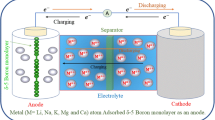
Abstract
Electrode materials with appropriate mechanical, electronic and structural attributes are prerequisites for next generation renewable energy technology. An essential stage in development of batteries to achieve superior performance is selecting an appropriate anode material. In this research, application of B3S monolayer for anode materials has been investigated employing first-principles-based DFT. For B3S monolayer, as an anode material, it is anticipated to have high performance with a low sodium diffusion barrier (Ea < 0.45 eV), low open-circuit voltage (OCV∼0.12 V), and high storage capacity (1855 mA h g−1). In addition, metallicity of B3S monolayer has been maintained at the end of Na adsorption, which reveals a favorable battery operating cycle and electrical conductivity. Our findings elucidate that these outstanding attributes cause B3S monolayer to be an attractive option for anode materials in sodium-ion batteries (NIBs).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Today, a fundamental challenge is a serious concern about finding renewable and clean energy resources because of ongoing decrease of non-renewable energy resources such as fossil fuels and their harmful effects on environment [1,2,3,4,5]. Therefore, myriad endeavors have been dedicated to development of energy storage devices and electrochemical energy conversion. Electronic devices, like electric vehicles, mobile phones [6], and laptops are increasingly using Li-ion batteries (LIBs) for rechargeable ion batteries [7], which are battery technologies of future [8,9,10,11] Nevertheless, it has been replaced by low-cost sodium-ion batteries (NIBs) due to scarcity of lithium and its rapidly increasing price [12,13,14,15] and also other metal ions battery such as Ca, Zn, Mg and etc. [16,17,18].
Recently, rechargeable ion batteries have been centered of considerable investigation efforts. Despite low specific capacity (372 mA h g−1), graphite is the most widely applied anode material for lithium-ion batteries that has good electrical conductivity and is low-cost and stable [19,20,21,22]. Since Na atoms have a larger atomic radius than Li, they have difficult intercalation into graphite. Thus, the use of graphite as the anode of NIBs faces limitations [23, 24]. Likewise, lithium–titanium-oxide (LTO) is another commercialized anode material that, despite its low capacity (175 mA h−1) and high cost, has remarkable thermal stability and cycle life [25]. The theoretical storage capacity of Si is up to 4200 mAhg−1, which is high and has been offered to replace graphite as an anode material. Nevertheless, delithiation/lithiation processes experience a great volume contraction/expansion (up to 300%), which cause structural degradation [26,27,28,29]. Hence, finding high-performance anode materials for NIBs have attracted attention in recent years from materials science perspective.
The interest in studying two-dimensional (2D) materials has been stimulated by discovery of graphene [30,31,32,33,34]. Due to confinement effects and attendance of electron–electron coupling, atomically thin 2D materials have delivered outstanding mechanical, optical, and electronic features [35,36,37,38]. Research groups have been motivated by extensive applications and fascinating attributes to explore for novel 2D materials for electrocatalytic reactions [39,40,41,42], solar energy conversion [43], photocatalysis [44,45,46,47,48], and energy storage [49,50,51,52,53]. The enhancing demand for next-generation NIBs can only be realized by developing performance of electrode materials. Cathode materials have improved faster than anode materials. Cost-effective graphite has favorable cycling stability and is extensively employed as anode material in most current NIBs [54]. However, low capacity of graphite, combined with its relatively weak interaction with Na atoms, limits its further application [55]. Poor bonding is a severe issue and endeavors were done to overwhelm this problem by forming defects on pristine graphene surface and introducing dopants. Unluckily, local structural alteration strategies affect slightly on improvement of anode material as they suffer mobility and stability challenges following Na adsorption, because of band gap opening [56,57,58,59,60,61]. As a result, next-generation NIB anodes should be made from 2D materials without structural distortions.
In present work, a novel binary compound (monolayer B3S) with relatively low symmetric honeycomb structure, flexible bonding ability, and remarkable electronic configuration has been proposed [62,63,64,65]. Via a global structural explore in conjunction with DFT computations, this novel 2D material with low sodium diffusion barrier and high theoretical capacity has been identified. Moreover, diffusion energy barriers for various feasible ways of sodium on B3S monolayer have been assessed employing nudged-elastic-band (NEB) method. Finally, for various adsorption concentrations of sodium on B3S monolayer, theoretical specific storage capacity and OCV have been computed and compared with formerly reported monolayers.
