Abstract
Rationale
Parenting experiences with caregivers play a key role in neurodevelopment. We recently reported that adolescents reared by a single-mother (SM) display an anxiety-prone phenotype and drink more alcohol, compared to peers derived from a biparental (BP) rearing condition.
Objectives
To investigate if SM and BP offspring infant mice exhibit differential sensitivity to ethanol-induced locomotor activity and differential activity patterns in brain areas related to anxiety response. We also analyzed anxiety response and ethanol-induced anxiolysis in SM and BP adolescents.
Methods
Mice reared in SM or BP conditions were assessed for (a) ethanol-induced locomotor activity at infancy, (b) central expression of Fos-like proteins (likely represented mostly by FosB, a transcription factor that accumulates after chronic stimuli exposure and serves as a molecular marker of neural plasticity) and cathecolaminergic activity, and (c) anxiety-like behavior and ethanol-induced anxiolysis in adolescence.
Results
Infant mice were sensitive to the stimulating effects of 2.0 g/kg alcohol, regardless parenting structure. SM mice exhibited, relative to BP mice, a significantly greater number of Fos-like positive cells in the central amygdala and basolateral amygdala nuclei. Ethanol treatment, but not parenting condition, induced greater activation of dopaminergic neurons in ventral tegmental area. SM, but not BP, adolescent mice were sensitive to ethanol-induced anxiolysis.
Conclusions
These results highlight the complex relationship between parenting experiences and neurodevelopment. The SM parenting may result in greater neural activation patterns in brain areas associated with anxiety response, potentially contributing to increased basal anxiety and alcohol sensitivity.
Highlights
-
Infant mice exhibited ethanol-induced motor stimulation.
-
Parenting experience leads to a differential activation of amygdaloid nuclei.
-
Single-mother parenting induced a higher anxiety-like behavioral profile.
-
Single-mother parenting induced an increased response to ethanol-induced anxiolysis in adolescent mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early attachment to caregivers plays a key role in the neurodevelopment. Generally, females take most of the toll of the caregiving. However, male parental care is, depending on cultural and ecological factors (Geary 2000; Lamb and Lewis 2010), relatively common in certain species. Such involvement has been linked to long-term physiological effects on offspring (Lamb and Lewis 2010). In species with biparental care the father assumes a significant role in rearing the young, and in most of these cases the absence of the father cannot be compensated by the mother (Gubernick and Alberts 1987).
C57BL/6J mice do not form selective social bonds between mates, and the male does not spontaneously provide alloparental care, as observed in other monogamous rodent species. In these mice, however, paternal behavior becomes evident after cohabitation, following mating during pregnancy and lactation (Ferreyra et al. 2020). In a recent study we assessed anxiety-like and risk assessment behaviors, and ethanol (EtOH) intake, in mice reared under single-mother (SM) or biparental (BP) conditions. Parental presence in the nest was significantly greater in the BP than in the SM condition, and was much less frequent to find a nest unattended in the BP than in the SM condition. Furthermore, BP parents displayed significantly more pup-directed behaviors (i.e., licking and grooming, nest building, nursing, huddling over the pups) than the mother alone in the SM condition. Moreover, SM-reared mice exhibited an anxiety-prone phenotype, and -compared to BP counterparts- fewer risk-associated behaviors and greater levels of ethanol consumption.
These results conflict with those of a pioneer study by Anacker et al. (2012), who reported a lack of effect of early life family structure on ethanol drinking, in a monogamous biparental rodent species (prairie voles). These authors, however, measured ethanol drinking at adulthood, whereas we focused on infancy and adolescence, developmental stages characterized by heightened sensitivity to the reinforcing effects of ethanol [see (Miranda-Morales et al. 2014; Pautassi et al. 2009) for infants; (Spear 2018) for adolescents)].
The present study scrutinized potential behavioral, pharmacological, and neurobiological differences emerging from SM and BP rearing conditions. More in detail, we assessed, in infant or adolescent C57BL/6J mice, ethanol-induced locomotor stimulation and anxiolysis, as well as baseline level of anxiety responses and neuronal activation in brain areas related to reward and processing of emotional stimuli (i.e., nucleus accumbens, striatum, amygdala, paraventricular hypothalamic nuclei, supraoptic nucleus, prelimbic cortex and ventral tegmental area). Ethanol-induced locomotor activation is a proxy of ethanol-induced positive reinforcement (Camarini and Pautassi 2016) and, in infant or adolescent rats, is sensitive to pharmacological antagonism that also disrupts ethanol-induced reinforcement and drinking (Pautassi et al. 2011). Neuronal activation was measured via Fos-like protein immunoreactivity. Some of these proteins, like FosB, accumulate in response to chronic exposure to natural and drug-related rewards (Wallace et al. 2008). Interestingly, the fosb gene has also been implicated in maternal behavior (Brown et al. 1996). We also measured baseline and ethanol induced cathecolaminergic activity (colocalization of Fos and tyrosine hydroxylase) in ventral tegmental area (VTA), a key nuclei of the mesorticolimbic pathway (Koob and Nestler 1997).
