Abstract
Rationale
Opioid injection drug use (IDU) has been linked to a more severe pattern of use (e.g. tolerance, overdose risk) and shorter retention in treatment, which may undermine abstinence attempts.
Objectives
This secondary data analysis of four human laboratory studies investigated whether current opioid IDU modulates subjective abuse liability responses to high-dose hydromorphone during intermediate-dose buprenorphine stabilization (designed to suppress withdrawal but allow surmountable agonist effects), and whether hydromorphone response magnitude predicts latency of return to opioid use during buprenorphine dose-tapering.
Methods
Regular heroin users not currently seeking treatment (n = 54; 29 current injectors, 25 non-injectors) were stabilized on 8-mg/day sublingual buprenorphine and assessed for subjective responses (e.g. ‘liking’, craving) to hydromorphone 24-mg intramuscular challenge (administered 16-hr post-buprenorphine) under randomized, double-blinded, controlled conditions. A subgroup (n = 35) subsequently completed a standardized 3-week outpatient buprenorphine dose-taper, paired with opioid-abstinent contingent reinforcement, and were assessed for return to opioid use based on thrice-weekly urinalysis and self-report.
Results
During buprenorphine stabilization, IDU reported lower ‘liking’ of buprenorphine and post-hydromorphone peak ‘liking’, ‘good effect’ and ‘high’ compared to non-IDU. Less hydromorphone peak increase-from-baseline in ‘liking’ (which correlated with less hydromorphone-induced craving suppression) predicted significantly faster return to opioid use during buprenorphine dose-tapering.
Conclusions
In these buprenorphine-stabilized regular heroin users, IDU is associated with attenuated ‘liking’ response (more cross-tolerance) to buprenorphine and to high-dose hydromorphone challenge and, in turn, this cross-tolerance (but not IDU) predicts faster return to opioid use. Further research should examine mechanisms that link cross-tolerance to treatment response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment of opioid use disorder (OUD) is an urgent challenge internationally, but particularly in the United States, where fewer than 15% of individuals with OUD receive any approved medication for OUD (Krawczyk et al. 2022; Substance Abuse and Mental Health Services Administration 2021). Buprenorphine, a partial µ-opioid receptor agonist, is a first-line medication for treating OUD. In persons diagnosed with OUD, buprenorphine maintenance produces dose (or concentration)-dependent occupancy of µ-opioid receptors that (at intermediate doses) reliably suppresses spontaneous opioid withdrawal signs/symptoms, and (at higher doses) can attenuate the reinforcing and respiratory depressant effects of experimental opioid challenge, and reduce outpatient opioid use (Greenwald et al. 2014; Laffont et al. 2022; Nasser et al. 2016).
Injection drug use (IDU) is associated with worse overall outcomes among people affected by OUD. Compared to non-IDU, those who inject opioids exhibit greater addiction severity (Darke et al. 2004; Dinwiddie et al. 1996; Strang et al. 1999), are more likely to experience non-fatal overdoses (Darke et al. 2004; Greenwald et al. 2023a; Kerr et al. 2007) and to die from overdose (Hall et al. 2022), remain in OUD treatment for shorter duration (Hillhouse et al. 2013; Potter et al. 2013; but see Darke et al. 2005), and manifest greater polysubstance use (Betts et al. 2016; Carpenter et al. 1998; Fischer et al. 2006; Highfield et al. 2007; Karamouzian et al. 2022; Makarenko et al. 2018).
Few studies have examined mechanisms that could underlie disparate OUD treatment outcomes between IDU and non-IDU. First, pharmacokinetic/pharmacodynamic interactions could play a role. Injection use delivers opioids directly into the bloodstream, bypassing liver metabolism, leading to higher brain concentration of opioids (Balyan et al. 2020). Rapid increases in opioid concentration may intensify drug reinforcing efficacy, making it more difficult to achieve successful treatment outcomes. Second, rapid onset of opioid effects via IDU may be accompanied by rapid offset of effects including increased withdrawal symptoms. Third, IDU could increase opioid tolerance (leading to escalation of use) and cross-tolerance beyond that produced by non-injection use of opioids. For instance, rapid kinetics and higher peak effects produced by injection of µ-receptor agonists (especially those acting via β-arrestin), or sequelae of IDU such as neuroinflammation (Pahan and Xie 2023; Zhou et al. 2021), could potentially alter µ-receptor signaling in ways that non-IDU might not (Adhikary and Williams 2022; Bailey and Connor 2005); these effects could modulate response to OUD medication treatment. Finally, injection and non-injection opioid users might differ on characteristics beyond polysubstance use including hepatic disease which can increase opioid exposure via impaired metabolism (Soleimanpour et al. 2016), or psychiatric disturbance (Connor et al. 2008; Fischer et al. 2006; Le et al. 2020; Wang and Liu 2012), which could modulate the efficacy of OUD medications.