2 Computational method
2.1 DFT calculation
By applying B3LYP functional and 6-31G (d,p) basis set, electronic analysis, structural optimizations, and energy computations have been carried out. In order to anticipate weak interaction properly, dispersion term of Grimme “D” has been used [66]. According to literature review, B3LYP is an acceptable functional to compute structural and electronic features of various nanomaterials [67,68,69,70,71,72,73]. In present research, GAMESS software has been applied to execute all of the computations [74].
2.2 Calculation formula
By binding energy of Na atoms on B3S, adsorption strength can be determined. Binding energy (Eb) of adsorbed Na atoms on B3S is calculated as followings [75]:
Herein, energy of an isolated Na in vacuum, energy of B3S, total energy of adsorbed Na atom on B3S, and total number of adsorbed Na atoms on B3S are represented by ENa, EB3S, EB3S+Na, and n, respectively. Basis set superposition error energy is represented by EBSSE, which is computed by counterpoise approach. OCV of Na-ions batteries anode materials is determined as below [17, 76, 77]:
Herein, electronic charge of Na ions, difference in number of Na ions at two different compositions in charge/discharge process, and difference in Gibbs free energy are represented by e (e = 1), ΔN, and ΔG, respectively.
ΔG can be approximated to ΔE due to negligible contribution of entropy and volume terms [78]. Hence, following formula can be used to simplify OCV:
The Eb and OCV of Na atoms adsorbed on a B3S have been calculated as a function of sodium concentration. To model ion transport in batteries, researchers have used DFT to calculate the activation energy along diffusion pathways. The Modified Climbing Image Nudged Elastic Band (CI-NEB) method is often used to simulate diffusion kinetics. DFT has been employed to simulate the transport mechanism of ions in batteries by calculating the activation energy along the ion's diffusion paths [79]. The NEB method is a computational approach used in molecular dynamics simulations to study the motion of atoms along reaction pathways. It involves dividing the pathway into a series of discrete steps or images, optimizing the configuration of each image, and nudging neighboring images along the pathway to minimize the total energy of the system.
3 Results and discussion
3.1 Electronic features and Structural parameters of B3S monolayer
Following an extensive global structure search involving the binary combination of three Boron (B) and one Sulfur (S) as well as a comparison with previous research [80], we were able to identify three energetically favorable hexagonal B3S monolayers that displayed no buckling in the sheet. Nevertheless, it has been reported that the most stable B3S monolayer corresponds to the orthogonal primitive cell [81], as presented in Fig. 1a. The optimized structure of the B3S monolayer is shown in Fig. 1a. With lattice constants of a = 5.25 Å and b = 6.06 Å, B3S includes an orthogonal primitive cell (space group PMMA). Bond distances of boron–boron and boron–sulfur are between 1.662 and 1.678 Å and 1.815 to 1.849 Å, respectively. Also, B–B–B, B–B–S, and B–S–B bond angles are 125.8º, 114.7º, and 120.3º, respectively. Unlike graphene, these structural attributes cause B3S monolayer to be anisotropic. For B3S monolayer, isosurface plot of electron localization function (ELF) is shown in Fig. 1b, which red zone represents powerful covalent bonds are formed between boron–sulfur and boron–boron, as well as it has sp2 hybridization. It is interesting to note that value of ELF diagrams is around 0.5 in six-membered ring (hollow) zone, except for center of hollow, where there is not any electron distribution, which reveal that electrons in hollow will be unpaired and discrete. B3S monolayer hollow sites are therefore potential metal decoration sites. Because of valence band passing via Fermi level, B3S monolayer has metallic attributes. Also, the reported band gap value for B3S monolayer is about 0.74 eV, which agrees well with the value of 0.79 eV (seen Fig. 1S) obtained in our work [63, 82].
