of EGFR, PDGFR, FGFR and VEGFRMediated signaling pathways as potential anticancer agents. Curr Med Chem 29(2):212–234
Rosenkranz AA, Slastnikova TA (2020) Epidermal growth factor receptor: key to selective intracellular delivery. Biochemistry (Mosc) 85(9):967–1092
Kaufman NEM et al (2021) Molecular targeting of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR). Molecules 26(4):1076
Normanno N et al (2001) The role of EGF-related peptides in tumor growth. Front Biosci 6(3):D685-707
Spano JP et al (2005) Impact of EGFR expression on colorectal cancer patient prognosis and survival. Ann Oncol 16(1):102–108
Tripathi SK et al (2020) Recent updates on the resistance mechanisms to epidermal growth factor receptor tyrosine kinase inhibitors and resistance reversion strategies in lung cancer. Med Res Rev 40(6):2132–2176
Nandi S, Bagchi MC (2011) In silico design of potent EGFR kinase inhibitors using combinatorial libraries. Mol Simul 37(03):196–209
Durgapal J et al (2018) QSAR and structure-based docking studies of aryl pyrido [2, 3-d] pyrimidin-7 (8H)-ones: an attempt to anticancer drug design. Int J Quantitat Struct-Property Relationships (IJQSPR) 3(1):43–73
da Rosa R, Schenkel EP, Campos Bernardes LS (2020) Semisynthetic and newly designed derivatives based on natural chemical scaffolds: moving beyond natural products to fight Trypanosoma cruzi. Phytochem Rev 19:105–122
Tan S, Lu R, Yao D, Wang J, Gao P, Xie G, Yao X (2023) Identification of LRRK2 inhibitors through computational drug repurposing. ACS Chem Neurosci 14(3):481–493
Ligand-based S-b (2023) Computer-aided drug design. Curr Drug Synth p 339
Lin X, Li X, Lin X (2020) A review on applications of computational methods in drug screening and design. Molecules 25(6):1375
Bertaccini EJ (2023) Anesthesia, coming of age in the world of modern in silico drug design. Anesthesiology 138(2):129–131
Cohen NC (ed) (1996) Guidebook on molecular modeling in drug design. Gulf Professional Publishing
Keith JA et al (2021) Combining machine learning and computational chemistry for predictive insights into chemical systems. Chem Rev 121(16):9816–9872
Metwaly AM et al (2023) Preparation and characterization of patuletin-loaded chitosan nanoparticles with improved selectivity and safety profiles for anticancer applications. J Chem 2023:6684015
Moroy G, Martiny VY, Vayer P, Villoutreix BO, Miteva MA (2012) Toward in silico structure-based ADMET prediction in drug discovery. Drug Discov Today 17(1–2):44–55
Metwaly AM, Elkaeed EB, Alsfouk BA, Saleh AM, Mostafa AE, Eissa IH (2022) The computational preventive potential of the rare flavonoid, patuletin, isolated from tagetes patula, against SARS-CoV-2. Plants 11(14):1886
Tielens F et al (2020) Characterization of amorphous silica based catalysts using DFT computational methods. Catal Today 354:3–18
Pracht P, Bohle F, Grimme S (2020) Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys Chem Chem Phys 22(14):7169–7192
Eissa IH et al (2023) A theobromine derivative with anticancer properties targeting VEGFR-2: semisynthesis, in silico and in vitro studies. ChemistryOpen 12(10):e202300066
Chalkha M et al (2022) Crystallographic study, biological assessment and POM/Docking studies of pyrazoles-sulfonamide hybrids (PSH): identification of a combined antibacterial/antiviral pharmacophore sites leading to in-silico screening the anti-Covid-19 activity. J Mol Struct 1267:133605
Eissa IH, Yousef RG, Elkady H, Alsfouk AA, Alsfouk BA, Husein DZ, Metwaly AM (2023) A new anticancer semisynthetic theobromine derivative targeting EGFR protein: CADDD study. Life 13(1):191
Elkaeed EB, Yousef RG, Elkady H, Alsfouk AA, Husein DZ, Ibrahim IM, Eissa IH (2022) A new theobromine-based EGFRWT and EGFRT790M inhibitor and apoptosis inducer: design, semi-synthesis, docking, DFT, MD simulations, and in vitro studies. Processes 10(11):2290
Elkaeed EB, Yousef RG, Elkady H, Alsfouk AA, Husein DZ, Ibrahim IM, Eissa IH (2022) New anticancer theobromine derivative targeting egfrwt and egfrt790m: design, semi-synthesis, in silico, and in vitro anticancer studies. Molecules 27(18):5859
Sobh EA et al (2023) Design, synthesis, docking, MD simulations, and anti-proliferative evaluation of thieno[2,3-d]pyrimidine derivatives as new EGFR inhibitors. J Enzyme Inhib Med Chem 38(1):2220579
