Abstract
The adsorption of dibenzyl disulfide (DBDS) on a Cu(111) surface model was investigated by using density functional calculations, considering energetic and electronic aspects. Several complexes were generated, where the bridge, hollow hcp, hollow fcc and top adsorption sites were considered. The results show that the Cu–S interaction guides the final complexes, and a secondary π–Cu weak interaction confers an extra stability. The complexes were grouped as physi- or chemisorption according to their adsorption energy applying a distortion decomposition model, with a preference by a double interaction of S with Cu (i.e., hollow hcp and bridge sites). A degree of disulfide bond dissociation was observed in the complexes, being correlated with adsorption energies. From an electronic aspect, it was found that the electronic flow from copper to DBDS occurs in the most stable complexes, checked with charge analysis. These results are in agreement with experimental revelations of copper corrosion on power transformers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The adsorption of sulfur-containing molecules on gold surfaces has attracted much attention, especially since the discovery of the self-assembly of alkyl thiols on Au(111) [1–3]. Thiols adsorption has also been studied on the other coinage metals [4–7]. Thiols [4, 8–15], disulfides [16–19], methionines [20–24], cysteines [25–33], etc., have been studied experimentally and theoretically. The reactivity toward sulfur-containing molecules decreases going down the column of the coinage metals, explaining why molecular adsorption has been successfully observed in the perfectly ordered thiol chains on Au(111) (see reviews on the topic cited higher). On Cu(111), the more reactive surface of the three metals (Cu, Ag and Au), a different picture of the adsorption is expected and observed [5, 34, 35]. The surface itself will reconstruct, and the adsorbed molecules easily dissociated.
Thus, understanding the mechanism of interaction of sulfur-containing molecules is expected to be of high relevance for opening new perspectives toward improving the reactivity or stability of materials used in different applications [36]. Concerning the copper surfaces, sulfur-containing molecules induce copper metal corrosion, which is a chemical phenomenon that triggers serious failures in industrial applications, and especially in power transformers [37, 38].
Dibenzyl disulfide (DBDS) is an additive with antioxidant properties, frequently used in insulating mineral oil employed in power transformers. Under operation conditions, DBDS is known to further copper corrosion in electric equipment, by forming copper (I) sulfide Cu2S as the main product, with the subsequent production of other sulfur compounds such as benzyl sulfide and dibenzyl sulfide. In spite of successive studies about the chemical phenomenon, the mechanism remains unclear [39–42]. Since DBDS is related to the commonly used thiol molecules, in the well-known thiol self-assembled monolayers (SAM) on metal surfaces [9, 43–45], the analysis of the metal–sulfur bond is important to complete the already known information on the chemical properties of this type of systems.
In the present paper, the nature of the interaction between Cu(111) and DBDS is theoretically studied. A slab model is used to simulate the copper surface at reasonable accuracy, i.e., using periodic PBE-D2. In particular, we clarify some aspects associated with copper corrosion by DBDS at the DFT level by accounting geometric, energetic and electronic properties that govern the interaction between DBDS and copper. The main focus of attention is to characterize locally the copper–sulfur interaction/bonding.
2 Computational details
2.1 Computational method
Calculations were performed in the frame of periodic DFT by means of the Vienna Ab Initio Simulation Package (VASP 5.3) [46, 47]. The electron–ion interactions were described by the projector augmented wave (PAW) method [48, 49], representing the valence electrons. The convergence of the plane-wave expansion was obtained with a cutoff of 400 eV. The Perdew–Burke–Ernzerhof (PBE) [50, 51] generalized gradient approximation (GGA) functional was used.
The adsorption of DBDS (see Fig. 1) on Cu(111) was modeled using a c7 × 7 periodic unit cell, with the aim to study the adsorption of an isolated adsorbate molecule interacting with the surface. The Cu slab contains three layers, from which the bottom one was not allowed to relax and kept at the bulk positions. The lattice parameter was initially fixed at the experimental value, 3.61 Å, to build the initial slab [52]. Then, it was re-optimized, and a subestimation of 3 % from the original supercell was obtained. This small change is accepted because it is in good agreement with the experimental parameter and obtained theoretically from PBE [53].