3.2 Binding energy of a single Na atom on B3S monolayer
Our focus was on applicability of B3S monolayer as an anode material for current NIBs due to its planar hexagonal graphene-like honeycomb structure with intrinsic metallic property. In computation of Na binding energy, 6 unique sodium adsorption areas were regarded: two bridge sites of boron-boron and boron-sulfur, two separate on-top sites of sulfur and boron atoms, and A1 and A2 inequivalent hollow sites, as indicated in Fig. 2. A1 and A2 sites have binding energies of − 1.34 eV and − 1.12 eV respectively, showing that Na atom prefers hollow sites. Vertical distances between B3S monolayer and Na at A1 and A2 sites are 1.67 Å and 1.89 Å, respectively. The energy values of absorption at B-sites were very weak, in addition, positive energy absorption values were obtained at T-sites. By p-orbital distribution, differences in binding length and binding energy of Na can be investigated. As hollow sites interact with the π-electron cloud, Na adsorption becomes stable (Table 1). In this case, π-electron cloud is predominantly formed by boron atom's pz orbital. There is a stronger interaction with the atom Na and higher π-electron cloud density at hollow sites with more boron atoms. As a result, A1 site may have better interaction with Na atom than the A2 site in terms of orbital interaction due to its more boron atoms. B3S monolayer delivers a relatively greater binding energy for sodium than foresaid anode materials (see Table 2), which indicate that structural stability of sodium is superior. By preventing metal clustering or dendrites forming on electrode surfaces, it can be more effective. Therefore, B3S monolayer can serve as an efficient anode material for Na-ion batteries.
3.3 Diffusion attributes of Na ions on B3S monolayer
Across B3S monolayer, mobility of sodium ions impact on charge/discharge rate and plays a critical role in performance of battery. Hence, various feasible diffusion paths of sodium ions on B3S monolayer have been investigated and displayed in Fig. 3. A small sodium diffusion barrier is required to increase fast charge/discharge process. Diffusion barrier has been computed between closest adjacent inequivalent sites A1 and A2 employing nudged elastic band (NEB) approach. Three sodium diffusion paths (A1 → A2 → A1 → A1 indicated in Fig. 3) are obtainable from anticipated B3S monolayer. In order to describe applicability of electrode materials, vacancy and sodium diffusion barriers should be computed at high and low state of charge (SOC). In a low state of charge, calculated sodium diffusion barriers are 0.50, 0.48, and 0.41 eV for paths A1 → A2 → A1 → A1 (indicated in Fig. 3b) between hollow sites. Single vacancy at A2 is more stable than A1 when state of charge is high, which reveals Na prefers A1. Thus, vacancy diffusion path on one side of B3S monolayer must be A2 → A1 → A1 → A2, and related diffusion barriers are 0.81, 0.73, and 0.65 eV, respectively. In comparison to vacancy diffusion barrier, sodium diffusion barrier is low at a various state of charge. Moreover, vacancy and sodium diffusion barriers have been computed when contrary side of B3S monolayer is entirely sodium adsorbed. Comparable diffusion barriers reveal that attendance of sodium on other side of B3S monolayer has no effect on vacancy and sodium diffusions. For anode material purposes, Na and vacancy diffusions on B3S monolayer are comparable to those at Na diffusion barrier on graphite [83]. Hence, B3S may be more suitable anode material for NIBs.
3.4 Open-circuit voltage and storage capacity
In addition to factors such as diffusion energy barrier, electronic and structural attributes, open circuit voltage and storage capacity of B3S monolayer are also critical for efficient anode materials. Specific capacity is defined according to number of adsorbed atoms on surface of layered structure. By enhancing Na concentration, Na adsorption has been studied to assess fully Na adsorbed of B3S structure. The number of Na ions n used for this analysis was performed for n = 1, 2, 3, 4, 5, and 6. The B3S increases the specific capacity when a larger number of Na ions is adsorbed on the B3S matrix. At each Na concentration, all probable Na adsorption configurations were generated and maximum Na adsorption with exothermic negative Eads were estimated. With desirable Eads, maximum four Na atoms per B3S stoichiometric formula (Na4B3S) are adsorbable on two sides of B3S based on our computations. Unlike graphene, there are Na atoms in all hollow sites of B3S in both single-sided and dual-sided Na2B3S structures. Consequently, all hollow sites in Na-adsorbed systems can be filled by Na atoms because of larger lattice parameter in B3S and decreased Na–Na repulsion. NIB applications will therefore require high capacity of B3S. Then, specific capacity of the B3S structure for Na4B3S was assessed as below:
Herein, Q is theoretical capacity, n is number of Na atom adsorbed, z is the number of ions in the electrochemical reaction, F is Faraday constant (96,485 C/mol), and M is molecular weight of B4S2 ring. A theoretical Na adsorption capacity of 1855 mA h g−1 has been computed for B3S monolayer, which is the highest reported value for two-dimensional monolayer anodes for NIBs. By comparing the results presented in Table 2, it can be concluded that the monolayer B3S has higher capacitance and lower OCV compared to other reported monolayers, making it suitable for sodium battery applications [84]. However, its diffusion barriers energy value is higher than some of the monolayers.
