Abstract
The reaction of toluene (T) with ·OH produces addition products as well as the benzyl radical (TR). TR can react with ·OH or O2 to produce oxygenated species, for many of which there is no experimental information available. We present here theoretically determined heats of formation (HFs) of 17 such species using the non-isodesmic reactions on the potential energy surface of TR + O2 and T + ·OH +O2. For those species the experimental HFs of which are known, we obtained a good correlation between experimental and theoretical values at the G4 (r2 = 0.999) and M06/cc-pVQZ (r2 = 0.997) levels, thus showing the goodness of the methods used. Experimentally unknown HFs of other radicals (benzyloxyl, spiro [1,2-dioxetane benzyl], hydroxyphenyl and benzylperoxyl) and closed-shell species (salicylic alcohol, benzo[b]oxetane and p-hydroxy cyclohexa-2,5-dienone) were later determined using those methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gas-phase tropospheric chemistry of organic species is a sub-area of research within atmospheric chemistry that has received considerable attention in recent times [1]. Because of the ubiquity of the hydroxyl radical, ·OH, its reaction with organic compounds (especially aromatics) in the atmosphere is of particular interest [2,3,4].
Toluene is the simplest aromatic molecule including an aliphatic moiety, which allows competitive hydrogen abstraction and ring addition of ·OH to occur. It is also one of the main anthropogenic aromatic molecules in the atmosphere, due to car’s exhaust, solvent use and biomass burning. Therefore, its reactions have been studied repeatedly, both experimentally and theoretically. A non-exhaustive list of recent studies can be found in Refs [5,6,7,8,9,10,11]. We have recently performed a detailed study of the possible routes for the reaction of toluene (T) with ·OH, and further reactions of the intermediate radicals with both ·OH and O2 [12, 13]. Several intermediates were identified theoretically, for which scarce or no experimental information is available. Among other things, there is no information about their enthalpies of formation. At the same time, other intermediates have been well characterized before, and their experimental enthalpies of formation are available. Since all these species lie on the same potential energy surface (PES), we considered interesting to assess the accuracy of some theoretical methods that can be applied to this system, using already known experimental enthalpies of formation for some of them. We used afterward the most accurate of those methods to predict the experimentally unknown enthalpies of formation of the rest of the compounds.
In this paper, we report a study on the enthalpies of formation of such species, using several methods and non-isodesmic reactions of formation, either from toluene, hydroxyl radical and oxygen, or benzyl radical and oxygen. In some cases, water molecules are also participating in the mechanisms and have been included in the reactions employed to calculate the enthalpies of formation of the species.
2 Computational methods
Very accurate molecular properties can be obtained if, for instance, the CCSD(T) method is used at the complete basis set (CBS) limit. This method, however, is only possible for small molecules, and less accurate approximations have often to be used. The simplest post-Hartree–Fock method including dynamical correlation energy is the second-order Møller–Plesset (MP2) perturbation theory [14, 15 and references therein]. However, several failures of this method have been reported [16, 17 and references therein] during the many years it has been in use. We used MP2 in this work in combination with a sufficiently large Pople’s 6-311 ++G(3df,2pd) basis set, in order to evaluate whether it is accurate enough for the calculation of critical points on the aforementioned PES.
Special combinations of methods and basis sets, generally described as chemical models or model chemistries, have been developed to approximate CCSD(T)/CBS calculations, resorting to additive single-point contributions to the total energy, which take into account the effects of basis set enlargement and improved methods for calculating the correlation energy. These models are accurate enough for the description of chemical reactions (affording relative energies accurate to 1 or 2 kcal/mol, which is generally known as chemical precision). Two of these chemical models have been used in this work to obtain the enthalpies of formation of the species shown in Figs. 1, 2 and 3. (Species studied are highlighted in the mechanism of Fig. 1.) On the one hand, the CBS-QB3 method of Peterson et al. [18, 19] uses B3LYP [20] optimized geometries and frequencies, adding corrections to the complete basis set limit, and correlation effects at the single-point CCSD(T) level. Both an empirical and a spin correction are added to obtain the final result. On the other hand, the G4 method of Curtiss et al. [21] was used. In this method, geometries and frequencies are obtained at the B3LYP/6-31G(2df,p) level and the basis set effect is computed including diffuse and polarization functions in single-point calculations. Correlation contributions are taken into account through a series of single-point MP4 and CCSD(T) calculations and, finally, a semiempirical high-level correction (HLC) is added to the results. Both the CBS-QB3 and G4 methods that estimated average errors in several properties for a large series of molecules are around 1 kcal/mol, and recent papers have been published on benchmark calculations using these methods for calculating enthalpies of formation of closed-shell molecules and radicals [22, 23].
Finally, density functional theory (DFT) [24] was used to obtain optimum geometries and thermodynamic functions. Many different DFT methods exist, and several papers have been published recently on the accuracy of such procedures [25,26,27,28,29,30,31,32,33,34]. Based on our previous experience [35, 36], we have chosen the M06 method [37], one of the components of the Minnesota functional suite, by Truhlar and collaborators. The method depends on a series of parameters optimized with different basis sets. It is known that DFT methods exhibit a smaller dependence on the basis sets than post-Hartree–Fock methods. However, the effect may be non-negligible. Since this is a factor that may influence our own results, we tried a limited variety of basis sets. Analogously to the MP2 calculations, we chose the 6-311 ++G(3df,2pd) basis set as the standard option, but we performed also calculations using a smaller basis, 6-31 + G(d,p) and a more extended one, the cc-pVQZ Dunning basis set, to assess the dependence of our results on the basis set [38]. Due to the fact that for the largest basis sets used scaling factors used for correcting the ZPE and harmonic frequencies are always larger than 0.95, the unmodified values were used in this paper, without the introduction of any scale factor for the molecular orbital or DFT calculations.
