Abstract
Rationale
Increasing evidence shows that imidazoline I2 receptor agonists enhance opioid-induced analgesia, suggesting that the combination of I2 receptor agonists with opioids could be a favorable strategy for pain control. However, the effect of I2 receptor agonists on the abuse liability of opioids is unknown. This study examined the impact of the I2 receptor agonist 2-BFI on some abuse-related behavioral effects of the opioid morphine in rats.
Objectives
The von Frey filament test was used to determine the antinociceptive effects of 2-BFI (intravenous, i.v.) in a rat model of complete Freund’s adjuvant (CFA)-induced inflammatory pain. IV self-administration was used to assess the reinforcing effects of 2-BFI alone and to assess the effects of non-contingent injections of 2-BFI (i.p.) on morphine self-administration. A two-lever drug discrimination paradigm in which rats were trained to discriminate 3.2 mg/kg morphine (i.p.) from saline was used to examine whether 2-BFI or another I2 receptor agonist 2-(4,5-dihydroimidazol-2-yl)quinoline hydrochloride (BU224) affected the discriminative stimulus effects of morphine.
Results
2-BFI could not maintain reliable self-administration behavior in rats with no pain or CFA-treated inflammatory pain. However, pretreatment with 2-BFI (i.p.) produced dose-dependent decreases in the dose-effect curve of morphine self-administration. Both 2-BFI and BU224 did not substitute for morphine but significantly attenuated the discriminative stimulus effects of morphine.
Conclusions
These results suggest that I2 receptor agonists do not enhance, but in fact appear to decrease, the abuse liability of opioids, further supporting the potential utility of I2 receptor agonist-opioid combination therapy for pain control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the search for a non-addictive painkiller commenced nearly 90 years ago, effective alternatives to opioids have still not been found. In fact, despite the enormous health care challenge that chronic pain presents, affecting over 100 million adults in the USA and imposing an economic burden of approximately $635 billion dollars each year (NIH 2013), no mechanistically novel painkillers have entered the market for the past 50 years (Kissin 2010). This is problematic since opioids, the current standard treatment for pain conditions, are not only poorly suited to long-term pain management (Chou et al. 2015; Sullivan and Howe 2013) but are also subject to a range of adverse effects including sedation, constipation, tolerance, dependence, and abuse liability. The recent opioid pandemic and the surge of opioid overdose-related deaths exacerbate this situation. Thus, novel painkillers are desperately needed.
Numerous recent studies have shown that ligands for the imidazoline I2 receptor (I2R) produce analgesia when administered alone (Li et al. 2014; Siemian et al. 2016a) but also additively or synergistically enhance opioid analgesia which indicates their potential for combination therapy with opioids (Lanza et al. 2014; Li et al. 2011; Siemian et al. 2016b; Thorn et al. 2015, 2011). These compounds have also been demonstrated to prevent tolerance and physical dependence to morphine as well as alleviate opioid withdrawal symptoms in rats (Thorn et al. 2016). Despite these findings, one concern that still exists for combination therapy is that I2R ligands may simultaneously enhance opioid abuse liability, a possibility which has not yet been tested.
This study sought to address this concern and examined the effects of selective I2R agonists on the abuse liability of the opioid morphine in rats. The reinforcing effects of morphine or 2-(2-benzofuranyl)-2-imidazoline hydrochloride (2-BFI) alone were assessed, as were the effects of 2-BFI pretreatment on the maintenance of morphine self-administration. Additionally, the discriminative stimulus effects of morphine alone and in combination with 2-BFI or 2-(4,5-dihydroimidazol-2-yl)quinoline hydrochloride (BU224) were examined.
Methods
Subjects
Male (n = 61 rats) Sprague-Dawley rats (Harlan, Indianapolis, IN) approximately 12 weeks old at experiment onset were individually housed on a 12/12-h light/dark cycle with behavioral experiments conducted during the light period. Subjects had free access to water, except during test sessions. Rats used in pain tests (n = 12) had free access to standard rodent chow in their home cages. Rats used in drug discrimination studies (n = 8) had slightly restricted access to food after their daily sessions such that their bodyweights were maintained at approximately 90% of their free-feeding counterparts. Rats used in self-administration studies (n = 41) had free access to food with the exception of animals pre-trained to respond for food, which were given restricted access to food similar to the drug discrimination group, followed by free access to food following the pre-training. Animals were maintained and experiments were conducted in accordance with guidelines of the International Association for the Study of Pain (Zimmermann 1983) and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington, DC) and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York (Buffalo, NY).