OCV of electrode materials also affects battery performance. NIB joints to cathodes require low open-circuit voltages on anodes in order to operate at their maximum voltages. Since open-circuit voltage is computed by chemical potential alteration of Na atom (μNa) based on SOC, it is essential to describe intermediate structures throughout charge/discharge process. At high and low states of charge, computed open-circuit voltage is 0.12 V and 1.53 V, respectively (see Fig. 4). Attendance of several Na atoms at high state of charge, causes repulsion between them and decreases attraction with monolayer. Hence, interactions between B3S monolayer and Na are weakened and voltage is decreased from low to high state of charge. OCV value for B3S (0.12 V) makes it a suitable option for NIB applications.
4 Conclusions
An investigation of B3S monolayer electrochemical, electronic, and structural attributes was carried out in present study in order to determine if it could be applied as anodes in NIBs based on first-principles DFT computations. Designed structure contains large lattice constants, which increase specific capacity with high Eads of Na. In addition, by applying DFT, Na diffusion barrier and OCV of B3S monolayer were computed. Based on unit formula of Na4B3S, theoretical storage capacity of B3S monolayer for NIBs reaches 1855 mA h g−1. Mean value of OCV is 1.53 V, which is less than conventional anode material and regarded as average value for anode materials. Obtained findings affirm that B3S monolayer is a promising and new anode material for NIB purposes. It can be expected that present work will provide a new motivation for further experimental and theoretical investigation on designed two-dimensional materials with acceptable absorption behavior.
Data and material availability
Confirm.
References
Chien F (2022) How renewable energy and non-renewable energy affect environmental excellence in N-11 economies? Renew Energy 196:526–534
Chodvadiya D, Som NN, Jha PK, Chakraborty B (2021) Enhancement in the catalytic activity of two-dimensional α-CN by B, Si and P doping for hydrogen evolution and oxygen evolution reactions. Int J Hydrogen Energy 46:22478–22498
Abbasi KR, Shahbaz M, Zhang J, Irfan M, Alvarado R (2022) Analyze the environmental sustainability factors of China: the role of fossil fuel energy and renewable energy. Renew Energy 187:390–402
Liao Q, Li S, Xi F, Tong Z, Chen X, Wan X, Ma W, Deng R (2023) High-performance silicon carbon anodes based on value-added recycling strategy of end-of-life photovoltaic modules. Energy 281:128345
Li T, Yu Y, Pei M (2023) Dual-atom doping carbon materials as highly efficient electrocatalysts for lithium–sulfur batteries: bimetallic cooperation mechanism. J Phys Chem C 127:6271–6279
Zhang X, Tang Y, Zhang F, Lee CS (2016) A novel aluminum–graphite dual-ion battery. Adv Energy Mater 6:1502588
Cai X, Shadike Z, Cai X, Li X, Luo L, An L, Yin J, Wei G, Yang F, Shen S (2023) Membrane electrode assembly design for lithium-mediated electrochemical nitrogen reduction. Energy Environ Sci 16:3063–3073
Sharma L, Adiga SP, Alshareef HN, Barpanda P (2020) Fluorophosphates: next generation cathode materials for rechargeable batteries. Adv Energy Mater 10:2001449
Zheng T, Kramer D, Mönig R, Boles ST (2022) Aluminum foil anodes for Li-ion rechargeable batteries: the role of Li solubility within β-LiAl. ACS Sustain Chem Eng 10:3203–3210
Ming J, Cao Z, Wu Y, Wahyudi W, Wang W, Guo X, Cavallo L, Hwang J-Y, Shamim A, Li L-J (2019) New insight on the role of electrolyte additives in rechargeable lithium ion batteries. ACS Energy Lett 4:2613–2622