In the clinical setting, these mechanisms are challenging to address due to many uncontrolled factors. The present study aimed to determine in a controlled setting among individuals not currently seeking treatment for OUD: (Aim 1) whether current IDU or related heroin-use features (e.g. amount of use) influence abuse liability indices (especially ‘liking’ of) experimental high-dose hydromorphone challenge during stabilization on buprenorphine at a moderate dose intended to suppress withdrawal symptoms but allow for surmountable agonist effects of hydromorphone and evaluation of cross-tolerance [Strain et al. 1997]); and (Aim 2) whether hydromorphone response magnitude predicts latency to return to illicit opioid use during a three-week outpatient buprenorphine dose taper. We predicted that IDUs would exhibit lower-magnitude positive response to hydromorphone (more cross-tolerance), and less hydromorphone suppression of heroin craving, compared to non-IDUs. We further hypothesized that hydromorphone response magnitude (level of cross-tolerance) would be associated with latency to return to opioid use (time to recurrence). Establishing phenotypes and/or mechanisms underlying treatment response in persons with OUD could help improve pharmacotherapy outcomes.
Materials and methods
Participants
The local Institutional Review Board approved this research, which was conducted according to the Declaration of Helsinki. Volunteers in four source studies, all registered on ClinicalTrials.gov (Greenwald and Hursh 2006 [NCT00218309]; Greenwald and Steinmiller 2009 [NCT00218361]; Greenwald 2010 [NCT00608504]; Greenwald et al. 2013 [NCT00684840]), were recruited using advertisements and word-of-mouth referrals. All participants provided informed consent.
Participant selection criteria and general procedures were identical across studies, and there were no duplicate participants across studies. Male and female volunteers, aged 18 to 55 years, self-identified as regular heroin users who were not currently seeking treatment for their opioid or other substance use. All participants met criteria for OUD, based on the Semi-Structured Clinical Interview for DSM-IV (First et al. 1996), which was updated using DSM-5 criteria (SCID; American Psychiatric Association 2015). Candidates were excluded if they met DSM-5 criteria for current moderate or severe substance use disorders other than opioid and tobacco (e.g. cocaine, alcohol, sedatives or cannabis), serious lifetime mental health problems (psychosis, bipolar, major depression that was not substance-induced, or PTSD), or reported antisocial/violent history. Candidates were excluded if they reported significant current health problems or were taking medications for such conditions (e.g. hepatitis, high blood pressure, pulmonary diseases, diabetes or other systemic diseases, chronic pain); had abnormal medical screening tests (e.g. electrocardiogram, liver enzymes, tuberculosis test with positive X-ray); or, because of required experimental hydromorphone injections, if they reported significant needle phobia as defined by a score > 15 on the 10-item Injection and Blood Withdrawal Phobia subscale of the Medical Fear Survey (Kleinknecht et al. 1999). Candidates whose urine samples at screening tested negative for opioids (< 300 ng/ml), or positive for methadone (≥ 300 ng/ ml), benzodiazepines (≥ 300 ng/ml), barbiturates (≥ 200 ng/ml) or whose breath sample was positive for alcohol (≥ 0.02%) were ineligible, whereas samples positive for cocaine (≥ 300 ng/ml) or cannabinoids (≥ 50 ng/ml) were allowed. Notably, these source studies were conducted prior to the widespread adulteration of the heroin supply with fentanyl (and its congeners), benzodiazepines, and other substances (e.g. xylazine). Thus, exclusions for urine drug screen results were likely based on intentional use and not a contaminated supply.
Design and procedures
The experimental protocol for these studies was described in Woodcock et al. (2015). Briefly, participants first underwent outpatient stabilization on 8-mg/day SL buprenorphine tablets (Subutex™, Reckitt-Benckiser, Hull, UK; supplied by Research Triangle Institute, Research Triangle Park, NC, USA), then dosing (8-mg/day SL) continued each evening (8pm) on a residential unit under monitored conditions. Stabilization on this moderate-dose buprenorphine was intended to suppress spontaneous opioid withdrawal symptoms while allowing for surmountable agonist effects of hydromorphone and evaluation of cross-tolerance under controlled conditions.
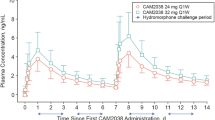
Phase 1. On days 1 and 2 of the residential stay, each participant was administered two double-blinded, intramuscular (deltoid) doses of hydromorphone (Dilaudid-HP™ in 10 mg/mL ampoules, purchased commercially) in counterbalanced order and constant volume (2.4 mL) at 12 noon (i.e. 16-hr after the prior-evening buprenorphine dose). On one session day of all source studies the dose was 24-mg; on the other session day, the dose was either 0-mg (physiological saline) or 12-mg, depending on the source study. For this Aim 1 analysis, only 24-mg session data are reported. Subjective responses were assessed 30-min pre-hydromorphone (11:30 am baseline) and at 30, 60, 90, 120, 150 and 180 min post-drug (ending at 3 pm). Participants were asked to attend to effects produced by each dose because, in later sessions, they could choose to take these drugs (results previously reported).