All calculations have been performed using Gaussian 09 [39]. Tight thresholds were used for geometry optimizations, and the ultrafine grid was used for the numerical evaluation of integrals. The standard rigid rotator/harmonic oscillator approximation was used to compute thermochemical properties. Due to their cost and the relatively small contribution to the energies, no anharmonicity corrections were included in the calculations. All optimized structures were checked to be true minima by inspection of the eigenvalues of the Hessian.
3 Results and discussion
3.1 Simple reactions
Since in this work we need to provide a reasonable description of the electronic structure of closed-shell molecules (like toluene or benzaldehyde) and open-shell species, we performed some tests of the chemical models used, addressing some very simple reactions intended to represent situations like the ones found in the real systems. Enthalpies of formation of the closed-shell water and formaldehyde molecules, and of free radicals HOO· and ·CH3, were calculated employing the reactions
Since these are all small systems, we included also calculations at the QCISD(T) [40, 41] and CCSD(T, Full) [40–42 and references therein] levels which are normally more accurate than MP2. All species were assumed to be in their ground states and the geometries were optimized using each chemical model independently. The experimental enthalpies of formation of reactants and products needed to calculate \( \Delta_{\text{f}} H_{298}^{0} \) of the four species are taken from Ref. [43 and references therein]. The errors of each calculation with respect to the experimental \( \Delta_{\text{f}} H_{298}^{0} \) of the species are collected in Table 1.
Values in Table 1 are organized as follows. For each of the four species considered, a column shows the absolute value of the difference between the theoretical and experimental \( \Delta_{\text{f}} H_{298}^{0} \) for each method and the corresponding basis set. The final columns show the average deviation for each model, over the whole set of eleven reactions, and the error on this value obtained as two times the standard deviation. These parameters are used to appraise the average accuracy of the methods used. At the bottom of the table, the average with respect to all methods for each reaction is taken, including and excluding MP2 results which in most cases are the worst ones. These numbers are used to appraise the average errors of the theoretical predictions for each reaction.
As expected, the error varies with the chemical model and the reaction considered. In general, MP2 calculations are not very reliable for the radicals; errors as high as 11.2 kcal/mol were found. There is no systematic way of assessing the error for these specific reactions, but in general, the addition of a larger fraction of correlation energy in QCISD(T) and CCSD(T) calculation lowers the average error and its dispersion. Careful addition of basis set and correlation energy effects in composite methods lower the average error and its spread, from 4.3 kcal/mol in MP2 to 0.7 kcal/mol in G4. For the reactions studied, the G4 error varies in the interval [0.0–1.9] kcal/mol. Analogously, the M06 calculations using the 6-311 ++G(3df,2pd) basis set perform G4, with errors in the interval [0.1–2.0] kcal/mol. However, notice that the enlargement of the basis set does not assure a better result, something that seems to be a general feature of DFT methods (see, for instance, reference [35]). According to the results in this table, one could expect that results at the G4 and M06 levels would be mostly in the range [− 2, + 2] kcal/mol around the experimental data.
3.2 Enthalpy of formation of the benzyl radical TR
The mechanisms studied in our work on the reaction between the benzyl radical and O2 are shown in Fig. 1 [12, 13]. (The benzyl radical was obtained by abstraction of a hydrogen from the CH3 group in toluene by the ·OH radical.) We framed there those species that are studied in this paper, in black for those with known experimental enthalpy of formation and in red for the other ones.
Since our ultimate goal is to assess the expected accuracy of our methods on the PES for the reaction starting from T and ·OH and proceeding further from the benzyl radical (TR), T and TR were the species we studied first. We have collected in Fig. 2 some important parameters of their optimum geometries. In the case of T, the first entry corresponds to the experimental geometry determined by Amir-Ibrahimi et al. [44]. There is no accurate experimental information on TR. However, Noble-Eddy attempted a mixed procedure in his PhD thesis [45], using electron diffraction data to refine MP2/6-311 ++G(d,p) calculations. As far as we are aware, the best theoretically optimized geometries were those obtained by Kortyna et al. [46] at the CCSD(T)-f12b/cc-pVTZ-f12 level. Both sets of results are included in Fig. 2.