Wang K, Wan J, Xiang Y, Zhu J, Leng Q, Wang M, Xu L, Yang Y (2020) Recent advances and historical developments of high voltage lithium cobalt oxide materials for rechargeable Li-ion batteries. J Power Sources 460:228062
Mu X, Pan H, He P, Zhou H (2020) Li–CO2 and Na–CO2 batteries: toward greener and sustainable electrical energy storage. Adv Mater 32:1903790
Khan K, Tareen AK, Aslam M, Mahmood A, Zhang Y, Ouyang Z, Guo Z, Zhang H (2020) Going green with batteries and supercapacitor: Two dimensional materials and their nanocomposites based energy storage applications. Prog Solid State Chem 58:100254
Cai S, Yan F, Zhao Y, Li M, Chen Y, He X, Wang C (2022) Hierarchical micro-composite assembled from Bi spheres and expanded graphite flakes as anodes for sodium-ion half/full cells with excellent comprehensive electrochemical performance. Chem Eng J 430:132938
Chen M, Liu Q, Wang SW, Wang E, Guo X, Chou SL (2019) High-abundance and low-cost metal-based cathode materials for sodium-ion batteries: problems, progress, and key technologies. Adv Energy Mater 9:1803609
Wang M, Jiang C, Zhang S, Song X, Tang Y, Cheng H-M (2018) Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat Chem 10:667–672
Xie J, Wei X, Bo X, Zhang P, Chen P, Hao W, Yuan M (2023) State of charge estimation of lithium-ion battery based on extended Kalman filter algorithm. Front Energy Res 11:1180881
Chen L, Bao J, Dong X, Truhlar D, Wang Y, Wang C, Xia Y (2017) Aqueous Mg-ion battery based on polyimide anode and prussian blue cathode. ACS Energy Lett 2:1115–1121
Liu T, Zhang Y, Jiang Z, Zeng X, Ji J, Li Z, Gao X, Sun M, Lin Z, Ling M (2019) Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ Sci 12:1512–1533
Yang W, Lu Y, Zhao C, Liu H (2020) First-principles study of black phosphorus as anode material for rechargeable potassium-ion batteries. Electron Mater Lett 16:89–98
Chang G, Zhao Y, Dong L, Wilkinson DP, Zhang L, Shao Q, Yan W, Sun XA, Zhang J (2020) A review of phosphorus and phosphides as anode materials for advanced sodium-ion batteries. J Mater Chem A 8:4996–5048
Huang G, Kong Q, Yao W, Wang Q (2023) High proportion of active nitrogen-doped hard carbon based on mannich reaction as anode material for high-performance sodium-ion batteries. Chemsuschem 16:e202202070
Hou H, Qiu X, Wei W, Zhang Y, Ji X (2017) Carbon anode materials for advanced sodium-ion batteries. Adv Energy Mater 7:1602898
Zhao Z, Wu Y, Hu R, Lu J, Chen D, Li T, Guo Y, Zhang L, Chen H, Ye Z, Zhang C (2023) Intercalation pseudocapacitance in 2D VS2/Ti3C2Tx MXene hybrids for all-climate and long-cycle sodium-ion batteries. Adv Funct Mater. https://doi.org/10.1002/adfm.202307794
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18:252–264
Raugei M, Winfield P (2019) Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J Clean Prod 213:926–932
Refino AD, Yulianto N, Syamsu I, Nugroho AP, Hawari NH, Syring A, Kartini E, Iskandar F, Voss T, Sumboja A (2021) Versatilely tuned vertical silicon nanowire arrays by cryogenic reactive ion etching as a lithium-ion battery anode. Sci Rep 11:1–15
Guo J, Zhai W, Sun Q, Ai Q, Li J, Cheng J, Dai L, Ci L (2020) Facilely tunable core-shell Si@ SiOx nanostructures prepared in aqueous solution for lithium ion battery anode. Electrochim Acta 342:136068
Mu S, Liu Q, Kidkhunthod P, Zhou X, Wang W, Tang Y (2021) Molecular grafting towards high-fraction active nanodots implanted in N-doped carbon for sodium dual-ion batterie. Nat Sci Rev 8(7):178
Zhang L, Gong T, Wang H, Guo Z, Zhang H (2019) Memristive devices based on emerging two-dimensional materials beyond graphene. Nanoscale 11:12413–12435