Phase 2. After the inpatient phase, participants in three of the four source studies (Greenwald and Steinmiller 2009; Greenwald 2010; Greenwald et al. 2013) completed a standardized three-week, double-blinded, outpatient buprenorphine dose-tapering schedule (4-mg/day during week one, 2-mg/day during week two, and 0-mg during week three). We previously reported that opioid abstinence-contingent reinforcement ($30 per consecutive opioid-negative urine samples, measured thrice weekly M-W-F) significantly slowed the speed of return to opioid use during the buprenorphine dose taper (Greenwald 2008), in comparison to the first source study (Greenwald and Hursh 2006) which did not have this contingency. Due to this important methodological difference, only the later three studies had comparable paired data for the Aim 2 analysis (hydromorphone response predicting return to opioid use during buprenorphine dose tapering with the opioid-abstinence contingency), thus, data from the first source study (Greenwald and Hursh 2006) were excluded from the Aim 2 analysis.
Measures
We monitored several vital signs: breathing rate, oxygen saturation, heart rate, and systolic and diastolic blood pressure. Given that respiratory depression is important for safety, and our experience from prior studies is that hydromorphone has minimal effect on cardiovascular responses during buprenorphine stabilization, we report only results on breathing rate and oxygen saturation in this analysis. However, we did not hypothesize that route of administration would differentially affect respiratory depression.
Heroin craving was assessed with a 10-item Brief Form (S.T. Tiffany, personal communication, 11/23/99) of the Heroin Craving Questionnaire (Schuster et al. 1995); each item was scored on a 1–7 Likert scale, such that total heroin craving scores could range from 10 (low) to 70 (high). Seven visual analog scale (VAS, 0-100) ratings were obtained: Any Drug Effect, Good Drug Effect, Bad Drug Effect, High, Like the Drug Effect, Stimulated, and Sedated. Opioid agonist and withdrawal symptoms were assessed using a 32-item Opioid Symptom Questionnaire (Schuster et al. 1995), with 16 Agonist scale items and 16 Withdrawal scale items. Each item was scored on a scale from 0 (not at all) to 4 (extremely), yielding total scores ranging from 0 to 64.
A modified Multiple Choice Procedure (Griffiths et al. 1993) was used to assess reinforcing value of the 24-mg hydromorphone injection, and is an alternative measure of abuse liability. After the final (3-hr) post-hydromorphone assessment, the participant used a questionnaire to make 44 independent choices between the drug dose and increasing money amounts ranging from $0.25 to $25.00. Money values increased by $0.25 steps from the lowest amount until $2.00, by $0.50 steps until $15.00, and by $1.00 steps until the highest amount. The amount at which the participant switched from choosing drug to money was defined as the reinforcing value of the hydromorphone dose.
Days to return to opioid use (range: 0–19; where 19 days indicated completion of dose taper without use) was quantified by combining two data sources: urinalysis and self-reported opioid use (see Woodcock et al. 2015).
Data analysis
This report is based on secondary analysis of data across the four source studies. Analyses were conducted using SPSS v.28 (IBM, Armonk NY). Criterion to reject the null hypothesis was p < .05. Descriptive statistics presented are mean (M) ± one standard deviation (SD). Analyses of variance (ANOVAs) and chi-square tests were used to evaluate IDU vs. non-IDU group differences.
Notably, opioid (cross) tolerance could occur to buprenorphine and/or hydromorphone in this context. Thus, we report scores for pre-hydromorphone baseline levels that reflect the residual agonist effect of buprenorphine (administered 16-hr before hydromorphone); unadjusted post-hydromorphone peak responses that reflect the additive agonist effects of buprenorphine and hydromorphone; and the post-hydromorphone peak subtracted from pre-hydromorphone baseline, which offers an estimate of the incremental agonist effect of hydromorphone. Hydromorphone responses (primarily focusing on subjective ‘liking’, ‘good effect’, and ‘high’; and secondarily breathing rate and oxygen saturation as indices of respiratory depression) were analyzed using mixed-model ANCOVAs, using session time points as repeated measures, IDU as group factor, and adjusting for covariates including randomized hydromorphone injection order (24-mg first or second) and other factors that differed between IDU groups. Huynh-Feldt adjusted P values were used for sphericity violations.
Hierarchical multiple regression analysis (with stepwise entry) was used to assess predictors of peak increase in hydromorphone liking (Aim 1) and return to opioid use (Aim 2). In Block 1, we entered relevant pre-experimental variables from Table 1 including age, race, injection use status, duration of heroin use, and lifetime heroin-use consequences; in Block 2, we entered baseline (buprenorphine-related) liking, as well as the peak increase in hydromorphone liking and hydromorphone-induced maximum decrease in heroin craving. Survival curve analysis was then conducted in GraphPad Prism v.9 (Boston, MA) to depict outpatient days to opioid use recurrence (following inpatient discharge) as a function of grouping factor.