Materials and methods
Experimental animals and rearing conditions
In Experiment 1, 59 infant C57BL/6J mice (30 males, 29 females; derived from 8 SM and 8 BP litters) were assessed for ethanol-induced locomotor activity. Experiment 2 assessed blood ethanol levels (BELs) in 20 infant mice (males and females) derived from 10 litters (5 SM, 5 BP). Experiment 3 obtained immunohistochemistry measurements from 5 SM and 5 BP male mice from Experiment 1. Experiment 4 measured anxiety-like (and ethanol-induced anxiolysis) behavior of 107 adolescent mice, derived from 16 litters (8 SM and 8 BP).
The mice were born and reared in the vivarium of the Instituto M. M. Ferreyra (INIMEC-CONICET-Universidad Nacional de Córdoba, Córdoba, Argentina). The colony was maintained at 22 ± 1 °C with a 12 h/12 h light/dark cycle (lights on at 08:00 AM). Animals had ad libitum access to water and food (Cooperativa irradiated balanced food for rodents; Capital Federal, Argentina) and were housed in standard semi-transparent (caramel color) cages for mice (396 mm length × 215 mm wide × 172 mm height) with corncob used as bedding. The experiments began at 10:00 AM, and complied with the ARRIVE guidelines, the Guide for the Care and Use of Laboratory Animals as endorsed by the NIH/EU. They were also approved by the Institutional Animal Care and Use Committee at INIMEC-CONICET-UNC.
Mice of the BP condition were derived from couples that had been living together since mating (postnatal day 60, PD 60). Couples belonging to the SM condition were housed together at mating and separated at gestational day (GD 18). This was the first time these mice underwent mating. Day of birth was denoted as PD 0. Litter size for this strain typically ranges from 2 to 8 pups. Litters exceeding 8 pups were culled to 8. Pups were weaned at PD 21 and housed in same-sex cages. The animals were left undisturbed until testing at infancy or adolescence (PD 16 or 28, respectively). Figure 1 presents a schematic timeline detailing animal manipulation and experiments conducted in this study. No more than one male and one female from each litter was assigned to each cell of the design. Animals were habituated to handling before experiments but no habituation to injections was conducted.
Schematic representation of the timeline of experimental procedures for each experiment. Timeline details the number of animals employed in each experiment, along with the procedures or manipulations conducted. BP: biparental mating condition; SM: single-mother condition; GD: gestational day; PD: postnatal day; BEL: blood ethanol levels; LMA: locomotor activity; IHC: immunohistochemistry
Measurement of ethanol-induced locomotor activity and BELs
The mice were assessed on PDs 16–18 and on PD 20. On PD 16 they were separated from their mothers and placed in pairs in a holding cage (396 mm length × 215 mm wide × 172 mm height) lined with corncob bedding. After a 30-minute acclimation period, body weights were registered and the pups received an intraperitoneal (i.p.) injection of 0.0–2.0 g/kg ethanol. This is a standard dose for the analysis of ethanol-induced behavioral stimulation in mice (Phillips et al. 1994). Locomotor activity was assessed for 5 min after ethanol administration, in a novel environment, i.e., a circular and white Plexiglas container (240 mm diameter). The floor was divided into sixteen quadrants, and an experimenter blind to subject treatment counted the number of entries into the quadrants. An entry was counted if the mouse introduced its head and forepaws into a quadrant. The same protocol was repeated at PDs 17, 18 and 20.
In parallel, at PD 16, a separate group of male and female infant mice were given 2.0 g/kg ethanol (i.p.) and trunk blood samples were collected at post-administration time 7.5 min. The samples were stored at -80 °C until the determination of BELs, which was performed via a head-space gas chromatography [see Pepino et al. (1998) for details on the protocol]. BELs were expressed as milligrams of ethanol per deciliter (mg/dl = mg%).
Immunohistochemical measurement of Fos-like proteins and Fos/TH levels
Ninety minutes after termination of the ethanol-induced locomotor activity test, the mice were anesthetized (Chloral hydrate: 0.001 ml/g of a 30% v/v solution) and perfused transcardially with 0.9% heparinized saline (10 U/ml) followed by 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4). The brains were removed and left overnight in PFA and transferred to 30% sucrose for at least 72 h. The protocol followed that described in Wille-Bille et al. (2017). The 40-µm thick coronal brain sections were incubated free-floating overnight at RT under continuous agitation, with a polyclonal antibody (1:1000, c-fos K-25, Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted in 0.1 M PB, containing 0.3% Triton X-100 (Sigma Chemical Co., St. Louis, MO, USA) plus 2% NHS. This antibody is relatively nonspecific, recognizing several Fos-like proteins. It should be noted, however, that the c-Fos response tends to habituate to repeated treatments, whereas FosB shows higher stability, prolonged presence and accumulation with chronic stimulation (Hope et al. 1994; Melia et al. 1994; Nestler 2008). Therefore, it can be assumed that the Fos-like immunoreactivity observed in this experiment after repeated treatment is mostly represented by FosB rather than c-Fos. Western blotting analyses have shown that proteins exhibiting a molecular weight matching FosB can be identified using this antibody (Perrotti et al. 2004, 2005).
