Abstract
The persistent use of opioids leads to profound changes in neuroplasticity of the brain, contributing to the emergence and persistence of addiction. However, chronic opioid use disrupts the delicate balance of the reward system in the brain, leading to neuroadaptations that underlie addiction. Chronic cocaine usage leads to synchronized alterations in gene expression, causing modifications in the Nucleus Accumbens (NAc), a vital part of the reward system of the brain. These modifications assist in the development of maladaptive behaviors that resemble addiction. Neuroplasticity in the context of addiction involves changes in synaptic connectivity, neuronal morphology, and molecular signaling pathways. Drug-evoked neuroplasticity in opioid addiction and withdrawal represents a complicated interaction between environmental, genetic, and epigenetic factors. Identifying specific transcriptional and epigenetic targets that can be modulated to restore normal neuroplasticity without disrupting essential physiological processes is a critical consideration. The discussion in this article focuses on the transcriptional aspects of drug-evoked neuroplasticity, emphasizing the role of key transcription factors, including cAMP response element-binding protein (CREB), ΔFosB, NF-kB, Myocyte-enhancing factor 2 (MEF2), Methyl-CpG binding protein 2 (MeCP2), E2F3a, and FOXO3a. These factors regulate gene expression and lead to the neuroadaptive changes observed in addiction and withdrawal. Epigenetic regulation, which involves modifying gene accessibility by controlling these structures, has been identified as a critical component of addiction development. By unraveling these complex molecular processes, this study provides valuable insights that may pave the way for future therapeutic interventions targeting the mechanisms underlying addiction and withdrawal.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug addiction, particularly opioid addiction, is a complex and multifaceted disorder that poses a significant public health challenge worldwide. Addiction, which is defined as the persistent desire for and use of drugs despite negative effects or a lack of self-control, results from long-term alterations in certain brain areas (Hyman et al. 2006). Opioid addiction has reached epidemic proportions, with alarming morbidity and mortality rates. The pharmacological effects of opioids are produced by binding to particular brain receptors, mainly mu-opioid receptors, which results in feelings of reward, pleasure, and analgesia (Nestler 2013). However, chronic opioid use disrupts the delicate balance of the reward system of the brain, leading to neuroadaptations and neuroplasticity of the brain, which plays a key role in fostering the onset and persistence of addiction. The brain’s remarkable capacity to change its shape and function in response to new information, including drugs of abuse, is known as neuroplasticity (Nestler 2001a, Russo et al. 2010). Understanding the molecular mechanisms underlying drug-evoked neuroplasticity in opioid addiction and withdrawal is crucial for developing effective treatments and interventions to combat this devastating disorder. Although repeated drug exposure may cause addiction in some people, others can use drugs in moderation without developing dependency. Neuroplasticity in the context of addiction involves changes in synaptic connectivity, neuronal morphology, and molecular signaling pathways. These changes contribute to the persistent craving for opioids, the inability to control drug-seeking behavior, and the occurrence of withdrawal symptoms upon cessation of drug use (Kalivas and Volkow 2005; Kauer and Malenka 2007). Unraveling the transcriptional and epigenetic mechanisms underlying drug-evoked neuroplasticity is essential for elucidating the molecular basis of addiction and for developing targeted therapeutic strategies. During opioid addiction and withdrawal, genetic, epigenetic, and environmental variables interact to cause drug-evoked neuroplasticity.
Molecular mechanisms of opioid addiction
Chronic opioid exposure alters gene expression and affects neuronal function and synaptic plasticity. CREB and AP-1 transcription factors regulate gene expression in addiction-related neuroplasticity (Robison and Nestler 2011; McClung and Nestler 2003). Epigenetic changes such as DNA methylation and histone modifications mediate long-term opioid addiction-related gene expression patterns. DNA methylation, especially at CpG dinucleotides in gene promoters, can alter gene expression and cause addiction-related symptoms (Maze et al. 2010; Renthal and Nestler 2008). Histone changes, including acetylation, methylation, and phosphorylation, affect gene transcription by altering chromatin shape and accessibility (Tsankova et al. 2004). Non-coding RNAs, particularly microRNAs (miRNAs), also regulate neuroplasticity and addiction. These small RNA molecules can target messenger RNAs (mRNAs) and either degrade or inhibit their translation, leading to the post-transcriptional regulation of gene expression. Dysregulation of specific miRNAs has been observed in the context of opioid addiction, suggesting their involvement in neuroplasticity modulation (Smith et al., 2018). The complex relationship between these epigenetic and transcriptional processes leads to maladaptive changes in synaptic plasticity and the neural circuitry observed in opioid addiction. Understanding the molecular pathways and signaling cascades altered by chronic opioid exposure is crucial for developing effective therapeutic interventions. The dopaminergic, glutamatergic, and GABAergic systems, as well as neurotrophic factors, play integral roles in addiction-related neuroplasticity (Kalivas and Volkow 2005; Lüscher and Malenka 2011). By targeting transcriptional and epigenetic mechanisms, it may be possible to reverse the drug-evoked neuroplastic changes associated with opioid addiction and facilitate recovery and abstinence. Therapeutic interventions aimed at restoring normal gene expression patterns and modifying epigenetic marks hold promise for attenuating the craving for opioids, reducing drug-seeking behavior, and preventing relapse (Nestler 2008, 2022). The development of novel therapies to target drug-evoked neuroplasticity faces several challenges. The complexity of the brain’s neuroplasticity mechanisms, individual variability in genetic and epigenetic profiles, and chronic nature of addiction necessitate a comprehensive approach. Additionally, identifying specific transcriptional and epigenetic targets that can be modulated to restore normal neuroplasticity without disrupting essential physiological processes is critical (Renthal and Nestler 2008). By understanding the transcriptional and epigenetic pathways of drug-evoked neuroplasticity in opioid addiction and withdrawal, we can pave the way for innovative and effective treatments that address underlying molecular alterations.
This review comprehensively investigates various well-defined changes that have been shown to play a role in specific aspects of the behavioral syndrome of addiction. Additionally, we focused on alterations caused by drugs in transcription factors, which are nuclear proteins that attach to the regulatory regions of particular genes and control their transformation into mRNA. The focus on transcription factors is based on the idea that changes in gene expression caused by drugs can help explain the long-lasting behavioral abnormalities seen in addiction and the mechanisms responsible for drug-induced neuroplasticity.
Synaptic resilience: unveiling Neuroplasticity’s crucial nexus in the genesis of opioid dependency
Target areas of brain
Neuroplasticity refers to long-lasting alterations in neural circuits and synaptic connections evoked by the chronic consumption of drugs of abuse (Koob 2013). These alterations in the structure and function of the brain play a crucial role in the development of addiction and contribute to the persistence of drug-seeking behaviors, even after prolonged periods of abstinence (Volkow and Morales 2015; Lovinger and Kash 2015). Drug-evoked neuroplasticity involves multiple brain regions, including the mesolimbic dopaminergic system, which plays a key role in reward and reinforcement processing (Dong and Nestler 2014). Within this system, the primary target for drugs of abuse, such as opioids, is the NAc, which is rich in dopamine receptors and densely interconnected with other regions of the brain that are involved in reward and motivation (Russo et al. 2010; Lüscher and Malenka 2011). Several brain regions, such as the prefrontal cortex, amygdala, and hippocampus send signals to the NAc. This allows the NAc to combine information about drug-related cues and motivation-driven control behaviors (Volkow et al. 2002, 2003).
Role of neurotransmitters and their signaling mechanisms
A crucial cellular process underlying drug-evoked alterations in the brain is the activation of the dopamine system. Addictive drugs such as cocaine, amphetamines, and opioids cause the NAc to release more dopamine, producing an intense feeling of reward (Volkow et al. 2019; Covey et al. 2014). Dopaminergic neurons in the ventral tegmental area (VTA) are altered by drugs, resulting in elevated levels of dopamine release (Bartas et al. 2022). Long-term drug use changes how these neurons work, causing them to fire more often and release more dopamine due to chronic drug exposure. These changes are caused by alterations in the production and function of various molecules, including dopamine receptors, transporters, and proteins involved in cellular signaling (Nestler 2022). When dopamine receptors in the NAc are activated, they set off a chain of cellular signaling pathways that ultimately lead to long-lasting changes in function and brain connections (Wolf et al. 2003; Thomas and Malenka 2003). The cyclic adenosine monophosphate (cAMP) pathway is an important signalling pathway. Addictive drugs increase the level of cAMP in NAc, activating the protein kinase A (PKA) and modifying various other molecules (Yan et al. 2016; Boudreau et al. 2009). Modifying transcription factors, such as cAMP response element-binding protein (CREB), by adding phosphates promotes the expression of genes involved in changing brain connections and behaviors related to reward (Chowdhury et al. 2023) (Fig. 1). Additionally, PKA-mediated phosphorylation of ion channels and neurotransmitter receptors alters their functions, further contributing to drug-evoked neuroplasticity (Lüscher and Malenka 2011). Another important mechanism contributing to drug-evoked neuroplasticity is the remodeling of dendritic spines, which are small protrusions on the surface of neurons where most excitatory synapses are formed. Chronic drug exposure also modifies spine density and morphology in the NAc and other brain areas (Ethell and Pasquale 2005). Cocaine administration enhances the number of dendritic spines in the NAc, whereas chronic opioid exposure results in decreased spine density (Russo et al. 2010). These changes in spine morphology are regulated by a complex interplay of molecular mechanisms involving the cytoskeleton, synaptic proteins, and intracellular signaling pathways. Actin cytoskeleton dynamics, regulated by small GTPases such as RhoA and Cdc42, play a key role in spine remodeling (Russo et al. 2010). Epigenetic mechanisms also contribute to the drug-evoked neuroplasticity. Histone acetylation and DNA methylation are two examples of epigenetic changes that control gene expression, and can be influenced by chronic drug exposure. Cocaine administration alters DNA methylation patterns in the NAc, leading to altered gene expression profiles. In addition, histone acetylation, which is regulated by enzymes such as histone acetyltransferases (HATs) and histone deacetylases (HDACs), is an essential component of drug-induced neuroplasticity (Sadri-Vakili 2015). Prolonged drug exposure can lead to alterations in histone acetylation patterns, resulting in alterations in gene expression that contribute to long-term synaptic plasticity and addiction-related behaviors (Robison and Nestler 2011; Sadri-Vakili 2015). In addition to these molecular mechanisms, drug-evoked neuroplasticity involves changes in neurotransmitter systems other than dopamine. For example, chronic opioid exposure leads to adaptations in the endogenous opioid system, including alterations in the expression and function of opioid receptors. These alterations contribute to the development of tolerance and withdrawal symptoms associated with opioid addiction (Kalivas and Volkow 2005). Glutamate, the principal excitatory neurotransmitter in the brain, is another important factor in the circuit of drug-evoked neuroplasticity. Chronic drug use leads to dysregulation of glutamatergic signaling, leading to long-lasting changes in synaptic strength and plasticity. Drugs of abuse can modulate glutamate release, alter the expression and function of glutamate receptors, and disrupt glutamate homeostasis. These changes contribute to the remodeling of excitatory synapses and the development of addiction-related behaviors (Kalivas 2009). Drug-induced neuroplasticity is a complex process that involves multiple molecular mechanisms that interact with each other. The interplay between dopaminergic, glutamatergic, and other neurotransmitter systems, along with the involvement of intracellular signaling pathways and epigenetic modifications, leads to persistent changes in the neural circuits observed in addiction (Mews and Calipari 2017). By targeting specific molecules or signaling pathways involved in drug-induced neuroplastic changes, it may be possible to restore normal brain function and reduce drug cravings and relapses. Several potential targets have been identified, including dopamine receptors, glutamate receptors, and various intracellular signaling molecules. Additionally, epigenetic modifications provide a promising avenue for therapeutic intervention as they can be reversible and may be targeted using pharmacological approaches. Additional investigations are required to clarify the exact mechanisms that cause drug-induced changes in the brain’s ability to adapt and create successful therapies for addiction.
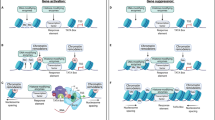
Role of Neuroplasticity in addiction development. Chronic opioid exposure activates the brain’s reward system. Hyperactivation of dopaminergic neurons in the Nucleus Accumbens (NAc) leads to increased cAMP levels and PKA activation. PKA phosphorylates CREB, ultimately promoting gene expression and neuroplastic changes in the brain
Transcriptional tapestry of various mechanisms involved in opioid dependence and withdrawal induced neuroplastic changes
Genetic regulation of neuronal adaptations and evolutionary dynamics
Regulation of neuronal adaptation is a complex and fascinating process that involves various genetic and epigenetic factors. Genetic mutations or variations associated with neuronal plasticity can change the potency and effectiveness of synaptic connections, influencing cognitive function and behavior (Sweatt 2016). One crucial aspect of the genetic regulation of neuronal adaptation is the influence of specific genes. Several genes have been reported to play a role in neural plasticity, including those involved in the growth and maintenance of neuronal structures such as dendrites and spines (Sala and Segal, Levy et al. 2014). One example is the neurotrophin family of genes, which includes brain-derived neurotrophic factor (BDNF), and has been linked to the control of synaptic plasticity (Benarroch 2015; Waterhouse and Xu 2009). BDNF promotes neuronal proliferation and maintenance, and facilitates neuronal connections (Baydyuk and Xu 2014; Koshimizu et al. 2009). Unlike genetic changes, which directly affect the DNA code, epigenetic changes affect gene expression rather than the DNA sequence. These changes, particularly DNA methylation, where a methyl group is attached to DNA, can influence neuronal adaptation. Histone modifications may involve changes in the proteins that envelop DNA, and can also affect gene expression and neuronal adaptations (Cheng et al. 2023). Epigenetic and genetic variables interact significantly to regulate neuronal adaptations. Genetic factors can influence epigenetic alterations, and certain genetic variations can affect the susceptibility of DNA to epigenetic modifications. For instance, genetic variations in DNA methylation can affect the regulation of neuronal plasticity (Cheng et al. 2015). Chronic morphine exposure alters tRNA cytosine methylation in the medial prefrontal cortex, affecting gene expression and neuronal adaptation. Nsun2 knockout disrupts reward-seeking behavior, suggesting that the tRNA machinery is a potential therapeutic target for addiction (Blaze et al., 2024). Transcriptional pathways and chromatin mechanisms play crucial roles in neuronal adaptation to opioid addiction, withdrawal, and relapse. Chronic opioid exposure alters BDNF gene expression in the VTA through epigenetic modifications, affecting neuronal adaptations during opioid addiction and withdrawal (Koo et al. 2015). Research indicates that genetic factors play a significant role in opioid addiction, particularly through the effects of vital genes, such as BDNF and OPRM1. Animal knockout studies have demonstrated that BDNF is crucial for the development of addiction-related behaviors, and its absence leads to altered responses to opioids and reduced reward sensitivity (Carter et al. 2024). Additionally, variations in the OPRM1 gene have been linked to differences in opioid receptor function, which influences susceptibility to addiction (Gaddis et al. 2022). Human studies have identified polymorphisms in genes associated with the dopaminergic and opioid systems, which correlate with addiction phenotypes. For example, specific OPRM1 variants have been associated with an increased risk of opioid dependence (Lemen et al. 2022). However, the complexity of gene-environment interactions suggests that, while these genetic factors are significant, they do not act in isolation, and further research is needed to fully elucidate their mechanisms in the context of opioid addiction (Gaddis et al. 2022; Guo et al., 2022). Epigenetic interventions have been shown to significantly influence drug addiction and withdrawal. Research indicates that alterations in DNA methylation and histone modifications can affect gene expression related to addiction pathways, thereby affecting behavioral responses to drugs and withdrawal symptoms (Shaik et al. 2023). For instance, specific epigenetic modifications have been linked to the development of addiction and severity of withdrawal, suggesting that targeting these modifications could provide therapeutic avenues for addiction treatment (Fanfarillo et al. 2024). Moreover, studies have highlighted that pharmacological agents that modify epigenetic marks can mitigate withdrawal symptoms and reduce relapse rates, indicating the potential for epigenetic therapies in addiction management (Do 2024). However, the complexity of epigenetic regulation and individual variability in response to interventions pose challenges, necessitating further research to optimize these strategies for clinical applications (Koijam et al. 2024). Further research is ongoing to unravel the intricacies of genetic regulation in neuronal adaptations and their implications for brain function and behavior. By understanding the genetic and epigenetic mechanisms involved, scientists hope to gain insights into how changes in neuronal adaptations lead to various neurological disorders, and how they can be potentially targeted for therapeutic interventions. Therefore, genetic variations and epigenetic modifications can affect the strength and efficiency of synaptic connections, which, in turn, influences cognitive function and behavior. Additional investigations are needed to completely understand the molecular mechanisms underlying these processes and their implications in various neurological and psychiatric conditions.