Results
Participant characteristics
Fifty-four participants completed the hydromorphone challenge procedure. Table 1 presents characteristics of these individuals, grouped by current heroin injection-use status. Those who reported current injection heroin use were significantly more likely to be younger, self-identify their race as white, and to report more lifetime heroin-use adverse consequences (including an 11-fold higher rate of non-fatal overdose). Injection-use groups did not significantly differ on other baseline demographic or substance-use variables.
Thirty-five participants who completed the hydromorphone challenge subsequently underwent buprenorphine dose tapering with opioid abstinent-contingent reinforcement, and provided data for return to opioid use. Characteristics of these participants were similar to the overall sample, suggesting there was no selection bias in this subgroup.
Hydromorphone responses
Session baseline (pre-hydromorphone) drug liking scores, reflecting buprenorphine agonist effect, were significantly lower among IDU than non-IDU. Injection status groups did not significantly differ on other measures prior to hydromorphone administration.
ANCOVAs, controlling for race and age, examined the effect of current IDU vs. non-IDU group on the session time course of hydromorphone responses. Drug ‘high’ ratings increased (from baseline) significantly less for IDU than non-IDU, Group X Time F(1,49) = 2.83, p = .039. There was no significant effect of injection status on the time course of hydromorphone-induced ‘liking’ or ‘good effect’, craving, opioid agonist symptoms, nor respiratory depression.
Session time-course and hysteresis analysis (Supplemental Fig. 1) found that drug liking significantly increased 30-min post-hydromorphone and remained elevated, whereas craving (which was at moderately low levels) non-significantly decreased 30-min post-hydromorphone and did not change thereafter.
Absolute peak post-hydromorphone responses (increase [maximum] for subjective drug-effect ratings; and decrease [minimum] for heroin craving, respiration rate and oxygen saturation), reflecting the combined agonist effects of buprenorphine and hydromorphone, were examined as a function of injection status group. Absolute peak scores for ‘liking’, ‘good effect’ and ‘high’ were all significantly lower for IDU than non-IDU (Table 1). Analysis of peak change-from-baseline scores, reflecting the incremental effects of hydromorphone, found that only ‘high’ ratings were significantly lower for IDU than non-IDU (Table 1).
IDU vs. non-IDU status did not significantly affect the monetary reinforcing value of hydromorphone, as measured with the Multiple Choice Procedure (Table 1). However, hydromorphone reinforcing value significantly correlated with pre-hydromorphone baseline (i.e. buprenorphine-related) liking scores (r = .40, p = .003) and with absolute peak hydromorphone liking scores (r = .32, p = .019), but not with peak increase-from-baseline in liking (r = .06, p = .67).
Pearson correlations were computed between hydromorphone responses, followed by hierarchical multiple regression, with the goal of predicting hydromorphone peak increase-from-baseline in liking. As Fig. 1 (left panel) shows, bivariate analysis found that maximum hydromorphone-induced decrease in craving, younger age, female sex, and lower baseline (buprenorphine-related) liking were each significantly associated with greater hydromorphone-induced peak liking increase. In the hierarchical multiple regression model, younger age (β = − 0.407, ∆r2 = 0.137), female sex (β = 0.238, ∆r2 = 0.101), and greater hydromorphone-induced craving suppression (β = − 0.269, ∆r2 = 0.066; Fig. 1 right panel) predicted greater hydromorphone-induced peak liking increase, F(3,50) = 7.28, p < .001, adjusted r2 = 0.262. In contrast to peak change-from-baseline liking scores, absolute post-hydromorphone peak drug liking was significantly associated with current IDU, pre-hydromorphone baseline (i.e. buprenorphine-related) liking and monetary reinforcing value of hydromorphone (MCP), and was not significantly associated with age or craving (Supplemental Fig. 2).
Inter-relationships among experimental variables. A: Yellow box indicates hydromorphone challenge measures (maximum decrease in craving, maximum increase in drug liking). Variables outside the box are baseline factors, except “days to opioid use recurrence” during the buprenorphine dose-taper. Red lines depict significant (p < .05) zero-order negative correlations; dark blue lines depict significant positive correlations. Female sex and baseline drug liking (related to buprenorphine residual agonist effect) were associated with greater peak increase in hydromorphone liking, but did not survive in the regression (dashed lines), i.e. only younger age and greater hydromorphone-induced maximum decrease-from-baseline in craving significantly predicted greater hydromorphone-induced liking (solid lines). B: Less hydromorphone-induced craving suppression correlated with less peak increase-from-baseline in liking
Predicting speed of return to opioid use
Using bivariate Pearson correlations, hydromorphone-induced peak liking increase-from-baseline (r = .37, p = .015) and maximum decrease-from-baseline in craving (r = − .29, p = .043) were each associated with longer delay (more days) to opioid use recurrence during buprenorphine dose-tapering (Fig. 1 left panel). In hierarchical multiple regression analysis, pre-experimental variables in Block 1 (including injection use group) did not predict return to opioid use; only the relationship between peak liking increase from baseline (Block 2) predicted days to opioid use, F(1,33) = 5.12, p = .030, β = 0.367, r2 = 0.134. Thus, less hydromorphone liking (suggesting cross-tolerance) predicted more rapid (fewer days to) return to opioid use. Figure 2 left panel presents the correlation between hydromorphone peak increase in liking and return to opioid use. Figure 2 right panel presents the same data as an opioid abstinence survival function for higher, intermediate and lower ‘liking peak increase’ strata. The survival curves for these subgroups significantly differed, log-rank (Mantel-Cox) test, χ2(2) = 6.38, p = .041.