Later, the sections were incubated for 60 min in biotinylated secondary antibody (1:500 Jackson Laboratories, West Grove, PA, USA) with 0.05% NHS, followed by another three washes in 0.01 M PB. To visualize the staining, sections were incubated for 5 min with a solution containing 0.05% 3–3’ diamino-benzidine tetra hydrochloride (DAB, Sigma Aldrich, St. Louis, MO, USA) with 0.01% H2O2 + 1% ClCo 1.0% + 1% de SO4ClNi resulting in a black Fos-like mark (see Fig. 3). The same protocol was repeated using an antibody against tyrosine hydroxilase (TH) (1:1000, Millipore, Billerica, MA, USA). The bodies of dopaminergic neurons of the mesocorticolimbic circuit are located in the VTA (Koob and Nestler 1997). It is known that 60–75% of VTA neurons are dopaminergic, and the rest are, mostly, GABAergic (Barrot et al. 2012). Therefore, it can be assumed that TH staining in the present study represents dopaminergic neurons in VTA. To distinguish the TH mark from the Fos-like mark, we revealed the sections with a solution containing 0.05% 3–3’ DAB to obtain a brown staining (Fig. 3). The brain sections were then mounted, dehydrated and covered with DPX. Brain sections photographs were obtained with an Axicam Microscope camera (Zeiis, Germany) connected to a Primo Star iLed microscope. The Fos-like and Fos/TH positive cells were counted on 0.04 mm2 squares at 10X, in both hemispheres, using the software FIJI Is Just Image J (Schindelin et al. 2012). Data from three sections were selected per animal from each brain region and averaged for the statistical analysis.
The coronal coordinates of each area analyzed (Paxinos and Franklin 2004) were, from bregma, as follows: prelimbic cortex (PrL) 1.94 mm; nucleus accumbens shell (AcbSh) and Core (AcbC) 1.10 mm; dorsomedial (StrD) and lateral striatum (StrL) 0.98 mm; basolateral (BLA) and central amygdala (CEA) -1.06 mm; magnocellular (PvnM) and parvocellular paraventricular hypothalamic nucleus (PvnP); supraoptic nucleus (SN) -0.82 mm and ventral tegmental area (VTA) -3.16 mm.
Elevated plus maze (EPM) test
The maze was constructed from black Plexiglas and consisted of two open (450 mm × 50 mm) and two closed arms (450 mm length × 50 mm width × 450 mm height) that extended from a central platform (50 mm × 50 mm) elevated 500 mm above the floor. The mice were injected with ethanol (0.0, 0.5, 1.0–2.0 g/kg; 0.015 ml/g, i.p.) and, five or ten minutes after the administration (see LDB section), were placed in the center of the EPM. The following dependent variables were registered for 5 min: (i) time spent in and number of entries into the open arms; (ii) stretching (stretched-attend postures toward the open arm, indicative of risk assessment and cautious exploration); (iii) total number of arm entries, which reflects the overall activity level, and (iv) head dipping (an exploratory behavior accounting for both activity and reduced anxiety). An entry into an arm was computed when the mouse crossed into that arm with all four paws at the same time. We also calculated the average time (s) spent in each visit to the open arms (i.e., total time spent in the open arms divided by the frequency of entries into those arms).
Light-dark box (LDB) test
The LDB was made of Plexiglas and had a white (245 mm × 250 mm × 250 mm, 400 lx illumination) and a black compartment (175 mm × 250 mm × 250 mm, 0 lx illumination). Both areas were connected by an opening at floor level, allowing free movement between them. For evaluation, each mouse was injected with ethanol (0.0, 0.5, 1.0–2.0 g/kg; 0.015 ml/g, i.p.) and, five or ten minutes after the administration, placed in the white compartment. The test duration was 5 min and we measured the latency to first exit from white compartment, and the average time per visit to the white compartment (i.e., total time spent in the white section of the LDB divided by the frequency of entries into that section).
Evaluation in the EPM and the LDB was performed at the same day. Each animal was assigned to be evaluated first in the EPM or in the LDB in a random manner, so that the order of evaluation was counterbalanced. After completing one test, the mice were immediately transferred to the other test. The counterbalanced measurement helped reduce the possibility that the order of assessment adversely influenced the results.
Experimental design and data analysis
Experiment 1 assessed ethanol-induced locomotor stimulation (i.e., entries into quadrants in the OF) via a 2 [rearing condition (SM, BP)] × 2 [ethanol treatment (0.0, 2.0 g/kg)] × 2 [sex (male or female)] factorial design. Experiment 2 evaluated BELs through a two-way factorial design [rearing condition × sex]. In Experiment 3, number of Fos-like positive cells was analyzed through a rearing condition × ethanol treatment factorial. Experiment 4 assessed performance in the EPM and LDB, and employed a 2 [rearing condition] × 2 [sex] × 4 [ethanol treatment (0.0, 0.5, 1.0–2.0 g/kg)] factorial.
The data were analyzed using one-way, factorial or repeated-measures analysis of variance (ANOVA). More in detail, the body weights registered prior to the OF tests at PDs 16, 17, 18 and 20, and the locomotor activity in the OF, were analyzed via separate three-way repeated-measures ANOVA. BELs were analyzed through a factorial ANOVA. Fos immunoreactivity (Fos-ir) at each brain area was analyzed with a factorial ANOVA. The variables registered in the EPM and LDB were analyzed through separate factorial ANOVAs. Least significant difference (LSD) pairwise post hoc tests analyzed the loci of the significant main effects or significant interactions yielded by the ANOVAs. The alpha level was 0.05. Across analyses, the main effects or interactions that did not achieve statistical significance are not mentioned in the Results Section.
