Abstract
Rational
Patients experience post-stroke cognitive impairment during aging. To date, no specific treatment solution has been reported for this disorder.
Objective
The purpose of this study was to evaluate the effects of exercise training and coenzyme Q10 supplementation on middle cerebral artery occlusion (MCAO) induced behavioral impairment, long-term potentiation inhibition and cerebral infarction size in aging rats.
Methods
Fifty aging male rats underwent MCAO surgery and were randomly distributed in to the following groups: 1-Sham, 2- control, 3- Coenzyme Q10, 4- Exercise training and 5- Exercise training with Q10 supplementation (Ex + Q10). Aerobic training groups were allowed to run on a treadmill for 12 weeks. Q10 (50 mg/kg) was administered intragastrically by gavage. Morris water maze, shuttle box and elevated plus maze tests were used to evaluate cognitive function. The population spike (PS) amplitude and slope of excitatory postsynaptic potentials (EPSP) in the dentate gyrus area were recorded as a result of perforant pathway electrical stimulation.
Results
Our study showed that Q10 and aerobic training alone ameliorate spatial memory in the acquisition phase, but have no effect on spatial memory in the retention phase. Q10 and exercise training synergistically promoted spatial memory in the retention phase. Q10 and exercise training separately and simultaneously mitigated cerebral ischemia-induced passive avoidance memory impairment in acquisition and retention phases. The EPSP did not differ between the groups, but exercise training and Q10 ameliorate the PS amplitude in hippocampal responses to perforant path stimulation. Exercising and Q10 simultaneously reduced the cerebral infarction volume.
Conclusion
Collectively, the findings of the present study imply that 12 weeks of aerobic training and Q10 supplementation alone can simultaneously reverse cerebral ischemia induced neurobehavioral deficits via amelioration of synaptic plasticity and a reduction in cerebral infarction volume in senescent rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing quality of life in developed and developing countries has led to an increase in life expectancy and an increase in the number of individuals aged 65 years and older (Collaboration 2008). Cerebral ischemia is one of the world’s most significant health problems. Today, cerebral stroke is the third leading cause of death after cardiovascular disease and cancer, and the second leading cause of death in elderly individuals; moreover; cerebral stroke is the leading cause of adult disability worldwide (Boyko et al. 2013; Kim et al. 2020).
Recently, several researchers suggested that aging and cerebral stroke enhance each other’s harmful effects on brain function (Salehpour et al. 2019). Previous studies confirmed that following cerebral stroke, aging rats not only are more sensitive to hypoxia but also experience larger cerebral infarction sizes (Boyko et al. 2013; DiNapoli et al. 2008). Overall, 25-30% of ischemic stroke survivors suffer from cognitive dysfunction in the short and long term (Wen et al. 2018).A review paper reported that 80% of individuals with cerebral stroke suffer from cognitive impairments (Saa et al. 2019; Sun et al. 2014). The prevalence of cognitive disorders in elderly individuals is 10 to 20% and the risk of dementia increases exponentially with advancing age (Langa and Levine 2014).
Hypoxia-induced proapoptotic gene products, mitochondrial dysfunction and neuroinflammation initiate a complex cascade of pathogenetic events that lead to neurodegeneration and thus brain dysfunction including cognitive decline and memory loss (Kim et al. 2020). Both cerebral infarction (Yu et al. 2013) and aging (Boric et al. 2008) have detrimental effects on synaptic plasticity. A reduction in synaptic plasticity is one of the effective factors for the development of cerebral ischemia-induced behavioral deficits during aging. It seems logical that any factor that can reduce cerebral ischemia-induced oxidative stress and inflammation is an effective therapeutic strategy for the treatment of cognitive disorders caused by cerebral infarction.
The first intervention of interest is the administration of Coenzyme-Q10 (Co-Q10) or ubiquinone (2,3-dimethoxy-5-methyl-6-polyprenyl-1,4-benzoquinone) which is a powerful endogenous antioxidant in the mitochondrial inner membrane that plays a major role in the electron transport chain by contributing to energy production by carrying electrons. The evidence suggests that Co-Q10 is effective at protecting lipids from harmful oxidative damage, as is DNA and proteins. Co-Q10 deficiency is one of the changes that has been reported in Alzheimer’s disease (Manzar et al. 2020). Co-Q10 decreases during aging (Barcelos and Haas 2019), and a previous paper showed that Co-Q10 supplementation reverses age-related brain dysfunction and decreases protein oxidation (Shetty et al. 2013). Furthermore, Co-Q10, by reducing the levels of inflammatory cytokines reduces neurological defects and brain injury following brain ischemia (Ghasemloo et al. 2021). Recently, a behavioral, biochemical, and electrophysiological study demonstrated that Co-Q10 (50 mg/kg) supplementation for 4 weeks ameliorates memory and long-term potentiation (LTP) in aged β-amyloid-induced Alzheimer’s disease rats (Asadbegi et al. 2023).
The second potential intervention of interest is exercise training. There is a consensus that regular moderate exercise training is one of the best non pharmacological therapeutic strategies for age-related physiological and pathophysiological dysfunctions. Exercise training through anti-inflammatory and antioxidant effects decreases cerebral ischemia‒reperfusion injury (Hamakawa et al. 2013; Lu et al. 2021). Additionally, Zhang et al. (Zhang et al. 2017) indicated that early treadmill exercise significantly ameliorated cognitive dysfunction and anxiety-like behavior in an ischemic rat model. It has also been suggested that exercise training alleviates cerebral ischemia‒reperfusion-induced cognitive impairment in juvenile rats (Pan et al. 2021). In this regard, Li et al. showed that exercise training for 4 weeks after cerebral ischemia promoted synaptic plasticity in adult (8–10 weeks old) rats (Li et al. 2022a).
Therefore, a therapeutic strategy to reduce poststroke cognitive impairment and inhibit synaptic plasticity during the early period after infarction is important for decreasing morbidity and mortality during aging. Hence, the aim of the present study was to evaluate the synergistic effect of Co-Q10 and aerobic training for 12 weeks on transient middle cerebral artery occlusion-induced cognitive deficit and long-term potentiation suppression in senescent rats.