The sampling in the Brillouin zone was performed employing 4 and 13 k-points, resolved on 2 × 2 × 1 and 5 × 5 × 1 grids for the geometry optimizations (including dispersion corrections) and energy evaluation (at single PBE level), respectively.
Since our system involved organic molecules interacting through weak forces, the pure DFT energies obtained in periodic DFT PBE should be corrected. Therefore, we included Grimme D2 corrections [54], which can be calculated using the presently used VASP version, although D2 corrections overestimate the binding energy ([55, 56] and references therein).
The optimization procedure consisted of locating initially the DBDS molecule at 2.3 Å from the surface, at the beginning of the geometry relaxation. This distance was taken from chemi- and physisorption data reported in several studies involving the adsorption of sulfur molecules on copper surfaces [34, 35, 57, 58].
2.2 Calculation of the adsorption energy and charge transfer
Each complex can be characterized by its adsorption energy, ΔE ads, which is calculated from the total energy of the ground-state optimized geometries of the complex, Cu(111) slab and DBDS as follows:
Equation (2) includes the dispersion contribution D2, explicitly for each term.
However, this term can be scaled to obtain a better energetic estimation, avoiding the most part of the overestimation of empirical of the metal bulk. A commonly used correction is that the adsorption energy is approximated to include only the dispersion from the top layer of the surface and the adsorbate (DBDS), defining the term ΔE ads.D2.1layer as:
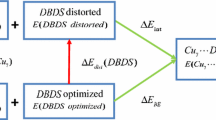
Moreover, an energetic decomposition scheme can be inspected, to describe energetically the change along the adsorption process, through a two-part scheme of deformation/interaction [59]. This partition scheme consists of two contributions, deformation (∆E def) and interaction energy (∆E int), related as:
where
in which ΔE def represents an energetic measure of perturbation over the gas-phase equilibrium geometry of each molecule, to obtain their respective geometry in the complex. It is clear that ΔE int represents the interaction energy between the deformed molecules in the complex and can be easily computed, from Eqs. (4) and (5).
The amount of total electronic charge transferred between DBDS and the Cu surface was quantified by a global charge transfer descriptor (GCT), which corresponds to the sum of atomic charges (q A ) on each molecule (A), i.e., the DBDS or the surface:
For this purpose, the Bader Charge Analysis [60, 61] was used.
Additionally, the electron localization function (ELF) was studied to characterize the existence of the S–S bond, while the DBDS interacts with the surface. The ELF of Becke and Edgecombe [62] provides an orbital-independent description of the electron localization based on strong physical arguments regarding the Fermi hole. The ELF is defined in terms of the excess of local kinetic energy density due to the Pauli exclusion principle and the Thomas–Fermi kinetic energy density. Its numerical values are conveniently mapped on the interval (0, 1), facilitating its analysis. According to the interpretation of the ELF, a region of the space with a high value of ELF corresponds to a region where it is more probable to localize a pair of electrons of opposite spin. The topological analysis of the ELF gradient field [63–65] provides a mathematical model enabling the partition of the molecular position space in a set of continuous and non-overlapping basins of attractors that present in principle a one-to-one correspondence with electron pairs.
In this way, an accurate calculation of chemical local objects such as bonds, lone pairs or atomic shells can be achieved. The basins are either core basins labeled C(A) or valence basins V(A,…) belonging to the outermost shell. Valence basins are characterized by their coordination number (synaptic order) with core [66]. The original work of Silvi and Savin on the ELF generated a fruitful field of applications in a variety of chemical problems, ranging from structural and chemical reactivity studies as well as the study of chemical reactions [67–70]. This scheme will be applied to evaluate the S–S bond in the complex.
3 Results and discussion
3.1 Geometric and energetic aspects
In this work, 20 optimized adsorption complexes were defined combining the different possible S adsorption sites on Cu(111) surface (top, hollow hcp, hollow fcc and bridge) with the three isoenergetic DBDS gas-phase conformations, obtained from a previous study [71] (see Fig. 1).
Using PBE, the structure B was more stable than A and C conformers by 0.03 and 0.07 eV, respectively. It confirms the isoenergetic character of three conformers. In the succeeding optimization procedures, the adsorption complexes between DBDS and Cu(111) result with different interaction sites, mainly noticed by S–Cu geometrical parameters. A schematic representation of showing selected geometrical parameters is presented in Fig. 2.