Results
Open field test and BELs
The ANOVA for body weights indicated that infants significantly increased their body weight at PD 20 in comparison with any other day (F3,153 = 94.82, p < .001). This was not affected by rearing condition, sex or ethanol treatment. The analysis of motor activity scores revealed significant main effects of ethanol and days of assessment (F1,51=30.58; F3,153=11.88, p’s < 0.001). Of particular interest, the interaction between rearing condition, alcohol treatment and days reached significance (F3,153=2.82, p < .05). The post-hoc tests indicated that the ethanol-treated animals exhibited significantly greater activity than saline-treated counterparts. The post-hoc tests revealed that this ethanol-induced behavioral stimulation was, at PD 18, significantly greater in BP vs. SM counterparts. These results are in Fig. 2.
Locomotor activity (frequency of entries into quadrants of an open field) of infant mice reared by a single mother (SM) or by both parents (BP). The offspring was treated with 2.0 g/kg Ethanol (EtOH) or vehicle (Saline) during postnatal days 16, 17, 18 and 20. The asterisk (*) sign indicates significant differences between ethanol and saline treatment, for a given day of testing. The pound (#) sign indicates significant differences between BP and SM for ethanol treatment, at PD18. Vertical bars represent mean ± SEM
BELs at PD 16 were not significantly affected by rearing conditions or sex. The mice had an average of 246.85 ± 10.19 mg/dl of ethanol in their blood at 7.5 min post administration time (i.e., halfway through the OF test).
Immunohistochemical measurement
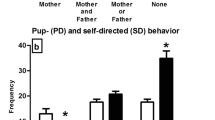
Fos-ir at nucleus accumbens (core and shell), prelimbic cortex, striatum (dorsomedial and lateral), supraoptic nucleus, paraventricular hypothalamic nucleus (magnocellular and parvocellular) or ventral tegmental area were not significantly affected by sex, rearing condition or ethanol treatment, nor any interaction between these factors was significant. The ANOVAs for Fos-ir in BLA and CEA indicated greater Fos-ir in SM than in BP animals (F1,15=5.33 and F1,15=5.05, p < .05, respectively). Furthermore, the ANOVA for Fos/TH positive cells in VTA yielded a borderline effect of ethanol treatment (F1,15=4.25, p = .057), with greater neural activation of dopaminergic neurons after ethanol than after vehicle. Figures 3 and 4 illustrate these patterns.
Representative photomicrographs (10×) of Fos-like positive cells, likely represented mostly by FosB, in basolateral amygdala (BLA) and central amygdala (CEA) or cells expressing both Fos-like and tyrosine hydroxylase in ventral tegmental area (VTA). The brain areas were derived of infant mice reared by a single mother (SM) or reared by both parents (BP). The offspring was treated with 2.0 g/kg ethanol or vehicle during postnatal days 16, 17, 18 and 20. White arrows in VTA photomicrographs indicate cells expressing Fos-like and tyrosine hydroxylase
Total number of Fos-like positive cells, likely represented mostly by FosB, in basolateral amygdala (BLA) and central amygdala (CEA) or total number of cells expressing both Fos-like and tyrosine hydroxylase (TH), in ventral tegmental area (VTA) of infant mice reared by a single mother (SM) or by both parents (BP). The offspring was treated with 2.0 g/kg ethanol or vehicle during postnatal days 16, 17, 18 and 20. The asterisk indicates a significant main difference between rearing conditions. The data are expressed as the number of Fos-like positive cells, likely represented mostly by FosB, per 0.04 mm2. Vertical bars represent the mean ± SEM
EPM and LDB tests
Time spent in the open arms of the EPM was significantly affected by ethanol (F3,91=7.42, p < .0001), and there was a significant interaction between ethanol and rearing condition (F3,91=2.78, p < .05). The post hoc tests showed significantly greater time spent in the open arms in SM mice treated with 2.0 g/kg ethanol, when compared to most of the groups (i.e., except vs. BP-1.0, p = .077). This pattern was similar to that found for the number of entries into the open arms of the EPM. The analysis of this variable yielded a significant main effect of ethanol treatment (F3,91=15.18, p < .0001), whereas the rearing condition × alcohol treatment interaction neared significance (F3,91=2.53, p = .06). Visual inspection of Fig. 5 suggests that SM adolescents treated with 2.0 g/kg ethanol visited the open arms more frequently than the other groups.
Behavioral activity in the elevated plus maze, of adolescent mice reared by a single mother (SM) or by both parents (BP). The offspring was treated with 0.5, 1.0, 2.0 g/kg Ethanol (EtOH) or vehicle (Saline), before the test. The upper panel depicts time (s) spent in the open arms of the maze and frequency of visits to the open arms. The middle panel describes frequency of stretching and head dipping behaviors and the lower panel depicts frequency of total entries (to open and closed arms) and mean time per visit to the open arms (s). The pound sign indicates a significant difference between the SM-EtOH 2.0 group and the other groups. The asterisk signs indicate a significant difference between EtOH 2.0 groups and saline group, regardless rearing conditions. Vertical bars represent the mean ± SEM
The ANOVA for stretching revealed a borderline effect of ethanol treatment (p = .080). Mice given 2.0 g/kg ethanol tended to display less stretching than saline-control adolescents. In contrast, head dipping (F3,91=9.83, p < .0001) and total arms entries (F3,91=2.95, p < .05) were significantly lower in saline-control than in animals given 2.0 g/kg ethanol. The analysis for time spent in each visit to the open arms indicated a significant main effect of ethanol treatment (F3,91=5.57, p < .01).