In hierarchical multiple regression analysis (A), only hydromorphone-induced increase in liking (see Fig. 1) predicted significantly slower return to opioid use during buprenorphine dose-tapering (correlation shown). In survival curve analysis (B), the percentages of participants who remained opioid abstinent (no return to opioid use) were greater for those with higher hydromorphone peak liking increase (∆ liking of 66–100, 35–65 and ≤ 34 points on the visual analog scale; tertile groups)
When this analysis was repeated using absolute peak post-hydromorphone liking scores to predict speed of return to opioid use, the results did not change (Supplemental Fig. 3).
Discussion
In this secondary data analysis of individuals with OUD who were not currently interested in treatment, opioid IDU (compared to non-IDU) reported significantly less ‘liking’ of buprenorphine prior to hydromorphone (administered 16-hr after daily 8-mg buprenorphine stabilization). Furthermore, IDU reported significantly less absolute ‘liking’, ‘good effect’ and ‘high’, but not drug reinforcing value (MCP) or respiratory depressant responses to 24-mg IM hydromorphone (which is equipotent to ≈ 160-mg morphine and ≈ 1.6-mg fentanyl [Bhatnagar and Pruskowski 2023]). These smaller absolute subjective responses reflect additive agonist effects of hydromorphone and buprenorphine, thus, a plausible interpretation of these findings is that IDU may exhibit tolerance to both opioids. However, when adjusting for pre-hydromorphone baseline (buprenorphine-related) ‘liking’ levels and covariates (injection status, sex, age, maximum hydromorphone-induced decrease in heroin craving, pre-experimental level of heroin use, and the severity measure of lifetime heroin-use consequences), only younger age and greater maximum decrease in craving predicted greater peak increase from baseline in hydromorphone ‘liking’ (or conversely, older age and less maximum craving decrease predicted less liking increase). Notably, smaller absolute and peak change in ‘liking’ responses (but not IDU status nor other factors including pre-experimental level of heroin use and lifetime heroin-use consequences) each predicted faster return to opioid use during buprenorphine dose-tapering coupled with an opioid abstinence incentive. Therefore, contrary to hypothesis, IDU only had an indirect effect on speed of return to opioid use (through its effect on peak liking).
Attenuated response to high-dose hydromorphone during buprenorphine stabilization could be interpreted to mean: (1) that buprenorphine is providing effective opioid blockade for the individual, (2) that the subject is cross-tolerant to hydromorphone, compared to his/her pre-experimental heroin use, or (3) both. We have found that parenteral administration of high-dose hydromorphone during stabilization on intermediate-dose buprenorphine (8 mg/day) (Greenwald and Hursh 2006; Greenwald and Steinmiller 2009; Greenwald 2010), or fentanyl administration during intermediate-dose methadone (60–70 mg/day) (Greenwald 2005), reliably and dose-dependently increases drug liking but does not markedly reduce opioid craving. This suggests a potential difference in the sensitivity of these two measures to acute agonist challenge. In the present analysis, less peak liking increase correlated with less craving suppression (or conversely, greater peak liking increase correlated with more craving suppression). This novel finding illustrates a relationship between the negative reinforcing effect (craving suppression) and positive reinforcing effect (liking) of hydromorphone, and potentially other opioids, that may occur during buprenorphine maintenance.
The empirical link between negative and positive reinforcement in this study challenges our understanding of the theoretical distinction between ‘wanting’ (craving) and ‘liking’ (Bechara et al. 2019; Berridge et al. 2009); specifically, the present findings suggest there may be instances, for example, in persons who are physically dependent on opioids or other substances, where these two processes interact. However, as this finding is a correlation, it is unclear whether (1) craving and liking responses are coincident (not causally related) and instead, may be modulated by extraneous factors not accounted for here, (2) suppression of craving (and associated interoceptive state) facilitates increased liking, or (3) increased liking (and associated interoceptive state) facilitates craving suppression. Session time-course and hysteresis analysis found that drug liking markedly increased 30-min post-hydromorphone and remained elevated, whereas craving levels (which were moderately low, likely due to buprenorphine stabilization) non-significantly decreased 30-min post-hydromorphone and did not change thereafter. Thus, while the first hypothesis above may seem plausible and parsimonious, it remains possible that buprenorphine suppression of craving may have created a floor effect for some individuals, such that hydromorphone could not substantially reduce craving further. Regardless, the interplay of craving suppression and drug liking has potential to explain risk of recurrent opioid use in those with OUD and should be investigated further.