Role of transcriptional factors in neuroplasticity orchestration
Regular use of substances such as cocaine and other opioids can lead to alterations in gene expression in a specific brain region, that is, the NAc and medium spiny neurons (MSNs). The primary mechanism proposed is that cocaine increases dopamine (DA) levels at the synapse, activating two types of receptors, D1 and D2, in different subgroups of MSNs (Teague and Nestler 2022). These receptors initiate separate signaling pathways that lead to the activation or suppression of multiple transcription factors. These changes can occur through post-translational alterations or changes in the overall expression levels of various transcription factors. Transcription factors are protein molecules that can attach to specific DNA sequences called response elements or motifs (Teague and Nestler 2022). These elements are typically 6–12 base pairs long and are located in gene regulatory areas such as enhancers and promoters. Once bound to these DNA sequences, transcription factors facilitate the recruitment of various secondary regulatory proteins. This recruitment process ultimately results in the inhibition or activation of transcription, which is the initial step in the gene expression process (Teague and Nestler 2022). By modifying synaptic communication within the mesocorticolimbic system, drugs of abuse can profoundly affect reward processing and reinforcement. However, for long-term modifications to neuronal activity to occur, new protein synthesis is necessary (Kandel 2001). Repeated exposure to addictive substances can lead to specific changes in gene expression in certain regions, which may contribute to persistent behavioral abnormalities associated with addiction (Nestler 2001b, Robinson and Kolb 2004). The control of gene expression by addictive substances can occur through various mechanisms, such as the activation or inhibition of transcription factors, epigenetic processes, and induction of non-coding RNAs (Teague and Nestler 2022). Transcription factors mediate the regulation of gene expression at the transcriptional level in cellular processes. These proteins act as molecular switches in response to internal changes and bind to specific regions of target genes. Within the designated promoter area, these regulatory regions reside outside the coding region of the gene. These sequences are recognized and are referred to as cis-regulatory elements. The mechanisms that regulate gene expression, and ultimately result in functional protein production, may lead to the development of addiction (Teague and Nestler 2022). Drug-evoked changes in addiction-related gene expression may generate persistent synaptic plasticity. These molecules can be regulated at various stages, including the organization of DNA into nucleosomes and compact units controlled by histones that make up the chromatin. Therefore, basal transcription is halted, unless gene accessibility is regulated. Epigenetic regulation, which involves changes in the availability of genes through regulation of these structures, has been identified as a critical component of addiction development (Nestler 2014).
Interpreting the minutiae of CREB-mediated transcriptional control
CREB is a crucial transcription factor that orchestrates diverse processes within the nervous system, ranging from the formation of connections during development to solidifying memories and adapting to new experiences (Carlezon et al. 2005; Shaywitz et al., 1999). It controls the activity of thousands of genes by forming pairs with itself and binding to specific sequences of DNA known as cAMP response elements (CREs). Typically, CREB is bound to its target genes in an inactive state. However, when stimulated, it undergoes phosphorylation at serine 133. This allows the recruitment of CREB-binding protein (CBP), an enzyme that adds acetyl groups to histones and other regulatory proteins (Teague and Nestler 2022). Interestingly, a previous study showed that mice lacking the phosphorylation site at serine 133 still retained certain functions of CREB in the hippocampus, suggesting additional complexities in its regulatory mechanisms (Briand et al. 2015). The initial connection between CREB and drug addiction emerged from its established involvement in cAMP signaling, and growing evidence suggests an increase in the activity of the cAMP pathway in various regions of the brain in response to commonly abused psychostimulant or opioid drugs (Rezayof et al., 2023). Abuse-related drugs can lead to abrupt control of molecular signaling pathways, which can change the activity of transcription factors. For instance, numerous addictive substances have been shown to acutely upregulate cAMP levels in numerous addiction-related brain areas. CREB has attracted significant attention and is thought to play a role in drug-induced learning. Numerous intracellular signaling mechanisms can trigger CREB, which is widespread in the brain and is ultimately phosphorylated at serine 133 (Mayr and Montminy 2001). Calcium-calmodulin-dependent kinase (CaMK), protein kinase A (PKA), and other kinases phosphorylate CREB in response to elevated cAMP levels (Mayr and Montminy 2001). The production of numerous downstream genes is facilitated by phosphorylated CREB (pCREB), which promotes CBP induction. When exposed to psychostimulants, pCREB is quickly triggered in the striatum, and it is believed that this is a balancing process that prevents behavioral reactions to addictive substances (Wang et al. 2003). In a conditioned place preference (CPP) model, the reinforcing qualities of cocaine are reduced when CREB is overexpressed in the NAc shell. In contrast, the opposite was observed when CREB was inhibited in this area (Pliakas et al. 2001). Studies have shown that CREB activation, such as during methamphetamine self-administration, increases gene expression of c-fos, Fosb, BDNF, and TrkB. (Krasnova et al. 2013). The investigation of addiction-related behaviors and general brain development has long involved BDNF and its target, TrkB (Li and Wolf 2015). It has been identified that CREB plays a key role in late-phase types of long-term potentiation, which further implicates it as a modulator of plasticity. This study showed that phosphorylation of CREB in the NAc is crucial for the pleasing effects of opioids. Inhibition of CREB phosphorylation in the NAc attenuates opioid-induced reward and prevents the progression of morphine-induced conditioned place preference (Morón et al. 2010). CREB is identified as necessary for the development of opioid withdrawal. Inhibition of CREB activity in the locus coeruleus is involved in withdrawal symptoms and attenuates the expression of opioid withdrawal (Barrot et al. 2002). Therefore, these studies suggest that the expression of genes regulated by CREB may play an essential role in the adaptability of neurons in response to both short and long-term alterations in brain equilibrium caused by opioids.
Unscrambling the role of transcriptional master regulator ΔFosB
ΔFosB, a unique transcription factor derived from the fosB gene, has features similar to those of its family counterparts, such as c-Fos, FosB, Fra1, and Fra2 (Nestler 2008). These factors can partner with Jun family members like c-Jun, JunB, or JunD to form the active “activator protein-1” (AP-1) complexes, which regulate gene expression. The resulting AP-1 complex binds to specific AP-1 sites in gene promoters, thereby regulating gene transcription (Nestler 2008) after an initial dose of diverse addictive drugs. In particular, the brain experiences a quick and temporary surge in the activity of various Fos family proteins. However, chronic drug exposure leads to distinct responses (Nestler 2001). Interestingly, ΔFosB, unlike other Fos family members, accumulates biochemically modified isoforms after repeated drug exposure in the same brain regions. This ΔFosB accumulation has been observed across a wide range of drugs of abuse (Muller and Unterwald 2005; McDaid et al. 2006). Evidence suggests that AP-1 DNA binding increases after long-term potentiation (LTP) stimulation in the dentate gyrus region, proving its link to long-term plasticity and late-phase LTP (Williams et al. 2000). Following acute stimulation, all Fos family proteins are quickly activated in various brain areas; their induction becomes less prominent with chronic treatments. In contrast, the FosB gene has a splice variant called ΔFosB, which exhibits exceptional stability and accumulates with chronic treatments, implying its importance in inducing and maintaining long-term plasticity (McClung et al. 2004). Chronic stimuli, such as repeated drug exposure or stress, induce ΔFosB in specific brain regions (McClung et al. 2004; Perrotti et al. 2004). Intriguingly, the function of ΔFosB changes over time; initially repressing gene expression at AP-1 sites during short-term treatments, it transitions to activation as its levels increase with chronic exposure (McClung and Nestler 2003). Knockout mice lacking both FosB and ΔFosB displayed abnormal drug responses and heightened anxiety/depression, particularly under stress, highlighting their crucial role in modulating both reward and stress pathways. This suggested that this gene plays an essential role in regulating these responses (Zhu et al. 2007). Furthermore, behaviors such as addiction are induced by the persistent overexpression of ΔFosB, particularly in the NAc. However, this addiction-like trait is countered by the existence of a dominant-negative form of c-Jun, known as Dc-Jun, which inhibits AP-1 activity (Peakman et al. 2003; Zachariou et al. 2006). Moreover, voluntary wheel running and food-reinforced instrumental performance were improved by the long-term expression of ΔFosB in the NAc. This implies that ΔFosB participates in both the reaction to natural rewards and response to pharmacological rewards (Werme et al. 2002; Olausson et al. 2006). Research studies have revealed that casein kinase 2 (CK2) phosphorylates ΔFosB at Serine 27. This phosphorylation process increases the transcriptional activity of ΔFosB and inhibits its degradation (Ulery and Nestler 2007). Considering the increased CK2 activity observed during LTP induction in the hippocampus, it is plausible that this phosphorylation event serves as a mechanism to augment the activity and stability of ΔFosB, leading to long-term alterations in gene expression associated with plasticity (McClung and Nestler 2008). The complete FosB protein and other Fos family proteins lack two degron domains, contributing to the exceptional stability of ΔFosB as they are known to degrade quickly (Carle 2007). This transcription factor acts as a “molecular switch, " which increases drug incentive motivation and addiction. Although it was initially thought to be affected only by immediate morphine administration, further research has revealed that other addictive drugs lead to dopamine-mediated alterations in the regulation of this factor in brain regions related to addiction (Oliver and Perrone-Bizzozero 2017). The upregulation of ΔFosB in mesolimbic structures during withdrawal is significant for understanding relapse, as it indicates that this factor may contribute to the intense drug cravings that characterize addiction.
Exploring the role of nuclear factor Kappa B
Synaptic plasticity and memory have been linked to the critical transcription factor, nuclear factor kappa B (NF-κB), which is known for its role in immune responses and inflammation (Kaur et al. 2022; Meffert and Baltimore 2005). Studies have demonstrated that following repeated cocaine administration, NF-κB is induced in the NAc, where it causes medium spiny neurons in the nucleus accumbens to develop dendritic spines. This process is associated with sensitization to the rewarding effects of drugs, highlighting the role of NF-κB in addiction (Ang et al. 2001; Russo et al. 2009). Researchers are currently focusing on identifying the specific genes targeted by NF-κB to induce cellular and behavioral plasticity after cocaine use. The induction of NF-κB by cocaine is mediated through ΔFosB, a transcription factor explained earlier, which is involved in the actions of drugs. Increased levels of ΔFosB in the NAc result in activation of NF-κB (Peakman et al. 2003; Ang et al. 2001). Furthermore, NF-κB is associated with the neurotoxic effects of methamphetamine in striatal areas (Shah et al. 2012). Research findings indicate that NF-κB is involved in the creation of new spines in medium spiny neurons in models of stress and depression. Given the common association between depression and addiction (Christoffel et al. 2011), and the well-known link between stress and drug misuse relapse, this conclusion is significant. NF-κB has two forms, dormant and active, which are found in neurons (Kaltschmidt et al. 2006). The active form is found in the nucleus, whereas the inactive form is retained in the cytoplasm via interactions with IκB, an inhibitory protein (Kaltschmidt et al. 2006). When stimulated by various factors, IκB degrades, allowing the NF-κB to move into the nucleus and become active. Additionally, upon stimulation, the RelA subunit of NF-κB undergoes a modification called acetylation, preventing interaction with IκBα (Chen et al. 2001). Conversely, histone deacetylase 3 (HDAC3) removes the acetyl group from RelA, promoting its export from the nucleus and binding to IκBα (Chen et al. 2001). This reversible acetylation process controls the duration of nuclear NF-κB activation. NF-κB activation occurs in response to cellular stress, neurodegeneration, or trauma. Regulating genes involved in cell survival helps neurons stay alive (Mattson 2005; Behl et al. 2021). Additionally, NF-κB activity is induced following LTP in the hippocampus and amygdala (Yeh et al. 2002; Romano et al. 2006). Studies have shown that blocking NF-κB activity hinders LTD and disrupts LTP in the hippocampus and amygdala (Yeh et al. 2002; Albensi et al., 2000). Glutamate receptor stimulation increases DNA binding in the hippocampus of certain members of the NF-κB family. The late phase of LTD is impaired in mice lacking c-Rel (O’Riordan et al. 2006). Interestingly, mice with neurons that overexpress a protein that inhibits NF-κB activity (IkB-AA) exhibit learning and memory impairments (Kaltschmidt et al. 2006). These mice also have problems with late-phase hippocampal LTP and cannot undergo LTD (Kaltschmidt et al. 2006). This study identified a protein called the PKA catalytic α-subunit as a target gene for NF-κB. When NF-κB is inactive, phosphorylation of CREB by PKA is reduced (Kaltschmidt et al. 2006). Thus, these mice may have learning and memory problems due to a lack of activated CREB. Chronic cocaine may activate NF-κB in the NAc, which may be controlled by ΔFosB, in addition to its involvement in memory (Ang et al. 2001). NF-κB signaling plays a crucial role in opioid-induced neuroplastic changes associated with dependence and withdrawal, regulating the expression of genes critical for opioid responses in cells (Chen et al. 2006). TLR4-mediated NF-κB activation in the spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia, indicating the involvement of NF-κB in opioid dependence and withdrawal-induced neuroplastic changes (Bai et al. 2014). The IKK/NF-κB signaling pathway plays a critical role in the extinction of morphine-induced conditioned place aversion memory in rats, suggesting its involvement in opioid withdrawal-associated neuroplastic changes (Yang et al. 2012). This indicates that NF-κB may be involved in the plasticity associated with addiction, and further research is needed to explore this possibility.