Most relevant geometrical parameters for T and TR (C2v symmetry in both cases). The fourth entry for T is the experimental values from Ref. [44] (dark red, italic), first to third entries (black) are MP2/6-311 ++G(3df,2pd), G4 and M06/cc-pVQZ values calculated in this work, respectively. The first to third entries for T black are our own MP2, G4 and M06 results; the fourth entry (dark red, italic) are the semi-experimental values of Ref. [45], the fifth entry (dark red, italic) are the CCSD(T)-f12b/cc-pVTZ-f12 results of Ref. [46]. Distances are in Å and bond angles in degrees
All the theoretical results for T are in qualitative agreement among themselves concerning the order of the bond lengths. The C1–C7 bond length is the longest, followed by C1–C2 and C2–C3. C3–C4 is almost equal to C2–C3 but slightly longer. The theoretical calculations provide shorter bond lengths than the experimental ones, with the M06/cc-pVQZ results systematically being the smallest ones. For TR, the situation is different. The mixed experimental-theoretical results of Noble-Eddy [46] suggest the same order of bond lengths than in T, namely C1–C7 > C1–C2 > C2–C3 < C3–C4. However, only the MP2 theoretical calculations exhibit this ordering. The best theoretical results of Kortyna et al. [24], as well as our own DFT calculations, suggest that C1–C7 is considerably shorter than C1–C2. Moreover, the semi-experimental values for these two bonds are much larger than those obtained from the theoretical calculations. It is possible that Noble-Eddy semi-experimental results are not very accurate, and therefore probably further experimental work is needed on this subject.
The reactions employed to obtain the enthalpy of formation of the benzyl radical are as follows:
Reaction (13) is isodesmic, but reaction (12) is not. We showed above that the theoretically calculated values obtained for H2O and CH4 employing these radicals are reasonably accurate. For both these reasons, one expects to get reasonable error cancellation and to obtain reasonable values of \( \Delta_{\text{f}} H_{298}^{0} \). Experimental values needed to calculate the enthalpies of formation of TR were obtained from the thermochemical data tables [43] in the case of ·OH (8.96 kcal/mol), H2O (− 57.80 kcal/mol), ·CH3 (35.00 kcal/mol), CH4 (− 17.81 kcal/mol) and T (12.01 kcal/mol). The experimental value of the enthalpy of formation of TR (49.71 ± 0.41 kcal/mol) is taken from Ref. [47] and used to evaluate the error of the calculations. The value preferred by Ruscic et al. [47] was actually obtained as the weighted average of both experimental and theoretically calculated values, but it is almost identical with the average of the experimental values only. It should be taken into account that the experimental value is not unique, but lies on a range from 48.5 ± 1.4 kcal/mol [48] to 50.3 ± 1.0 kcal/mol [49], so that the experimental interval can be considered to be [47.1–51.3] kcal/mol. At present, the most widely accepted value is that recommended by Tsang [50], 49.4 ± 1.0 kcal/mol (Table 2).
The first observation is that, as expected on the basis of the above calculations on simple molecules and radicals, MP2 results are extremely bad. This is a consequence of two things. On the one side, the lack of enough electronic correlation is in the representation of the geometrical and electronic structure of TR. This conclusion is supported by the CBS-QB3 and G4 results. Although the geometry optimization is performed at the B3LYP level in both cases, the inclusion of higher orders of correlation energy (MP4, CCSD(T)) improves the energy results, giving errors below the 2.0 kcal/mol limit. The second reason is the high spin contamination of the UMP2 calculations. In fact, the UMP2/6-311 ++G(2df,2pd) calculation of TR at the optimum minimum geometry affords a \( \left\langle {s^{2} } \right\rangle \) value of 1.26 for S = 0.73, before annihilation of the first spin contaminant, and \( \left\langle {s^{2} } \right\rangle = 0.98 \) after it. These values differ markedly from the correct \( \left\langle {s^{2} } \right\rangle = 0.75 \) for the doublet radical (S = 0.5). On the other side, the concept of spin contamination is not completely meaningful at the DFT level, because of the absence of a comparable wavefunction. Even so, an analogous M06 calculation gives values of \( \left\langle {s^{2} } \right\rangle = 0.79 \) for S = 0.52, i.e., much nearer to the theoretical value. Although it is then clear that MP2 calculations will not be useful for the study, we keep also these results to point out some fortuitous agreement of the calculations with the experiments. In order not to clutter the paper with nonsignificant results, we included all the MP2 calculations (except for TR) in Table SI1 of the Supplementary Information section.
All the values obtained for \( \Delta_{\text{f}} H_{298}^{0} \) (TR) with the other methods are within the experimental range. M06 values are generally as good as the CBS-QB3 or G4 results, independently of the basis set used. The choice of the reaction is not indifferent. While the CBS-QB3 and G4 results are nearer to the experimental data when reaction (2) is used rather than (1), the opposite is true for the M06 calculations. In general, the former methods overestimate the enthalpy of formation of TR, while the M06 calculations underestimate it, considering the average experimental value. These trends suggest the empirical working hypothesis that the average of G4 and M06/cc-pVQZ values should provide a reasonable approximation to the experimental enthalpy of formation. In the case of TR, this average gives 50.4 and 49.5 kcal/mol using reactions (1) and (2), respectively, in both cases within 1.0 kcal/mol from the average experimental value. Moreover, the W1 value communicated privately by Martin to Ruscic et al. [47] was 49.3 ± 1.0 kcal/mol, in nice agreement with our own values.
3.3 Enthalpy of formation of species on the PES of TR + [·OH, O2]
Figure 3 shows the structures of the 16 radical and closed-shell structures considered in the proposed mechanism. Their geometric and energetic characteristics will be briefly described in the following.