Patra A, More MA, Late DJ, Rout CS (2021) Field emission applications of graphene analogous two dimensional materials: recent developments and future perspectives. J Mater Chem C 9(34):11059–11078
Liu Y, Qin J, Lu L, Xu J, Su X (2023) Enhanced microwave absorption property of silver decorated biomass ordered porous carbon composite materials with frequency selective surface incorporation. Int J Miner Metall Mater 30:525–535
Ren R, Lai F, Lang X, Li L, Yao C, Cai K (2023) Efficient sulfur host based on Sn doping to construct Fe2O3 nanospheres with high active interface structure for lithium-sulfur batteries. Appl Surf Sci 613:156003
Liu Y, Fan B, Xu B, Yang B (2023) Ambient-stable polyethyleneimine functionalized Ti3C2Tx nanohybrid corrosion inhibitor for copper in alkaline electrolyte. Mater Lett 337:133979
Ugeda MM, Bradley AJ, Shi SF, da Jornada FH, Zhang Y, Qiu DY, Ruan W, Mo SK, Hussain Z, Shen ZX, Wang F, Louie SG, Crommie MF (2014) Giant bandgap renormalization and excitonic effects in a monolayer transition metal dichalcogenide semiconductor. Nat Mater 13:1091–1095
Dolui K, Quek SY (2015) Quantum-confinement and structural anisotropy result in electrically-tunable dirac cone in few-layer black phosphorous. Sci Rep 5:11699
Huang Z, Luo P, Zheng H, Lyu Z, Ma X (2023) Novel one-dimensional V3S4@ NC nanofibers for sodium-ion batteries. J Phys Chem Solids 172:111081
Lu S, Ban Y, Zhang X, Yang B, Liu S, Yin L, Zheng W (2022) Adaptive control of time delay teleoperation system with uncertain dynamics. Front Neurorobot 16:928863
Yu Q, Luo Y, Mahmood A, Liu B, Cheng H-M (2019) Engineering two-dimensional materials and their heterostructures as high-performance electrocatalysts. Electrochem Energy Rev 2:373–394
Li Z, Zhang X, Cheng H, Liu J, Shao M, Wei M, Evans DG, Zhang H, Duan X (2020) Confined synthesis of 2D nanostructured materials toward electrocatalysis. Adv Energy Mater 10:1900486
Zhang X, Chen A, Chen L, Zhou Z (2022) 2D materials bridging experiments and computations for electro/photocatalysis. Adv Energy Mater 12:2003841
Zhang Z, Feng L, Liu H, Wang L, Wang S, Tang Z (2022) Mo 6+–P 5+ co-doped Li 2 ZnTi 3 O 8 anode for Li-storage in a wide temperature range and applications in LiNi 0.5 Mn 1.5 O 4/Li 2 ZnTi 3 O 8 full cells. Inorg Chem Front 9:35–43
Cui H, Guo Y, Ma W, Zhou Z (2020) 2 D materials for electrochemical energy storage: design, preparation, and application. Chemsuschem 13:1155–1171
Hu R, Liao G, Huang Z, Qiao H, Liu H, Shu Y, Wang B, Qi X (2021) Recent advances of monoelemental 2D materials for photocatalytic applications. J Hazard Mater 405:124179
Agarwal A, Goverapet Srinivasan S, Rai B (2021) Data-driven discovery of 2D materials for solar water splitting. Front Mater 8:679269
Mortazavi B, Shahrokhi M, Cuniberti G, Zhuang X (2019) Two-dimensional SiP SiAs, GeP and GeAs as promising candidates for photocatalytic applications. Coatings 9:522
Zhu X, Liu M, Qi X, Li H, Zhang YF, Li Z, Peng Z, Yang J, Qian L, Xu Q (2021) Templateless, plating-free fabrication of flexible transparent electrodes with embedded silver mesh by electric-field-driven microscale 3D printing and hybrid hot embossing. Adv Mater 33:2007772
Liu Z, Fan B, Zhao J, Yang B, Zheng X (2023) Benzothiazole derivatives-based supramolecular assemblies as efficient corrosion inhibitors for copper in artificial seawater: formation, interfacial release and protective mechanisms. Corros Sci 212:110957
Chen A, Zhang X, Zhou Z (2020) Machine learning: accelerating materials development for energy storage and conversion. InfoMat 2:553–576
Jeong GH, Sasikala SP, Yun T, Lee GY, Lee WJ, Kim SO (2020) Nanoscale assembly of 2D materials for energy and environmental applications. Adv Mater 32:1907006