Interpreting the role of a key player myocyte-enhancing factor 2 (MEF2)
Although transcription factors have been extensively studied, CREB and ΔFosB are involved in addiction, and numerous other transcription factors likely influence plasticity and behavior related to addiction. Among these factors, myocyte-enhancing factor 2 (MEF2) has emerged as an intriguing candidate that is specifically linked to cocaine-induced structural plasticity (Oliver and Perrone-Bizzozero 2017, Pulipparacharuvil et al. 2008). In the brain, including NAc medium spiny neurons (MSNs), multiple isoforms of MEF2 proteins are expressed. These isoforms form homodimers and heterodimers, recruiting various proteins such as co-repressors referred to as class II histone deacetylases (HDACs) and co-activator p300 to activate or repress gene transcription (Yagishita et al. 2014; Alvarez and Sabatini 2007). Remarkably, experimental evidence has demonstrated that MEF2 is necessary and sufficient for increasing the dendritic spine count, the physical synaptic structures implicated in addiction-related plasticity (Yagishita et al. 2014; Alvarez and Sabatini 2007). Additionally, MEF2 controls a large network of genes linked to plasticity, presenting exciting opportunities for further investigation into its role in addiction-related plasticity and behavior (Flavell et al. 2008). Brain MEF2 variants have been found in the MSNs of the NAc. The interactions of these variants with specific proteins determine whether the gene transcription is activated or suppressed. Research studies indicate that long-term cocaine use can hinder MEF2 activity in the nucleus accumbens by interfering with the function of calcineurin, which is suppressed by the D1 receptor and cyclic AMP. (Pulipparacharuvil et al. 2008; Nestler 2012). Cocaine may also inhibit MEF2 activity through induction of Cdk5, a gene regulated by ΔFosB (Bibb et al. 2001). This reduction in MEF2 activity seems to be necessary for the increased dendritic spine number observed in MSNs after cocaine exposure. However, it may also impede behavioral drug sensitization (Pulipparacharuvil et al. 2008). Although these results demonstrate the critical function of MEF2 in cocaine-induced structural and behavioral alterations, they also reveal a seemingly contradictory phenomenon: increased spine numbers in MSNs despite the absence of behavioral sensitization (Russo et al. 2010). Ethanol lowers MEF2 expression in rat cardiomyocytes, although its effect on brain MEF2 function remains unclear (Chen et al. 2010). The effects of drug abuse on brain MEF2 function are unknown (Robison and Nestler 2011). MEF2 transcription factors play a crucial role in neuroplastic changes induced by addictive drugs such as cocaine. However, their specific involvement in opioid dependence and withdrawal remains unclear (Dietrich et al., 2013). Further research could illuminate MEF2’s role in addiction-related plasticity and the molecular mechanisms underlying the brain response to various drugs.
Regulatory role of Methyl-CpG binding protein 2 (MeCP2)
Prolonged exposure to cocaine has been shown to significantly affects the expression of the transcription factor methyl-CpG-binding protein 2 (MeCP2) in the dorsal striatum of rats (Im et al. 2010). MeCP2 recruits histone deacetylases (HDACs) to act as transcriptional repressors and other repressor molecules, thereby inhibiting target gene expression. Intriguingly, MeCP2 has been associated with miR-212 regulation in addition to brain-derived neurotrophic factor (BDNF), which is closely associated with cocaine-related behaviors (Graham et al. 2007). This suggests that MeCP2 may serve as a crucial mediator of molecular pathways underlying cocaine addiction. Moreover, MeCP2 and miR-212 engage in a negative feedback loop that aids in maintaining homeostasis. The disruption of MeCP2 activity, specifically within the dorsal striatum, impedes the increase in drug intake in rats, ultimately resulting in a decline in their motivation to respond to cocaine (Im et al. 2010). This highlights the significance of MeCP2 in the regulation of drug-seeking behaviors, providing an alternative perspective to the well-studied roles of CREB and ΔFosB. In contrast to CREB and ΔFosB, which primarily function as transcriptional activators, MeCP2 acts as a transcriptional repressor in the dorsal striatum (Madsen et al. 2012). This variation in the transcriptional regulatory systems complicates our understanding of the molecular processes involved in addiction. More research on MeCP2 has illuminated its role in addiction-related plasticity and behavior. Chronic cocaine exposure causes long-term behavioral and synaptic changes mediated by MeCP2. MeCP2 deficiency inhibits cocaine behavioral sensitization, suggesting its importance in neuroplastic adaptation to repeated drug exposure (Im et al. 2010). MeCP2 plays a significant role in opioid dependence and withdrawal by regulating the expression of GluA1 in the central amygdala (CeA). Reduced repression during persistent pain contributes to neuroplastic changes that sustain morphine-seeking behavior, highlighting a potential target for therapeutic interventions in opioid addiction (Hou et al. 2015). MeCP2 regulates opioid dependence by repressing G9a, which leads to increased expression of BDNF in the central amygdala, influencing the pain and reward pathways. This mechanism enhances sensitivity to morphine-induced reward behaviors, particularly in conditions of persistent pain. Experimental manipulations have confirmed that MeCP2 activity is crucial for neuroplastic changes associated with opioid exposure (Zhang et al. 2014). MeCP2 has also been linked to the control of synaptic plasticity in the dorsal striatum, contributing to the rewiring of neural circuits underlying addictive behaviors (Madsen et al. 2012). Further investigation into the intricate interplay between MeCP2, miR-212, BDNF, and other molecular factors within the dorsal striatum could enhance our understanding of the mechanisms governing addiction-related plasticity. Additionally, studying MeCP2 and related pathways may help develop new substance use disorder treatments.
Decoding the role of E2F3a transcription factor
E2F transcription factors were predicted by examining the reward circuitry of the brain following cocaine self-administration. The NAc is related to addiction-like behaviors, and these variables are important upstream regulators of genes whose expression changes during chronic cocaine use or withdrawal (Walker et al. 2018). A study that used NAc Chromatin Immunoprecipitation followed by sequencing (ChIP-seq) data to examine histone modifications after prolonged cocaine use also found involvement of the E2F family. It has also been found that cocaine regulates transcription and alternative splicing via E2F proteins (Feng et al. 2014). Despite their high expression in neurons, E2F proteins have not been previously linked to addiction-related phenomena, and their study of neuronal function is limited. E2F3 is the most expressed NAc E2F family member. E2F3a and E2F3b are forms that can be produced by alternative splicing. These isoforms activate or repress transcription in other tissues (Julian et al. 2013). E2F3a was exclusively expressed in MSNs in chronic cocaine-treated animals, whereas E2F3b was unchanged (Cates et al. 2018). In NAc neurons, E2F3a overexpression reproduced many transcriptional alterations in alternative splicing and chronic cocaine-induced gene expression (Cates et al. 2018). Overexpression of cocaine and NAc E2F3a regulated Fgfr1, Tle2, and Ptbp1. These findings indicate that E2F3a is essential for chronic cocaine-induced behavioral and transcriptional plasticity. E2F3a may enhance cocaine-induced changes in the brain to stimulate ΔFOSB. The FosB gene contains an E2F binding site at 500 base pairs before the transcription start site (TSS). Prolonged cocaine use increases the binding of E2F3 to this site in the NAc. Enhanced E2F3a levels in the NAc lead to elevated FosB and ΔFosB mRNA expression, whereas reducing its levels partially hinders the induction of ΔFOSB caused by cocaine (Cates et al. 2019a). These findings indicate that E2F3a regulates ΔFOSB upstream of the NAc. Cocaine regulates E2F3b in the prefrontal cortex (PFC), which has transcriptional and behavioral effects different from E2F3a in the NAc (Cates et al., 2019b). Although the specific role of E2F3a in opioid addiction and withdrawal has not been directly addressed, the general mechanisms of neuroadaptation in opioid addiction, including synaptic plasticity and signaling pathways, are likely relevant. These findings emphasize the importance of studying drug addiction transcription in brain reward regions other than the NAc.
Transcriptional control mechanisms of FOXO3a
Laboratory experiments using ChIP-seq and de novo motif analysis have shown that chronic cocaine administration enriched the FOXO transcription factor family motif in SIRT1-bound genes in the NAc (Ferguson et al. 2015). This discovery revealed that cocaine increased the brain’s most abundant FOXO family member, FOXO3a, and its NAc target genes (Ferguson et al. 2015). The roles of other FOXO family members in cocaine-evoked neuroplasticity in the NAc remain unknown (Hoekman et al. 2006). Similar to SIRT1, virally induced FOXO3a overexpression in the NAc enhances cocaine reward (Ferguson et al. 2013, 2015). These findings, along with previous evidence linking ΔFOSB to cocaine-induced SIRT1 in the NAc, suggest that FOXO3a is a component of a larger transcriptional regulation network mediated by ΔFOSB (Ferguson et al. 2013; Renthal et al. 2009). SIRT1 overexpression in the NAc may directly deacetylate FOXO3a. This decrease in acetylation promotes FOXO3a transcription. Chronic cocaine use increased NAc H4K15ac acetylation and decreased SIRT1 genomic binding. Instead of binding to DNA directly, SIRT1 interacts with transcription factors and other DNA-binding proteins when recruited to particular genes (Teague and Nestler 2022). These findings suggest that SIRT1 regulates H4K15ac levels and activates FOXO3a-dependent transcriptional programs to influence cocaine-induced plasticity in the NAc (Teague and Nestler 2022). FOXO-mediated transcription affects drug-induced neuroplasticity in opioid addiction and withdrawal; therefore, more research is needed to identify FOXO3a target genes during opioid exposure. This is an intriguing topic for future research.
Other transcription factors involved in opioid-induced neuroplasticity
Chronic cocaine self-administration increased NAC-1 mRNA expression in the NAc of rats (Korutla et al. 2005). This may contribute to cocaine sensitization (Li et al. 2022; Mackler et al. 2000). After repeated cocaine administration, virally overexpressing NAC-1 in the NAc of rats reduced locomotor sensitization but not expression, suggesting a homeostatic and compensatory role similar to CREB (Chandrasekar and Dreyer 2010). The accumbent elevated NAC-1 levels may regulate gene transcription to affect drug addiction behaviors, particularly psychomotor stimulant-related paranoia (Chandrasekar and Dreyer 2010). NURR1, which activates dopamine transporter transcription in vitro, is another important transcription factor. Midbrain dopamine neurons in human cocaine abusers had significantly lower NURR1 mRNA levels, suggesting that repeated drug exposure may decrease dopamine transporter gene transcription (Bannon et al. 2002). Other transcription factors involved in cocaine and other drug abuse include the glucocorticoid receptor, early growth response factors (EGRs), and signal transducers and activators of transcription (STATs) (Nestler 2001, Robison and Nestler 2011). Dopamine receptor-expressing neurons require the glucocorticoid receptor to seek cocaine, but not morphine molecular and behavioral responses (Ambroggi et al. 2009; Barik et al. 2010). This gene polymorphism may also cause teen alcohol abuse events (Desrivières et al. 2011). Research suggests that E2F3a regulates cocaine action in the NAc (Cates et al. 2018), along with transcription factors like Npas4, PGC1-α, SMAD3, Egr3, and BRG1 (Taniguchi et al. 2017; Chandra et al. 2015, 2017; Gancarz et al. 2015; Wang et al. 2016). Cocaine-evoked neuroplasticity in NAc MSNs and the desire for self-administration of cocaine are mediated by the TGF-B transcription factor SMAD3. Cocaine activates activin receptors that phosphorylate SMAD3, which associates with BRG1 to orchestrate transcriptional alterations in the NAc that drive cocaine-seeking during withdrawal (Gancarz et al. 2015; Wang et al. 2016). Nur77, Nurr1, and Nor-1 are transcription factors and early genes that transmit dopaminergic signals to the striatum. In addition, transcription factor dysregulation may lead to various addictive phenotypes (Bannon et al. 2002; Campos-Melo et al. 2013). Finally, the activity-dependent transcription factor NPAS4 enhances inhibitory signaling and may rewire neuronal connections to facilitate inhibitory responses. Cocaine and morphine induce NPAS4 expression in the NAc, making it a promising candidate for studying novel transcriptional programs in addiction (Guo et al. 2012; Martin et al. 2012; Piechota et al. 2010).
Post-transcriptional regulatory mechanisms
Post-transcriptional control refers to the regulatory processes that occur after transcription and continue until the mRNA is converted into a functional protein. This type of regulation encompasses various methods, including mRNA processing, splicing, editing, stability, and translation (Oliver and Perrone-Bizzozero 2017). Among these processes, mRNA stability and translation have been the most widely studied, particularly in the context of addiction. As nearly 20% of brain genes are regulated by mRNA stability, research indicates that it may be crucial for brain function (Bolognani et al. 2010). The nervous system contains several small non-coding RNAs that control neurogenesis, synapse development, and brain plasticity (Dreyer 2010).
Understanding the role of polyadenylation factors
Apart from the permanent modifications produced to DNA and transcription factors that control neural plasticity, evidence suggests the involvement of post-transcriptional mechanisms. Many mRNAs are delivered to synapses, where they replicate in a particular region of synapses by undergoing localized translation. Several studies have identified mRNA-binding proteins that control mRNA translation at the synapse, including cleavage and polyadenylation specificity factor and the cytoplasmic polyadenylation element-binding protein (CPEB) (McClung and Nestler 2008; Hansen et al. 2013). In Drosophila, mutations in orb, a homolog of CPEB, result in a deficiency in long-term memory (Dubnau et al. 2003). Moreover, the activation of NMDA receptors leads to the phosphorylation of CPEB by Aurora kinase, causing the dissociation of CPEB from a repressor protein known as Maskin. CPEB may bind to translation initiation factors, such as eIF4G and eIF4E, on specific mRNAs (Huang et al. 2002; Si et al. 2003). This action stabilizes the synaptic changes associated with plasticity at the respective sites. Aplysia CPEB shows prion-like traits, being modular and transportable, and able to alter the structure of other proteins. (Si et al. 2003). The active state of CPEB is its prion-like aggregated form, which can be induced by chronic stimulation, resulting in long-lasting and localized synaptic alterations. Few studies have focused on the effect of polyadenylation factors on opioid addiction and withdrawal. However, opioid receptor activation can also result in changes in gene expression. This change involves blocking adenylyl cyclase, resulting in a decrease in cAMP and adjustment of calcium and potassium ion channels (Tabanelli et al. 2023).