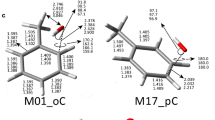
Structure of all the radicals and closed-shell species which enthalpies of formation were calculated with the methods used in this paper. Important optimized geometrical parameters are shown for the MP2/6-311 ++G(3df,2pd), G4 and M06/cc-pVQZ calculations (first three entries); bond distances are given in Å and angles in degrees. Important theoretical or experimental information for the geometries are given as the last entries for some of the molecules: phenyl radical [50, 51], phenoxyl radical [52] and benzaldehyde [53]. The spin density for the important atoms in the molecule has been included in the figure next to the position of the atom
The geometry of the simplest aryl radical, phenyl radical (I), was determined only recently by Martinez et al. [51]. They used a combination of rotational spectroscopy of singly substituted isotopic species and vibrational corrections calculated theoretically, to obtain what they called a semi-experimental structure. Large CCSD(T)/cc-CV5Z calculations were also performed, allowing the comparison with purely theoretical results. It is clear from the calculated parameters (see I in Fig. 3) that these CCSD(T)/cc-CV5Z calculations give a geometrical structure very similar to the experimental one, but also that the G4 and M06/cc-pVQZ calculations give comparable results. MP2 calculations, on the contrary, give poor geometries.
In this paper, we used the non-isodesmic reactions
to obtain the enthalpy of formation of (I) at all levels of theory. The experimental value of the enthalpy of formation was recently corrected by Stevens et al. [54] to 80.5 ± 0.1 kcal/mol. The theoretical values we obtained from reactions (14) and (15) are in general lower than the experimental one. G4, particularly using reaction (15), is closer than M06 to the experimental result. Notice that reaction (14) has an open-shell radical on the right-hand side which may unbalance it, while the errors related to triplet oxygen atom may cancel with those on the triplet oxygen molecule on the left-hand side. Reaction (15), with one open-shell radical on both sides of the equation, should be better balanced, a conjecture supported by the G4 calculations, but not by the M06 ones.
Benzoyl radical (II) has been recently studied by Sebbar et al. [55]. They performed B3LYP/6-311G(d,p) and G3MP2B3 calculations of the reaction of II with O2. In the course of this study, they determined values of the enthalpy of formation of II as 30.70 ± 2.1 kcal/mol (B3LYP) and 30.12 ± 0.56 kcal/mol, respectively. The experimental value was determined by Simões and Griller [56] using photoacoustic calorimetry, obtaining \( \Delta_{\text{f}} H_{298}^{0} \) = 27.72 ± 2.60 kcal/mol, which overlaps with the theoretical value. Our own values for this radical (see Table 3) were obtained as 28.8 ± 2.2 kcal/mol (G4) and 32.6 ± 4.6 kcal/mol (M06/cc-pVQZ). Both values are larger than the experimental value, but they overlap when the error bars are considered. They also overlap with the theoretical values of Sebbar et al. [55] obtained as averages of values derived from the enthalpies of several reactions. In our case, the enthalpies of formation were obtained as the average of the results from the non-isodesmic reactions
The G4 result for reaction (16) differs by 0.4 kcal/mol from the experimental one, while the error of reaction (17) is a bit larger. This does not occur with the M06 method, which gives the same value for both reactions, although it is about 5 kcal/mol too high.
The phenoxyl radical (III) was also studied experimentally by Walker and Tsang [48] who found a formation enthalpy of 13.22 kcal/mol. A careful experimental and theoretical study performed recently by Simões et al. [57], afforded a formation enthalpy of 13.26 ± 0.57 kcal/mol, very near to the previous one. Our own results were obtained using the non-isodesmic reactions
As seen in Table SI1, MP2 again fails badly for reaction (19). However, it gives a surprisingly good enthalpy of formation from reaction (18), undoubtedly a fortuitous result. (The same as observed for the phenyl radical.) G4 gives results within 1 kcal/mol from the experimental value, while the M06 calculations give much too low values.
The rest of the species considered, whose experimental values are known, are closed-shell molecules, namely benzaldehyde (IV), benzyl alcohol (V), phenol (VI), p-benzoquinone (VII), catechol (VIII) and benzoic acid (IX). Experimental results, references and the reactions from which we obtained the enthalpies of formation in this work are the following:
IV, benzaldehyde, \( \Delta_{\text{f}} H_{298}^{0} \) = 8.83 ± 0.22 kcal/mol [43],
V, benzyl alcohol, \( \Delta_{\text{f}} H_{298}^{0} \) = − 22.6 ± 0.72 kcal/mol [58, 63]
VI, phenol, \( \Delta_{\text{f}} H_{298}^{0} \) = − 23.04 ± 0.22 kcal/mol [59]. Dorofeeva and Ryzhova [64] recently re-examined the enthalpy of formation of phenol, reporting alleged inconsistencies between the theoretically derived values and Cox experimental one [59]. They recommended a lower value, − 21.94 ± 0.6 kcal/mol, but we used Cox’s value anyway for assessing the errors of our calculations.
VII, p-benzoquinone, \( \Delta_{\text{f}} H_{298}^{0} \) = − 27.7 ± 3.0 kcal/mol [60],
VIII, catechol, \( \Delta_{\text{f}} H_{298}^{0} \) = − 65.68 ± 0.29 kcal/mol [61]
IX, benzoic acid, \( \Delta_{\text{f}} H_{298}^{0} \) = − 73.01 kcal/mol [62] (obtained using the enthalpy of formation of the solid and the enthalpy of sublimation)
All the results we obtained for these molecules, including the errors with respect to the experimental values, are reported in Table 3. Considering these nine species plus the benzyl radical, the average error obtained with the G4 method was 1.1 kcal/mol and with the M06/cc-pVQZ, 2.3 kcal/mol. We assigned twice the average of these two errors as the expected accuracy of our predictions.