The ANOVAs for latency to escape from the white compartment of the LDB or mean time spent per visit to white compartment did not reveal significant main effects nor significant interactions. Nevertheless, visual inspection of these results (Fig. 6) revealed a pattern similar to that found in the EPM: SM-adolescents treated with 2.0 g/kg ethanol spent more time in the white compartment at the beginning of the test, and spent more time in the white compartment during each visit.
Behavioral activity in the light dark box test, of adolescent mice reared by a single mother (SM) or by both parents (BP). The offspring was treated with 0.5, 1.0, 2.0 g/kg Ethanol (EtOH) or vehicle (Saline), before the test. The upper panel depicts the first latency to escape (s) from the white compartment. The lower panel depicts mean time (s) per visit in the white compartment. Vertical bars represent the mean ± SEM
Discussion
Ethanol induced, in this study, motor stimulation in infant mice. This finding is consistent with a plethora of data, mostly coming from studies conducted in rats (Arias et al. 2009a, b, 2010b; Miranda-Morales et al. 2013; Miranda-Morales and Pautassi 2016; Molina et al. 2007; Pautassi et al. 2009, 2011), suggesting that sensitivity to the stimulating effect of ethanol is evident very early in development (i.e., around PD10 approximately). New information was that this ethanol-induced behavioral stimulation [a proxy of the rewarding effects of the drug, see (Camarini and Pautassi 2016)] was not significantly influenced by distinct parenting conditions experienced during infancy. Of great importance, SM mice exhibited increased stimulus-induced Fos-like activity in the amygdala nuclei; and ethanol-induced anxiolysis in the EPM. The BELs of the mice were not affected by the parenting structure, suggesting that differences in ethanol sensitivity were not due to pharmacokinetic differences.
Our previous study (Ferreyra et al. 2020) demonstrated distinct behavioral profiles associated with SM and BP parenting. There were significant differences in time spent in the nest and in pup-directed behaviors (i.e., significantly greater in BP vs. SM). At adolescence these differences were associated with significantly greater anxiety responses in SM, than in BP, mice. The present study explored further potential behavioral and neural differences derived from SM and BP parenting. Experiment 1 revealed, akin to previous studies in preweanling rats (Arias et al. 2009b, 2010a), sensitivity to ethanol-induced locomotor stimulation. This is, to the best of our knowledge, the first study to provide evidence of such effect in infant mice, and at a dose similar to that inducing behavioral stimulation in adolescent (Melón and Boehm 2011) or adult mice (Melón and Boehm 2011; Phillips et al. 1994).
Although many researchers link ethanol-induced locomotor to ethanol-induced positive reinforcement (see Camarini and Pautassi 2016; for a review), other researchers suggest that ethanol-induced stimulation could reflect anxiety or stress. Studies on adult mice (Pastor et al. 2008) and infant rats (Arias et al. 2010c) have shown that pharmacological blockade of CRFR1 signaling inhibits the expression of psychomotor sensitization and locomotor stimulation to ethanol, respectively. Both studies suggest the involvement of CRF/CRF1 and the HPA axis in these ethanol-related responses.
Many studies reported sensitization or tolerance to ethanol-induced motor stimulation (Camarini and Pautassi 2016). Nevertheless, our four-day administration/testing protocol was not sensitive to detect these phenomena. In infant rats, Castello et al. (2015) reported sensitization to ethanol only in young infant males (PDs 8–12), and when ethanol administration was not made contingent to the testing context. Older infants, of an age akin to that tested in our study, did not exhibit sensitization, but instead showed tolerance to ethanol-induced motor stimulation (Castello et al. 2015). Recently we found (preliminary, unpublished data) ethanol-induced sensitization in adolescent mice (PD 28–40), yet only after 10 days of ethanol administration (not contingent with testing context). In adolescent and adults C57 mice, it is more frequently to observe ethanol-induced sensitization after 7 or more days of drug administration (see Camarini and Pautassi 2016 for a review). It is possible that, in the present study, the shortness of the administration protocol, and the fact that drug administration was contingent with the testing context, may have interfered with the possibility of detecting ethanol-induced sensitization or tolerance.
In Ferreyra et al. (2020), we reported an anxiety-prone behavioral profile in SM mice, coupled with heightened ethanol intake. Our hypothesis was that SM-adolescents would be more sensitive to ethanol’s anxiolytic effects. The results gathered in Experiment 3 confirmed the hypothesis. SM adolescents exhibited, compared to BP counterparts, greater time spent in and frequency of entry into the anxiety-inducing section of the EPM, after the ethanol injection. A similar profile was observed in the LDB test, although it was not as pronounced as in the EPM. These results imply that ethanol drinking in SM mice may serve as a means to alleviate their elevated baseline anxiety, suggesting negative reinforcement.