Xu B, Qi S, Jin M, Cai X, Lai L, Sun Z, Han X, Lin Z, Shao H, Peng P (2020) roadmap on two-dimensional materials for energy storage and conversion. Chin Chem Lett 30(2019):2053–2064
Xia H, Zan L, Yuan P, Qu G, Dong H, Wei Y, Yu Y, Wei Z, Yan W, Hu JS (2023) Evolution of stabilized 1T-MoS2 by atomic-interface engineering of 2H-MoS2/Fe−Nx towards enhanced sodium ion storage. Angew Chem 135:e202218282
Du Y, Xie Y, Liu X, Jiang H, Wu F, Wu H, Mei Y, Xie D (2023) In-situ formed phosphorus modified gel polymer electrolyte with good flame retardancy and cycling stability for rechargeable lithium batteries. ACS Sustain Chem Eng 11:4498–4508
Guo JZ, Gu ZY, Zhao XX, Wang MY, Yang X, Yang Y, Li WH, Wu XL (2019) Flexible Na/K-ion full batteries from the renewable cotton cloth–derived stable, low-cost, and binder-free anode and cathode. Adv Energy Mater 9:1902056
Li Y, Lu Y, Adelhelm P, Titirici M-M, Hu Y-S (2019) Intercalation chemistry of graphite: alkali metal ions and beyond. Chem Soc Rev 48:4655–4687
Ling C, Mizuno F (2014) Boron-doped graphene as a promising anode for Na-ion batteries. Phys Chem Chem Phys 16:10419–10424
Hankel M, Ye D, Wang L, Searles DJ (2015) Lithium and sodium storage on graphitic carbon nitride. J Phys Chem C 119:21921–21927
Belasfar K, Houmad M, Boujnah M, Benyoussef A, Kenz AE (2020) First-principles study of BC3 monolayer as anodes for lithium-ion and sodium-ion batteries applications. J Phys Chem Solids 139:109319
Mikhaleva NS, Visotin MA, Kuzubov AA, Popov ZI (2017) VS2/graphene heterostructures as promising anode material for Li-ion batteries. J Phys Chem C 121:24179–24184
Joshi RP, Ozdemir B, Barone V, Peralta JE (2015) Hexagonal BC3: a robust electrode material for Li, Na, and K ion batteries. J Phys Chem Lett 6:2728–2732
Li S, Chen J, He X, Zheng Y, Yu C, Lu H (2023) Comparative study of the micro-mechanism of charge redistribution at metal-semiconductor and semimetal-semiconductor interfaces: Pt (Ni)-MoS2 and Bi-MoS2 (WSe2) as the prototype. Appl Surf Sci 623:157036
Yong Y, Hu S, Zhao Z, Gao R, Cui H, Lv Z (2021) Potential reversible and high-capacity hydrogen storage medium: Li-decorated B3S monolayers. Mater Today Commun 29:102938
Jana S, Thomas S, Lee CH, Jun B, Lee SU (2019) B 3 S monolayer: prediction of a high-performance anode material for lithium-ion batteries. J Mater Chem A 7:12706–12712
Shriram SR, Gourishetty R, Panda D, Das D, Dongre S, Saha J, Chakrabarti S (2022) Subsiding strain-induced In–Ga intermixing in InAs/InxGa1−xAs sub-monolayer quantum dots for room temperature photodetectors. Infrared Phys Technol 121:104047
Sah BK, Kundu S (2019) Behaviour of protein (BSA)-lipid (DMPA) mixed monolayer on the spreading order of the individual component. Chem Phys Lipid 225:104810
Grimme S (2004) Accurate description of van der Waals complexes by density functional theory including empirical corrections. J Comput Chem 25:1463–1473
Adhikari K, Ray AK (2011) Carbon- and silicon-capped silicon carbide nanotubes: an ab initio study. Phys Lett Sect A Gen At Solid State Phys 375:1817–1823
Peyghan AA, Tabar MB, Yourdkhani S (2013) A theoretical study of OH and OCH<inf>3</inf> free radical adsorption on a nanosized tube of BC<inf>2</inf>N. J Cluster Sci 24:1–10
Du J, Sun X, Jiang G (2010) Structures, chemical bonding, magnetisms of small Al-doped zirconium clusters. Phys Lett Sect A Gen At Solid State Phys 374:854–860
Liu X, Zhu B, Gao Y (2016) Structure stability of TiAu4 nanocluster with water adsorption. Phys Lett Sect A Gen At Solid State Phys 380:1971–1975
Bashiri S, Vessally E, Bekhradnia A, Hosseinian A, Edjlali L (2017) Utility of extrinsic [60] fullerenes as work function type sensors for amphetamine drug detection: DFT studies. Vacuum 136:156–162