It is interesting to make some comparisons between the experimental and calculated values of the enthalpies of formation for these species, particularly in those cases where different molecules show similar \( \Delta_{\text{f}} H_{298}^{0} \). The first observation is that all methods reported in Table 3 show the same ordering of the enthalpies of formation, with that of the phenyl radical being the most positive one, and that of benzoic acid the most negative. A comparison of the phenyl radical and benzaldehyde, with similar positive enthalpies of formation, shows that CBS-QB3, G4 and M06/cc-pVQZ exhibit the correct ordering, in agreement with experiment, whereas M06 with the smaller basis sets does not. This is another argument in favor of calculations with the larger cc-pVQZ set at the M06 level. Two other species which exhibit very similar enthalpies of formation are benzyl alcohol and phenol, which experimentally differ by only 0.4 kcal/mol in favor of phenol. One sees in the table that all methods predict correctly the ordering, although the difference is (in all cases except CBS-QB3) larger than in the experiment.
Figure 4 shows the correlation between the experimental and the theoretically derived enthalpies of formation. Each marker is located at the intersection of the experimental and theoretical value for each species and method. As mentioned before, the MP2 values (either with respect to TR or with respect to T, see Table SI1) are bad. The linear correlation shown in this figure deviates much from the X = Y diagonal corresponding to perfect agreement between computation and experiment. G4 and M06/cc-pVQZ calculations, on the other hand, show a very reasonable agreement with experiment. The regression lines for these two methods, in kcal/mol, obey the equations
Correlation between the experimental enthalpies of formation of the selected species and their theoretically calculated values. Each marker is placed at the intersection of the theoretical and experimental value for each species. The dashed line represents the perfect correlation (i.e., when the experimental and theoretical values are identical). Full lines represent the least squares alignment of the data for each theoretical method employed when the enthalpies of formation are calculated with respect to the benzyl radical. In the case of the MP2 calculations, the dotted line represents the alignment when the enthalpies of formation are calculated with respect to toluene. The corresponding lines for G4 and M06 are not shown because they coincide with the ones with respect to the benzyl radical. Values in kcal/mol
These equations can be used to correct the raw values for the enthalpies of formation of unknown species participating in the oxidation of aromatics by ·OH.
Table 4 contains the enthalpies of formation predicted for those species for which no experimental data are available. (The optimized structures are shown in Fig. 3.) The values given were obtained as averages of the extrapolated values obtained from the enthalpies of reaction with respect to TR and T, using Eqs. (21) and (22), only for those two methods that behaved consistently well. The quoted error, as said before, is given as twice the average of the errors for G4 and M06/cc-pVQZ enthalpies of formation of species (I)–(IX) and TR. The actual error is probably lower.
The species that predicted enthalpies of formation and reactions employed in each case are presented in Table 5.
To the best of our knowledge, the enthalpies of formation of these compounds have not been determined before, neither experimentally nor theoretically, except for the cases reported in the following.
For salicylic alcohol, a determination using the group-additivity Joback–Reid method [65] was published [66], giving a value of − 67.12 kcal/mol in good agreement with our recommended value. Da Silva and Bozzelli [67, 68], using a simpler theoretical method (G3B3), suggested a value of 31.1 kcal/mol for the benzyloxyl radical and 29.6 kcal/mol for the benzylperoxyl radical. Both values are larger than the best ones we found, and moreover, they suggest that the latter is more stable than the former, contrary to what observed. Additive group calculations were used also by Bounaceur et al. [11] to determine the enthalpies of formation of the benzyl, benzylperoxyl and benzoyl radicals. Their values (49.4, 26.9 and 26.0 kcal/mol) are close to the more accurate ones we present in this paper. Finally, Fenter et al. [69] determined the forward rate constant and the equilibrium constant for the association reaction of the benzyl radical with oxygen and derived an enthalpy of formation of 28.0 ± 1.4 kcal/mol for the benzylperoxyl radical, a value with which our theoretical result is in good agreement. All these related information lend support to our procedure and the set of proposed enthalpies of formation we derived in this paper.
Reaction (46) is particularly interesting, because experimental data on its enthalpy of reaction have been published [58, 69]. Adopting the same extrapolation procedure described before to correct the enthalpies of reaction at the G4 and M06/cc-pVQZ level and averaging these values, our result for the enthalpy of reaction is − 21.7 ± 1.6 kcal/mol. Elmaimouni et al. [58] studied the kinetics of the reaction of the benzyl radical with molecular oxygen between 393 and 433 K. From the data obtained, they predicted \( \Delta_{\text{r}} H_{298}^{0} \) = − 20 ± 1 kcal/mol. A little later, Fenter et al. [69] studied the kinetics and equilibrium of the benzyl radical association reaction with molecular oxygen and predicted \( \Delta_{\text{r}} H_{298}^{0} \) = − 21.8 ± 1.0 kcal/mol. Our theoretical value is in excellent agreement with both experimental results.
4 Conclusions
Quantum mechanical calculations have been performed on radicals and closed-shell species lying on the potential energy surface of the reaction of benzyl radical with O2, ·OH and H2O. These species arise in different channels in the reaction mechanism of toluene reaction with the ·OH radical (see, for instance, Metcalfe et al. [5], Murakami et al. [10], Bounaceur et al. [11] and ourselves [12, 13]), and the enthalpies of formation were known for about half of the 17 compounds studied. Non-isodesmic reactions connecting products and reactants on these reaction paths, starting either from toluene or the benzyl radical, were used to calculate the enthalpies of formation.