Typically, the adsorption site for both S atoms is found close to a bridge site. Other adsorption sites slightly higher in energy are also present, such as on top (close). Different adsorption geometries (mainly due to different benzyl conformations) showing different adsorption energies can adsorb on the same adsorption site. In order to rationalize the data, we defined the vertical spacing distance Δz and the shortest S–Cu distance, d1 and d2 (see Fig. 2; Table 1).
The most stable physisorbed geometries, shown in Fig. 3, can be divided into two groups: (a) having an S–Cu atop adsorption and (b) 2S–Cu atop adsorption. The first is slightly more favorable (structure B7). It should be noted that the benzyl groups tend to orient parallel to the Cu(111) surface.
From Table 1, the S–S distances (d3) between 2.0 and 2.2 Å correspond to physisorbed complexes and also correlate with a high average values of d1 and d2 (>2.56 Å), with adsorption sites that vary between bridge, top and hollow sites. In the other side, the group of structures having d3 distance larger than 3.4 Å and an average of d1 and d2 lower than 2.56 describes the chemisorption complexes, where sulfur atom prefers the adsorption on bridge (slightly to hollow) sites.
In summary, the most favorable adsorption sites were found to be bi-coordinated bridge in the case of physisorption and a bridge (slightly to hollow) for each dissociated fragment of benzylthiol, in the case of chemisorption (see Figs. 3, 5).
The Δz distance is related to the strength of the interaction of the sulfur atom with the Cu(111) surface. Moreover, the difference of the vertical spacing between the S atom and the closest Cu surface atom and Δz give a geometrical measure for the deformation of the surface. After adsorption, and especially with dissociative chemisorption, a Cu atom can be lifted up out of the surface and can be considered as a precursor state to the formation of an adatom. Similar results have been reported in the adsorption of thiols on Au and Ag surfaces, being less evident than Cu, attributable to the metal hardness. Looking at Δz in Table 1, one can conclude that the effect of chemisorption on the z coordinate of the surface copper atoms is particularly noticeable over ∆z1, where values of ∆z1 lower than 5.98 correspond to chemisorbed species, while higher than this value are related to physisorption complexes. The most affected copper atoms of the top layer are lifted up by 0.2 Å. The magnitude of surface deformation due to the tension generated by the adsorption of DBDS will certainly increase with increasing coverage, as is noticed in thiol/Au(111) SAMs [9, 72]. This surface tension is observed over a range of copper atoms in the neighborhood of the binding sulfur atom (see Fig. 4).
The adsorption energies (see Table 2) are in agreement with the geometrical parameters. Indeed, the adsorption complexes having the shortest distances between the DBDS sulfur atom and the Cu(111) surface (d1, d2) correlate with the highest (absolute value) adsorption energies. The adsorption energy helps to identify that the phenyl-Cu proximity shows a weak π–Cu interaction (see lower), leading to an extra stabilization of the adsorption complexes (structures B1, B2 and B3), which correspond to the chemisorbed species (see Fig. 5).
In general, the complexes formed from conformer types B and C (see Fig. 1) have more stable adsorption energies due to the proximity of the phenyl groups to the surface, favoring π–Cu interactions.
The deformation/interaction decomposition scheme displayed in Table 3 shows that in case of physisorbed complexes, the surface is slightly more perturbed than DBDS. The low values of ∆E def and almost the total contribution of ∆E int to ∆E ads explain the low geometrical effect in the adsorption. In the other side for chemisorbed complexes, the ∆E def is higher and particularly ∆E def (DBDS) is greater than the surface, and it represents more than 70 % of ∆E ads with the inclusion of an important part of the dissociative process. Additionally, ∆E def (DBDS) represents more than the 50 % of absolute value of ∆E int, while the ∆E def (Cu) is <5 % for ∆E int. Those assesses explain an important role of the geometry relaxation of DBDS related to dissociation over the adsorption.