Experimental procedures
Subjects
Fifty aging male Sprague‒Dawley rats (22–24 months, 380–420 g) were purchased from the animal laboratory of the Hamadan University of Medical Sciences. The subjects were housed in groups of 5 at standard conditions (light (8:00 a.m-20 p.m.)/dark (20p.m-8 a.m.), 50 ± 5% humidity and 20 ± 2 °C temperature) and given ad libitum access to water and food. All the experimental procedures were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (Garber 2011). The procedures were approved by the ethics committee of Hamedan University of Medical Science, and the study was carried out in compliance with the ARRIVE Guidelines.
Study design
Senescent rats were divided into 5 groups: 1-sham (sham, n = 10), 2-control (Con, n = 10), 3-coenzyme Q10 supplementation (Q10, n = 10), 4-exercise training (Ex, n = 10) and 5-exercise training with Co-Q10 supplementation (Ex + Q10, n = 10).
Senescent rats underwent middle cerebral artery occlusion surgery until cerebral ischemia induction. The sham group did not experience cerebral ischemia. One week after MCAO, the training group was subjected to aerobic exercise on a treadmill for 12 weeks. The Q10 mice were treated with coenzyme Q10 (50 mg/kg) by gavage every day at 12 a.m. The experimental timeline is shown in Fig. 1.
Transient middle cerebral artery occlusion
Cerebral ischemia was induced by focal middle cerebral artery occlusion according to previous methods (Longa et al. 1989). Briefly, rats were deeply anaesthetized with intraperitoneal injection of ketamine (60 mg/kg) and xylazine (15 mg/kg) under a stereo dissecting microscope (Nikon, Japan), and the right common carotid artery (CCA) and carotid bifurcation were exposed through midline neck incision (1.5–2 cm) freed from surrounding nerves and fascia without damaging the muscles or the vagus nerve or its collaterals. As a next step, the proximal ends of the CCA and the internal common artery (ICA) were temporally occluded with an artery clamp, and 6/0 silk sutures were used to tie 2 knots in the external common artery (ECA). An oblique incision was made between the ECC and bifurcation of the CCA, and then, a 4 − 0 monofilament nylon suture tip (total length of monofilament: 3 cm; silicon-coated tip length and diameter: 5 mm and 0.39 mm, respectively) was rounded by heating and inserted into the internal carotid artery through the external carotid artery, while the ICA clamp was removed and advanced 17–20 mm away from the carotid bifurcation until resistance was felt. The endoscope could occlude the origin and proximal part of the anterior cerebral artery for 30 min, after which the filament was carefully withdrawn to establish reperfusion. Notably, after the monofilament was inserted, the proximal ends of the ECA were loosely tied to 6/0 silk, and after monofilament removal during reperfusion, this tie was permanently closed to prevent bleeding. In the sham group, only the CCA and ECA were exposed and ligated, and no filament was inserted through the ECA. Finally, the skin was sutured, and the animals were returned to their individual recovery cages.
Neurological impairment
One day, 6 weeks and 12 weeks after cerebral hypoxia induction, the subjects underwent modified neurological severity score testing (mNSS) to evaluate neurological deficits. As previously described by Pan et al., the mNSS included balance ability (beam balance test), sensation (placement test and proprioceptive test), exercise (placing the rat on the floor and raising the rat by the tail) and reflexes (the pinna reflex, corneal reflex and starter reflex). The scale ranges from 0 to 18. At 0 for intact sham rats, 1–6 for mild injury, 7–12 for moderate injury rats and 13–18 for severe injury and maximum defects, a score of 18 revealed the greatest neurological impairment. All tests were performed by one person blinded to the groups (Pan et al. 2021).
Forced treadmill training
Exercise training began seven days after transient focal cerebral hypoxia induction. In the training groups, the rats were allowed to run on a rodent motorize treadmill at 18 m/min for 30 min/day for 5 days/week for 12 weeks. At the beginning of the exercise program, the duration and intensity of exercise gradually increased so that the rats ran at 18 m/min for 30 min until the last training session in the twelfth week. Exercise sessions began with warm up for 5 min at 10 m/min, followed by 30 min at 18 m/min and cooling for 5 min at 10 m/min (Al-Jarrah et al. 2007). During running, mild shock (0.05 mA) was administered to encourage the rats to move forward. Similarly, sedentary rats (sham, Con, and Co-Q10) were left on the off treadmill for the same amount of time.
Coenzyme-Q10 supplementation
Seven days after MCAO surgery, 50 mg/kg Co-Q10 (Sigma‒Aldrich, St. Louis, MO) was administered intragastrically via oral gavage needles once a day for 12 weeks.
Cognitive function measurement
To evaluate spatial (acquisition and retention) and aversive (acquisition and retention) learning and memory and anxiety after cerebral hypoxia, the Morris water maze (MWM), shuttle box test and elevated plus maze were used.
Elevated plus maze
The anxiolytic activity of rats with focal cerebral hypoxia was demonstrated by the elevated plus maze (EPM) test one day after the last mNSS test. This test was based upon the instinctive behavior of rats to stay in the dark compartment and avoid risky surroundings (open arm) (Sawantdesai et al. 2016). As previously described by our laboratory (Parsa et al. 2021b), the EPM apparatus was composed of two opposing closed arms (10 × 50 cm) aligned perpendicularly to two open arms (50 × 10 × 50 cm) connected by a central square (10 × 10 cm). This apparatus is located at a height of 80 cm above the ground. Each rat was placed in the center of the apparatus, facing between the open arm and closed arm, and allowed to search inside the apparatus for 5 min. The amount of time spent in the dark arms, indicating anxiety behavior, was recorded via a video camera. It should be noted that no animal was lost during the research.
Morris water maze
As previously described by our laboratory, the MWM test (one day after the EPM test) was used for evaluating learning and spatial memory ability (Zarrinkalam et al. 2018). Briefly, the MWM device included a circular black pool (180 cm in diameter, 60 cm in height) filled with water (22 ± 1 °C) to a depth of 30 cm that was located in a low light and sound insulated room with various visual cues. The pool had four quadrants with four starting lines, namely, north (N), east (E), south (S), and west (W), and an invisible Plexiglas platform (10 cm in diameter) centrally located 1 cm beneath the water in quadrant N. This test included an acquisition phase and a retention phase that lasted 4 and 1 day, respectively. In the acquisition phase, each day, there were two blocks with four trials (90 s). There was a 30 s gap between two trials on the platform, and the rest of the time between two consecutive blocks was 5 min. In each block, the starting position of the rats was changed quasirandomly during each trial. If the rat could not find the platform within 90 s, the experimenter placed the rat on the platform; on the other hand, if the rat found the platform, the experimenter allowed the rat to remain on the platform for 30 s. The time spent finding the platform was recorded by a camera (Nikon, Melville, New York, USA) located above the pool, which was connected to a computer. The time needed for subjects to find the platform was considered the escape latency. In the acquisition phase, the mean escape latency (mean escape latency of trials on each day) and mean total escape latency (mean escape latency of trials on the first, second, third and fourth days) were recorded. The retention phase (probe trial) was performed one day after the acquisition phase (i.e., on day five of MWM testing). In the probe trial, the platform was removed, the rat was allowed to swim for 60 s, and the ratio of time spent in the target quadrant to the number of times the rat crossed the platform in 60 trials was recorded and used for spatial retention memory evaluation.