In the present analysis, smaller hydromorphone peak ‘liking’ responses (but neither IDU status nor other factors) predicted faster return to illicit opioid use during outpatient buprenorphine dose-tapering coupled with an opioid abstinence incentive. The interpretation of this finding is complex. Minimal response to opioid use (blockade) is a desired feature of buprenorphine maintenance and typically obtained at higher treatment doses leading to greater brain and plasma concentrations (Greenwald et al. 2014; Laffont et al. 2022); yet, between-subject variability in opioid blockade remains poorly understood and, to our knowledge, no prior studies have addressed whether opioid blockade predicts risk or speed of return to use during agonist maintenance or dose-tapering. Notably, our finding does not align with the hypothesis that greater blockade of hydromorphone effect during buprenorphine maintenance protects against return to opioid use during buprenorphine dose tapering. However, it is essential to state, this does not argue against the role of higher-dose buprenorphine treatment to suppress withdrawal and protect against agonist effects of illicit opioid use during treatment (i.e. independent of dose tapering).
Here, we consider alternative (not mutually exclusive) hypotheses that could potentially explain the present findings, which are candidates for future study. The first hypothesis is that someone who uses an illicit opioid (mimicked by hydromorphone herein) during buprenorphine maintenance but experiences a smaller net increase in liking could be more opioid-tolerant and less able to experience agonist effects of opioids, including buprenorphine (especially as the maintenance dose is decreased during tapering); this lack of agonist effect could increase risk for return to illicit opioid use in these individuals. In this regard, we recently found in a large clinical trial, controlling for other factors, that IDU who were randomized to receive a higher dose of extended-release buprenorphine achieved more consistent opioid abstinence and were retained longer during maintenance treatment (Greenwald et al. 2023b). A second hypothesis is that repeated experiences of attenuated/blocked opioid response during buprenorphine maintenance (i.e. using an opioid with the goal of feeling better than at baseline, but not ‘liking’ or valuing the net change) achieves a partial extinction of opioid reinforcement; however, once buprenorphine dose-tapering occurs (leading to less baseline agonist effect and increased withdrawal symptoms), opioid motivation rebounds unless there are protective factors (e.g. supportive environment). A third hypothesis is that, in the studies that contributed data to this analysis, the concurrent opioid abstinence incentive during buprenorphine dose tapering – which delays the return to opioid use (Greenwald 2008) – could modulate the correlation between opioid (hydromorphone) blockade and opioid recurrence. Specifically, individuals who derive less pharmacological reinforcement from opioid agonist use during buprenorphine maintenance might also be less sensitive to non-drug rewards (i.e. abstinence-contingent money) which would increase vulnerability to recurrence of opioid use. This would be consistent with a ‘general reward-deficit’ hypothesis.
The present study has several limitations. First, although sample size for the parenteral high-dose hydromorphone challenge is larger than other published studies, the subsample for correlating hydromorphone response with return to opioid use is modest. Second, participants were recruited from a single urban area, so these results may not generalize to other locations. Third, these data were collected prior to the current wave in synthetic opioid use; patterns might differ for persons who use synthetic opioids such as fentanyl. Fourth, the maintenance dose of buprenorphine (8 mg/day) was intermediate and lower than optimal in clinical practice and was primarily intended to suppress opioid withdrawal symptoms; less overall hydromorphone response is expected with a higher buprenorphine dose, and more hydromorphone response would be observed with a lower buprenorphine dose (Greenwald et al. 2014). However, the aim of this research was to use a buprenorphine stabilization dose that would (1) suppress baseline opioid withdrawal (i.e. such that hydromorphone was not simply relieving an opioid deficit) and (2) allow a range of agonist effects from hydromorphone. We recognize these findings might not generalize across different buprenorphine and hydromorphone doses.
In conclusion, we found that in individuals with OUD who were not currently interested in treatment, younger age and less maximum decrease in craving predicted smaller peak increase from baseline in hydromorphone ‘liking’ (cross-tolerance) during buprenorphine maintenance which, in turn, predicted faster return to opioid use during buprenorphine dose-tapering. Further research should examine mechanisms that link cross-tolerance to treatment response.