Dang W, Liao S, Yang B, Yin Z, Liu M, Yin L, Zheng W (2023) An encoder-decoder fusion battery life prediction method based on Gaussian process regression and improvement. J Energy Storage 59:106469
Lu S, Ding Y, Liu M, Yin Z, Yin L, Zheng W (2023) Multiscale feature extraction and fusion of image and text in VQA. Int J Comput Intell Syst 16:54
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA Jr (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363
Hwang HJ, Koo J, Park M, Park N, Kwon Y, Lee H (2013) Multilayer graphynes for lithium ion battery anode. J Phys Chem C 117:6919–6923
Hankel M, Searles DJ (2016) Lithium storage on carbon nitride, graphenylene and inorganic graphenylene. Phys Chem Chem Phys 18:14205–14215
Hao W, Xie J (2021) Reducing diffusion-induced stress of bilayer electrode system by introducing pre-strain in lithium-ion battery. J Electrochem Energy Convers Storage 18:020909
Eftekhari A (2017) Low voltage anode materials for lithium-ion batteries. Energy Storage Mater 7:157–180
Wang Z, Deng M, Xia X, Gao Y, Shao G (2018) Fundamental basis for distinctive sensing of H2 in humid environment. Energy Environ Mater 1:174–178
Lu S, Cai Y, Hu X (2021) Tunable electronic and optical properties in buckling a non-lamellar B 3 S monolayer. Phys Chem Chem Phys 23:18669–18677
Jana S, Thomas S, Lee CH, Jun B, Lee SU (2019) B3S monolayer: prediction of a high-performance anode material for lithium-ion batteries. J Mater Chem A 7:12706–12712
Lu S, Cai Y, Hu X (2021) Tunable electronic and optical properties in buckling a non-lamellar B3S monolayer. Phys Chem Chem Phys 23:18669–18677
Tarascon J-M, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414:359–367
Bhauriyal P, Mahata A, Pathak B (2018) Graphene-like carbon-nitride monolayer: a potential anode material for Na- and K-ion batteries. J Phys Chem C 122:2481–2489
Lv X, Li F, Gong J, Gu J, Lin S, Chen Z (2020) Metallic FeSe monolayer as an anode material for Li and non-Li ion batteries: a DFT study. Phys Chem Chem Phys 22:8902–8912
Putungan DB, Lin S-H, Kuo J-L (2016) Metallic VS2 monolayer polytypes as potential sodium-ion battery anode via ab initio random structure searching. ACS Appl Mater Interfaces 8:18754–18762
Zhou Y, Zhao M, Chen ZW, Shi XM, Jiang Q (2018) Potential application of 2D monolayer β-GeSe as an anode material in Na/K ion batteries. Phys Chem Chem Phys 20:30290–30296
Bekeur CA, Mapasha RE (2023) Enhancement of electrochemical performance of monolayer SnS_2for Li/Na-ion batteries through a sulphur vacancy: a DFT study. J Solid State Electrochem 27:2445–2456
Chodvadiya D, Jha U, Śpiewak P, Kurzydłowski KJ, Jha PK (2022) Potential anodic application of 2D h-AlC for Li and Na-ions batteries. Appl Surf Sci 593:153424
Funding
No.
Author information
Authors and Affiliations
Contributions
MJS, MAA, AAL: Conceptualization, Methodology, Software, Writing, Conceptualization, Methodology, Management and responsibility for the research activity planning and execution; AK, SKH, AHS: Methodology, Software, Writing—review & editing; AAK, AM: Writing—original draft, Methodology, Software, review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not required.
Consent to participate
Confirm.
Consent for publication
Confirm.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saadh, M.J., Abbood, M.A., Lagum, A.A. et al. The B3S monolayer as a high-capacity anode material for sodium-ion batteries: First-principles density functional theory approach. Theor Chem Acc 142, 128 (2023). https://doi.org/10.1007/s00214-023-03070-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-023-03070-0