Induction of inflammatory pain
Inflammatory pain was induced by CFA inoculation as previously described (Li et al. 2014). Briefly, 0.1 ml of CFA (Sigma-Aldrich, St. Louis, MO) containing approximately 0.05 mg of Mycobacterium butyricum dissolved in paraffin oil was injected in the right hind foot pad of rats under isoflurane anesthesia (2% isoflurane mixed with 100% oxygen). Sufficient anesthesia was determined by the loss of righting and toe-pinch reflexes. Since neither the course of CFA-induced hypersensitivity (which lasts at least 1 month) nor repeated treatment with I2R ligands (which does not lead to tolerance) is expected to affect behavioral results (Li et al. 2014; Thorn et al. 2015), mechanical nociception tests were conducted between 2 and 8 days after CFA inoculation.
Mechanical hyperalgesia
Mechanical nociception was measured using von Frey filaments consisting of calibrated filaments (1.4–26 g; North Coast Medical, Morgan Hill, CA). Rats (n = 6) were placed in elevated plastic chambers with a wire mesh floor (IITC Life Science Inc., Woodland Hills, CA) and allowed to habituate prior to testing. Filaments were applied perpendicularly to the medial plantar surface of the hind paw from below the mesh floor in an ascending order of filament force, beginning with the lowest filament (1.4 g). A filament was applied until buckling occurred and maintained for approximately 2 s. The lowest force filament to elicit a paw withdrawal was recorded, with measurements taken immediately before and after intravenous (i.v.) 2-BFI injection as well as every subsequent 10 min until measurements returned to baseline. Experimenters were well-trained and treatments were blinded to the experimenter.
Catheterization and self-administration
Twelve standard operant chambers (Med Associates, St. Albans, VT) were used for all self-administration studies with details described elsewhere (An et al. 2012; Liu et al. 2016). The behavioral programs and data recording were controlled by Med-PC IV software (Med Associates, St. Albans, VT). For the saline, 2-BFI, and cocaine self-administration experiments, rats were first trained during five daily 1-h sessions to press the right (active) lever for 45 mg food pellets (BioServ, Frenchtown, NJ). A fixed ratio schedule was used which increased from FR1 to FR3 over the 5 days, with rats earning a maximum of 50 food pellets each day. Rats weighing 225–275 g were anesthetized with ketamine and xylazine (60 and 5 mg kg−1, respectively, i.p.) and implanted with chronic indwelling jugular catheters (Thorn et al. 2014). A 1-week recovery period following surgery was allowed during which catheters were flushed daily with 0.2 mL solution of enrofloxacin (4 mg/mL) in a heparinized saline solution (50 IU/mL in 0.9% sterile saline) to preserve catheter patency and prevent infection. Over the course of the experiments, catheter patency was periodically assessed via infusion of 0.5 mg kg−1 ketamine. A loss of muscle tone and righting reflex indicated catheter patency. If patency was lost, rats were re-catheterized using the left jugular vein. In some groups of rats, CFA was administered as described above 1 day prior to the beginning of training.
Upon recovery, rats began self-administration training. Due to prior food training, groups administering saline, 0.56 mg kg−1 infusion−1 2-BFI, or 0.75 mg kg−1 infusion−1 cocaine were trained on an FR3 schedule. Every third press on the active lever was accompanied by saline or drug infusion, a 5-s illumination of the stimulus light above the active lever, and a 10-s timeout period during which the house light was extinguished. Sessions ended after 2 h or 40 infusions had been earned, whichever occurred first.
Rats administering 0.3 mg kg−1 infusion−1 morphine were trained on an FR1 schedule that was gradually increased to FR5 according to each rat’s individual performance; no prior food training was used. Program settings were the same as used above with the exception that the number of maximum infusions was increased to 60. After stable responding for 0.3 mg kg−1 infusion−1 morphine at FR5 was achieved, test sessions began during which i.p. injections of saline, 5.6 mg kg−1 2-BFI, or 10 mg kg−1 2-BFI were administered 10 min prior to program initiation. Tests were given in an ascending order of 2-BFI dose with 2 days of stable responding separating each test. The completion of tests at this dose of morphine was followed by subsequent decreases in the maintenance dose to 0.1 and 0.03 mg kg−1 infusion−1 and then saline infusions. At least 5 days following maintenance dose transitions were allowed for responding to stabilize before tests began.