MicroRNA-Mediated pathways in neuroplasticity
Reduced expression of genes associated with addiction may arise due to the destabilization of various mRNAs (Oliver and Perrone-Bizzozero 2017). MicroRNAs (miRNAs) are small non-coding RNAs that control mRNA stability (Kim et al. 2009). The control of gene expression by drugs of abuse can be achieved through miRNAs. These miRNAs comprise 2–3% of the genes that are translated and encoded in the genome. They are approximately 20–22 nucleotide molecules long, but do not turn into proteins. miRNAs can target certain mRNAs by interacting with the 3’ untranslated region (UTR) of the mRNA via seed sections within the miRNA, including complementary sequences (Oliver and Perrone-Bizzozero 2017). Researchers have found that rats given prolonged access to self-administered cocaine showed signs of miRNAs being involved in the regulation of transcription (Hollander et al. 2010). While miRNAs can bind to mRNAs, the RNA-induced silencing complex (RISC) is a large protein complex responsible for the effects of this binding. By forming an association with RISC, miRNAs can degrade or suppress the translation of specific mRNAs (Bartel 2004). While an individual miRNA generally has a single seed region that binds to the mRNA, the mRNA may contain multiple sites complementary to multiple miRNAs. This indicates that a single miRNA can target multiple mRNAs, thereby influencing various cellular pathways and processes. This is similar to transcriptional regulation in which various gene types may have a specific upstream sequence regulated by a single transcription factor. It also indicates that miRNAs could regulate a network of genes associated with plasticity (Filipowicz et al. 2008). Many structural alterations linked to synaptic plasticity occur in the dendritic spines and branches (Schratt et al. 2006). Under normal conditions, miRNAs may inhibit protein translation at these synapses; however, stimulation relieves this. In the hippocampus, a brain-specific miRNA, miR-134, represses LIM-domain kinase 1 (Limk1) mRNA in the synapto-dendritic compartment of neurons to reduce dendritic spine growth (Schratt et al. 2006). Stimulation of neurons by brain-derived neurotrophic factor (BDNF) releases the repression of Limk1 by miR-134, resulting in spine growth (Schratt et al. 2006). Another Drosophila study found that synapses synthesize local proteins after an electric shock. At these synapses, RISC, which includes miRNA-associated protein Armitage, decreases mRNA activity (Ashraf and Kunes 2006; Ashraf et al. 2006; Chekanova and Belostotsky 2006). After stimulation, armitage degrades, allowing mRNA translation. This route targets plasticity proteins such as CaMKII and Staufen (Ashraf and Kunes 2006; Ashraf et al. 2006). Therefore, it is likely that the RISC complex plays a considerable role in neuroplasticity. Interestingly, extensive transcription factor-binding investigations have shown that CREB may regulate the production of 33 brain-specific miRNAs with CRE regulatory sequences. CREB targets the hippocampus-dominant miRNA MiR-132 (Vo et al. 2005; Wu and Xie 2006; Lukiw 2007). The transcriptional repressor RE1-silencing transcription factor, also known as neuron-restrictive silencer factor, recruits MeCP2 and HDACs to regulatory areas of certain miRNAs to suppress them in the long term (Wu and Xie 2006). Consequently, prolonged cocaine treatment can potentially modify the expression of these miRNAs by manipulating these transcription factors, resulting in either heightened or reduced translation of proteins at the local level that modifies synaptic connections. The fact that cocaine regulates the miR-8 family of genes suggests new ways in which drugs can modify the cytoskeletal and synaptic frameworks of neurons (Eipper-Mains et al. 2011). Future studies should focus on understanding this process in drug-induced control of dendritic morphology.
Role of RNA-binding proteins (RBPs)
RBPs are pivotal players in intricate gene regulation networks, exerting their influence by binding to specific sequences on mRNA molecules. Through their interactions with mRNA, RBPs control various aspects of mRNA metabolism, including stability, splicing, transport, and translation processes. In addiction research, RBPs have emerged as key regulators that modulate addiction-related mRNA translation in both directions, thereby shaping cellular responses to addictive substances (Oliver and Perrone-Bizzozero 2017). RBPs exhibit multifaceted functionality by interacting with miRNAs and various other regulatory factors, thereby orchestrating a complex interplay that influences gene expression and cellular processes. Such versatility has earned miRNAs and RBPs the moniker of “master switches” of gene regulation. Within the brain, this regulatory paradigm assumes particular significance, as a substantial proportion of approximately 15–20% of brain-specific transcripts contain AU-rich elements (AREs), implying their regulation via post-transcriptional mechanisms mediated by RBPs (Bolognani et al. 2010). Several RBPs regulate addiction-associated mRNAs and their subsequent translation into functional proteins. For instance, the RBP HuR (human antigen R) has been shown to govern the stability and translation of mRNAs encoding proteins crucial for drug addiction, including delta FosB and BDNF. Likewise, other RBPs, such as FMRP (fragile X mental retardation protein) and PTBP1 (polypyrimidine tract-binding protein 1), have also been implicated in the regulatory control of addiction-related mRNAs (Oliver and Perrone-Bizzozero 2017). Collectively, RBPs occupy a central position in the intricate web of addiction-related mRNA translation, suggesting a potential direction for therapeutic interventions in addiction studies. By targeting RBPs, it may be possible to modulate the translation of addiction-associated mRNAs, thereby offering novel strategies to combat addiction and related disorders. Further investigation into the precise mechanisms by which RBPs exert their regulatory effects will undoubtedly shed light on the intricacies of addiction biology, ultimately paving the way for innovative therapeutic approaches against addiction.
mRNA editing and neuroplasticity
mRNA editing represents a dynamic post-transcriptional mechanism essential for controlling gene expression (Glisovic et al. 2008). By modifying specific nucleotides, mRNA editing generates alternative protein forms without modifying the genetic code. One common form of mRNA editing is deamination, which involves the conversion of adenosine nucleotides into inosine. Adenosine Deaminase Acting on RNA (ADAR) enzymes catalyzes this modification process, which has been shown to affect protein function, including that of AMPA receptor (AMPAR) subunits crucial for synaptic signaling and neuroplasticity. Editing of the GluRA2 subunit of AMPAR in the NAc area of the brain by ADAR2 is of special interest in addiction research. Studies have demonstrated that this mRNA editing event can exert regulatory control over cocaine-seeking and relapse-like behaviors (Schmidt et al. 2015). This intriguing finding highlights the potential significance of mRNA editing as a target for modulating drug-induced plasticity and for the development of therapeutic interventions for addiction. The role of mRNA editing in neuroplasticity extends beyond addiction research. Numerous studies have explored the effects of mRNA editing on various aspects of neuronal function and synaptic plasticity. For instance, the editing of serotonin 2 C receptor (5-HT2CR) mRNA by ADAR enzymes has been shown to modulate serotonergic neurotransmission, influencing behaviors such as aggression, anxiety, and cognition (Werry et al. 2008; Weissmann et al. 2016). Additionally, mRNA editing of the GABA receptor subunit GABRA3 has been implicated in the regulation of inhibitory neurotransmission, with alterations in editing patterns potentially contributing to neuropsychiatric disorders (Tassinari et al., 2023). Understanding the mechanisms and functional effects of mRNA editing on neuroplasticity can improve brain function and targeted therapy. By elucidating the roles of specific ADAR enzymes and their editing targets, researchers may uncover novel avenues for interventions in neurological and neuropsychiatric disorders. Further investigations are warranted to unravel the intricate interplay among mRNA editing, synaptic plasticity, and disease pathogenesis, paving the way for innovative approaches to effectively treat and manage these conditions.
Epigenetic modifications in opioid addiction
Epigenetics: an overview
Epigenetics is a broad term encompassing the intricate interactions between genetic factors and the environment that contribute to developing specific biological traits and phenotypes (Bird 2007). The historical definition of “epigenetic” refers to heritable characteristics that are not directly determined by the DNA sequence but are rather influenced by cellular processes beyond the genomic code. A prime example illustrating the key role of epigenetic phenomena is in cellular differentiation, where specific cellular lineages are established and maintained (Feinberg 2007; Schuettengruber et al. 2007). Chromatin structure, which packages DNA and proteins, controls this epigenetic process. These heritable epigenetic processes may be mediated by chromatin structural changes that occur in adult post-mitotic neurons. This emphasizes the relevance of chromatin modification in various biological processes (Siegmund et al. 2007; Tsankova et al. 2007). Gene function is regulated by reversible but stable epigenetic processes that effectively turn genes on and off. This regulatory capacity extends to priming genes in response to triggers from the surroundings, such as drug exposure, where subsequent exposure can lead to enhanced expression of the gene’s mRNA or protein product (Damez-Werno et al. 2012). Consequently, epigenetic processes enable long-term control of gene expression without requiring mutagenic alterations within the DNA sequence itself. This extraordinary flexibility enables cellular responses and modifications by fine-tuning gene expression patterns using environmental signals. A common theory is that drug abuse causes long-term epigenetic changes that modify patterns of gene expression and augment neuroadaptive modifications during addiction. Additionally, these epigenetic changes may perpetuate relapse after abstinence, making treatment and recovery difficult (Pandey et al. 2008; Pascual et al. 2009; Schroeder et al. 2008). These epigenetic modifications are thought to act as molecular imprints, with a lasting impact on the neural circuitry and gene regulation, thereby shaping addictive behaviors and susceptibility to relapse. Among the diverse epigenetic processes known, the two most comprehensively understood mechanisms are DNA methylation and post-translational modification of histones, proteins that package and regulate DNA accessibility (Fig. 2). Both processes mediate the downstream neuroadaptive changes. Still, they can work together to shape gene expression patterns and cellular responses (Grewal and Moazed 2003; Sharma et al. 2020). In summary, epigenetics offers a profound perspective on the interplay between genes and the environment and illustrates how these interactions shape biological phenotypes. The dynamic nature of epigenetic processes, particularly their impact on chromatin structure and gene expression, provides a mechanism for long-term gene function control without reliance on genetic mutations. To understand addiction and develop effective treatments, epigenetic modifications such as those caused by drug abuse must be understood. The intricate link between heredity, environment, and gene expression will become clearer through research on epigenetic processes, including DNA methylation and histone changes.
Histone modifications and chromatin remodelling
Over the last ten years, a notable increase in research has focused on understanding how modifications to DNA and chromatin structure influence the regulation of transcriptional potential. This highlights the vital role of epigenetic changes in driving adaptations within the adult organism, with implications extending to various fields, including the study of drug addiction (Renthal and Nestler 2008; McQuown and Wood 2010). Histones wrap DNA strands into compact genetic material (Fig. 3). The tails of these histone proteins undergo covalent modifications, creating a complex “code” that controls genome accessibility to transcriptional machinery (Jenuwein and Allis 2001; LaPlant and Nestler 2011). Neural plasticity may be caused by long-term changes in chromatin structure that alter gene expression. The histone proteins H2A, H2B, H3, and H4, as well as DNA, form a tightly packed structure in which DNA is condensed and organized. Histone H1 helps to link these histone-DNA complexes, resulting in a highly compact configuration. Given this dense arrangement, gene expression is partly controlled by the regulation of transcriptional activator access to DNA (Felsenfeld and Groudine 2003; Li et al. 2007). Modifications to histone proteins can promote DNA unwinding, allowing the attachment of various transcription factors and subsequent gene activation. Conversely, other histone modifications can also prevent transcription factor binding and gene expression. Transcriptional machinery must access the chromatin-bound DNA for gene expression. Drugs of abuse cause gene- and region-specific histone modifications. These modifications also depend on whether the drug is administered acutely or chronically. Furthermore, the duration of drug exposure plays a role, as distinct modifications can be observed during periods of withdrawal (Wong et al. 2011; Maze and Nestler 2011).
Epigenetic dynamics of histone acetylation in addiction
Most studies of drug-evoked epigenetic alterations have focused on histone tail lysine residue acetylation. Acetylation opens chromatin and facilitates gene transcription by reducing histone-coiled DNA electrostatic tension in histone-coiled DNA. Opioids have been mostly studied for histone H3 tail acetylation. Preclinical research has shown that experimenter- and self-administered opioids increase mesolimbic dopamine system H3 global acetylation (Sheng et al. 2011; Wang et al. 2014). Interestingly, post-mortem tissue samples of heroin users showed similar findings (Egervari et al. 2017). The extent of global H3 hyperacetylation in the striatum of heroin users appears to correlate with heroin use duration, suggesting a link between heroin exposure and chromatin modification stabilization (Egervari et al. 2017). Hyperacetylation at H3K9, H3K14, H3K18, and H3K27 has been observed after repeated exposure to morphine or heroin in experimental or self-administered models (Egervari et al. 2017; Jalali et al., 2012; Wei et al. 2016; Wang et al. 2015; Chen et al. 2016). The most comprehensive investigation to date, which integrated both clinical and preclinical methodologies, elucidated the precise involvement of H3K27ac in opioid addiction (Egervari et al. 2017). This study also found elevated levels of H3K27ac in the striatum of heroin consumers and heroin self-administering rats, with a positive correlation with heroin use duration. The researchers also used ATAC-seq to map chromatin accessibility and found that this mark opens the chromatin. These results imply that increased amounts of H3K27ac may aid in the persistence of opioid addiction gene expression systems because this marker is typically enriched in enhancer regions of DNA. In line with the H3 results, one study discovered hyperacetylation at H4K5 and H4K8 in the NAc of heroin-seeking rats (Chen et al. 2016). These studies agree that opioids open chromatin through histone acetylation. In opioid addiction, this increases transcriptional activity, which induces the expression of plasticity-related genes (Fig. 3).
Understanding the role of histone methylation
The impact of opioids on histone methylation is poorly understood compared to the vast body of knowledge on histone acetylation. The majority of the research that has been done so far on this epigenetic modification has concentrated on how opioid treatment affects the methylation of one particular histone tail residue, H3K9. Sun et al., showed that while monomethylation and trimethylation are unaffected, repeated morphine treatment lowers NAc H3K9 dimethylation (H3K9me2) (Sun et al. 2012). After repeated opioid treatment, a comparable decrease in H3K9me2 levels has been observed in the central nucleus of the amygdala (Zhang et al. 2014). The long-term effects of drug exposure determine this decrease in H3K9me2 and seem to increase its transcriptional activity (Sun et al. 2012). Examination of the genome-wide deposition of H3K9me2 in the NAc was performed using ChIP-seq, and they found multiple genetic loci that showed differential enrichment of H3K9me2 after exposure to opioids (Sun et al. 2012). Remarkably, there was a decrease in H3K9me2 enrichment throughout the FosB. FosB is an essential transcription factor that contributes to drug addiction (Robison and Nestler 2011; Nestler et al. 2001). This shows that by lowering H3K9me2 deposition at the gene level, long-term morphine exposure may lessen the repression of FosB. Moreover, Ingenuity Pathway Analysis demonstrated modifications in the methylation of glutamatergic signaling-related genes, suggesting a possible function of H3K9me2 in controlling transcriptional programs linked to neuroplasticity. It is important to note that H3K9 methylation-induced transcriptional regulation induced by opioids may not be limited to the NAc. A week after stopping an escalating dosage regimen, a different study found that the ventral tegmental region and locus coeruleus had decreased H3K9 trimethylation (Jalali et al., 2012).