When available, experimentally known enthalpies of formation were compared to those calculated theoretically. It was determined that the MP2 values were very unreasonable when the enthalpies of formation were calculated with respect to the benzyl radical. The results improved when the toluene molecule was employed, but the results were still unsatisfactory. Only in few cases, e.g., hydroxyphenyl radical, the MP2 values are similar to the experimental ones, suggesting that this method is not adequate for the study of the oxidation of toluene (and possibly other aromatics) in atmospheric and combustion chemistry.
The other methods employed in this paper gave smaller errors. CBS-QB3 and G4 gave average errors between 1 and 2 kcal/mol, independently of whether T or TR were used as reference. The M06 errors depended on the basis set used and showed a non-systematic behavior. Taking either T or TR as reference, the results obtained with the 6-311 ++G(3df,2pd) basis set were worse than those obtained with the simpler 6-31 + G(d,p) basis set, perhaps because the former set, although larger, is not well balanced for DFT methods. The best results were obtained with Dunning’s cc-pVQZ basis set. Notwithstanding that the M06/cc-pVQZ average error was larger than either CBS-QB3 or G4, it is in the correct range (2.3 kcal/mol) and is independent of whether T or TR are used to determine the enthalpy of formation. Thus, M06/cc-pVQZ was determined to be an appropriate method for the study of the more complex PES.
Both G4 and M06/cc-pVQZ models were shown to perform pretty well for enthalpies of formation over a range of more than 120 kcal/mol. The correlation between theoretical and experimental values exhibits very large correlation coefficients (r2 = 0.999 for G4 and 0.997 for M06/cc-pVQZ), and the slope of the lines is almost unity (0.990 for G4 and 0.984 for M06/cc-pVQZ). Therefore, it is proposed that these regression lines can be used to correct the results obtained with those methods.
Using the knowledge derived from the study of the species with known experimental enthalpies of formation, a prediction of these values for a set of radicals and closed-shell species considered in the literature as intermediates or products in some of the reaction paths for the reaction of the benzyl radical with oxygen was performed. The values reported are the most accurate, and in most cases, the only ones available up to now in the literature.
In our opinion, the procedure outlined and the results obtained represent a further step toward a deeper knowledge of the atmospheric chemistry of toluene. The newly determined set of enthalpies of formation may be useful in studies of combustion and atmospheric chemistry of this species. Some of the tested theoretical methods show promise for the extension of these studies to more complex systems.
References
Atkinson R (1990) Gas-phase tropospheric chemistry of organic compounds: a review. Atmos Environ 24A:1–41
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem Rev 85:69–201
Yang Y, Shao M, Wang X, Nolscher AC, Kessel S, Guenther A, Williams J (2016) Towards a quantitative understanding of total OH reactivity: a review. Atmos Environ 134:147–161
Nayebzadeh M, Vahedpour M (2017) A review on reactions of polycyclic aromatic hydrocarbons with the most abundant atmospheric chemical fragments: theoretical and experimental data. Prof React Kinet Mech 42:201–220
Metcalfe WK, Dooley S, Dryer FI (2011) Comprehensive detailed chemical kinetic modeling study of toluene oxidation. Energy Fuels 25:4915–4936
Baltaretu CO, Lichtman EI, Hadler AB, Elrod MJ (2009) Primary atmospheric oxidation mechanism for toluene. J Phys Chem A 113:221–230
Hatipoglu A, Vione D, Yalçin Y, Minero C, Ҫinar Z (2010) Photo-oxidative degradation of toluene in aqueous media by hydroxyl radicals. J Photochem Photobiol A Chem 215:59–68
Wu R, Pan S, Li Y, Wang L (2014) Atmospheric oxidation mechanism of toluene. J Phys Chem A 118:4533–4547
Ji Y, Zhao J, Terazono H, Misawa K, Levitt NP, Li Y, Lin Y, Peng Y, Wang Y, Duan L, Pan B, Zhang F, Feng X, An T, Marrero-Ortiz W, Secrest J, Zhang AL, Shibuya K, Molina MJ, Zhang R (2017) Reassesing the atmospheric oxidation mechanism of toluene. Proc Natl Acad Sci 114:8169–8174
Murakami Y, Oguchi T, Hashimoto K, Nosaka Y (2007) Theoretical study of the benzyl + O2 reaction: kinetics, mechanism, and product branching ratios. J Phys Chem A 111:13200–13208
Bounaceur R, da Costa I, Fournet R, Billaud F, Battin-Leclerc F (2005) Experimental and modeling study of the oxidation of toluene. Chem Kinet 37:25–49
Salta Z, Kosmas AM, Segovia ME, Kieninger M, Ventura ON, Barone V. A reinvestigation of the deceptively simple reaction of toluene with ·OH, and the fate of the benzyl radical. I. A combined thermodynamic and kinetic study on the competition between HO-addition and H-abstraction reactions (unpublished)
Salta Z, Kosmas AM, Segovia ME, Kieninger M, Ventura ON, Barone V. A reinvestigation of the deceptively simple reaction of toluene with ·OH, and the fate of the benzyl radical. II. The “secret” routes to cresols and benzaldehyde (unpublished)