Shuttle box test
One day after the MWM test, the shuttle box test was used to assess passive avoidance memory in rats with cerebral hypoxia according to our previous papers (Parsa et al. 2021a; Zarrinkalam et al. 2018). Briefly, the shuttle box apparatus consisted of two dark and light compartments similar in dimension (20 × 20 × 30 cm), with a grid stainless-steel rod floor connected to a shock generator and a guillotine door separating the two compartments. This test consists of two phases, an acquisition trial and a retention trial, which are performed over 2 consecutive days. On the first day, the rats were placed in a light compartment, and the guillotine door was closed. Thirty seconds later, the door was manually opened, and the rats were returned to their home cages according to their natural preference in the dark. After 30 s, the rats were returned to their home cages. The same steps were repeated 30 min later. When all 4 paws were placed in the dark, the entrance latency to the dark section (step-through latency, STLa) was recorded. After the rats entered the dark section, the door was closed, after which a 0.8 mA electrical shock was applied for 2 s, after which the rats were returned to the home cage after 30 s. The test was repeated after 2 min. The rats were subjected to foot shock each time the dark section was re-entered. The test was finished when the rat remained in the light section for 120 s, and the number of trials was recorded.
On the second day of the retention phase, the rat was placed in the light compartment, the guillotine door was opened for 5 s, after which the step-through latency in the retention test (STLr) and the time spent in the dark section (TDC) were recorded for 10 min.
Synaptic plasticity and electrophysiological recording
Hippocampal synaptic plasticity was evaluated by LTP as a cellular mechanism of learning and memory. LTP induction was performed according to our previous works (Asadbegi et al. 2023; Ranjbar et al. 2022). Briefly, the animals were anaesthetized with urethane (1.5 gr/kg i.p.) and placed in a stereotaxic device for electrode implantation. Small holes were drilled on the right side of the skull, and stimulating electrodes were placed in the perforant path (AP: − 8.1 mm from bregma; ML: + 4.3 mm from midline; DV: 3.2 mm from the skull surface); additionally, recording electrodes in the dentate gyrus (DG) granular cell layer (AP: − 3.8 mm from bregma; ML: + 2.3 mm from midline; DV: 2.7–3.2 mm from the skull surface) were placed in the right hemisphere according to the Paxinos and Watson atlas of the rat brain (Paxinos and Watson 2005). Input–output response curves were generated by stimulating the perforant pathway (PP) to determine the stimulus intensity used for each animal. The perforant path was stimulated with monophasic square waves for 0.1 ms at 10 s intervals. To obtain the high population spike (PS) amplitude and field excitatory post synaptic potential (fEPSP) slope, electrodes were positioned. LTP was provoked using a high-frequency stimulation (HFS) protocol of 400 Hz (including 10 bursts of 20 stimuli, 0.2 ms stimulus duration, and 10 s interburst interval). HFS-induced changes in the fEPSP slope and PS amplitude were measured at 5, 30, and 60 min to evaluate the synaptic response of PP-DG neurons (Karimi et al. 2021; Omidi et al. 2020; Safari et al. 2021). The data analysis tool (eTrace, www.sciencebeam.com) was used to measure the fEPSP slope and PS amplitude.
Measurement of the evoked potentials
A trace of the recording electrodes (arrow) in the dentate gyrus (DG) is shown in 2a and 2b. The functional role of the fEPSP slope was evaluated on the basis of the slope of the line, through which the beginning of the first positive deflection of the evoked potential was connected to the peak of the second positive deflection. The PS amplitude was measured from the peak of the initial positive deflection of the evoked potential to the peak of the next negative potential (Fig.2c), as previously described by our laboratory (Komaki et al. 2017; Ranjbar et al. 2022). fEPSP slope and PS amplitude were calculated by following equations: fEPSP slope=∆V/∆T and PS amplitude= (∆V1+∆V2)/2. ΔV1 determined by the difference between points e and f; ΔV2 determined by difference between points f and g; ΔV calculated by difference between points c and d and ΔT was measured by difference between points a and b (Fig.2c). The magnitude of LTP was measured by calculating the average percentage increase in the slope of EPSP and PS compared to the base level. A successful induction of LTP was identified when the change in PS amplitude surpassed 25% (Ghaderi et al. 2023). In this paper, to evaluate the effects of aerobic training and Co-Q10 supplementation on the EPSP slopes and PS amplitudes of granular cells in the DG in a cerebral ischemia rat model, LTP was evaluated through the application of HFS to the perforant pathway.
Schematic drawing of a rat brain coronal section from Paxinos and Watson showing traces of recording electrodes (arrows) in the dentate gyrus (DG) (a). Cross-sectional view of the hippocampal area with the tip of the recording electrode (arrowhead) in the DG; sample on the right and atlas plate on the left (b). Scale bar: 2 mm. The arrows represent the PSs and the slope of the EPSP (c)
Cerebral infarction volume
To evaluate the infarction volume, 2, 3, 5 triphenyltetrazolium chloride (TTC) staining was used. Immediately after electrophysiological recording, the skull was broken, and the intact brain was kept at -20 °C for one hour. The brain was cut transversely and immersed in TCC (TTC; Sigma‒Aldrich, St. Louis, MO, USA) for 20 min at 37 °C. Then, the slices were fixed in 10% formaldehyde for 24 h. TTC reacts with dehydrogenases in viable cells, resulting in a red colour, and the white area indicates infarction (Ke et al. 2011). It should be noted that one rat from each group died during the research period.
Statistical analyses
All the statistical analyses were performed using SPSS version 23. The Shapiro‒Wilk test was used to confirm the normality of the data distributions. Statistically significant differences in neurological score, escape latency and LTP (EPSP slope and PS amplitude) between groups were tested by mixed repeated-measures ANOVA. For statistical analyses of the probe and other data, one-way ANOVA was used. Tukey’s post hoc test was used to compare the experimental groups. The data are presented as the mean ± SEM. Values were considered significant at P < 0.05.