3.2 Electronic aspects
3.2.1 Global charge transfer
A global charge transfer descriptor was introduced to quantify the relation between the adsorption energies and charge transfer between DBDS and the Cu(111) surface. Table 2 summarizes this quantity. It can be seen that the direction of the charge transfer is highly related to the kind of adsorption (physi- or chemisorption). In the most stable cases (B1, B2 and B3, i.e., chemisorbed species), the DBDS molecule acts as an electron acceptor. Most transferred charge is then allocated on the disulfide group, reaching a maximum when dissociation of the S–S bond occurs. Conversely, in the physisorption processes, the DBDS molecule acts as donor but with a lower rate of charge transfer to the Cu(111) surface (see Fig. 6).
Also, a spontaneous (barrier less) dissociation of the disulfide bond is observed for chemisorbed species, which increases the ability of DBDS to become an electron acceptor. In this case, the S–S distance increases from 2.0 to 2.2 Å and from 3.5 to 4.5 Å for physisorption and chemisorption, respectively. Finally, bi-coordinated interactions of the sulfur atoms with the Cu atoms and preferential orientation of the phenyl rings parallel to the copper surface were observed.
3.2.2 Electron localization function (ELF) analysis
In Fig. 7, a representation of the ELF basins is shown to help visualize the most relevant regions of valence electron density that characterize the DBDS in its adsorption process on copper surface.
The basin population analysis using pseudopotentials is not accurate enough, mainly to describe the population associated with sulfur atoms. However, the topological description gives us important information about the bonds in the different obtained complexes in this work.
In all kinds of formed complexes by physi- or chemisorption, each benzyl sulfide moiety of the adsorbed DBDS presents two kinds of C–H bonds, aromatic (6) and methylene (2), represented by disynaptic basins V(H,C). The six carbon–carbon aromatic bonds are represented by disynaptic V(C–C), the shape and the population, close to 3e, is characteristic for delocalized bonds in aromatic systems, and the one non-aromatic bond, V(C–C), has population close to 2.1 e. Around the sulfur atom is possible to visualize the disynaptic V(C–S) with population between 1 and 1.5e and two monosynaptic basins V(S) which characterize the electron lone pairs. At least one of these is located between sulfur and surface. But the main difference is centered in the disynaptic basin V(S–S), which describes the disulfide bond. In the physisorption complexes it is possible to visualize this basin, however in the chemisorption complexes the V(S-S) disynaptic basin disappears. It indicates the disulfide dissociation in the most stable formed complexes.
Corroboration of the topological description was made using the structure of isolated DBDS, with the geometry adopted in the complex. Initially, the level of calculation previously described was used and the topological description was confirmed by the all electron PBE/def2-TZVP level of calculation. The same description was obtained, and the disynaptic V(S–S) appears and disappears in the DBDS physi- and chemisorption complexes, respectively.
4 Conclusions
The molecular (physisorption) and dissociative (chemisorption) adsorption of DBDS on Cu(111) was studied by means of periodic DFT. Physisorption was investigated in detail by optimizing systematically all possible conformations. The adsorption site was found to be bi-coordinated bridge for physisorption and on simple bridge for chemisorption. The adsorption energies were calculated and decomposed, validating the high contribution of relaxation in the adsorption process. The charge transfer and the ELF function were used to describe the electronic structure of the adsorption complex. It was found that a charge transfer from the Cu(111) surface to the disulfide group occurs, reaching a maximum in the structures where S–S bond dissociation is observed, whereas in the physisorption processes, the DBDS molecule acts as donor but with a lower rate of charge transfer to the Cu surface. The ELF shows the absence and presence of the S–S bond in chemisorption and physisorption, respectively.
The dissociative adsorption of DBDS on Cu(111) can be considered as the first step of the copper corrosion phenomenon in which an oxidation process takes place on the surface.