Sobh EA, Dahab MA, Elkaeed EB, Alsfouk AA, Ibrahim IM, Metwaly AM, Eissa IH (2023) Discovery of new thieno [2, 3-d] pyrimidines as EGFR tyrosine kinase inhibitors for cancer treatment. Future Med Chem 15(13):1167–1184
Sobh EA, Dahab MA, Elkaeed EB, Alsfouk AA et al. (2023). Computer aided drug discovery (CADD) of a thieno [2, 3-d] pyrimidine derivative as a new EGFR inhibitor targeting the ribose pocket. J Biomol Struct Dynam pp 1–23
Eissa IH, Yousef RG, Elkady H, Elkaeed EB, Husein DZ, Ibrahim IM, Metwaly AM (2023) New theobromine derivative as apoptotic anti-triple-negative breast cancer targeting EGFR protein: CADD story. J Mol Struct 1294:136336
Eissa IH, Yousef GR, Elkady H, Alsfouk AA et al. (2023) New apoptotic anti-triple-negative breast cancer theobromine derivative inhibiting EGFRWT and EGFRT790M: in silico and in vitro evaluation. Mol Divers, pp 1-21
Eissa IH et al (2023) Anticancer derivative of the natural alkaloid, theobromine, inhibiting EGFR protein: computer-aided drug discovery approach. PLoS ONE 18(3):e0282586
Lee HYJ et al (2021) Medicinal herbs and bioactive compounds overcome the drug resistance to epidermal growth factor receptor inhibitors in non-small cell lung cancer. Oncol Lett 22(3):1–10
Salehi B et al (2018) Resveratrol: a double-edged sword in health benefits. Biomedicines 6(3):91
Zhu Y et al (2015) Resveratrol overcomes gefitinib resistance by increasing the intracellular gefitinib concentration and triggering apoptosis, autophagy and senescence in PC9/G NSCLC cells. Sci Rep 5(1):1–12
Lee J-Y et al (2011) Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: the versatile adjuvant for gefitinib therapy. PLoS ONE 6(8):e23756
Chen P et al (2019) Curcumin overcome primary gefitinib resistance in non-small-cell lung cancer cells through inducing autophagy-related cell death. J Exp Clin Cancer Res 38(1):1–17
Li X et al (2017) Shikonin inhibits gefitinib-resistant non-small cell lung cancer by inhibiting TrxR and activating the EGFR proteasomal degradation pathway. Pharmacol Res 115:45–55
Xu L et al (2018) Gambogenic acid inhibits fibroblast growth factor receptor signaling pathway in erlotinib-resistant non-small-cell lung cancer and suppresses patient-derived xenograft growth. Cell Death Dis 9(3):1–14
Wang Z et al (2016) Cordycepin induces apoptosis and inhibits proliferation of human lung cancer cell line H1975 via inhibiting the phosphorylation of EGFR. Molecules 21(10):1267
Hawley SA, Ross FA, Russell FM, Atrih A, Lamont DJ, Hardie DG (2020) Mechanism of activation of AMPK by Cordycepin. Cell Chem Biol 27(2):214–222
Wang Z et al (2010) Binding of Cordycepin monophosphate to AMP-activated protein kinase and its effect on AMP-activated protein kinase activation. Chem Biol Drug Des 76(4):340–344
Wei C et al (2019) Cordycepin inhibits drug-resistance non-small cell lung cancer progression by activating AMPK signaling pathway. Pharmacol Res 144:79–89
Nasser AA et al (2020) Discovery of new pyrimidine-5-carbonitrile derivatives as anticancer agents targeting EGFR WT and EGFR T790M. Org Biomol Chem 18(38):7608–7634
Elkaeed EB et al (2022) New anticancer theobromine derivative targeting egfrwt and egfrt790m: design, semi-synthesis, in silico, and in vitro anticancer studies. Molecules 27(18):5859
Husein DZ, Hassanien R, Khamis M (2021) Cadmium oxide nanoparticles/graphene composite: synthesis, theoretical insights into reactivity and adsorption study. RSC Adv 11(43):27027–27041
Nandi S et al (2013) Quantitative structure-activation barrier relationship modeling for Diels-Alder ligations utilizing quantum chemical structural descriptors. Chem Cent J 7(1):1–13
Wang T, Husein DZ (2022) Novel synthesis of multicomponent porous nano-hybrid composite, theoretical investigation using DFT and dye adsorption applications: disposing of waste with waste. Environ Sci Pollut Res 30(4):8928–8955
Nandi S, Bagchi MC (2010) 3D-QSAR and molecular docking studies of 4-anilinoquinazoline derivatives: a rational approach to anticancer drug design. Mol Diversity 14:27–38
Nossier ES, Alasfoury RA, Hagras M, El-Manawaty M, Sayed SM, Ibrahim IM, Elzahabi HS (2022) Modified pyrido [2, 3-d] pyrimidin-4 (3H)-one derivatives as EGFRWT and EGFRT790M inhibitors: design, synthesis, and anti-cancer evaluation. J Mol Struct 1270:133971
Lipinski CA et al (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23(1–3):3–25