Results
Neurological scores
As shown in Fig. 3, neurological scores at 1 day, 6 weeks and 12 weeks after cerebral ischemia induction were different between the groups. The neurological score at 1 day after cerebral ischemia was not different between MCAO groups (p = 0.8). Neurological scores decreased over time following experimentally induced stroke (p < 0.05). The reduction in neurological scores was greater in the treatment groups than in the the control group. The neurological score reductions in the first 6 weeks in the Con, Co-Q10, Ex and Ex + Q10 groups were 8%, 18%, 32% and 34%, respectively, and in the last 6 weeks were 11%, 34%, 30% and 37%, respectively. Notably, neurological score reductions in the first 6 weeks and last 6 weeks were not significantly different between the treatment groups. The neurological score reductions over time in the Q10, Ex and Ex + Q10 groups were 46%, 52% and 57%, respectively. Although the neurological score was greater in the Ex + Q10 group, there was no significant difference between the treatment groups.
Anxiety-like behavior
The percent of time spent in the close arms (F(4,40) = 29.36, P = 0.0001) and the number of close arms entries (F(4,40) = 3.5, p = 0.02) in the elevated plus maze were different between the groups. Percent of time spent in the close arms was greater in the transient middle cerebral arterial occlusion rat model than in the healthy control rat (these rats underwent surgery even if they did not undergo cerebral ischemia, p = 0.0001); but percent of time spent in the close arms was not different between the groups with MCAO (Fig.4). Furthermore number of close arms entries was greater in cerebral ischemia rats than in the sham rats (p = 0.03). Additionally, the number of close arms entries did not differ between the different ischemia groups (Fig.5).
Spatial learning and memory
The Mean escape latency in the acquisition phase was different between the experimental groups (F = 6.2, p = 0.004, Fig.6). Pair-wise comparison showed that mean escape latency in control groups was more than sham group (p = 0.04), but treatments groups (Q10, Ex and Ex + Q10) were not different compared to the sham group (p > 0.05). Similarly, the mean escape latency did not differ between the treatment groups. In this regard, the mean of total escape latency differed between groups (F(4,40) = 7.6, p = 0.001). Mean of total escape latency in the control group was greater than that in the sham group (p = 0.003). Aerobic training (p = 0.02) and Q10 (p = 0.02) supplementation for 12 weeks alone and simultaneously (p = 0.001) decreased mean of total escape latency in the transient middle cerebral arterial occlusion rat model (Fig.7).
The mean escape latency was different between groups at D1-D4 in the acquisition phase of the Morris water maze test. Co-Q10 and aerobic training with and without each other decreased the mean escape latency in the MWM test. The data are expressed as the mean ± SEM
* = significant difference vs. Con group
The time spent in the target quadrant in the retention phase (probe day) of the MWM test was different among the five groups (F(4,40) = 4.7, p = 0.008). The time spent in the target quadrant in the retention phase was shorter in the control group than in the sham group (p = 0.03; Fig.8). Co-Q10 supplementation for 12 weeks did not significantly increase the amount of time spent in the target quadrant (p = 0.1). Additionally, exercise training for 12 weeks after cerebral ischemia non significantly promoted the amount of time spent in the target quadrant (p = 0.9). Importantly, exercise training and Q10 supplementation synergistically promoted the time spent in the target quadrant (p = 0.02). The amount of time spent in the target quadrant in the Ex + Q10 group was not significantly greater than that in the Ex (p = 0.08) and sham groups. As shown in Fig. 9, the number of platform crossings on the probe day in the MWM test was different between the groups (F(4,40) = 3.9, p = 0.016). There was less crossover after cerebral ischemia in the sedentary group than in the sham group (p = 0.001). Exercise training and Co-Q10 alone did not significantly (p = 0.09 and p = 0.1, respectively) promote crossover from the removal platform. Notably, exercise training and Co-Q10 supplementation synergistically promoted the number of crossovers from the removal platform in a rat transient middle cerebral artery occlusion model (p = 0.02). There were no significant differences between the experimental groups (p > 0.05).
Passive avoidance memory
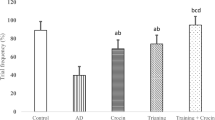
Statistical analyses also revealed that passive avoidance memory in the shuttle box test differed between the treatment groups. In this regard, the STLa was different between the experimental groups (F(4,40) = 6.3, p = 0.002). The results showed that the STLa in the acquisition phase was longer in the Con group than in the Sham group (p = 0.001). Twelve weeks of aerobic training and Co-Q10 supplementation alone (p = 0.019 and p = 0.02, respectively) and simultaneously (p = 0.011) decreased the STLa following transient middle cerebral artery occlusion. The STLa was not significantly different among the Q10, Ex and Ex + Q10 groups (Fig. 10). Additionally, the number of trials in the acquisition phase in the treatment groups was lower than that in the control groups (F(4,40) = 3.7, p = 0.01). The number of trials in the control group was not significantly greater than that in the sham group (p = 0.3). Exercise training with and without Co-Q10 supplementation decreased the number of trials in the acquisition phase (p = 0.02; Fig.11). The number of trials in which Co-Q10 was used decreased non significantly (p = 0.1). Notably, there were no differences between the treatment groups and the sham group.
As shown in Fig. 12 and 13, passive avoidance memory in the retention phase differed between the groups. The STLr (F(4,40) = 28.4, p = 0.0001) and time spent in the dark compartment (TDC) (F(4,40) = 10.6, p = 0.0001) were affected by transient middle cerebral arterial occlusion. There were two more STLr neurites in the sham group than in the control group (0.001). Exercise training and Co-Q10 supplementation returned the STLr to the normal level, but the effect of exercise training was greater (Fig. 12). In this regard, there was more TDC following cerebral ischemia than following healthy surgery (p = 0.0001). Exercise training (p = 0.2) and Co-Q10 supplementation (p = 0.09) alone and simultaneously (p = 0.5) did not significantly decrease TDC in the retention phase (Fig. 13).