Frisch MJ, Head-Gordon M, Pople JA (1990) Direct MP2 gradient method. Chem Phys Lett 166:275–280
Head-Gordon M, Head-Gordon T (1994) Analytic MP2 frequencies without fifth order storage: theory and application to bifurcated hydrogen bonds in the Water Hexamer. Chem Phys Lett 220:122–128
Coitiño EL, Ventura ON (1993) Isomerization of the formaldehyde radical cation and the failure of MP2. Chem Phys Lett 202:479–482
Soydaş E, Bozkaya U (2015) Assessment of orbital-optimized MP2.5 for thermochemistry and kinetics: dramatic failures of standard perturbation theory approaches for aromatic bond dissociation energies and barrier heights of radical reactions. J Chem Theory Comput 11:1564–1573
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822–2827
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5648–5652
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory. J Chem Phys 126:084108
Somers KP, Simmie JM (2015) Benchmarking compound methods (CBS-QB3, CBS-APNO, G3, G4, W1BD) against the active thermochemical tables: formation enthalpies of radicals. J Phys Chem A 119:8922–8933
Simmie JM, Somers KP (2015) Benchmarking compound methods (CBS-QB3, CBS-APNO, G3, G4, W1BD) against the active thermochemical tables: a litmus test for cost-effective molecular formation enthalpies. J Phys Chem A 119:7235–7246
Koch W, Holthausen MC (2001) A chemists guide to density functional theory. Wiley, New York
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol Phys 115:2315–2372
van Santen JA, DiLabio GA (2015) Dispersion corrections improve the accuracy of both noncovalent and covalent interactions energies predicted by a density-functional theory approximation. J Phys Chem A 119:6703–6713
Jones RO (2015) Density functional theory: its origins, rise to prominence, and future. Revs Mod Phys 87:897–923
Sengupta A, Raghavachari K (2017) Solving the density functional conundrum: elimination of systematic errors to derive accurate reaction enthalpies of complex organic reactions. Org Lett 19:2576–2579
Boese AD (2015) Density functional theory and hydrogen bonds: are we there yet? ChemPhysChem 16:978–985
Yu HS, He X, Li SL, Truhlar DG (2016) MN15: a Kohn–Sham global-hybrid exchange–correlation density functional with broad accuracy for multi-reference and single-reference systems and noncovalent interactions. Chem Sci 7:5032–5051
Lehtola S, Steigemann C, Oliveira MJT, Marques MAL (2018) Recent developments in libxc—a comprehensive library of functionals for density functional theory. SoftwareX 7:1–5
Grimme S, Schreiner PR (2018) Computational chemistry: the fate of current methods and future challenges. Angew Chem Int Ed 57:4170–4176
Gulans A, Kozhevnikov A, Draxl C (2018) Microhartree precision in density functional theory calculations. Phys Rev B 97:161105(R)
Hait D, Head-Gordon M (2018) How accurate is density functional theory at predicting dipole moments? An assessment using a new database of 200 benchmark values. J Chem Theory Comput 14:1969–1981
Irving K, Kieninger M, Ventura ON (2019) Basis Set Effects in the description of the Cl-O bond in ClO and XClO/ClOX isomers (X = H, O and Cl) using DFT and CCSD(T) methods. J Chem. https://doi.org/10.1155/2019/4057848
Petsis G, Salta Z, Kosmas AM, Ventura ON (2019) Theoretical study of the microhydration of 1-chloro and 2-chloro ethanol as a clue for their relative propensity toward dehalogenation. Int J Quantum Chem. https://doi.org/10.1002/qua.25931 (in press)
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Jensen F (2017) Introduction to computational chemistry, 3rd edn. Wiley, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian Inc., Wallingford
Pople JA, Head-Gordon M, Raghavachari K (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:35975
Purvis GD, Bartlett RJ (1982) A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J Chem Phys 76:1910–1919
Scuseria GE, Schaefer HF III (1989) Is coupled cluster singles and doubles (CCSD) more computationally intensive than quadratic configuration-interaction (QCISD)? J Chem Phys 90:3700–3703
Ruscic B, Pinzon RE, Morton ML, von Laszewski G, Bittner S, Nijsure SG, Amin KA, Minkoff M, Wagner AF (2004) Introduction to active thermochemical tables: several “key” enthalpies of formation revisited. J Phys Chem A 108:9979–9997
Amir-Ebrahimi V, Choplin A, Demaison J, Roussy G (1981) Microwave spectrum of the 13C-ring-monosubstituted toluenes and structure of toluene. J Mol Spectrosc 89:42–52
Noble-Eddy R (2009) Gas-phase electron diffraction studies of unstable molecules. A thesis presented for the degree of Doctor of Philosophy in the College of Science and Engineering at the University of Edinburgh. http://www.era.lib.ed.ac.uk/handle/1842/4101. Web site consulted Dec. 2017
Kortyna A, Samin AJ, Miller TA, Nesbitt DJ (2017) Sub-Doppler infrared spectroscopy of resonance stabilized hydrocarbon intermediates: ν 3/v4 CH stretch modes and CH2 internal rotor dynamics of benzyl radical. Phys Chem Chem Phys 19:29812–29821