A drawback of the hypothesis is that the ethanol intake scores in Ferreyra et al. (2020) should have yielded BELs much lower than those associated with the dose exerting anxiolytic effects in the EPM or LDB. It is worth noting, however, that preexisting (high) anxiety levels are key in the initiation and maintenance of ethanol-drinking [see Koob (2003), Pandey (2003)]. Moreover, clinical literature indicates that coping motives (i.e., the so called “drinking-to-cope”) facilitate ethanol intake, particularly among those that endure stress (Banks 2023), or that have reduced distress tolerance or emotion regulation abilities (Norton et al. 2023).
Preclinical literature has shown that maternal deprivation during the neonatal period (which shares some features with the SM condition) significantly augments anxiety-related behaviors, with these effects lingering until adulthood (Liu et al. 2000; Plotsky and Meaney 1993; Romeo et al. 2003). Intriguingly, precocious weaning (i.e., before PD 20–21) augmented anxiety and aggressiveness in mice and rats (Kanari et al. 2005; Kikusui et al. 2004). These findings point out that parent–pup interactions are critical for normal behavioral development. Our results extend this notion to the field of ethanol sensitivity. Specifically, the results suggest that the parenting structure modulates baseline level of anxiety which, in turn, affects sensitivity to ethanol-induced anxiolysis. A difference between this study and Ferreyra et al. (2020) is that the present study did not yield a significant main effect of rearing conditions on anxiety. The length of testing differed, however, between the studies. In Ferreyra et al. (2020), we employed a relatively long (20 min) assessment in an apparatus (the concentric square field) that, unlike the EPM or LDB tests, provides non-binary behavioral options (Augustsson and Meyerson 2004; Meyerson et al. 2006).
Studies on communal rearing in mice have consistently shown that such experience increases, compared to SM rearing, maternal licking, grooming, and nursing, and promotes enhanced growth rates (Branchi et al. 2006a; Sayler and Salmon 1969). Communal rearing in mice has also been associated with reductions in anxiety- and depressive-like behaviors and increased social interactions (Branchi et al. 2006a, b). As reported by Ferreyra et al. (2020), the BP offspring receive higher rates of parenting behavior, compared to the SM offspring. Consistent with this, the current results suggest that BP offspring display, compared to SM peers, lower anxiety and diminished response to ethanol-induced anxiolysis.
A limitation of our animal model is that, in the BP condition, dams exhibit receptivity (postpartum estrus) during certain hours after parturition. During this time, males can mate with the dam, potentially leading to pregnancy during the lactation period. This could result in a distinct hormonal status compared to SM dams, thereby differentially impacting maternal behavior, irrespective of parental structure. While this confounder cannot be disregarded, it is noteworthy that in Ferreyra et al. (2020), we compared maternal behavior between mothers from both rearing conditions. Pup-directed behavior, nest-building behavior, and lactation behavior were similar in SM and BP mothers. Differences in pup-directed behavior, time spent in the nest, and nest-building behavior were attributed to the presence of another adult caring for the litter (the father in this case).
Experiment 2 revealed greater Fos-like accumulation in the CEA and BLA of SM, compared to BP mice. It has been shown that chronic exposure to stress (e.g., restraint, unpredictable foot shock, electroconvulsive seizures) or drugs (alcohol, cocaine, amphetamine, nicotine, morphine, and antipsychotics), can lead to regional differences in FosB levels, a member of Fos-like proteins (McClung et al. 2004). It is important to consider that FosB exhibits unique stability, allowing for its prolonged presence in the brain compared to other Fos family proteins (Nestler 2008; Hope et al. 1994) such as c-Fos, whose response tends to habituate after repeated treatment (Melia et al. 1994; Hope et al. 1994). Although we assessed Fos-like accumulation in several brain regions, only the CEA and BLA exhibited differences in response to the parenting structure. These results are consistent with the anxiety-prone phenotype, found in SM mice. The central, medial, and basolateral nuclei of the amygdala have been implicated in the regulation of emotion and anxiety, traits which in turn modulate the emergence of alcohol use disorders (Koob and Volkow 2010; LeDoux 2000; Pandey et al. 2006).
Furthermore, after four days of ethanol injections SM mice displayed a pronounced, but not statistically significant, increase in Fos-like expression in the CEA and BLA, compared to their BP counterparts. Other studies have found a significant increase in Fos-like proteins (FosB) after repeated ethanol treatment. Ryabinin and Wang (1998) found, in adult low alcohol-preferring DBA/2J mice, an ethanol-induced increase in amygdaloid nuclei activation. They employed four days of administration but the ethanol dose was much higher (i.e., 4 g/kg) than ours. Other studies, such as that of Ozburn et al. (2012), have also demonstrated changes in neuronal plasticity underlying the motivational aspects of ethanol consumption, yet these researchers measured FosB levels after 72 days of continuous access to ethanol. Our group has also shown that long-term access (six weeks) to ethanol in adolescence results in drinking escalation and induction of FosB expression in central amygdala nucleus, capsular and basolateral amygdala, an effect not found when drinking takes place at adulthood (Wille-Bille et al. 2017). It seems that not only ethanol dose but, more importantly, a robust chronic exposure to the drug is a critical factor in order to observe significant changes in expression of the FosB protein. In conjunction with this prior literature, the present study pinpoints specific brain areas in which the early social environment seems to induce neuroplasticity changes that contribute to an anxiogenic profile.