References
Ulman A (1996) Chem Rev 96:1533–1554
Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM (2005) Chem Rev 105:1103–1169
Vericat C, Vela ME, Benitez G, Carro P, Salvarezza RC (2010) Chem Soc Rev 39:1805–1834
Cometto FP, Paredes-Olivera P, Macagno VA, Patrito EM (2005) J Phys Chem B 109:21737–21748
Gronbeck H (2010) J Phys Chem C 114:15973–15978
Laibinis PE, Whitesides GM, Allara DL, Tao YT, Parikh AN, Nuzzo RG (1991) J Am Chem Soc 113:7152–7167
Kacprzak KA, Lopez-Acevedo O, Hakkinen H, Gronbeck H (2010) J Phys Chem C 114:13571–13576
Fonticelli MH, Benitez G, Carro P, Azzaroni O, Salvarezza RC, Gonzalez S, Torres D, Illas F (2008) J Phys Chem C 112:4557–4563
Luque NB, Santos E, Andres J, Tielens F (2011) Langmuir 27:14514–14521
Sawaguchi T, Sato Y, Mizutani F (2001) J Electroanal Chem 496:50–60
Woodruff DP (2007) Appl Surf Sci 254:76–81
Woodruff DP (2008) Phys Chem Chem Phys 10:7211–7221
Tielens F, Costa D, Humblot V, Pradier CM (2008) J Phys Chem C 112:182–190
Tielens F, Santos E (2010) J Phys Chem C 114:9444–9452
Doneux T, Tielens F, Geerlings P, Buess-Herman C (2006) J Phys Chem A 110:11346–11352
Andreoni W, Curioni A, Gronbeck H (2000) Int J Quantum Chem 80:598–608
Cometto FP, Macagno VA, Paredes-Olivera P, Patrito EM, Ascolani H, Zampieri G (2010) J Phys Chem C 114:10183–10194
Di Felice R, Selloni A (2004) J Chem Phys 120:4906–4914
Gronbeck H, Curioni A, Andreoni W (2000) J Am Chem Soc 122:3839–3842
Humblot V, Tielens F, Luque NB, Hampartsoumian H, Methivier C, Pradier C-M (2014) Langmuir 30:203–212
Naitabdi A, Humblot V (2010) Appl Phys Lett 97:223112
Méthivier C, Humblot V, Pradier C-M (2015) Surf Sci 632:88–92
Schiffrin A, Riemann A, Auwarter W, Pennec Y, Weber-Bargioni A, Cvetko D, Cossaro A, Morgante A, Barth JV (2007) Proc Natl Acad Sci USA 104:5279–5284
Schiffrin A, Reichert J, Pennec Y, Auwaerter W, Weber-Bargioni A, Marschall M, Dell’Angela M, Cvetko D, Bavdek G, Cossaro A, Morgante A, Barth JV (2009) J Phys Chem C 113:12101–12108
De Renzi V, Lavagnino L, Corradini V, Biagi R, Canepa M, del Pennino U (2008) J Phys Chem C 112:14439–14445
Di Felice R, Selloni A, Molinari E (2003) J Phys Chem B 107:1151–1156
Gonella G, Terreni S, Cvetko D, Cossaro A, Mattera L, Cavalleri O, Rolandi R, Morgante A, Floreano L, Canepa M (2005) J Phys Chem B 109:18003–18009
Hoffling B, Ortmann F, Hannewald K, Bechstedt F (2010) Adsorption of cysteine on the Au(110)-surface: a density functional theory study. Springer, Berlin
Fischer S, Papageorgiou AC, Marschall M, Reichert J, Diller K, Klappenberger F, Allegretti F, Nefedov A, Woell C, Barth JV (2012) J Phys Chem C 116:20356–20362
Luque NB, Santos E (2012) Langmuir 28:11472–11480
Santos E, Avalle L, Poetting K, Velez P, Jones H (2008) Electrochim Acta 53:6807–6817
Santos E, Avalle LB, Scurtu R, Jones H (2007) Chem Phys 342:236–244
Marti EM, Methivier C, Pradier CM (2004) Langmuir 20:10223–10230
Rieley H, Kendall GK, Chan A, Jones RG, Ludecke J, Woodruff DP, Cowie BCC (1997) Surf Sci 392:143–152
Fan X-L, Liu Y, Ran R-X, Lau W-M (2013) J Phys Chem C 117:6587–6593
Barlow SM, Raval R (2003) Surf Sci Rep 50:201–341
Tumiatti V, Maina R, Scatiggio , Pompili M, Bartnikas R (2008) In: Conference Record of the 2008 IEEE International Symposium on Electrical Insulation, pp 284–286