Drug discrimination
Drug discrimination studies were performed in commercially available two-lever operant chambers located within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, PA) as described previously (Qiu et al. 2014, 2015). Data were collected through an interface using Graphic State 3.03 software (Coulbourn Instruments Inc., Whitehall, PA). Training protocols were used and are detailed elsewhere (Li et al. 2013; Qiu et al. 2015). Briefly, daily sessions consisted of a 10-min timeout during which the chamber was dark and responses had no programmed consequence, followed by a 5-min response period, during which a house light and a cue light above each lever were illuminated and signaled availability of reinforcers. Ten consecutive responses (fixed ratio (FR) 10) on the correct lever resulted in food delivery (45 mg; BioServ Inc., Frenchtown, NJ). The correct lever was predetermined by an injection (e.g., right, saline; left, 3.2 mg kg−1 morphine). Response periods ended after 5 min or after delivery of 10 food pellets, whichever occurred first. Sessions were conducted 7 days per week according to a roughly double alternation schedule (e.g., saline, saline, drug, drug). Rats had to achieve at least 90% of the total active period responses on the correct lever for five consecutive or six out of seven consecutive sessions to qualify for drug test sessions, which were identical to training sessions except that ten consecutive responses on either lever delivered a food pellet. Rats needed to pass two consecutive sessions (one saline training session and one morphine training session) by responding at least 90% on the correct lever during each active period before each test. Morphine or saline were administered 10 min before training or test programs were initiated, and 2-BFI or BU224 was administered 10 min prior to saline or morphine on test days.
Data analysis
Measurements of CFA-induced mechanical hyperalgesia are presented as PWT (in g). The mean values (± SEM) were calculated from group data, and two-way repeated measures ANOVA (treatment × time), with time as within-subject factor, was used to determine statistical significance.
In the studies of intravenous self-administration, the mean number (± SEM) of 2-BFI, saline, or cocaine infusions were calculated from group data and plotted as a function of session. One- or two-way repeated measures ANOVA (treatment × session), with session as the within-subject factor, was used to determine statistical significance. For morphine self-administration, the mean number (± SEM) of morphine infusions was calculated from group data and plotted as a function of morphine dose per infusion. Two-way repeated measures ANOVA (2-BFI treatment × morphine dose), with 2-BFI dose as the repeated measure factor, was used to determine statistical significance.
In the studies of drug discrimination, % morphine-associated lever responding was calculated by dividing the number of morphine-associated lever responses by the total number of responses on either lever and multiplying by 100% for each rat; the group mean (± SEM) was plotted as a function of dose. The ED50 for morphine-associated lever responding was calculated for each rat individually; these values were used to calculate a group mean ED50 (95% CL). Morphine dose ratios were calculated by within-subject comparison of the ED50 of morphine after I2R ligand pretreatment versus the ED50 of morphine alone. The dose ratios were averaged within each treatment condition; if the dose ratios did not include 1.0, the shift was considered significant. For response rate, group mean values (± SEM) were plotted as a function of dose, and one-way repeated measures ANOVA (I2R ligand or morphine), with dose as the within-subject factor, was used to determine statistical significance. For all experiments, p < 0.05 was considered statistically significant.
Drugs
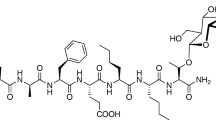
2-BFI hydrochloride was synthesized according to standard procedures (Ishihara and Togo 2007). Morphine sulfate and cocaine hydrochloride were provided by Research Technology Branch, National Institute on Drug Abuse, National Institutes of Health (Rockville, MD). All drugs were dissolved in 0.9% physiological saline.
Results
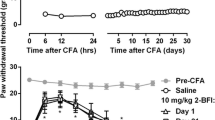
CFA injection into the hindpaw induced a marked hypersensitivity to mechanical stimuli that was stable over the 60-min test period (Fig. 1). Two-way repeated measures ANOVA revealed a significant main effect of CFA treatment (F (1, 112) = 101.12, p < 0.0001) but not time (F(7, 112) = 0.14, p > 0.05). Intravenous administration of 2-BFI dose-dependently increased the CFA-treated paw withdrawal threshold (Fig. 1). Two-way repeated measures ANOVA revealed significant treatment × time interaction (F(28, 175) = 3.26, p < 0.0001) with main effects of both 2-BFI treatment (F(4, 175) = 3.18, p < 0.05) and time (F(7, 175) = 19.76, p < 0.0001).