A possible mechanism underlying the present results may involve brain-derived neurotrophic factor (BDNF). Previous research by Pandey et al. (2006) demonstrated that decreased BDNF levels in the CEA and MEA are associated with anxiety-like behavior and increased alcohol intake. It is plausible to suggest, therefore, that the decreased BDNF levels in the amygdaloid nuclei could have been involved in the reported anxiety and ethanol-related responses. In parallel, oxytocin receptor levels have been found to be elevated in the amygdaloid nuclei of offspring derived from communal, compared to standard, rearing (Branchi et al. 2013). Accumulating evidence also suggests that oxytocin can modulate the effects of drugs of abuse in dopaminergic pathways associated with addiction (Peters et al. 2017). Future studies on these topics should, therefore, focus on oxytocinergic function.
Conclusions
Our study demonstrates that infant mice are responsive to the stimulating effects of alcohol, regardless of their parenting structure. The parenting experience led to a distinctive activation of amygdaloid nuclei that modulate anxiety responses during adolescence. Moreover, SM and BP adolescent mice exhibited substantial differences in sensitivity to ethanol-induced anxiolysis, suggesting a potential interaction between parenting structure and the shaping of alcohol responses during adolescence.
Traditionally, investigations into the influence of parenting on the brain and behavior have focused on adult outcomes, emphasizing long-term effects of variations in mother-infant interactions. In contrast, our study contributes to a growing body of evidence (Ahern et al. 2011; Ahern and Young 2009; Ferreyra et al. 2020) indicating that the consequences of parenting can emerge during late infancy or early adolescence. By focusing on these critical developmental periods, we shed light on the significance of parenting effects. Understanding how these early experiences impact offspring neurobiology and ethanol responses can offer valuable insights for the development of interventions and prevention strategies for anxiety and alcohol use disorders.
References
Ahern TH, Young LJ (2009) The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie Vole (microtus ochrogaster). Front Behav Neurosci 3:17
Ahern TH, Hammock EA, Young LJ (2011) Parental division of labor, coordination, and the effects of family structure on parenting in monogamous prairie voles (Microtus ochrogaster). Dev Psychobiol 53:118–131
Anacker AM, Ahern TH, Young LJ, Ryabinin AE (2012) The role of early life experience and species differences in alcohol intake in microtine rodents. PLoS ONE 7:e39753
Arias C, Mlewski EC, Miller S, Molina JC, Spear NE (2009a) Novelty modulates the stimulating motor effects of ethanol in preweanling rats. Pharmacol Biochem Behav 92:448–456
Arias C, Mlewski EC, Molina JC, Spear NE (2009b) Ethanol induces locomotor activating effects in preweanling Sprague-Dawley rats. Alcohol 43:13–23
Arias C, Mlewski EC, Hansen C, Molina JC, Paglini MG, Spear NE (2010a) Dopamine receptors modulate ethanol’s locomotor-activating effects in preweanling rats. Dev Psychobiol 52:13–23
Arias C, Molina JC, Spear NE (2010b) Differential role of micro, delta and kappa opioid receptors in ethanol-mediated locomotor activation and ethanol intake in preweanling rats. Physiol Behav 99:348–354
Arias C, Solari AC, Mlewski EC, Miller S, Haymal B, Spear NE, Molina JC (2010c) Social isolation and stress related hormones modulate the stimulating effect of ethanol in preweanling rats. Behav Brain Res 211(1):64–70
Augustsson H, Meyerson BJ (2004) Exploration and risk assessment: a comparative study of male house mice (Mus musculus musculus) and two laboratory strains. Physiol Behav 81:685–698
Banks AN (2023) Adverse childhood experiences and alcohol use among black college students: examining the mediating roles of depression and coping drinking motives. Substance use Misuse 1–7
Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC (2012) Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions. J Neuroscience Official J Soc Neurosci 32:14094–14101
Branchi I, D’Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E (2006a) Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry 60:690–696
Branchi I, D’Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E (2006b) Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and depression-like behavior. Journal of neuroscience research 83: 965 – 73
Branchi I, Curley JP, D’Andrea I, Cirulli F, Champagne FA, Alleva E (2013) Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology 38:522–532
Brown JR, Ye H, Bronson RT, Dikkes P, Greenberg ME (1996) A defect in nurturing in mice lacking the immediate early gene fosB. Cell 86:297–309
Camarini R, Pautassi RM (2016) Behavioral sensitization to ethanol: neural basis and factors that influence its acquisition and expression. Brain Res Bull 125:53–78
Castello S, Revillo DA, Molina JC, Arias C (2015) Ethanol-induced tolerance and sex-dependent sensitization in preweanling rats. Physiol Behav 139:50–58
Ferreyra E, Pasquetta L, Ramirez A, Wille-Bille A, Molina JC, Miranda-Morales RS (2020) Biparental care in C57BL/6J mice: effects on adolescent behavior and alcohol consumption. Psychopharmacology 237:1841–1850
Geary DC (2000) Evolution and proximate expression of human paternal investment. Psychol Bull 126:55–77
Gubernick DJ, Alberts JR (1987) The biparental care system of the California mouse, Peromyscus californicus. J Comp Psychol (Washington DC: 1983) 101:169–177
Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ (1994) Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13(5):1235–1244
Kanari K, Kikusui T, Takeuchi Y, Mori Y (2005) Multidimensional structure of anxiety-related behavior in early-weaned rats. Behav Brain Res 156:45–52
Kikusui T, Takeuchi Y, Mori Y (2004) Early weaning induces anxiety and aggression in adult mice. Physiol Behav 81:37–42