References
Adhikary S, Williams JT (2022) Cellular tolerance induced by chronic opioids in the central nervous system. Front Syst Neurosci 16:937126
American Psychiatric Association (2015) Diagnostic and statistical Manual of Mental disorders, 5th edn. American Psychiatric Association, Washington, DC
Bailey CP, Connor M (2005) Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol 5:60–68
Balyan R, Hahn D, Huang H, Chidambaran V (2020) Pharmacokinetic and pharmacodynamic considerations in developing a response to the opioid epidemic. Expert Opin Drug Metab Toxicol 16:125–141
Bechara A, Berridge KC, Bickel WK, Morón JA, Williams SB, Stein JS (2019) A neurobehavioral approach to addiction: implications for the opioid epidemic and the psychology of addiction. Psychol Sci 20:96–127
Berridge KC, Robinson TE, Aldridge JW (2009) Dissecting components of reward: ‘liking’, ‘wanting’ and learning. Curr Opin Pharmacol 9:65–73
Betts KS, Chan G, McIlwraith F, Dietze P, Whittaker E, Burns L, Alati R (2016) Differences in polysubstance use patterns and drug-related outcomes between people who inject drugs receiving and not receiving opioid substitution therapies. Addiction 111:1214–1223
Bhatnagar M, Pruskowski J (2023) Opioid equivalency. StatPearls [Internet]. Accessed 8/22/23 at: https://www.ncbi.nlm.nih.gov/books/NBK535402/
Carpenter MJ, Chutuape MA, Stitzer ML (1998) Heroin snorters versus injectors: comparison on drug use and treatment outcome in age-matched samples. Drug Alcohol Depend 53:11–15
Connor KR, Pinquart M, Duberstein PR (2008) Meta-analysis of depression and substance use and impairment among intravenous drug users (IDUs). Addiction 103:524–534
Darke S, Hetherington K, Ross J, Lynskey M, Teesson M (2004) Non-injecting routes of administration among entrants to three treatment modalities for heroin dependence. Drug Alcohol Rev 23:177–183
Darke S, Ross J, Teesson M (2005) Twelve-month outcomes for heroin dependence treatments: does route of administration matter? Drug Alcohol Rev 24:165–171
Dinwiddie SH, Cottler L, Compton W, Abdallah AB (1996) Psychopathology and HIV risk behaviors among injection drug users in and out of treatment. Drug Alcohol Depend 43:1–11
First MB, Spitzer RL, Gibbon M, Williams JBW (1996) Structured clinical interview for DSM–IV Axis disorders—Patient Edition (SCID-I/P, Version 2.0). New York State Psychiatric Institute, Biometrics Research Department, New York
Fischer B, Manzoni P, Rehm J (2006) Comparing injecting and non-injecting illicit opioid users in a multisite Canadian sample (OPICAN cohort). Eur Addict Res 12:230–239
Greenwald MK (2005) Opioid craving and seeking behavior in physically dependent volunteers: effects of acute withdrawal and drug reinforcement opportunity. Exp Clin Psychopharmacol 13:3–14
Greenwald MK (2008) Opioid abstinence reinforcement delays heroin lapse during buprenorphine dose tapering. J Appl Behav Anal 41:603–607
Greenwald MK (2010) Effects of experimental unemployment, employment and punishment analogs on opioid seeking and consumption in heroin-dependent volunteers. Drug Alcohol Depend 111:64–73
Greenwald MK, Hursh SR (2006) Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of unit price and pre-session drug supply. Drug Alcohol Depend 85:35–48
Greenwald MK, Steinmiller CL (2009) Behavioral economic analysis of opioid consumption in heroin-dependent individuals: effects of alternative reinforcer magnitude and post-session drug supply. Drug Alcohol Depend 104:84–93
Greenwald MK, Lundahl LH, Steinmiller CL (2013) Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals. Psychopharmacology 225:811–824
Greenwald MK, Comer SD, Fiellin DA (2014) Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: implications for clinical use and policy. Drug Alcohol Depend 144:1–11
Greenwald MK, Moses TEH, Lundahl LH (2023a) Prediction of lifetime heroin overdose: injection use, quit attempts, suicidal ideation, and hepatitis. Oral presentation at the 85th annual meeting of the College on Problems of Drug Dependence, Denver CO
Greenwald MK, Wiest K, Haight B, Laffont CM, Zhao Y (2023b) Examining the benefit of a higher maintenance dose of extended-release buprenorphine in opioid-injecting users treated for opioid use disorder. Harm Reduct J 20:173
Griffiths RR, Troisi JR, Silverman K, Mumford GK (1993) Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav Pharmacol 4:3–13
Hall EW, Rosenberg ES, Jones CM, Asher A, Valverde E, Bradley H (2022) Estimated number of injection-involved drug overdose deaths, United States, 2000–2018. Drug Alcohol Depend 234:109428
Highfield DA, Schwartz RP, Jaffe JH, O’Grady KE (2007) Intravenous and intranasal heroin-dependent treatment-seekers: characteristics and treatment outcome. Addiction 102:1816–1823
Hillhouse M, Canamar CP, Ling W (2013) Predictors of outcome after short-term stabilization with buprenorphine. J Subst Abuse Treat 44:336–342