Pain-free rats did not increase intravenous self-administration of saline (Fig. 2, top). Instead, the number of saline infusions decreased over the course of eight daily sessions (one-way repeated measures ANOVA: F(7, 48) = 9.58, p < 0.0001). Compared to the saline group, two-way repeated measures ANOVA revealed that 2-BFI-administering rats earned significantly less infusions with significant main effects of 2-BFI (F(1, 84) = 8.38, p < 0.05) and session number (F(7, 84) = 20.10, p < 0.0001). In contrast, cocaine-administering rats earned significantly more infusions as compared to the saline group with main effects of cocaine × session interaction (F(7, 70) = 4.50, p < 0.001), cocaine (F(1, 70) = 9.89, p < 0.05), and session number (F(7, 40) = 3.83, p < 0.01).
Effects of saline (n = 7), 2-BFI (n = 7), or cocaine (n = 5) on intravenous self-administration behavior in pain-free rats (top) and saline (n = 8), 2-BFI (n = 8), or cocaine (n = 6) in CFA-treated rats (bottom) in daily 2-h sessions. Bottom panel also shows effects of cocaine substitution for 2-BFI or saline in CFA-treated rats (sessions 11 through 17). Ordinates, mean (± SEM) number of infusions; abscissa, session number. *p < 0.05, **p < 0.01, ***p < 0.001
The number of saline infusions earned by CFA-treated rats did not vary significantly over eight daily sessions (Fig. 2, bottom). Two-way repeated measures ANOVA revealed that 2-BFI-administering rats did not significantly differ from the saline group in the number of infusions. However, cocaine-administering rats earned significantly more infusions as compared to the saline group with a main effect of cocaine (F(1, 84) = 12.87, p < 0.01) but not session number. After two additional sessions of self-administering saline and 2-BFI, both groups of rats were switched to cocaine self-administration for seven sessions (Fig. 2, bottom, sessions 11 through 17). Following this transition, the number of infusions earned increased significantly (one-way repeated measures ANOVA: F(6, 105) = 21.27, p < 0.0001).
Morphine maintained robust, dose-dependent self-administration behavior with a classical inverted U-shape dose-effect curve (Fig. 3; one-way repeated measures ANOVA: F(3, 44) = 7.92, p < 0.001). Pretreatments of 2-BFI dose-dependently decreased the number of morphine infusions earned with significant main effects of 2-BFI × morphine interaction (F(6, 88) = 3.38, p < 0.01), 2-BFI dose (F(2, 88) = 42.63, p < 0.0001), and morphine dose (F(3, 88) = 6.20, p < 0.01). Post hoc results were indicated with asterisk.
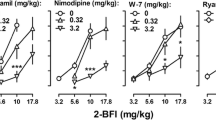
In rats trained to discriminate 3.2 mg kg−1 morphine from saline, morphine dose-dependently increased responding on the morphine-associated lever over a dose range of 0.32–3.2 mg kg−1 (Fig. 4, top; one-way repeated measures ANOVA: F(3, 28) = 17.54, p < 0.0001). The ED50 (95% CL) for morphine-associated lever responding was 0.74 (0.43, 1.27) mg kg−1 morphine. Within the same dose range, morphine did not significantly alter the response rate (Fig. 4, bottom). When administered alone, neither 2-BFI nor BU224 substituted for morphine within a 3.2–5.6 mg kg−1 dose range, but both drugs dose-dependently decreased the rate of responding (2-BFI: F(2, 21) = 17.61, p < 0.001; BU224: F(2, 21) = 10.33, p < 0.01). Injections of 3.2 and 5.6 mg kg−1 2-BFI as pretreatments produced ED50 s of 2.08 (1.26, 3.44) and 1.86 (1.25, 2.75) mg kg−1 morphine, respectively. Morphine dose ratios (95% CL) of 2.83 (1.73, 4.63) and 2.53 (1.42, 4.51) produced by 3.2 and 5.6 mg kg−1 2-BFI, respectively, revealed significant rightward shifts of the morphine dose–effect curve. One-way repeated measures ANOVA revealed that 5.6 mg kg−1 2-BFI in combination with morphine significantly altered the response rate as compared to vehicle (F(3, 28) = 6.07, p < 0.01) whereas 3.2 mg kg−1 2-BFI did not. Pretreatments of BU224 at 3.2 and 5.6 mg kg−1 resulted in ED50s of 1.90 (1.29, 2.80) and 2.81 (1.77, 4.47) mg kg−1 morphine, respectively. Morphine dose ratios (95% CL) of 2.59 (1.27, 5.27) and 3.83 (2.26, 6.47) produced by 3.2 and 5.6 mg kg−1 BU224, respectively, revealed that the rightward shifts of the morphine dose–effect curve were significant. Both doses in combination with morphine significantly reduced the response rate according to one-way repeated measures ANOVA (3.2 mg kg−1 BU224: F(3, 28) = 5.11, p < 0.01; 5.6 mg kg−1 BU224: F(3, 28) = 5.45, p < 0.01).