Koob GF (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–243
Koob GF, Nestler EJ (1997) The neurobiology of drug addiction. J Neuropsychiatr Clin Neurosci 9:482–97
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology Official Publication of the American College of Neuropsychopharmacology 35:217–38
Lamb ME, Lewis C (2010) The role of the father in child development, 5th edn. Wiley, Hoboken, NJ
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ (2000) Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol 12:5–12
McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ (2004) DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 132:146–154
Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC (1994) Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci 14(10):5929–5938
Melón LC, Boehm SL 2nd (2011) Role of genotype in the development of locomotor sensitization to alcohol in adult and adolescent mice: comparison of the DBA/2J and C57BL/6J inbred mouse strains. Alcohol Clin Exp Res 35:1351–1360
Meyerson BJ, Augustsson H, Berg M, Roman E (2006) The concentric square field: a multivariate test arena for analysis of explorative strategies. Behav Brain Res 168:100–113
Miranda-Morales RS, Pautassi RM (2016) Pharmacological characterization of the nociceptin/orphanin FQ receptor on ethanol-mediated motivational effects in infant and adolescent rats. Behav Brain Res 298:88–96
Miranda-Morales RS, Nizhnikov ME, Waters DH, Spear NE (2013) Participation of the nociceptin/orphanin FQ receptor in ethanol-mediated locomotor activation and ethanol intake in preweanling rats. Behav Brain Res 245:137–144
Miranda-Morales RS, Nizhnikov ME, Waters DH, Spear NE (2014) New evidence of ethanol’s anxiolytic properties in the infant rat. Alcohol 48:367–374
Molina JC, Pautassi RM, Truxell E, Spear N (2007) Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol 41:41–55
Nestler EJ (2008) Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci 363:3245–3255
Norton EO, Hailemeskel R, Bravo AJ, Pilatti A, Kaimner D, Conway CC, Mezquita L, Hogarth L (2023) Childhood traumatic experiences and negative alcohol-related consequences in adulthood: a cross-cultural examination of distress tolerance and drinking to cope. Subst Use Misuse 58:804–811
Ozburn AR, Mayfield RD, Ponomarev I, Jones TA, Blednov YA, Harris RA (2012) Chronic self-administration of alcohol results in elevated ∆FosB: comparison of hybrid mice with distinct drinking patterns. BMC Neurosci 13:130
Pandey SC (2003) Anxiety and alcohol abuse disorders: a common role for CREB and its target, the neuropeptide Y gene. Trends Pharmacol Sci 24:456–460
Pandey SC, Zhang H, Roy A, Misra K (2006) Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci Off J Soc Neurosci 26:8320–8331
Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ (2008) Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci USA 105(26):9070–9075
Pautassi RM, Nizhnikov ME, Spear NE (2009) Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci Biobehav Rev 33:953–974
Pautassi RM, Nizhnikov ME, Acevedo MB, Spear NE (2011) Naloxone blocks ethanol-mediated appetitive conditioning and locomotor activation in adolescent rats. Behav Brain Res 216:262–269
Paxinos G, Franklin KBJ (2004) The mouse brain in stereotaxic coordinates. Gulf Professional Publishing
Pepino MY, Kraebel KS, López MF, Spear N, Molina E JC (1998) Behavioral detection of low concentrations of ethanol in the preweanling rat. Alcohol 15:337–353
Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ (2004) Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci 24:10594–10602
Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M (2005) DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur J Neurosci 21:2817–2824
Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID (2017) Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens. Addict Biol 22:702–711
Phillips TJ, Dickinson S, Burkhart-Kasch S (1994) Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci 108:789–803
Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res 18:195–200
Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG (2003) Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav 43:561–567
Ryabinin AE, Wang YM (1998) Repeated alcohol administration differentially affects c-Fos and FosB protein immunoreactivity in DBA/2J mice. Alcohol Clin Exp Res 22:1646–1654
Sayler A, Salmon M (1969) Communal nursing in mice: influence of multiple mothers on the growth of the young. Science 164:1309–1310
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682
Spear LP (2018) Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19:197–214
Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA (2008) The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neuroscience: Official J Soc Neurosci 28:10272–10277
Wille-Bille A, de Olmos S, Marengo L, Chiner F, Pautassi RM (2017) Long-term ethanol self-administration induces DeltaFosB in male and female adolescent, but not in adult, Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry 74:15–30
Acknowledgements
This work was supported by grants PICT 2016–2461 and PICT 2019 − 0574 to RSMM, PICT 2019 − 0827 to JCM and RSMM of Agencia Nacional de Promoción Científica y Tecnológica (FONCyT). The funders had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We declare having no competing interest nor conflict of interest related to our MS or its results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on Spanning the spectrum of social behavior: towards more translationally relevant animal models
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pasquetta, L., Ferreyra, E., Wille-Bille, A. et al. C57BL/6J offspring mice reared by a single-mother exhibit, compared to mice reared in a biparental parenting structure, distinct neural activation patterns and heightened ethanol-induced anxiolysis. Psychopharmacology (2024). https://doi.org/10.1007/s00213-024-06627-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00213-024-06627-4