The step-through latency in the retention phase (STLr) returned to the normal level in response to Co-Q10 and exercise training for 12 weeks in the shuttle box test in a transient middle cerebral arterial occlusion rat model. The data are expressed as the mean ± SEM
=† significant difference vs. sham group
* =significant difference vs. Con group
HFS impact on the EPSP slope
As shown in fig.14 the significant effects of time (F = 11.07, p = 0.0001) and insignificant effect of group (F = 0.18, p = 0.24) and Time*group interaction (F = 1.27, p = 0.25) were found on the EPSP slope of the DG granular cells after HFS. Notably, EPSP, 5 min after HFS was not different between the experimental groups (F(4,40) = 1.55, p = 0.22, Fig.15), morover, EPSP 30 min after HFS did not differ between groups (F(4,40) = 1.67, p = 0.19, Fig.16), nor did EPSP in 60 min after HFS (F(4,40) = 1.44, p = 0.25, Fig.17). The EPSP slope in the control group was significantly lower than that in the sham group at all three time points (5, 30 and 60 min after HFS), but these decreases were not significant. In this regard, exercise training and Co-Q10 insignificantly promoted the EPSP slope in cerebral infarction.
HFS impact on the PS amplitude
The results showed that the changes in PS amplitude after HFS in the different groups were related to time (F = 9.4, p = 0.0001). Furthermore, the results indicated a significant effect of time (F = 124.87, p = 0.0001). Additionally, statistical analyses showed that the PS amplitude was different between the experimental groups (F = 46.3, p = 0.0001; Fig.18). One-way ANOVA revealed that the PS amplitude 5 min after HFS was different between the groups (F(4,40) = 37.7, p = 0.0001; Fig.19). A post hoc test demonstrated that the PS amplitude in the sham groups was greater than that in the control group (p = 0.0001); moreover, aerobic training had no effect on the PS amplitude, but Co-Q10 supplementation with and without exercise training promoted PS compared to that in the control group. Notably, exercise training and Co-Q10 synergistically ameliorate the PS amplitude 5 min after HFS. Furthermore, the results showed that the PS amplitude 30 min after HFS in the perforant pathway was different between the groups (F(4,40) = 40, p = 0.0001; Fig.20). Pair-wise comparison of the two groups revealed that middle cerebral artery occlusion decreased the PS amplitude compared to that in the sham group (0.0001); additionally, aerobic training had no effect on the PS amplitude (p = 0.9), but Co-Q10 and exercise training synergistically elevated the PS amplitude 30 min after HFS. Moreover, the PS amplitude 60 min after HFS was different between the groups (F(4,40) = 11.7, p = 0.0001; Fig.21). Cerebral ischemia decreased the PS amplitude compared to that in the sham group (p = 0.01). Neither Co-Q10 nor exercise training for 12 weeks had any effect on the PS amplitude (p = 0.055 and p = 1, respectively). At 2 additional time points, exercise training and Co-Q10 supplementation for 12 weeks synergistically ameliorated the PS amplitude 60 min after HFS compared with that in the control group (p = 0.0001).
Time-dependent alterations in hippocampal responses to perforant path stimulation after high-frequency stimulation. Exercise training and Co-Q10 synergistically ameliorate PS amplitude in 5, 30 and 60 min after HFS. Data are expressed as Mean±SEM
=† significant difference vs. sham group
* = significant difference vs. control group
#= significant difference vs. Ex group
=‡ significant difference vs. Q10 group
Time-dependent alterations in hippocampal responses to perforant path stimulation after high-frequency stimulation. Exercise training and Co-Q10 synergistically ameliorate PS amplitude in 5, 30 and 60 min after HFS. Data are expressed as Mean±SEM
=† significant difference vs. sham group
* = significant difference vs. control group
#= significant difference vs. Ex group
=‡ significant difference vs. Q10 group
Time-dependent alterations in hippocampal responses to perforant path stimulation after high-frequency stimulation. Exercise training and Co-Q10 synergistically ameliorate PS amplitude in 5, 30 and 60 min after HFS. Data are expressed as Mean±SEM
=† significant difference vs. sham group
* = significant difference vs. control group
#= significant difference vs. Ex group
=‡ significant difference vs. Q10 group
Time-dependent alterations in hippocampal responses to perforant path stimulation after high-frequency stimulation. Exercise training and Co-Q10 synergistically ameliorate PS amplitude in 5, 30 and 60 min after HFS. Data are expressed as Mean±SEM
=† significant difference vs. sham group
* = significant difference vs. control group
#= significant difference vs. Ex group
=‡ significant difference vs. Q10 group
Cerebral infarction volume
Statistical analyses also revealed that the infarct volume was different between the experimental groups (F(4,40) = 146.8, p = 0.0001). The cerebral infarction volume was 32.24% in the con group and 27.02% in the training group (p = 0.012). Co-Q10, like exercise training but with more impact power, decreased the infarction volume (0.0002). The cerebral infarction volume in the Co-Q10 group was 24.3%. Furthermore, exercise training and Co-Q10 supplementation synergistically decreased the infarction volume by 10% (p = 0.0001) (Fig.22). The cerebral infarction volume in the Ex + Q10 group was 22.1%.
Discussion
Focal cerebral ischemia is one of the most common cerebral vascular diseases and is prevalent in elderly people (Thal et al. 2012). Neurobehavioral deficits are among the most important consequences of this disease. Poststroke exercise training, as a noninvasive therapy, plays a critical role in neurobehavioral function recovery (Geng et al. 2021; Xing et al. 2018). The aim of this study was to evaluate the procognitive effect of exercise training and Co-Q10 supplementation on focal cerebral ischemia-induced neurobehavioral impairment and synaptic plasticity inhibition in senescent rats. Briefly, the results showed that short- and long-term spatial memory, passive avoidance memory and anxiety were impaired following cerebral ischemia in senescent rats. Poststroke cognitive impairment is well documented (Jokinen et al. 2015; Sun et al. 2014). A review of studies in this field revealed that neuronal loss (Li and Fan 2021; Zhang and Bi 2020), brain edema (Lin et al. 2016), and synaptic dysfunction are fundamental reasons for this process. In addition, our findings are in agreement with those of previous studies showing that cerebral infarction causes impairments in synaptic plasticity in the hippocampus (Gáspárová et al. 2008).