Lukic JM, Milosavljevic SB, Orlovic AM (2010) Ind Eng Chem Res 49:9600–9608
Toyama S, Tanimura J, Yamada N, Nagao E, Amimoto T (2009) IEEE Trans Dielectr Electr Insul 16:509–515
Ahmed Khan F, Sundara Rajan J, Ansari MZA, Asra PS (2012) In: 2012 International Conference on Advances in Power Conversion and Energy Technologies (APCET), pp 1–4
Oweimreen GA, Jaber AMY, Abulkibash AM, Mehanna NA (2012) IEEE Trans Dielectr Electr Insul 19:1962–1970
Maina R, Tumiatti V, Pompili M, Bartnikas R (2009) IEEE Trans Dielectr Electr Insul 16:1655–1663
Tielens F, Humblot V, Pradier C-M (2008) Int J Quantum Chem 108:1792–1795
Tielens F, Humblot V, Pradier CM, Calatayud M, Illas F (2009) Langmuir 25:9980–9985
Tielens F, Santos E (2010) J Phys Chem C 114:9444–9452
Kresse G, Hafner J (1993) Phys Rev B 47:558–561
Kresse G, Hafner J (1994) Phys Rev B 49:14251–14269
Blochl PE (1994) Phys Rev B 50:17953–17979
Kresse G, Joubert D (1999) Phys Rev B 59:1758–1775
Hammer B, Hansen LB, Norskov JK (1999) Phys Rev B 59:7413–7421
Perdew JP, Burke K, Ernzerhof M (1997) Phys Rev Lett 78:1396–1396
Straumanis ME, Yu LS (1969) Acta Crystallogr Sect A 25:676–682
Gattinoni C, Michaelides A (2015) Faraday Discuss 180:439–458
Grimme S (2006) J Comput Chem 27:1787–1799
Almora-Barrios N, Carchini G, Blonski P, Lopez N (2014) J Chem Theory Comput 10:5002–5009
Klimes J, Michaelides A (2012) J Chem Phys 137:120901
Ferral A, Patrito EM, Paredes-Olivera P (2006) J Phys Chem B 110:17050–17062
Crutcher ER, Warner K, Northwest M, electric-connectionco.com
Scaranto J, Mallia G, Harrison NM (2011) Comput Mater Sci 50:2080–2086
Henkelman G, Arnaldsson A, Jónsson H (2006) Comput Mater Sci 36:354–360
Tang W, Sanville E, Henkelman G (2009) J Phys: Condens Matter 21:084204
Becke AD, Edgecombe KE (1990) J Chem Phys 92:5397
Savin A, Becke AD, Flad J, Nesper R, Preuss H, von Schnering HG (1991) Angew Chem Int Ed Engl 30:409–412
Savin A, Jepsen O, Flad J, Andersen OK, Preuss H, von Schnering HG (1992) Angew Chem Int Ed Engl 31:187–188
Silvi B, Savin A (1994) Nature 371:683–686
Silvi B (2002) J Mol Struct 614:3–10
Melin J, Fuentealba P (2003) Int J Quantum Chem 92:381–390
Fuentealba P, Chamorro E, Santos JC (2007) In: Toro-Labbe A (ed) Theoretical aspects of chemical reactivity, vol 19, pp 57–85
Santos JC, Andres J, Aizman A, Fuentealba P, Polo V (2005) J Phys Chem A 109:3687–3693
Santos JC, Tiznado W, Contreras R, Fuentealba P (2004) J Chem Phys 120:1670–1673
Saavedra-Torres M, Jaque P, Tielens F, Santos JC (2015) Theoret Chem Acc 134:73
Bedford E, Humblot V, Méthivier C, Pradier CM, Gu F, Tielens F, Boujday S (2015) Chem Eur 21:14555–14561
Acknowledgments
The authors acknowledge the financial support by FONDECYT through the Project Number 1120785 and Universidad Andres Bello Grant DI-497-14/R. M.S.-T. thanks CONICYT for a Ph.D. scholarship and Universidad Andres Bello for support through grant DI40/12-I. F.T. thanks FONDECYT for several appointments as Invited Professor (2013 and 2015).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Saavedra-Torres, M., Tielens, F. & Santos, J.C. Dibenzyl disulfide adsorption on Cu(111) surface: a DFT study. Theor Chem Acc 135, 7 (2016). https://doi.org/10.1007/s00214-015-1763-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-015-1763-y