DNA methylation in response to opioid exposure
DNA methylation, especially 5-methylation of cytosines at cytosine-guanine dinucleotides (5mC), which often serves as a quiet gene, physically blocks RNA polymerase II and gene transcription. In contrast, transcriptional activation is linked to alternative types of DNA methylation, such as 5-hydroxymethylation of cytosine (5hmC), which is more prevalent in the brain (Fig. 3). Most studies pertaining to DNA methylation and opioid addiction have concentrated on 5mC and have only been tested in a limited number of opioid-exposed animal models, postmortem human brain tissue, and blood samples from clinical populations. Leukocytes from heroin addicts have higher levels of methylation at LINE-1 retrotransposon sites than those from control participants, suggesting that long-term heroin use increases DNA methylation throughout the genome (Doehring et al. 2013; Kozlenkov et al. 2017). Examination of neurons in the frontal brains of heroin users revealed distinct intragenic region methylation patterns (Kozlenkov et al. 2017). Moreover, increased levels of methylation are observed in CpG-rich islands within the mu-opioid receptor gene OPRM1 in the blood and brain tissue of heroin addicts (Doehring et al. 2013; Nielsen et al. 2009; Chorbov et al. 2011). Prolonged opioid exposure through long-term opioid therapy (LTOT) can lead to pharmacological changes in the body that manifest in ways comparable to individuals who are not taking opioids, highlighting the complexity and effects of opioid therapy (Doehring et al. 2013; Wyse et al. 2024). Unfortunately, preclinical studies have difficulty replicating the impact of opioids on DNA methylation, especially in the reward system. Overall, DNA methylation in the brain remains unchanged after long-term, stable, or increasing doses of heroin or morphine are administered (Fragou et al. 2013; Chao et al. 2014). Furthermore, studies have demonstrated that cocaine can alter DNA methylation globally in the PFC and NAc, but morphine therapy or heroin self-administration did not affect DNA methylation in the rodent mesocorticolimbic dopamine system (Imperio et al. 2018; Tian et al. 2012; Wright et al. 2015; Massart et al. 2015). Nonetheless, a study that exposed rats to prolonged morphine administration discovered various alterations in global or promoter-specific levels of 5mC and 5hmC in several brain areas (Barrow et al. 2017). The functional effects of these modifications on behavior and gene expression remain unknown. More efficient and reliable preclinical research, along with studies that concentrate on particular alterations in DNA methylation at specific loci, is necessary to completely understand opioid addiction and the complexity of DNA methylation. Once these changes are discovered, genes affected by DNA methylation can be cross-referenced with locations influenced by histone modifications to gain a comprehensive understanding of how opioid-induced epigenetic modifications interact and regulate gene expression (Browne et al. 2020).
Interplay between transcriptional regulation and epigenetic mechanisms
A study conducted over 16 weeks on 145 treatment-seeking AUD patients found that methylation of genes linked to opioid signaling, such as the µ-opioid receptor (OPRM1), can affect the efficacy of opioid antagonists, such as naltrexone, in treating AUD (Schacht et al. 2022). This suggests that epigenetic modifications of opioid-related genes can predict the effectiveness of certain drugs in modifying heavy drinking behaviors. Another study found that abstinent, addicted, and methadone-maintained subjects showed significantly increased NR3B subunit mRNA expression (Sedaghati et al. 2010). This suggests that opioid abuse causes the long-term overexpression of this receptor subunit, implying epigenetic changes in opioid addiction. The presence of a functional genetic polymorphism of the µ-opioid receptor gene (OPRM1) has been found to significantly affects cerebral pain processing in fibromyalgia (Ellerbrock et al. 2021). G-allele carriers showed increased posterior cingulate cortex (PCC) activation, indicating that different OPRM1 genotypes have distinct pain-modulatory mechanisms and implicate epigenetic factors in pain modulation. Based on genetic-epigenetic interactions, the human µ-opioid receptor variation 118 A > G (rs1799971) decreases the effectiveness and expression of opioid receptor signaling (Walter et al. 2013). This genetic variant reduces exogenous opioid effects; however, its small effect size makes it insignificant in clinical practice. Opioids can modify both genetic and epigenetic processes, suggesting a complex interplay between these mechanisms in mediating neuroplasticity in opioid addiction and in pain modulation. However, the exact mechanisms by which these changes occur and interact remain unclear and warrant further investigation. Using specialized protein domains, certain chromatin markers can be directly bound by certain chromatin-remodeling enzymes (Walter et al. 2013; Kouzarides 2007), whereas others interact with specific transcription factors to target chromatin. CREB is a well-known transcription factor that modulates cocaine-induced behavior. CREB is crucial in directing chromatin-modifying enzymes to gene promoters (Ashok et al. 2020). When phosphorylated, CREB interacts with CBP, a histone acetyltransferase (HAT) that acetylates neighboring histones to activate target genes (Mayr and Montminy 2001). The cocaine behavior also involves ΔFosB, another transcription factor. The bound AP-1 domains in the promoter regions of genes responsive to cocaine are the stable shortened splice product of the fosB gene, ΔFosB (McClung et al. 2004; Savell et al., 2023). Under certain conditions, ΔFosB can activate cdk5 and repress c-Fos (McClung and Nestler 2003; Renthal 2008). Studies have shown how ΔFosB regulates gene expression. Transcriptional activators such as BRG1 are recruited by ΔFosB when they bind to the promoter region of cdk5, which is increased by chronic cocaine exposure (Kumar et al. 2005). Following repeated cocaine injections in the animal’s home cage, ΔFosB binds to the promoter of c-fos and enlists HDAC1 to deacetylate adjacent histones (Renthal et al. 2008). Importantly, overexpression of ΔFosB alone upregulates cdk5 and represses c-fos, demonstrating that chromatin-modifying enzymes can be accurately directed to specific genes by a single transcription factor. Despite progress in understanding the gene regulatory effects of ΔFosB, its gene-specific mechanisms remain unknown. Neighboring promoter regulatory regions that recruit distinct transcription factors or undergo distinct post-translational modifications may be the underlying cause of these effects. The activating or repressive effects of ΔFosB may also be affected by drug-related environmental cues such as glutamatergic cerebral cortex inputs. When rats were repeatedly administered cocaine injections in an unfamiliar setting, c-Fos induction was higher (Hope et al. 2006). Gene regulatory processes are elucidated by the intricate interplay between gene transcription factors and chromatin remodeling enzymes. Thus, the development of small molecules that selectively stabilize or disrupt transcription factor-chromatin remodeling enzyme interactions could be a promising approach to modulate the effects of ΔFosB and potentially treat diseases associated with its dysregulation.
Neuroplasticity in opioid withdrawal, reversibility and potential targets
When people stop taking opioids after prolonged use (withdrawal), their brains undergo changes that contribute to cravings and relapse (Lefevre et al. 2023). One key adaptation involves decreased activity in a specific brain cell type (D1-MSNs) linked to rewards (Lefevre et al. 2023). Additionally, chronic opioid use leads to long-term changes in the brain circuits involving the neurotransmitter GABA, resulting in reduced dopamine levels and negative emotional states during withdrawal (Wang et al. 2023). Negative emotional behaviors can result from the morphological, functional, and molecular remodeling of nucleus accumbens neuron subtypes caused by withdrawal from opioids such as fentanyl (Fox et al., 2022). Withdrawing opioids causes transcriptional alterations and decreases dopamine release in the NAc (Fox et al., 2022). Research indicates that long-term drug abuse first alters the brain areas associated with emotions and pain, and these changes become more pronounced (sensitized) with repeated withdrawal and drug seeking, ultimately contributing to persistent cravings and addictive behavior, particularly for opioids (Koob 2020). Drug withdrawal triggers negative affective states, including depressive symptoms, and the lateral habenula plays a crucial role in driving these states (Meye et al. 2017). Acute and extended opioid withdrawal can induce endogenous LTP and LTD in the subicular NAc pathway, thereby impeding the subsequent initiation of neuroplasticity. These findings highlight the adaptive modifications in NAc functionality that transpire during opioid withdrawal (Dong et al. 2007). Observations of neurogenesis and neurotrophin activity during opioid withdrawal imply that neuroplasticity may be involved in the process (Bhalla et al. 2016). Conditioned withdrawal in opioid addiction can facilitate memory consolidation through neuroplasticity in the extended amygdala (Baidoo and Leri 2022). Glucocorticoids (GCs) are involved in gene expression changes in stress-related areas following chronic morphine exposure. Morphine dependence and withdrawal alters dopaminergic and transcription factors (García, 2015). Neuroplastic changes in the brain following opioid withdrawal are reversible. Continuous administration of morphine results in adaptations that reduce the activity of D1-MSNs, which are believed to facilitate reward-associated behaviors. The interruption of constant exposure to morphine does not decrease the functional output of D1-MSNs to the same degree, potentially augmenting behavioral responses to subsequent exposure to opioids (Lefevre et al. 2023). When fentanyl is administered in vivo, nociceptors exhibit neuroplasticity that persists even in culture, indicating that peripheral protein translation and nociceptor µ-opioid receptor-mediated calcium signaling are involved in opioid-induced hyperalgesia (OIH) (Khomula et al. 2019). Hyperkatifeia results from the engagement of brain stress systems and disruption of important neurochemical circuits within the system. This is characterized by an upsurge in negative emotional or motivational signals and symptoms of withdrawal. Following opioid exposure, the spinal cord undergoes reversible neuroplastic changes. Additionally, interactions between NMDA and opioid receptors may result in degenerative neuronal changes linked to the development of opioid tolerance (Christie 2008). The possible therapeutic targets for symptoms of opioid withdrawal include receptors of calcitonin gene-related peptide (CGRP), receptors of N-methyl-D-aspartate (NMDA), receptors of gamma-aminobutyric acid (GABA), potassium channels that are inwardly rectifying and gated by G-proteins (GIRK), and calcium channels (Rehni et al., 2013; Rehni et al. 2008). In addition, the endocannabinoid system, specifically the activation of CB1 receptors through the increase of N-arachidonoylethanolamine (anandamide; AEA) and 2-arachidonylglycerol (2-AG), has shown promise in reducing naloxone-precipitated morphine withdrawal symptoms (Behl et al. 2020; Ramesh et al. 2011). Additional possible targets encompass the glutamatergic, endocannabinoid, and orexin signaling systems, which have been linked to the emergence, sustenance, and manifestation of behaviors resembling addiction in animal models of opioid addiction (Chalhoub and Kalivas 2020). Furthermore, the NK1 receptor system, which is involved in various behaviors including nociception and affective dysfunction, has been shown to interact with opioid-related responses, suggesting that NK1 antagonists could be used to help patients who take opioids on a long-term basis avoid developing tolerance and physical dependence (Walsh et al. 2013).
Clinical implications and therapeutic avenues
The utilization of neuroplasticity as a treatment strategy is a promising approach in the field of psychiatry. Numerous studies indicate that treating mental illnesses, such as depression, may result in fewer symptoms if neuroplasticity is improved or modulated (Katz and Dwyer 2021). These treatments target brain disturbances and aim to promote brain plasticity, which involves interactions between neuronal network systems in the brain (Kargbo 2023). Neurotrophic substances produced from stem cell treatments have shown potential in targeting impaired neural plasticity associated with addiction (Tsai et al. 2019). Psychedelics are promising candidates for psychiatric therapies because they have been shown to decrease inflammation, improve oxidative stress, and stimulate neurogenesis and gliogenesis (Wilkinson et al. 2019; Khan et al. 2021). Cognitive and behavioral interventions can be used to improve long-term clinical outcomes by using enhanced neuroplasticity. Overall, utilizing neuroplasticity in treatment strategies holds promise for improving the therapeutic efficacy in mental disorders. In the last two decades, extensive neuropsychological investigations have consistently revealed the presence of cognitive impairments in individuals with alcohol or drug dependency. Specifically, deficiencies in goal-directed behaviors, decision-making, and executive functioning have surfaced as primary manifestations of these impairments, with the potential to impede the process of recovery (Manning et al. 2017; Bates et al. 2013). The theoretical underpinnings of addiction, as elucidated by neuroscientific models (Deutsch and Strack 2006; Lubman et al. 2004), underscore the concept that addictive disorders stem from an intricate interplay between two distinct yet interconnected information processing systems. These systems underactivate regulated or reflective processes and overactivate instinctive, appetitive, or impulsive processes. Investigating therapies that specifically target aberrant neurocognitive processes may result in novel therapeutic modalities and improve clinical outcomes (Manning et al. 2017). Importantly, given the burgeoning body of evidence supporting the concept of neuroplasticity, research endeavors have unveiled the capacity of cognitive deficits to recuperate during periods of abstinence and potentially be mitigated through the application of extremely specific cognitive therapies. Review articles dedicated to neurocognitive interventions further underscore the malleability of prefrontal regions associated with cognitive control (Zilverstand et al. 2016), shedding light on the circumstances under which approach bias modification is most efficacious (Gladwin et al. 2016). Extensive preclinical research supports the therapeutic potential of enriched environments comprising cognitive, social, and physical stimuli in reducing drug cravings and intake. Emphasizing the ‘cognitive’ component within these enriching interventions is crucial, as physical activity alone can also produce significant effects (Sampedro-Piquero et al. 2019). Preclinical evidence suggests a synergistic relationship between cognitive stimulation and exercise, suggesting the potential for more robust outcomes when both are combined. This realization has spurred the creation of innovative clinical interventions that combine rigorous education and physical exercise to improve mental health. Such strategies may prove valuable in treating substance use disorders (SUD) (Shors et al. 2014). Clinical studies have focused on strengthening cognitive function in SUD patients through structured training, but this approach has not been mirrored in preclinical studies (Sampedro-Piquero et al. 2019). The two common addiction behavior assessment models are drug self-administration and conditioned place preference (CPP). Research findings suggest that training animals in complex cognitive tasks can reduce addiction-like behaviors. Cognitive training can also accelerate the extinction of self-administered drugs by activating the PFC (Baratta et al. 2015). However, not all cognitive training tasks yielded the same results, suggesting that the specific cognitive demands of a task are important. Cognitive training induces neuroplastic changes in brain regions, such as the PFC and hippocampus, which can potentially modulate addiction-related behaviors. Preliminary preclinical data suggest that cognitive training may shield against the neurobehavioral effects of medications, providing insights into its potential in the treatment of SUD, although further research is required (Sampedro-Piquero et al. 2019).
Clinical status
Numerous clinical trials have explored neuroplasticity in the context of addiction. To enhance therapeutic guidance, conclusive clinical evidence derived from randomized prospective and observational studies would prove beneficial. Several clinical trials focusing on the exploration of neuroplasticity in addiction are currently in various stages, and their selection is detailed in Table 1.
Challenges and future directions
Opioid-evoked neuroplasticity presents several challenges and directions for future studies. The effectiveness of opioid analgesics is greatly diminished by the emergence of opioid tolerance (a negative indicator of cellular adaptation) and opioid-induced pain sensitivity (a positive indicator of cellular adaptation). The mechanisms of pain-related neuroplasticity in the brain, particularly in the endogenous opioid system, remain poorly understood. The supraspinal actions of opioids can both increase and decrease activity in descending pathways, indicating the potential for neuroplasticity in these pathways. The fact that memories associated with both good and negative opioid experiences play a critical role in promoting compulsive opioid-seeking and opioid-taking behaviors as well as relapse highlights the significance of neuroplasticity in addiction. Future research should focus on understanding the optimal functioning of the endogenous opioid system and using computational models to provide a rational basis for the treatment and prevention of chronic pain. Precision medicine approaches have the potential to address opioid-evoked neuroplasticity by targeting prevention and treatment strategies based on individual variabilities in genotype, environment, and lifestyle. The emerging field of precision medicine aims to develop individualized treatment and prevention plans based on the specificity of personal genetics, the environment, and lifestyle. By utilizing informatics and algorithms, precision medicine can assist healthcare providers in managing information and in making evidence-based decisions in medical contexts.