The main findings of the present study were that aerobic training and Co-Q10 supplementation, administered separately or in combination, ameliorated learning and memory deficits, reestablished synaptic plasticity and reduced infarction volume after transient middle cerebral arterial occlusion in senescent rats. In line with our findings, Salehpour et al. showed that Co-Q10 (500 mg/kg by gavage) administered for 2 weeks after cerebral ischemia improved spatial and episodic memory in artificially aged mice via a reduction in reactive oxygen species and a decrease in neuroinflammatory responsiveness (Salehpour et al. 2019). Additionally, Shetty et al. confirmed that, unlike a low dose of Co-Q10 (96 mg/kg/day), a high dose of Co-Q10 (457 mg/kg/day) for 15 weeks reverses age-related impairments in spatial learning and lowers protein oxidation and oxidative damage in the brain (Shetty et al. 2013). Surprisingly, analysis of the data confirmed that Co-Q10 has no effect on spatial memory in the retention phase, which is thought to be a measure of strength and accuracy. These findings are in agreement with those of Omidi et al., who reported that a low dose of Co-Q10 (100 mg/kg) for 90 days has no effect on retention memory in healthy or diabetic rats (Omidi et al. 2019). These findings demonstrated that Co-Q10 promotes learning but does not affect learning accuracy; this finding showed that Co-Q10 may affect attention or motivation rather than spatial performance.
However, the mechanisms underlying the procognitive effect of Co-Q10 supplementation following cerebral ischemia have not been elucidated. The main mechanism of the beneficial effect of Co-Q10 supplementation therapy relies on the mitigation of stress and inflammation. Oxidative stress and neuroinflammation play a cardinal role in hippocampal nerve cell apoptosis induced by ischemia and subsequent cognitive decline during ageing. In this regard, previous studies confirmed that the senescent brain is more sensitive to oxidative stress and inflammation caused by ischemic injury than the young brain is (Salehpour et al. 2019). Li et al. demonstrated that 28 days of Co-Q10 supplementation (1200 mg/kg/day) via gavage exerted neuroprotective effects by decreasing the infarction volume in the ischemic hemisphere induced by cerebral ischemia injury in aged mice (Li et al. 2007). Likewise, a large body of evidence demonstrates that Co-Q10 rescues mitochondrial function and prevents brain damage induced by middle cerebral arterial occlusion (Dong et al. 2015; Salehpour et al. 2019). It should be noted that starting time (age) and the amount and duration of intake of Co-Q10 are critical determinants of functional outcome (Shetty et al. 2013).
Moreover, the magnitude of cerebral ischemia-induced neurobehavioral deficit mitigation induced by exercise training in the present study was similar to that induced by Co-Q10 supplementation, but the protective effect of Co-Q10 on ischemia-induced synaptic plasticity impairment was greater than that of exercise training. The molecular mechanism of the procognitive effect of exercise training after focal cerebral ischemia in senescent rats is not yet clear. In line with our findings, Nie et al. demonstrated that poststroke exercise promoted by synaptic plasticity decreased cognitive impairment (Nie and Yang 2017). Additionally, Tang et al. reported that gradual aerobic training for 2 weeks promoted the number of microvessels surrounding the area of hippocampus ischemia by upregulating the expression of MT1-MMP, downregulating the expression of RECK on vascular endothelial cells around the cerebral ischemic area and alleviating neurological function after cerebral ischemia in adult male rats (Tang et al. 2018). On the other hand, 2 weeks of moderate-intensity aerobic training improved poststroke cognitive impairment through the caveolin-1/VEGF pathway partially by enhancing angiogenesis, neurogenesis and synaptic plasticity in adult male mice (Chen et al. 2019). Running wheel exercise training and skilled reaching training for 6 weeks ameliorated motor and cognitive functions in transient middle cerebral arterial occlusion rats through a mechanism probably associated with the axonal growth inhibitor pathway (suppressing the expression of the NgR1/RhoA/ROCK axon growth inhibitors and increasing the expression of the endogenous antagonists LOTUS/LGI1) in young rats (Li et al. 2022b). Furthermore, exercise training for 4 weeks increased the opening conductance level, time and probability of NMDA receptor channel activation and accelerated the formation of learningdependent longterm potentiation after cerebral ischemia in young rats (Yu et al. 2013). The duration of training is a key factor in increasing the efficiency of training in the recovery of patients. Many studies have used training in the short term, and studies with longer durations are limited. In this regard, Mankhong et al. reported that 12 weeks of aerobic training improved motor, balance, and memory functions via the inhibition of tau modification, especially tau acetylation, following infarction in a young rat MCAO model (Mankhong et al. 2020). Our results showed that exercise training and Co-Q10 for 12 weeks synergistically reinstated neurobehavioral deficits after transient middle cerebral artery occlusion via amelioration of synaptic plasticity in senescent rats.
How these two treatments put together could decreases infarction size, promoted synaptic plasticity and reduced behavioral deficits after cerebral ischemia during aging, is not yet clear. It is likely that exercise training and Co-Q10 supplementation both reinforce each other’s anti-inflammatory and antioxidatant effects. In this regard, it has been shown that Co-Q10 supplementation in exercised rats led to a decrease in cholesterol, triglyceride, lipid peroxidation (MDA) and heat shock proteins (HSP60, HSP70 and HSP90)(Pala et al. 2018). Another possible hypothesis is that Co-Q10 and exercise training amplified each other anti-apoptotic effects. Although, Astani et al. (2022) reported that high intensity interval training and Co-Q10 supplementation for 12 weeks insignificantly decreased Bax/Bcl2, Caspase 3 and Caspase 8 expression in the myocardial of obese rats (Astani et al. 2022). More studies are needed regarding the underlying molecular mechanism contributing to synergistic effects of chronic exercise training and Co-Q10 supplementation against cerebral ischemia-induced behavioral deficits. It should to be noted that one of the limitations of this research is that gavage-induced stress was not justified between groups; in fact there was no vehicle control for the Co-Q10 administration in the groups that did not received gavage. This lack of control may reduce the effectiveness of the Co-Q10 administration. It is possible that part of the effectiveness of the Co-Q10 administration was spent on eliminating the side effects of stress gavage-induced stress. Therefore, conceivable that Co-Q10 administration has more effects than what is reported in the findings.
Conclusion
In general, the results of this study showed that Co-Q10 and aerobic training for 12 weeks alone and synergistically ameliorated transient middle cerebral artery occlusion-induced memory and learning deficits and suppressed synaptic plasticity and reduced cerebral infarction volume and neurological score in senescent rats.
Data availability
Data will be made available on request.