Chuang KV, Gunsalus LM, Keiser MJ (2020) Learning molecular representations for medicinal chemistry: miniperspective. J Med Chem 63(16):8705–8722
Ferreira LLG, Andricopulo AD (2019) ADMET modeling approaches in drug discovery. Drug Discov Today 24(5):1157–1165
Idakwo G et al (2018) A review on machine learning methods for in silico toxicity prediction. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 36(4):169–191
Kruhlak NL et al (2012) (Q)SAR modeling and safety assessment in regulatory review. Clin Pharmacol Ther 91(3):529–534
Obeng E (2020) Apoptosis (programmed cell death) and its signals-A review. Braz J Biol 81:1133–1143
Wyllie AH (1980) Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 284:555–556
Taghour MS et al (2022) Benzoxazole derivatives as new VEGFR-2 inhibitors and apoptosis inducers: design, synthesis, in silico studies, and antiproliferative evaluation. J Enzyme Inhib Med Chem 37(1):2063–2077
Elwan A et al (2022) Modified benzoxazole-based VEGFR-2 inhibitors and apoptosis inducers: design, synthesis, and anti-proliferative evaluation. Molecules 27(15):5047
Suleimen YM, Jose RA, Mamytbekova GK, Suleimen RN, Ishmuratova MY, Dehaen W, Metwaly AM (2022) Isolation and in silico inhibitory potential against SARS-CoV-2 RNA polymerase of the rare Kaempferol 3-O-(6 ″-O-acetyl)-Glucoside from Calligonum tetrapterum. Plants 11(15):2072
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1:19–25
Brooks BR, Brooks CL III, Mackerell AD Jr, Nilsson L, Petrella RJ, Roux B, Karplus M (2009) CHARMM: the biomolecular simulation program. J Comput Chem 30(10):1545–1614
Jo S, Cheng X, Islam SM, Huang L, Rui H, Zhu A, Im W (2014) CHARMM-GUI PDB manipulator for advanced modeling and simulations of proteins containing nonstandard residues. Advances Protein Chem Struct Biol 96:235–265
Tuccinardi T (2021) What is the current value of MM/PBSA and MM/GBSA methods in drug discovery? Expert Opin Drug Discov 16(11):1233–1237
Valdés-Tresanco MS, Valdés-Tresanco ME, Valiente PA, Moreno E (2021) gmx_MMPBSA: a new tool to perform end-state free energy calculations with GROMACS. J Chem Theory Comput 17(10):6281–6291
Amadei A, Linssen AB, Berendsen HJ (1993) Essential dynamics of proteins. Proteins Struct, Funct Bioinf 17(4):412–425
Papaleo E, Mereghetti P, Fantucci P, Grandori R, De Gioia L (2009) Free-energy landscape, principal component analysis, and structural clustering to identify representative conformations from molecular dynamics simulations: the myoglobin case. J Mol Graph Modell 27(8):889–899
Biovia DS (2017) Discovery studio modeling environment, Release
Yousef RG et al (2022) (E)-N-(3-(1-(2-(4-(2, 2, 2-Trifluoroacetamido) benzoyl) hydrazono) ethyl) phenyl) nicotinamide: a novel pyridine derivative for inhibiting vascular endothelial growth factor receptor-2: synthesis, computational, and anticancer studies. Molecules 27(22):7719
Alley MC, Scudiero DA, Monks A, Hursey ML, Czerwinski MJ, Fine DL, Boyd MR (1988) Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res 48(3):589–601
Van de Loosdrecht AA, Beelen RHJ, Ossenkoppele G, Broekhoven MG, Langenhuijsen MMAC (1994) A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. J Immunol Methods 174(1–2):311–320
Koch A et al (2005) Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J Ethnopharmacol 101(1–3):95–99
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number (PNURSP2023R116), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Research Center at AlMaarefa University for funding this work.
Author information
Authors and Affiliations
Contributions
A.M, I.E and E.E Planned the work, A.M and I.E supervised the expemints, R.Y, M.A., and H.E made the synthesis and molecular docking, DH made the DFT, I.I made the MD simulations, A.A, E.E, participated in writing, revision and Funding. All authors revised and approved the final version of manuscript
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest to be declared.
Institutional review board statement
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eissa, I.H., G.Yousef, R., Elkady, H. et al. A new anticancer derivative of the natural alkaloid, theobromine, as an EGFR inhibitor and apoptosis inducer. Theor Chem Acc 143, 1 (2024). https://doi.org/10.1007/s00214-023-03071-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03071-z