Ruscic B, Boggs JE, Burcat A, Csaszar AG, Demaison J, Janoschek R, Martin JML, Morton ML, Rossi MJ, Stanton JF, Szalay PG, Westmoreland PR, Zabel F, Berces T (2005) IUPAC critical evaluation of thermochemical properties of selected radicals. Part I. J Phys Chem Ref Data 34:573–656
Walker JA, Tsang W (1990) Single-pulse shock tube studies on the thermal decomposition of n-butyl phenyl ether, n-pentylbenzene, and phenetole and the heat of formation of phenoxyl and benzyl radicals. J Phys Chem 94:3324–3327
Hippler H, Troe J (1990) Thermodynamic properties of benzyl radicals: enthalpy of formation from toluene, benzyl iodide, and dibenzyl dissociation equilibria. J Phys Chem 94:3803–3806
Tsang W (1996) Heats of formation of organic free radicals by kinetic methods. In: Simões JAM, Greenberg A, Liebman JF (eds) Energetics of organic free radicals. Blackie Academic and Professional, London, pp 22–58
Martinez O Jr, Crabtree KN, Gottlieb CA, Stanton JF, McCarthy MC (2015) An accurate molecular structure of phenyl, the simplest aryl radical. Angew Chem Int Ed 54:1808–1811
Cheng C-W, Lee Y-P, Witek HA (2008) Theoretical investigation of molecular properties of the first excited state of the phenoxyl radical. J Phys Chem A 112:2648–2657
Borisenko KB, Bock ChW, Hargittai I (1996) Molecular geometry of benzaldehyde and salicylaldehyde: a gas-phase electron diffraction and ab initio molecular orbital investigation. J Phys Chem 100:7426–7434
Stevens WR, Ruscic B, Baer T (2010) Heats of formation of C6H5, C6H5 + , and C6H5NO by threshold photoelectron photoion coincidence and active thermochemical tables analysis. J Phys Chem A 114:13134–13145
Sebbar N, Bozzelli JW, Bockhorn H (2011) Thermochemistry and reaction paths in the oxidation reaction of benzoyl radical: c6H5C (=O). J Phys Chem A 115:11897–11914
Simões JAM, Griller D (1989) Enthalpy of formation of the benzoyl radical by photoacoustic calorimetry. Chem Phys Lett 158:175–177
Simões RG, Agapito F, Diogo HP, Minas da Piedade ME (2014) Enthalpy of formation of anisole: implications for the controversy on the O − H bond dissociation enthalpy in phenol. J Phys Chem A 118:11026–11032
Elmaimouni L, Minetti R, Sawerysyn JP, Devolder P (1993) Kinetics and thermochemistry of the reaction of benzyl radical with 02: investigations by discharge flowhaser induced fluorescence between 393 and 433 K. Int J Chem Kinet 25:399–413
Cox JD (1961) The heats of combustion of phenol and the three cresols. Pure Appl Chem 2:125–128
Fattahi A, Kass SR, Liebman JF, Matos MAR, Miranda MS, Morais VMF (2005) The enthalpies of formation of o -, m -, and p -Benzoquinone: gas-phase ion energetics, combustion calorimetry, and quantum chemical computations combined. J Am Chem Soc 127:6116–6122
Sabbah R, Buluku ENLE (1991) Thermodynamic study of three isomers of dihydroxybenzene. Can J Chem 69:481–488
Goton R, Whalley E (1956) Thermodynamic properties of benzoic acid. Can J Chem 34:1506–1507
Papina TS, Pimenova SM, Luk’yanova VA, Kolesov VP (1995) Standard enthalpies of formation of benzyl alcohol and α, α, α-trichlorotoluene. Russ J Phys Chem (Engl Transl) 69:1951–1953
Dorofeeva OV, Ryzhova ON (2016) Enthalpy of formation and O–H bond dissociation enthalpy of phenol: inconsistency between theory and experiment. J Phys Chem A 120:2471–2479
Joback KG, Reid RC (1987) Estimation of pure-component properties from group-contributions. Chem Eng Commun 57:233–243
Cheméo website, see https://www.chemeo.com/cid/64-057-8/Salicyl%20alcohol. Visited 23rd Dec 2017
da Silva G, Hamdan MR, Bozzelli JW (2009) Oxidation of the benzyl radical: mechanism, thermochemistry, and kinetics for the reactions of benzyl hydroperoxide. J Chem Theor Comput 5:3185–3194
da Silva G, Bozzelli JW (2009) Kinetic modeling of the benzyl + HO2 reaction. Proc. Combust. Instit. 32:287–294
Fenter FF, Noziere B, Caralp F, Lesclaux R (1994) Study of the kinetics and equilibrium of the benzyl-radical association reaction with molecular oxygen. Int J Chem Kinet 26:171–189
Acknowledgements
PEDECIBA (Uy), CSIC (UdelaR, Uy) and ANII (Uy) are gratefully acknowledged for sustained funding of the theoretical atmospheric chemistry program in which the toluene project is included. Some of the calculations reported in this paper were performed in ClusterUY, a newly installed platform for high-performance scientific computing at the National Supercomputing Center, Uruguay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles derived from the 11th Congress on Electronic Structure: Principles and Applications (ESPA-2018).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ventura, O.N., Kieninger, M., Salta, Z. et al. Enthalpies of formation of the benzyloxyl, benzylperoxyl, hydroxyphenyl radicals and related species on the potential energy surface for the reaction of toluene with the hydroxyl radical. Theor Chem Acc 138, 115 (2019). https://doi.org/10.1007/s00214-019-2500-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2500-8