Change history
14 June 2024
A Correction to this paper has been published: https://doi.org/10.1007/s00213-024-06636-3
References
Al-Jarrah M, Pothakos K, Novikova L, Smirnova IV, Kurz MJ, Stehno-Bittel L, Lau Y-S (2007) Endurance exercise promotes cardiorespiratory rehabilitation without neurorestoration in the chronic mouse model of parkinsonism with severe neurodegeneration. Neuroscience 149:28–37
Asadbegi M, Komaki H, Faraji N, Taheri M, Safari S, Raoufi S, Kourosh-Arami M, Golipoor Z, Komaki A (2023) Effectiveness of coenzyme Q10 on learning and memory and synaptic plasticity impairment in an aged Aβ-induced rat model of Alzheimer’s disease: a behavioral, biochemical, and electrophysiological study. Psychopharmacology: 1–17
Astani K, Bashiri J, Pourrazi H, Nourazar MA (2022) Effect of high-intensity interval training and coenzyme Q10 supplementation on cardiac apoptosis in obese male rats. ARYA Atherosclerosis 18:1
Barcelos IPd, Haas RH (2019) CoQ10 and aging. Biology 8:28
Boric K, Muñoz P, Gallagher M, Kirkwood A (2008) Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J Neurosci 28:8034–8039
Boyko M, Kutz R, Gruenbaum BF, Cohen H, Kozlovsky N, Gruenbaum SE, Shapira Y, Zlotnik A (2013) The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cogn Affect Behav Neurosci 13:847–859
Chen Z, Hu Q, Xie Q, Wu S, Pang Q, Liu M, Zhao Y, Tu F, Liu C, Chen X (2019) Effects of treadmill exercise on motor and cognitive function recovery of MCAO mice through the caveolin-1/VEGF signaling pathway in ischemic penumbra. Neurochem Res 44:930–946
Collaboration ATC (2008) Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 372:293–299
DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL (2008) Early disruptions of the blood–brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging 29:753–764
Dong T, Zhang Q, Hamblin MR, Wu MX (2015) Low-level light in combination with metabolic modulators for effective therapy of injured brain. J Cereb Blood Flow Metabolism 35:1435–1444
Garber JC (2011) GUIDE FOR THE CARE AND USE OF LABORATORY ANIMALS. USA
Gáspárová Z, Jariabka P, # 138tolc S (2008) Effect of transient ischemia on long-term potentiation of synaptic transmission in rat hippocampal slices. Neuroendocrinol Lett 29:702
Geng X, Wang Q, Lee H, Huber C, Wills M, Elkin K, Li F, Ji X, Ding Y (2021) Remote ischemic postconditioning vs. physical exercise after stroke: an alternative rehabilitation strategy? Mol Neurobiol 58:3141–3157
Ghaderi S, Komaki A, Salehi I, Basir Z, Rashno M (2023) Possible mechanisms involved in the protective effects of chrysin against lead-induced cognitive decline: an in vivo study in a rat model. Biomed Pharmacother 157:114010
Ghasemloo E, Oryan S, Bigdeli MR, Mostafavi H, Eskandari M (2021) The neuroprotective effect of MicroRNA-149-5p and coenzymeQ10 by reducing levels of inflammatory cytokines and metalloproteinases following focal brain ischemia in rats. Brain Res Bull 169:205–213
Hamakawa M, Ishida A, Tamakoshi K, Shimada H, Nakashima H, Noguchi T, Toyokuni S, Ishida K (2013) Repeated short-term daily exercise ameliorates oxidative cerebral damage and the resultant motor dysfunction after transient ischemia in rats. J Clin Biochem Nutr : 12–72
Jokinen H, Melkas S, Ylikoski R, Pohjasvaara T, Kaste M, Erkinjuntti T, Hietanen M (2015) Post-stroke cognitive impairment is common even after successful clinical recovery. Eur J Neurol 22:1288–1294
Karimi SA, Komaki S, Taheri M, Omidi G, Kourosh-Arami M, Salehi I, Komaki A (2021) Effects of the hydroalcoholic extract of Rosa Damascena on hippocampal long-term potentiation in rats fed high-fat diet. J Physiological Sci 71:1–9
Ke Z, Yip SP, Li L, Zheng X-X, Tong K-Y (2011) The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS ONE 6:e16643
Kim H, Seo JS, Lee S-Y, Ha K-T, Choi BT, Shin Y-I, Yun YJ, Shin HK (2020) AIM2 inflammasome contributes to brain injury and chronic post-stroke cognitive impairment in mice. Brain Behav Immun 87:765–776
Komaki H, Saadat F, Shahidi S, Sarihi A, Hasanein P, Komaki A (2017) The interactive role of CB1 receptors and L-type calcium channels in hippocampal long-term potentiation in rats. Brain Res Bull 131:168–175
Langa KM, Levine DA (2014) The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312:2551–2561
Li M, Fan H (2021) Progress on the study of fibroblast growth factors as novel therapeutics in post-stroke cognitive impairment. Aging Pathobiology Ther 3:48–55
Li G, Zou L, Jack CR Jr, Yang Y, Yang ES (2007) Neuroprotective effect of Coenzyme Q10 on ischemic hemisphere in aged mice with mutations in the amyloid precursor protein. Neurobiol Aging 28:877–882
Li C, Hu J, Liu W, Ke C, Huang C, Bai Y, Pan B, Wang J, Wan C (2022a) Exercise intervention modulates synaptic plasticity by inhibiting excessive microglial activation via Exosomes. Front Cell Neurosci 16
Li C, Sun R, Chen J, Hong J, Sun J, Zeng Y, Zhang X, Dou Z, Wen H (2022b) Different training patterns at recovery stage improve cognitive function in ischemic stroke rats through regulation of the axonal growth inhibitor pathway. Behav Brain Res 421:113730
Lin R, Yu K, Li X, Tao J, Lin Y, Zhao C, Li C, Chen LD (2016) Electroacupuncture ameliorates post-stroke learning and memory through minimizing ultrastructural brain damage and inhibiting the expression of MMP-2 and MMP-9 in cerebral ischemia-reperfusion injured rats. Mol Med Rep 14:225–233
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Lu J, Wang J, Yu L, Cui R, Zhang Y, Ding H, Yan G (2021) Treadmill Exercise attenuates cerebral ischemia–reperfusion Injury by promoting activation of M2 Microglia via Upregulation of Interleukin-4. Front Cardiovasc Med 8:735485
Mankhong S, Kim S, Moon S, Lee K-H, Jeon H-E, Hwang B-H, Beak J-W, Joa K-L, Kang J-H (2020) Effects of Aerobic Exercise on Tau and related proteins in rats with the Middle cerebral artery occlusion. Int J Mol Sci 21:5842