Karamouzian M, Pilarinos A, Hayashi K, Buxton JA, Kerr T (2022) Latent patterns of polysubstance use among people who use opioids: a systematic review. Int J Drug Policy 102:103584
Kerr T, Fairbairn N, Tyndall M, Marsh D, Li K, Montaner J, Wood E (2007) Predictors of non-fatal overdose among a cohort of polysubstance-using injection drug users. Drug Alcohol Depend 87:39–45
Kleinknecht RA, Kleinknecht EE, Sawchuck CN, Lee T, Lohr JM (1999) Medical fear survey: psychometric properties. Behav Therapist 22:109–119
Krawczyk N, Rivera BD, Jent V, Keyes KM, Jones CM, Cerdá M (2022) Has the treatment gap for opioid use disorder narrowed in the U.S.? A yearly assessment from 2010 to 2019. Int J Drug Policy 110:103786
Laffont CM, Ngaimisi E, Gopalakrishnan M, Ivaturi V, Young M, Greenwald MK, Heidbreder C (2022) Buprenorphine exposure levels to optimize treatment outcomes in opioid use disorder. Front Pharmacol 13:1052113
Le SM, Trouiller P, Thi HD et al (2020) Daily heroin injection and psychiatric disorders: a cross-sectional survey among people who inject drugs (PWID) in Haiphong, Vietnam. Drug Alcohol Depend 216:108334
Makarenko I, Mazhnaya A, Marcus R et al (2018) Concurrent drug injection during opioid agonist treatment among people who inject drugs in Ukraine. J Subst Abuse Treat 87:1–8
Nasser AF, Greenwald MK, Vince B, Fudala PJ, Twumasi-Ankrah P, Liu Y, Jones JP III, Heidbreder C (2016) Sustained-release buprenorphine (RBP-6000) blocks the effects of opioid challenge with hydromorphone in subjects with opioid use disorder. J Clin Psychopharmacol 36:18–26
Pahan P, Xie JY (2023 April) 26, online ahead of print) microglial inflammation modulates opioid analgesic tolerance. J Neurosci Res. https://doi.org/10.1002/jnr.25199
Potter JS, Marino EN, Hillhouse MP, Nielsen S, Wiest K, Canamar CP, Martin JA, Ang A, Baker R, Saxon AJ, Ling W (2013) Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (START). J Stud Alcohol Drugs 74:605–613
Schuster CR, Greenwald MK, Johanson CE, Heishman SJ (1995) Measurement of drug craving during naloxone-precipitated withdrawal in methadone-maintained volunteers. Exp Clin Psychopharmacol 3:424–431
Soleimanpour H, Safiri S, Nia KS, Sanaie S, Alavian SM (2016) Opioid drugs in patients with liver disease: a systematic review. Hepat Mon 16:e32636
Strain EC, Walsh SL, Preston KL, Liebson IA, Bigelow GE (1997) The effects of buprenorphine in buprenorphine-maintained volunteers. Psychopharmacology 129:329–338
Strang J, Griffiths P, Powis B, Gossop M (1999) Heroin chasers and heroin injectors: differences observed in a community sample in London, UK. Am J Addict 8:148–160
Substance Abuse and Mental Health Services Administration (2021) Key substance use and mental health indicators in the United States: results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA. Accessed 28 June 2023 https://www.samhsa.gov/data/sites/default/files/reports/rpt35325/NSDUHFFRPDFWHTMLFiles2020/2020NSDUHFFR1PDFW102121.pdf
Wang Q-L, Liu Z-M (2012) Characteristics of psychopathology and the relationship between routes of drug administration and psychiatric symptoms in heroin addicts. Subst Abuse 33:130–137
Woodcock EA, Lundahl LH, Greenwald MK (2015) Predictors of buprenorphine initial outpatient maintenance and dose taper response among non-treatment-seeking heroin dependent volunteers. Drug Alcohol Depend 146:89–96
Zhou J, Ma R, Jin Y, Fang J, Du J, Shao X, Liang Y, Fang J (2021) Molecular mechanisms of opioid tolerance: from opioid receptors to inflammatory mediators. Exp Ther Med 22:1004
Funding
NIH R01 DA015462 (MKG) from the National Institute on Drug Abuse, the Gertrude Levin Endowed Chair in Addiction and Pain Biology (MKG), the Michigan Department of Health and Human Services (Helene Lycaki/Joe Young, Sr. funds), and the Detroit Wayne Integrated Health Network supported this research. Funding sources had no role in the design or conduct of this study, nor in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MKG oversaw all aspects of the project including conceptualization, data collection and management, analyses, and drafting/editing the manuscript. TS assisted with literature review, interpreting findings and drafting sections of the manuscript. TEHM assisted with initial study conceptualization and data analysis, and edited the manuscript.
Corresponding author
Ethics declarations
conflicts of interest
MKG is a consultant for Indivior Inc., which makes buprenorphine products; however, Indivior played no role in this study. The authors declare no conflict of interest with respect to the conduct or content of this work.
Disclaimer
The contents of the paper are solely the responsibility of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Greenwald, M.K., Sogbesan, T. & Moses, T.E. Relationship between opioid cross-tolerance during buprenorphine stabilization and return to opioid use during buprenorphine dose tapering. Psychopharmacology 241, 1151–1160 (2024). https://doi.org/10.1007/s00213-024-06549-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-024-06549-1