Conclusion
In this study, we provide a thorough examination of the well-established and well-researched transcriptional and epigenetic pathways by which drugs of abuse affect the transcription of genes in the reward system. These mechanisms contribute to the modulation of cellular and behavioral responses, ultimately affecting neuroplasticity. By gaining a deeper understanding of these underlying mechanisms, it is possible to selectively enhance or inhibit specific forms of plasticity, depending on the desired outcome. For instance, the reversal of chromatin modifications induced by chronic drug exposure may be achieved by targeting the transcription factors or enzymes involved in this process. By understanding the roles of transcription factors in these phenomena, which subsequently lead to alterations in gene expression and neuronal function, opportunities may arise for pharmacological interventions aimed at preventing or reversing drug-induced neurochemical changes that contribute to frequent relapses in drug addiction. The insights gained from this analysis have significant implications for the development of future therapeutic strategies that focus on the molecular mechanisms of opioid addiction and withdrawal. Such interventions have the potential to enhance treatment outcomes and improve patients’ overall well-being. Further research in this field is crucial to unravel the intricate complexities of drug-induced neuroplasticity and pave the way for more effective interventions within the realm of addiction neuroscience.
Data availability
Not applicable.
References
Albensi BC, Mattson MP (2000) Evidence for the involvement of TNF and NF-κB in hippocampal synaptic plasticity. Synapse 35(2):151–159
Alvarez VA, Sabatini BL (2007) Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci 30:79–97
Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, Van Der Veen R, Maroteaux G, Lemberger T, Schütz G (2009) Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci 12(3):247–249
Ang E, Chen J, Zagouras P, Magna H, Holland J, Schaeffer E, Nestler EJ (2001) Induction of nuclear factor-κB in nucleus accumbens by chronic cocaine administration. J Neurochem 79(1):221–224
Ashok C, Selvam M, Ponne S, Parcha PK, Raja KM, Baluchamy S (2020) CREB acts as a common transcription factor for major epigenetic repressors; DNMT3B, EZH2, CUL4B and E2F6. Med Oncol 37(8):68
Ashraf SI, Kunes S (2006) A trace of silence: memory and microRNA at the synapse. Curr Opin Neurobiol 16(5):535–539
Ashraf SI, McLoon AL, Sclarsic SM, Kunes S (2006) Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124(1):191–205
Bai L, Zhai C, Han K, Li Z, Qian J, Jing Y, Zhang W, Xu JT (2014) Toll-like receptor 4-mediated nuclear factor-κB activation in spinal cord contributes to chronic morphine-induced analgesic tolerance and hyperalgesia in rats. Neurosci Bull 30:936–948
Baidoo N, Leri F (2022) Extended amygdala, conditioned withdrawal and memory consolidation. Prog Neuropsychopharmacol Biol Psychiatry 113:110435
Bangar A, Khan H, Kaur A, Dua K, Singh TG Understanding mechanistic aspect of the therapeutic role of herbal agents on neuroplasticity in cerebral ischemic-reperfusion injury. J Ethnopharmacol 2023 Sep 17:117153
Bannon MJ, Pruetz B, Manning-Bog AB, Whitty CJ, Michelhaugh SK, Sacchetti P, Granneman JG, Mash DC, Schmidt CJ (2002) Decreased expression of the transcription factor NURR1 in dopamine neurons of cocaine abusers. Proceedings of the National Academy of Sciences. 99(9):6382–5
Baratta MV, Pomrenze MB, Nakamura S, Dolzani SD, Cooper DC (2015) Control over stress accelerates extinction of drug seeking via prefrontal cortical activation. Neurobiol Stress 2:20–27
Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Dzib JF, Bocquet O, Bailly A, Benecke A, Tronche F (2010) Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biol Psychiatry 68(3):231–239
Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V (2002) CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proceedings of the National Academy of Sciences. 99(17):11435–40
Barrow TM, Byun HM, Li X, Smart C, Wang YX, Zhang Y, Baccarelli AA, Guo L (2017) The effect of morphine upon DNA methylation in ten regions of the rat brain. Epigenetics 12(12):1038–1047
Bartas K, Hui M, Derdeyn P, Beier KT (2022) Mapping long-term changes in inputs to the ventral tegmental area after a single drug exposure. bioRxiv. 17:2022–2012
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Bates ME, Buckman JF, Nguyen TT (2013) A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev 23:27–47
Baydyuk M, Xu B (2014) BDNF signaling and survival of striatal neurons. Front Cell Neurosci 8:254
Behl T, Bungau S, Kumar K, Zengin G, Khan F, Kumar A, Kaur R, Venkatachalam T, Tit DM, Vesa CM, Barsan G (2020) Pleotropic effects of polyphenols in cardiovascular system. Biomed Pharmacother 130:110714
Behl T, Kaur D, Sehgal A, Singh S, Sharma N, Zengin G, Andronie-Cioara FL, Toma MM, Bungau S, Bumbu AG (2021) Role of monoamine oxidase activity in Alzheimer’s disease: an insight into the therapeutic potential of inhibitors. Molecules 26(12):3724
Benarroch EE (2015) Brain-derived neurotrophic factor: regulation, effects, and potential clinical relevance. Neurology 84(16):1693–1704
Bhalla S, Andurkar SV, Gulati A (2016) Neurobiology of opioid withdrawal: role of the endothelin system. Life Sci 159:34–42
Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ (2001) Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410(6826):376–380
Bird A (2007) Perceptions of epigenetics. Nature 447(7143):396
Blaze J, Browne CJ, Futamura R, Javidfar B, Zachariou V, Nestler EJ, Akbarian S tRNA epitranscriptomic alterations associated with opioid-induced reward-seeking and long-term opioid withdrawal in male mice. Neuropsychopharmacol 2024 Feb 8:1–9
Bolognani F, Contente-Cuomo T, Perrone-Bizzozero NI (2010) Novel recognition motifs and biological functions of the RNA-binding protein HuD revealed by genome-wide identification of its targets. Nucleic Acids Res 38(1):117–130
Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME (2009) Signaling pathway adaptations and novel protein kinase a substrates related to behavioral sensitization to cocaine. J Neurochem 110(1):363–377
Briand LA, Lee BG, Lelay J, Kaestner KH, Blendy JA (2015) Serine 133 phosphorylation is not required for hippocampal CREB-mediated transcription and behavior. Learn Mem 22(2):109–115
Browne CJ, Godino A, Salery M, Nestler EJ (2020) Epigenetic mechanisms of opioid addiction. Biol Psychiatry 87(1):22–33
Campos-Melo D, Galleguillos D, Sánchez N, Gysling K, Andrés ME (2013) Nur transcription factors in stress and addiction. Front Mol Neurosci 6:44
Carle TL (2007) Absence of conserved C-terminal degron domain contributes to ∆FosB’s unique stability. Eur J Neurosci 25:3009
Carlezon WA, Duman RS, Nestler EJ (2005) The many faces of CREB. Trends Neurosci 28(8):436–445
Carter JK, Quach BC, Willis C, Minto MS, Hancock DB, Montalvo-Ortiz J, Corradin O, Logan RW, Walss-Bass C, Maher BS, Johnson EO (2024) Identifying novel gene dysregulation associated with opioid overdose death: a meta-analysis of differential gene expression in human prefrontal cortex. medRxiv. Jan 13
Cates HM, Heller EA, Lardner CK, Purushothaman I, Peña CJ, Walker DM, Cahill ME, Neve RL, Shen L, Bagot RC, Nestler EJ (2018) Transcription factor E2F3a in nucleus accumbens affects cocaine action via transcription and alternative splicing. Biol Psychiatry 84(3):167–179
Cates HM, Lardner CK, Bagot RC, Neve RL, Nestler EJ (2019a) Mar Fosb induction in nucleus accumbens by cocaine is regulated by E2F3a. Eneuro. 6(2)
Cates HM, Bagot RC, Heller EA, Purushothaman I, Lardner CK, Walker DM, Peña CJ, Neve RL, Shen L, Nestler EJ (2019b Mar) A novel role for E2F3b in regulating cocaine action in the prefrontal cortex. Neuropsychopharmacology 44(4):776–784
Chalhoub RM, Kalivas PW (2020) Non-opioid treatments for opioid use disorder: rationales and data to date. Drugs 80(15):1509–1524
Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, Lobo MK (2015) Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci 35(20):7927–7937
Chandra R, Engeln M, Francis TC, Konkalmatt P, Patel D, Lobo MK (2017) A role for peroxisome proliferator-activated receptor gamma coactivator-1α in nucleus accumbens neuron subtypes in cocaine action. Biol Psychiatry 81(7):564–572
Chandrasekar V, Dreyer JL (2010) The brain-specific neural zinc finger transcription factor 2b (NZF-2b/7ZFMyt1) suppresses cocaine self-administration in rats. Front Behav Neurosci. :14
Chao MR, Fragou D, Zanos P, Hu CW, Bailey A, Kouidou S, Kovatsi L (2014) Epigenetically modified nucleotides in chronic heroin and cocaine treated mice. Toxicol Lett 229(3):451–457
Chekanova JA, Belostotsky DA (2006) MicroRNAs and messenger RNA turnover. MicroRNA Protocols. :73–85
Chen LF, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293(5535):1653–1657
Chen YL, Law PY, Loh HH (2006) Nuclear factor κB signaling in opioid functions and receptor gene expression. J Neuroimmune Pharmacol 1:270–279
Chen L, Wang F, Sun X, Zhou J, Gao L, Jiao Y, Hou X, Qin C, Zhao J (2010) Chronic ethanol feeding impairs AMPK and MEF2 expression and is associated with GLUT4 decrease in rat myocardium. Exp Mol Med 42(3):205–215
Chen WS, Xu WJ, Zhu HQ, Gao L, Lai MJ, Zhang FQ, Zhou WH, Liu HF (2016) Effects of histone deacetylase inhibitor sodium butyrate on heroin seeking behavior in the nucleus accumbens in rats. Brain Res 1652:151–157
Cheng MC, Hsu SH, Chen CH (2015) Chronic methamphetamine treatment reduces the expression of synaptic plasticity genes and changes their DNA methylation status in the mouse brain. Brain Res 1629:126–134
Cheng J, He Z, Chen Q, Lin J, Peng Y, Zhang J, Yan X, Jie Y, Niu S (2023) Histone modifications in cocaine, methamphetamine and opioids. Heliyon. May 19
Chorbov VM, Todorov AA, Lynskey MT, Cicero TJ (2011) Elevated levels of DNA methylation at the OPRM1 promoter in blood and sperm from male opioid addicts. J Opioid Manag 7(4):258
Chowdhury MA, An J, Jeong S (2023) The pleiotropic face of CREB family transcription factors. Mol Cells 46(7):399–413
Christie MJ (2008) Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol 154(2):384–396
Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ (2011) IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31(1):314–321
Covey DP, Roitman MF, Garris PA (2014) Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci 37(4):200–210
Damez-Werno D, LaPlant Q, Sun H, Scobie KN, Dietz DM, Walker IM, Koo JW, Vialou VF, Mouzon E, Russo SJ, Nestler EJ (2012) Drug experience epigenetically primes Fosb gene inducibility in rat nucleus accumbens. J Neurosci 32(30):10267–10272
Desrivières S, Lourdusamy A, Müller C, Ducci F, Wong CP, Kaakinen M, Pouta A, Hartikainen AL, Isohanni M, Charoen P, Peltonen L (2011) Glucocorticoid receptor (NR3C1) gene polymorphisms and onset of alcohol abuse in adolescents. Addict Biol 16(3):510–513
Deutsch R, Strack F (2006) Reflective and impulsive determinants of addictive behavior. Handb Implicit Cognition Addict 16:45–57
Dietrich JB (2013) The MEF2 family and the brain: from molecules to memory. Cell Tissue Res 352(2):179–190
Do J (2024) The role of epigenetics and contributing impact of stress, multigenerational, and developmental factors in Opiate Addiction. Cureus. 16(2)
Doehring A, Oertel BG, Sittl R, Lötsch J (2013) Chronic opioid use is associated with increased DNA methylation correlating with increased clinical pain. PAIN® 154(1):15–23
Dong Y, Nestler EJ (2014) The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 35(8):374–383
Dong Z, Cao J, Xu L (2007) Opiate withdrawal modifies synaptic plasticity in subicular–nucleus accumbens pathway in vivo. Neuroscience 144(3):845–854
Dreyer JL (2010) New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med 2(12):1–7
Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, Broger C (2003) The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol 13(4):286–296
Egervari G, Landry J, Callens J, Fullard JF, Roussos P, Keller E, Hurd YL (2017) Striatal H3K27 acetylation linked to glutamatergic gene dysregulation in human heroin abusers holds promise as therapeutic target. Biol Psychiatry 81(7):585–594
Eipper-Mains JE, Kiraly DD, Palakodeti D, Mains RE, Eipper BA, Graveley BR (2011) microRNA-Seq reveals cocaine-regulated expression of striatal microRNAs. RNA 17(8):1529–1543
Ellerbrock I, Sandström A, Tour J, Kadetoff D, Schalling M, Jensen KB, Kosek E (2021) Polymorphisms of the µ-opioid receptor gene influence cerebral pain processing in fibromyalgia. Eur J Pain 25(2):398–414
Ethell IM, Pasquale EB (2005) Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol 75(3):161–205
Fanfarillo F, Ferraguti G, Lucarelli M, Fuso A, Ceccanti M, Terracina S, Micangeli G, Tarani L, Fiore M (2024) The impact of Alcohol-Induced epigenetic modifications in the treatment of Alcohol Use disorders. Curr Med Chem
Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447(7143):433–440
Felsenfeld G, Groudine M (2003) Controlling the double helix. Nature 421(6921):448–453
Feng J, Wilkinson M, Liu X, Purushothaman I, Ferguson D, Vialou V, Maze I, Shao N, Kennedy P, Koo J, Dias C (2014) Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol 15(4):1–8
Ferguson D, Koo JW, Feng J, Heller E, Rabkin J, Heshmati M, Renthal W, Neve R, Liu X, Shao N, Sartorelli V (2013) Essential role of SIRT1 signaling in the nucleus accumbens in cocaine and morphine action. J Neurosci 33(41):16088–16098
Ferguson D, Shao N, Heller E, Feng J, Neve R, Kim HD, Call T, Magazu S, Shen L, Nestler EJ (2015) SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J Neurosci 35(7):3100–3111
Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9(2):102–114
Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME (2008) Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron 60(6):1022–1038
Fox ME, Wulff AB, Franco D, Choi E, Calarco CA, Engeln M, Turner MD, Chandra R, Rhodes VM, Thompson SM, Ament SA (2022 May) Negative emotional behavior during fentanyl abstinence is mediated by adaptations in nucleus accumbens neuron subtypes. bioRxiv 15:2022–2005
Fragou D, Zanos P, Kouidou S, Njau S, Kitchen I, Bailey A, Kovatsi L (2013) Effect of chronic heroin and cocaine administration on global DNA methylation in brain and liver. Toxicol Lett 218(3):260–265