Manzar H, Abdulhussein D, Yap TE, Cordeiro MF (2020) Cellular consequences of coenzyme Q10 deficiency in neurodegeneration of the retina and brain. Int J Mol Sci 21:9299
Nie J, Yang X (2017) Modulation of synaptic plasticity by exercise training as a basis for ischemic stroke rehabilitation. Cell Mol Neurobiol 37:5–16
Omidi G, Karimi SA, Rezvani-Kamran A, Monsef A, Shahidi S, Komaki A (2019) Effect of coenzyme Q10 supplementation on diabetes induced memory deficits in rats. Metab Brain Dis 34:833–840
Omidi G, Rezvani-Kamran A, Ganji A, Komaki S, Etaee F, Asadbegi M, Komaki A (2020) Effects of Hypericum Scabrum extract on dentate gyrus synaptic plasticity in high fat diet-fed rats. J Physiological Sci 70:1–8
Pala R, Beyaz F, Tuzcu M, Er B, Sahin N, Cinar V, Sahin K (2018) The effects of coenzyme Q10 on oxidative stress and heat shock proteins in rats subjected to acute and chronic exercise. J Exerc Nutr Biochem 22:14
Pan G, Cheng J, Shen W, Lin Y, Zhu A, Jin L, Xie Q, Zhu M, Liu C, Tu F (2021) Intensive treadmill training promotes cognitive recovery after cerebral ischemia-reperfusion in juvenile rats. Behav Brain Res 401:113085
Parsa H, Ghasemi F, Ranjbar K, Komaki A (2021a) The Effect of Co-administration of Portulaca Oleracea and Plantago Psyllium plus Submaximal Swimming Training on Memory Deficit in Streptozotocin/Nicotinamide-Induced type 2 Diabetic rats
Parsa H, Moradi-Khaligh Z, Rajabi S, Ranjbar K, Komaki A (2021b) Swimming training and Plantago psyllium ameliorate cognitive impairment and glucose tolerance in streptozotocin–nicotinamide-induced type 2 diabetic rats. J Physiological Sci 71:1–12
Paxinos G, Watson C (2005) The Rat Brain in Stereotaxic Coordinates. 2005: Elsevier Academic Press. San Diego
Ranjbar K, Zarrinkalam E, Asl SS, Salehi I, Taheri M, Komaki A (2022) The effect of different exercise training modes on dentate gyrus neurodegeneration and synaptic plasticity in morphine-dependent rats. Neurochem Int 155:105304
Saa JP, Tse T, Baum C, Cumming T, Josman N, Rose M, Carey L (2019) Longitudinal evaluation of cognition after stroke–A systematic scoping review. PLoS ONE 14:e0221735
Safari S, Ahmadi N, Mohammadkhani R, Ghahremani R, Khajvand-Abedeni M, Shahidi S, Komaki A, Salehi I, Karimi SA (2021) Sex differences in spatial learning and memory and hippocampal long-term potentiation at perforant pathway-dentate gyrus (PP-DG) synapses in Wistar rats. Behav Brain Funct 17:1–11
Salehpour F, Farajdokht F, Mahmoudi J, Erfani M, Farhoudi M, Karimi P, Rasta SH, Sadigh-Eteghad S, Hamblin MR, Gjedde A (2019) Photobiomodulation and coenzyme Q10 treatments attenuate cognitive impairment associated with model of transient global brain ischemia in artificially aged mice. Front Cell Neurosci 13:74
Sawantdesai NS, Kale PP, Savai J (2016) Evaluation of anxiolytic effects of aripiprazole and hydroxyzine as a combination in mice. J Basic Clin Pharm 7:97
Shetty RA, Forster MJ, Sumien N (2013) Coenzyme Q10 supplementation reverses age-related impairments in spatial learning and lowers protein oxidation. Age 35:1821–1834
Sun J-H, Tan L, Yu J-T (2014) Post-stroke cognitive impairment: epidemiology, mechanisms and management. Annals of translational medicine 2
Tang Y, Zhang Y, Zheng M, Chen J, Chen H, Liu N (2018) Effects of treadmill exercise on cerebral angiogenesis and MT 1-MMP expression after cerebral ischemia in rats. Brain Behav 8:e01079
Thal DR, Grinberg LT, Attems J (2012) Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 47:816–824
Wen T, Zhang X, Liang S, Li Z, Xing X, Liu W, Tao J (2018) Electroacupuncture ameliorates cognitive impairment and spontaneous low-frequency brain activity in rats with ischemic stroke. J Stroke Cerebrovasc Dis 27:2596–2605
Xing Y, Yang S-D, Dong F, Wang M-M, Feng Y-S, Zhang F (2018) The beneficial role of early exercise training following stroke and possible mechanisms. Life Sci 198:32–37
Yu Q, Li X, Wang J, Li Y (2013) Effect of exercise training on long–term potentiation and NMDA receptor channels in rats with cerebral infarction. Experimental Therapeutic Med 6:1431–1436
Zarrinkalam E, Ranjbar K, Salehi I, Kheiripour N, Komaki A (2018) Resistance training and hawthorn extract ameliorate cognitive deficits in streptozotocin-induced diabetic rats. Biomed Pharmacother 97:503–510
Zhang X, Bi X (2020) Post-stroke cognitive impairment: a review focusing on molecular biomarkers. J Mol Neurosci 70:1244–1254
Zhang Q, Zhang J, Yan Y, Zhang P, Zhang W, Xia R (2017) Proinflammatory cytokines correlate with early exercise attenuating anxiety-like behavior after cerebral ischemia. Brain Behav 7:e00854
Author information
Authors and Affiliations
Contributions
K. R. conceptualization, methodology and data collection, Corresponding author, A. K. supervision, B. F. writing- original draft preparation, E. Z. data collection and writing- reviewing and editing
Corresponding authors
Ethics declarations
Consent for publication
All the authors agreed to publish this manuscript.
Competing interests
The authors confirm that the content of the present article has no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: This article was originally published an error related to data handling and labeling during the preparation of the representative images in figure 22 resulted in incorrect placement of figure panels in the original article.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ranjbar, K., Komaki, A., Fayazi, B. et al. Coenzyme Q10 and exercise training reinstate middle cerebral artery occlusion-induced behavioral deficits and hippocampal long-term potentiation suppression in aging rats. Psychopharmacology 241, 1577–1594 (2024). https://doi.org/10.1007/s00213-024-06583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-024-06583-z