Gaddis N, Mathur R, Marks J, Zhou L, Quach B, Waldrop A, Levran O, Agrawal A, Randesi M, Adelson M, Jeffries PW (2022) Multi-trait genome-wide association study of opioid addiction: OPRM1 and beyond. Sci Rep 12(1):16873
Gancarz AM, Wang ZJ, Schroeder GL, Damez-Werno D, Braunscheidel KM, Mueller LE, Humby MS, Caccamise A, Martin JA, Dietz KC, Neve RL (2015) Activin receptor signaling regulates cocaine-primed behavioral and morphological plasticity. Nat Neurosci 18(7):959–961
García Pérez D (2015) Opiate addiction: neuronal plasticity on brain reward system and emotional memory-related areas = Adicción a opiáceos: plasticidad neuronal en los circuitos neuronales de recompensa y en áreas de memoria emocional. Proyecto de investigación:. Dec 1
Gladwin TE, Wiers CE, Wiers RW (2016) Cognitive neuroscience of cognitive retraining for addiction medicine: from mediating mechanisms to questions of efficacy. Prog Brain Res 224:323–344
Glisovic T, Bachorik JL, Yong J, Dreyfuss G (2008) RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett 582(14):1977–1986
Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW (2007) Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci 10(8):1029–1037
Grewal SI, Moazed D (2003) Heterochromatin and epigenetic control of gene expression. Science 301(5634):798–802
Guo ML, Xue B, Jin DZ, Liu ZG, Fibuch EE, Mao LM, Wang JQ (2012) Upregulation of Npas4 protein expression by chronic administration of amphetamine in rat nucleus accumbens in vivo. Neurosci Lett 528(2):210–214
Guo S, Yang J, Zhang Y An investigation into whether single nucleotide polymorphisms (Snps) of OPRM1 influence on opioid addiction. InInternational Conference on Biomedical and Intelligent Systems (IC-BIS 2022) 2022 Dec 6 (Vol. 12458, pp. 359–365). SPIE
Hansen KF, Karelina K, Sakamoto K, Wayman GA, Impey S, Obrietan K (2013) miRNA-132: a dynamic regulator of cognitive capacity. Brain Struct Function 218:817–831
Hoekman MF, Jacobs FM, Smidt MP, Burbach JP (2006) Spatial and temporal expression of FoxO transcription factors in the developing and adult murine brain. Gene Expr Patterns 6(2):134–140
Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ (2010) Striatal microRNA controls cocaine intake through CREB signalling. Nature 466(7303):197–202
Hope BT, Simmons DE, Mitchell TB, Kreuter JD, Mattson BJ (2006) Cocaine-induced locomotor activity and Fos expression in nucleus accumbens are sensitized for 6 months after repeated cocaine administration outside the home cage. Eur J Neurosci 24(3):867–875
Hou YY, Cai YQ, Pan ZZ (2015) Persistent pain maintains morphine-seeking behavior after morphine withdrawal through reduced MeCP2 repression of GluA1 in rat central amygdala. J Neurosci 35(8):3689–3700
Huang YS, Jung MY, Sarkissian M, Richter JD (2002) N-methyl-D-aspartate receptor signaling results in Aurora kinase-catalyzed CPEB phosphorylation and αCaMKII mRNA polyadenylation at synapses. EMBO J 21(9):2139–2148
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598
Im HI, Hollander JA, Bali P, Kenny PJ (2010) MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci 13(9):1120–1127
Imperio CG, McFalls AJ, Hadad N, Blanco-Berdugo L, Masser DR, Colechio EM, Coffey AA, Bixler GV, Stanford DR, Vrana KE, Grigson PS (2018) Exposure to environmental enrichment attenuates addiction-like behavior and alters molecular effects of heroin self-administration in rats. Neuropharmacology 139:26–40
Jalali Mashayekhi F, Rasti M, Rahvar M, Mokarram P, Namavar MR, Owji AA (2012) Expression levels of the BDNF gene and histone modifications around its promoters in the ventral tegmental area and locus ceruleus of rats during forced abstinence from morphine. Neurochem Res 37:1517–1523
Jenuwein T, Allis CD (2001) Translating the histone code. Science 293(5532):1074–1080
Julian LM, Vandenbosch R, Pakenham CA, Andrusiak MG, Nguyen AP, McClellan KA, Svoboda DS, Lagace DC, Park DS, Leone G, Blais A (2013) Opposing regulation of Sox2 by cell-cycle effectors E2f3a and E2f3b in neural stem cells. Cell Stem Cell 12(4):440–452
K Rehni A, Jaggi S, Singh A (2013) Opioid withdrawal syndrome: emerging concepts and novel therapeutic targets. CNS & neurological disorders-drug targets (formerly current drug Targets-CNS & neurological disorders). 12(1):112–125
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10(8):561–572
Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162(8):1403–1413
Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prüllage M, Pfeiffer J, Lindecke A, Staiger V, Israël A, Kaltschmidt C (2006) NF-κB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol 26(8):2936–2946
Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294(5544):1030–1038
Kargbo RB (2023) Psychedelic-assisted neuroplasticity for the Treatment of Mental Health Disorders. ACS Med Chem Lett 14(2):133–135
Katz DI, Dwyer B Clinical neurorehabilitation: using principles of neurological diagnosis, prognosis, and neuroplasticity in assessment and treatment planning. In Seminars in Neurology 2021 Mar 4 (Vol. 41, No. 02, pp. 111–123). 333 Seventh Avenue, 18th Floor, New York, NY 10001, USA: Thieme Medical Publishers, Inc
Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8(11):844–858
Kaur A, Singh TG, Khan H, Kumar M, Singh N, Abdel-Daim MM (2022) Neuroprotective effect of piclamilast-induced post-ischemia pharmacological treatment in mice. Neurochem Res 47(8):2230–2243
Khan H, Gupta A, Singh TG, Kaur A (2021) Mechanistic insight on the role of leukotriene receptors in ischemic–reperfusion injury. Pharmacol Rep 73:1240–1254
Khomula EV, Araldi D, Levine JD (2019) In vitro nociceptor neuroplasticity associated with in vivo opioid-induced hyperalgesia. J Neurosci 39(36):7061–7073
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10(2):126–139
Koijam AS, Singh KD, Nameirakpam BS, Haobam R, Rajashekar Y (2024) Drug addiction and treatment: an epigenetic perspective. Biomed Pharmacother 170:115951
Koo JW, Mazei-Robison MS, LaPlant Q, Egervari G, Braunscheidel KM, Adank DN, Ferguson D, Feng J, Sun H, Scobie KN, Damez-Werno DM (2015) Epigenetic basis of opiate suppression of Bdnf gene expression in the ventral tegmental area. Nat Neurosci 18(3):415–422
Koob GF (2013) Addiction is a reward deficit and stress surfeit disorder. Front Psychiatry 4:72
Koob GF (2020) Neurobiology of opioid addiction: opponent process, hyperkatifeia, and negative reinforcement. Biol Psychiatry 87(1):44–53
Korutla L, Champtiaux N, Shen HW, Klugman M, Kalivas PW, Mackler SA (2005) Activity-dependent subcellular localization of NAC1. Eur J Neurosci 22(2):397–403
Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A (2009) Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain 2(1):1–9
Kouzarides T (2007) Chromatin modifications and their function. Cell 128(4):693–705
Kozlenkov A, Jaffe AE, Timashpolsky A, Apontes P, Rudchenko S, Barbu M, Byne W, Hurd YL, Horvath S, Dracheva S (2017) DNA methylation profiling of human prefrontal cortex neurons in heroin users shows significant difference between genomic contexts of hyper-and hypomethylation and a younger epigenetic age. Genes 8(6):152
Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S, Quintero C, Brannock C, Barnes C, Adair JE, Lehrmann E (2013) CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis 58:132–143
Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, LaPlant Q, Sasaki TS, Whistler KN, Neve RL (2005) Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 48(2):303–314
LaPlant Q, Nestler EJ (2011) CRACKing the histone code: cocaine’s effects on chromatin structure and function. Horm Behav 59(3):321–330
Lefevre EM, Gauthier EA, Bystrom LL, Scheunemann J, Rothwell PE (2023) Differential patterns of synaptic plasticity in the Nucleus Accumbens caused by continuous and interrupted Morphine exposure. J Neurosci 43(2):308–318
Lemen P, Hatoum A, Dickson P, Mittleman G, Agrawal A, Reiner B et al (2022) Opiate responses are controlled by interactions of Oprm1 and Fgf12 loci in rodents: correspondence to human GWAS findings. bioRxiv
Levy AD, Omar MH, Koleske AJ (2014) Extracellular matrix control of dendritic spine and synapse structure and plasticity in adulthood. Front Neuroanat 8:116
Li X, Wolf ME (2015) Multiple faces of BDNF in cocaine addiction. Behav Brain Res 279:240–254
Li B, Carey M, Workman JL (2007) The role of chromatin during transcription. Cell 128(4):707–719
Li Y, Wang X, Ge SN, Wang XL (2022) Alterations in neurotransmitters targeted metabolomics from the key nuclei of brain reward circuits in cocaine-induced behavioral sensitization for self-administering rats. Available SSRN 4037936
Lovinger DM, Kash TL (2015) Mechanisms of neuroplasticity and ethanol’s effects on plasticity in the striatum and bed nucleus of the stria terminalis. Alcohol Res 37(1):109–124
Lubman DI, Yücel M, Pantelis C (2004) Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction 99(12):1491–1502
Lukiw WJ (2007) Micro-RNA speciation in fetal, adult and Alzheimer’s disease hippocampus. NeuroReport 18(3):297–300
Lüscher C, Malenka RC (2011) Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69(4):650–663
Mackler SA, Korutla L, Cha XY, Koebbe MJ, Fournier KM, Bowers MS, Kalivas PW (2000) NAC-1 is a brain POZ/BTB protein that can prevent cocaine-induced sensitization in the rat. J Neurosci 20(16):6210–6217
Madsen HB, Brown RM, Lawrence AJ (2012) Neuroplasticity in addiction: cellular and transcriptional perspectives. Front Mol Neurosci 5:99
Manning V, Verdejo-Garcia A, Lubman DI (2017) Neurocognitive impairment in addiction and opportunities for intervention. Curr Opin Behav Sci 13:40–45
Martin TA, Jayanthi S, McCoy MT, Brannock C, Ladenheim B, Garrett T, Lehrmann E, Becker KG, Cadet JL (2012) Methamphetamine causes differential alterations in gene expression and patterns of histone acetylation/hypoacetylation in the rat nucleus accumbens. PLoS ONE 7(3):e34236
Massart R, Barnea R, Dikshtein Y, Suderman M, Meir O, Hallett M, Kennedy P, Nestler EJ, Szyf M, Yadid G (2015) Role of DNA methylation in the nucleus accumbens in incubation of cocaine craving. J Neurosci 35(21):8042–8058
Mattson MP (2005) NF-κB in the survival and plasticity of neurons. Neurochem Res 30:883–893
Mayr B, Montminy M (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2(8):599–609
Maze I, Nestler EJ (2011) The epigenetic landscape of addiction. Ann N Y Acad Sci 1216(1):99–113
Maze I, Covington HE III, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y (2010) Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327(5962):213–216
McClung CA, Nestler EJ (2003) Regulation of gene expression and cocaine reward by CREB and ∆FosB. Nat Neurosci 6(11):1208–1215
McClung CA, Nestler EJ (2008) Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology 33(1):3–17
McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ (2004) ∆FosB: a molecular switch for long-term adaptation in the brain. Mol Brain Res 132(2):146–154
McDaid J, Graham MP, Napier TC (2006) Methamphetamine-induced sensitization differentially alters pCREB and ∆FosB throughout the limbic circuit of the mammalian brain. Mol Pharmacol 70(6):2064–2074
McQuown SC, Wood MA (2010) Epigenetic regulation in substance use disorders. Curr Psychiatry Rep 12:145–153
Meffert MK, Baltimore D (2005) Physiological functions for brain NF-κB. Trends Neurosci 28(1):37–43
Mews P, Calipari ES (2017) Cross-talk between the epigenome and neural circuits in drug addiction. Prog Brain Res 235:19–63
Meye FJ, Trusel M, Soiza-Reilly M, Mameli M (2017) Neural circuit adaptations during drug withdrawal—spotlight on the lateral habenula. Pharmacol Biochem Behav 162:87–93
Morón JA, Gullapalli S, Taylor C, Gupta A, Gomes I, Devi LA (2010) Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology 35(4):955–966
Muller DL, Unterwald EM (2005) D1 dopamine receptors modulate ∆FosB induction in rat striatum after intermittent morphine administration. J Pharmacol Exp Ther 314(1):148–154
Nestler EJ (2008) Transcriptional mechanisms of addiction: role of ∆FosB. Philosophical Trans Royal Soc B: Biol Sci 363(1507):3245–3255
Nestler EJ (2012) Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci 10(3):136
Nestler EJ (2013) Cellular basis of memory for addiction. Dialogues Clin Neurosci 15(4):431–443
Nestler EJ (2014) Epigenetic mechanisms of drug addiction. Neuropharmacology 76:259–268
Nestler EJ (2022) Cellular basis of memory for addiction. Dialogues in clinical neuroscience. Apr 1
Nestler EJ Molecular basis of long-term plasticity underlying addiction. Nature reviews neuroscience. 2001a Feb 1;2(2):119 – 28
Nestler EJ Molecular neurobiology of addiction. American Journal on Addictions. 2001b Jan 1;10(3):201 – 17
Nestler EJ, Barrot M, Self DW (2001) ∆FosB: a sustained molecular switch for addiction. Proc Natl Acad Sci 98(20):11042–11046
Nielsen DA, Yuferov V, Hamon S, Jackson C, Ho A, Ott J, Kreek MJ (2009) Increased OPRM1 DNA methylation in lymphocytes of methadone-maintained former heroin addicts. Neuropsychopharmacology 34(4):867–873
O’Riordan KJ, Huang IC, Pizzi M, Spano P, Boroni F, Egli R, Desai P, Fitch O, Malone L, Ahn HJ, Liou HC (2006) Regulation of nuclear factor κB in the hippocampus by group I metabotropic glutamate receptors. J Neurosci 26(18):4870–4879
Olausson P, Jentsch JD, Tronson N, Neve RL, Nestler EJ, Taylor JR (2006) ∆FosB in the nucleus accumbens regulates food-reinforced instrumental behavior and motivation. J Neurosci 26(36):9196–9204
Oliver RJ, Perrone-Bizzozero NI Molecular Mechanisms of Drug-Induced Plasticity. InSynaptic Plasticity 2017 Jun 21. IntechOpen
Pandey SC, Ugale R, Zhang H, Tang L, Prakash A (2008) Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci 28(14):3729–3737
Pascual M, Boix J, Felipo V, Guerri C (2009) Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108(4):920–931
Peakman MC, Colby C, Perrotti LI, Tekumalla P, Carle T, Ulery P, Chao J, Duman C, Steffen C, Monteggia L, Allen MR (2003) Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res 970(1–2):73–86
Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ (2004) Induction of ∆FosB in reward-related brain structures after chronic stress. J Neurosci 24(47):10594–10602
Piechota M, Korostynski M, Solecki W, Gieryk A, Slezak M, Bilecki W, Ziolkowska B, Kostrzewa E, Cymerman I, Swiech L, Jaworski J (2010) The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol 11:1–21
Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA (2001) Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci 21(18):7397–7403
Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P (2008) Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 59(4):621–633
Ramesh D, Ross GR, Schlosburg JE, Owens RA, Abdullah RA, Kinsey SG, Long JZ, Nomura DK, Sim-Selley LJ, Cravatt BF, Akbarali HI (2011) Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J Pharmacol Exp Ther 339(1):173–185
Rehni AK, Singh I, Kumar M (2008) Tramadol-induced seizurogenic effect: a possible role of opioid‐dependent γ‐aminobutyric acid inhibitory pathway. Basic Clin Pharmacol Toxicol 103(3):262–266
Renthal W, Nestler EJ (2008) Epigenetic mechanisms in drug addiction. Trends Mol Med 14(8):341–350
Renthal W, Carle TL, Maze I, Covington HE, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ (2008) ∆FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci 28(29):7344–7349
Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, Maze I, Sikder D, Robison AJ, LaPlant Q, Dietz DM, Russo SJ (2009) Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron 62(3):335–348
Rezayof A, Ghasemzadeh Z, Sahafi OH Addictive drugs modify neurogenesis, synaptogenesis and synaptic plasticity to impair memory formation through neurotransmitter imbalances and signaling dysfunction. Neurochemistry Int 2023 Jul 7:105572
Robinson TE, Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47:33–46
Robison AJ, Nestler EJ (2011) Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12(11):623–637
Romano A, Freudenthal R, Merlo E, Routtenberg A (2006) Evolutionarily-conserved role of the NF‐κB transcription factor in neural plasticity and memory. Eur J Neurosci 24(6):1507–1516
Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B (2009) Nuclear factor κB signaling regulates neuronal morphology and cocaine reward. J Neurosci 29(11):3529–3537
Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ (2010) The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci 33(6):267–276
Sadri-Vakili G (2015) Cocaine triggers epigenetic alterations in the corticostriatal circuit. Brain Res 1628:50–59
Sala C, Segal M (2014) Dendritic spines: the locus of structural and functional plasticity. Physiol Rev 94(1):141–188
Sampedro-Piquero P, de Guevara-Miranda DL, Pavón FJ, Serrano A, Suárez J, de Fonseca FR, Santín LJ, Castilla-Ortega E (2019) Neuroplastic and cognitive impairment in substance use disorders: a therapeutic potential of cognitive stimulation. Neurosci Biobehavioral Reviews 106:23–48
Savell KE, Hope BT (2023) Chronic Cocaine–Induced ∆FOSB: long-lasting and far-reaching. Biol Psychiatry 94(5):362–364
Schacht JP, Hoffman M, Chen BH, Anton RF (2022) Epigenetic moderators of naltrexone efficacy in reducing heavy drinking in Alcohol Use Disorder: a randomized trial. Pharmacogenomics J 22(1):1–8
Schmidt HD, McFarland KN, Darnell SB, Huizenga MN, Sangrey GR, Cha JH, Pierce RC, Sadri-Vakili G (2015) ADAR2-dependent GluA2 editing regulates cocaine seeking. Mol Psychiatry 20(11):1460–1466
Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439(7074):283–289
Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, Akbarian S (2008) Drug-induced activation of dopamine D1 receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology 33(12):2981–2992
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G (2007) Genome regulation by polycomb and trithorax proteins. Cell 128(4):735–745
Sedaghati M, Vousooghi N, Goodarzi A, Yaghmaei P, Mokri A, Zarrindast MR (2010) Expression of NR3B but not NR2D subunit of NMDA receptor in human blood lymphocytes can serve as a suitable peripheral marker for opioid addiction studies. Eur J Pharmacol 633(1–3):50–54
Shah A, Silverstein PS, Singh DP, Kumar A (2012) Involvement of metabotropic glutamate receptor 5, AKT/PI3K signaling and NF-κB pathway in methamphetamine-mediated increase in IL-6 and IL-8 expression in astrocytes. J Neuroinflamm 9:1–0
Shaik Z, Fatima SM, Suffah Naaz FM, Sreevani B (2023) Oct The epigenetics of Drug Addiction. Int J Pharm Sci Rev Res. 1–6
Sharma VK, Mehta V, Singh TG (2020) Alzheimer’s disorder: epigenetic connection and associated risk factors. Curr Neuropharmacol 18(8):740–753
Shaywitz AJ, Greenberg ME (1999) CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68(1):821–861
Sheng J, gang Lv Z, Wang L, Zhou Y, Hui B (2011) Histone H3 phosphoacetylation is critical for heroin-induced place preference. NeuroReport 22(12):575–580
Shors TJ, Olson RL, Bates ME, Selby EA, Alderman BL (2014) Mental and Physical (MAP) training: a neurogenesis-inspired intervention that enhances health in humans. Neurobiol Learn Mem 115:3–9
Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER (2003) A neuronal isoform of CPEB regulates local protein synthesis and stabilizes synapse-specific long-term facilitation in aplysia. Cell 115(7):893–904
Siegmund KD, Connor CM, Campan M, Long TI, Weisenberger DJ, Biniszkiewicz D, Jaenisch R, Laird PW, Akbarian S (2007) DNA methylation in the human cerebral cortex is dynamically regulated throughout the life span and involves differentiated neurons. PLoS ONE 2(9):e895
Smith ACW, Kenny PJ (2018) MicroRNAs regulate synaptic plasticity underlying drug addiction. Genes Brain Behav 17(3):e12424
Sun H, Maze I, Dietz DM, Scobie KN, Kennedy PJ, Damez-Werno D, Neve RL, Zachariou V, Shen L, Nestler EJ (2012) Morphine epigenomically regulates behavior through alterations in histone H3 lysine 9 dimethylation in the nucleus accumbens. J Neurosci 32(48):17454–17464
Sweatt JD (2016) Neural plasticity and behavior–sixty years of conceptual advances. J Neurochem 139:179–199
Tabanelli R, Brogi S, Calderone V (2023) Targeting opioid receptors in addiction and drug Withdrawal: where are we going? Int J Mol Sci 24(13):10888
Taniguchi M, Carreira MB, Cooper YA, Bobadilla AC, Heinsbroek JA, Koike N, Larson EB, Balmuth EA, Hughes BW, Penrod RD, Kumar J (2017) HDAC5 and its target gene, Npas4, function in the nucleus accumbens to regulate cocaine-conditioned behaviors. Neuron 96(1):130–144
Tassinari V, La Rosa P, Guida E, Colopi A, Caratelli S, De Paolis F, Gallo A, Cenciarelli C, Sconocchia G, Dolci S, Cesarini V (2023 Apr) Contribution of A-to-I RNA editing, M6A RNA methylation, and alternative splicing to physiological brain aging and neurodegenerative diseases. Mech Ageing Dev 5:111807
Teague CD, Nestler EJ (2022) Key transcription factors mediating cocaine-induced plasticity in the nucleus accumbens. Mol Psychiatry 27(1):687–709
Thomas MJ, Malenka RC (2003) Synaptic plasticity in the mesolimbic dopamine system. Philosophical Trans Royal Soc Lond Ser B: Biol Sci 358(1432):815–819
Tian W, Zhao M, Li M, Song T, Zhang M, Quan L, Li S, Sun ZS (2012) Reversal of cocaine-conditioned place preference through methyl supplementation in mice: altering global DNA methylation in the prefrontal cortex. PLoS ONE 7(3):e33435
Tsai ST, Liew HK, Li HM, Lin SZ, Chen SY (2019) Harnessing neurogenesis and neuroplasticity with stem cell treatment for addictive disorders. Cell Transplant 28(9–10):1127–1131
Tsankova NM, Kumar A, Nestler EJ (2004) Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci 24(24):5603–5610
Tsankova N, Renthal W, Kumar A, Nestler EJ (2007) Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 8(5):355–367
Ulery PG, Nestler EJ (2007) Regulation of ∆FosB transcriptional activity by Ser27 phosphorylation. Eur J Neurosci 25(1):224–230
Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S (2005) A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proceedings of the National Academy of Sciences. 102(45):16426–31
Volkow ND, Morales M (2015) The brain on drugs: from reward to addiction. Cell 162(4):712–725
Volkow ND, Fowler JS, Wang GJ, Goldstein RZ (2002) Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem 78(3):610–624
Volkow ND, Fowler JS, Wang GJ (2003) The addicted human brain: insights from imaging studies. J Clin Investig 111(10):1444–1451
Volkow ND, Michaelides M, Baler R (2019) The neuroscience of drug reward and addiction. Physiol Rev 99(4):2115–2140
Walker DM, Cates HM, Loh YH, Purushothaman I, Ramakrishnan A, Cahill KM, Lardner CK, Godino A, Kronman HG, Rabkin J, Lorsch ZS (2018) Cocaine self-administration alters transcriptome-wide responses in the brain’s reward circuitry. Biol Psychiatry 84(12):867–880
Walsh SL, Heilig M, Nuzzo PA, Henderson P, Lofwall MR (2013) Effects of the NK1 antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addict Biol 18(2):332–343
Walter C, Doehring A, Oertel BG, Lötsch J (2013) µ-opioid receptor gene variant OPRM1 118 A > G: a summary of its molecular and clinical consequences for pain. Pharmacogenomics 14(15):1915–1925
Wang JQ, Mao L, Parelkar NK, Tang Q, Liu Z, Sarwar S, Choe E (2003) Glutamate-regulated behavior, transmitter release, gene expression and addictive plasticity in the striatum: roles of metabotropic glutamate receptors. Curr Neuropharmacol 1(1):1–20
Wang Z, Yan P, Hui T, Zhang J (2014) Epigenetic upregulation of PSD-95 contributes to the rewarding behavior by morphine conditioning. Eur J Pharmacol 732:123–129
Wang Y, Lai J, Cui H, Zhu Y, Zhao B, Wang W, Wei S (2015) Inhibition of histone deacetylase in the basolateral amygdala facilitates morphine context-associated memory formation in rats. J Mol Neurosci 55:269–278
Wang ZJ, Martin JA, Mueller LE, Caccamise A, Werner CT, Neve RL, Gancarz AM, Li JX, Dietz DM (2016) BRG1 in the nucleus accumbens regulates cocaine-seeking behavior. Biol Psychiatry 80(9):652–660
Wang W, Xie X, Zhuang X, Huang Y, Tan T, Gangal H, Huang Z, Purvines W, Wang X, Stefanov A, Chen R (2023) Striatal µ-opioid receptor activation triggers direct-pathway GABAergic plasticity and induces negative affect. Cell Rep. 42(2)
Waterhouse EG, Xu B (2009) New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci 42(2):81–89
Wei L, Zhu YM, Zhang YX, Liang F, Barry DM, Gao HY, Li T, Huo FQ, Yan CX (2016) Microinjection of histone deacetylase inhibitor into the ventrolateral orbital cortex potentiates morphine induced behavioral sensitization. Brain Res 1646:418–425
Weissmann D, Van Der Laan S, Underwood MD, Salvetat N, Cavarec L, Vincent L, Molina F, Mann JJ, Arango V, Pujol JF (2016) Region-specific alterations of A-to-I RNA editing of serotonin 2c receptor in the cortex of suicides with major depression. Translational Psychiatry 6(8):e878
Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S (2002) ∆FosB regulates wheel running. J Neurosci 22(18):8133–8138
Werry TD, Loiacono R, Sexton PM, Christopoulos A (2008) RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharmacol Ther 119(1):7–23
Wilkinson ST, Holtzheimer PE, Gao S, Kirwin DS, Price RB (2019) Leveraging neuroplasticity to enhance adaptive learning: the potential for synergistic somatic-behavioral treatment combinations to improve clinical outcomes in depression. Biol Psychiatry 85(6):454–465
Williams JM, Beckmann AM, Mason-Parker SE, Abraham WC, Wilce PA, Tate WP (2000) Sequential increase in Egr-1 and AP-1 DNA binding activity in the dentate gyrus following the induction of long-term potentiation. Mol Brain Res 77(2):258–266
Wolf ME, Mangiavacchi S, Sun X (2003) Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann N Y Acad Sci 1003(1):241–249
Wong CC, Mill J, Fernandes C (2011) Drugs and addiction: an introduction to epigenetics. Addiction 106(3):480–489
Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, Mercer R, Feng J, Dietz DM, Lobo MK, Nestler EJ (2015) Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci 35(23):8948–8958
Wu J, Xie X (2006) Comparative sequence analysis reveals an intricate network among REST, CREB and miRNA in mediating neuronal gene expression. Genome Biol 7:1–4
Wyse JJ, Lindner S, Handley R, Morasco BJ, Carlson KF, Gordon AJ, Lovejoy TI (2024) Medications for opioid use disorder among patients with and without exposure to Long-Term Opioid Therapy for Chronic Pain. J Pain 25(4):71
Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H (2014) A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345(6204):1616–1620
Yan KU, Gao LN, Cui YL, Zhang YI, Zhou XI (2016) The cyclic AMP signaling pathway: exploring targets for successful drug discovery. Mol Med Rep 13(5):3715–3723
Yang CH, Liu XM, Si JJ, Shi HS, Xue YX, Liu JF, Luo YX, Chen C, Li P, Yang JL, Wu P (2012) Role of IKK/NF-κB signaling in extinction of conditioned place aversion memory in rats. PLoS ONE 7(6):e39696
Yeh SH, Lin CH, Lee CF, Gean PW (2002) A requirement of nuclear factor-κB activation in fear-potentiated startle. J Biol Chem 277(48):46720–46729
Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A (2006) An essential role for ∆FosB in the nucleus accumbens in morphine action. Nat Neurosci 9(2):205–211
Zhang Z, Tao W, Hou YY, Wang W, Kenny PJ, Pan ZZ (2014) MeCP2 repression of G9a in regulation of pain and morphine reward. J Neurosci 34(27):9076–9087
Zhu H, Lee M, Agatsuma S, Hiroi N (2007) Pleiotropic impact of constitutive fos B inactivation on nicotine-induced behavioral alterations and stress-related traits in mice. Hum Mol Genet 16(7):820–836
Zilverstand A, Parvaz MA, Moeller SJ, Goldstein RZ (2016) Cognitive interventions for addiction medicine: understanding the underlying neurobiological mechanisms. Prog Brain Res 224:285–304
Acknowledgements
The authors would like to thank Chitkara College of Pharmacy, Chitkara University Punjab India, and Aman Pharmacy College, Dholakhera Udaipurwati, Jhunjhunu, Rajasthan, India for providing facilities for the completion of this review.
Funding
Nil.
Author information
Authors and Affiliations
Contributions
Conceptualization: Heena Khan was involved in writing––original draft preparation and revising; Thakur Gurjeet Singh supervision; Shahid Nazir Wani was involved in writing––original draft preparation; Amarjot Kaur Grewal, Thakur Gurjeet Singh performed reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
All authors read and approved the final manuscript.
Consent to publication
Not applicable.
Conflict of interest
All the authors have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wani, S.N., Grewal, A.K., Khan, H. et al. Elucidating the molecular symphony: unweaving the transcriptional & epigenetic pathways underlying neuroplasticity in opioid dependence and withdrawal. Psychopharmacology (2024). https://doi.org/10.1007/s00213-024-06684-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00213-024-06684-9