Effects of 2-BFI (left panels) and BU224 (right panels) on discriminative stimulus effects (top panels) and response rate (bottom panels) of morphine in rats trained to discriminate 3.2 mg kg−1 morphine from saline. Ordinates, percentage responding on the morphine-associated lever (top panels) or responses per second (bottom panels); abscissa, morphine dose (mg kg−1, i.p.)
Discussion
The main findings of this study were threefold. First, the imidazoline I2R agonist 2-BFI was not self-administered in either pain-free or CFA-treated rats, suggesting that it does not produce reinforcing effect under normal conditions or via pain relief (negative reinforcement). Second, 2-BFI shifted the dose-effect curve of morphine self-administration downward which suggests that it may reduce, or at least does not enhance, the reinforcing effects of morphine. Third, both 2-BFI and another I2R agonist BU224 attenuated the discriminative stimulus effects of morphine. When taken in consideration with previous studies, these data support the notion that I2R agonists may have therapeutic utility in combination therapy with opioids, wherein they enhance opioid analgesia but reduce some adverse effects including tolerance, dependence, and abuse liability.
Chronic pain management is the largest current public health problem and is exacerbated by a lack of effective pharmacotherapies (Kissin 2010; Turk et al. 2011). Opioids such as morphine are the current gold standard to which all analgesics are compared, but they also come with a range of side effects that complicate and restrict their use including constipation, tolerance, dependence, and abuse potential. These concerns can on the one hand leave pain sufferers undertreated from ineffectiveness and non-compliance, but on the other hand also lead to the overuse and abuse of these drugs. Thus, novel analgesics that can function as opioid-alternative stand-alone therapies or enhance the analgesia of currently existing opioids are greatly needed. Recently, the I2R has been established as one such novel target for the development of new pain therapies. I2R agonists are effective in many preclinical pain models as monotherapy (Li et al. 2014; Siemian et al. 2016a) and also produce additive or synergistic interactions with opioids (Lanza et al. 2014; Li et al. 2011; Siemian et al. 2016b; Thorn et al. 2015) while decreasing opioid tolerance and dependence (Thorn et al. 2016). More importantly, the I2R agonist CR4056 is currently under clinical development and a Phase 2 clinical trial demonstrated significant analgesic efficacy to treat knee osteoarthritis (Rovati et al. 2020). However, one concern that had not yet been addressed by preclinical research was the potential enhancement of opioid abuse liability by I2R agonists.
To investigate this possibility, we first examined the reinforcing effects of the prototypical I2R agonist 2-BFI alone by using intravenous self-administration. 2-BFI and BU224 are two very selective and well-characterized I2R agonists. Both show ~ 1000-fold selectivity on I2 receptor over I1 receptor and adrenergic ⍺2 receptor (Thorn et al. 2012). Importantly, our previous studies have demonstrated that the antinociceptive effects of 2-BFI can be blocked by I2R antagonist idazoxan (Li et al. 2014). In pain-free rats, 2-BFI did not maintain self-administration behavior at a unit dose expected to be behaviorally active, whereas cocaine, as a positive control, was reliably self-administered. Since I2R agonists would be used as analgesics and since pain relief can be a powerful negative reinforcer, we next investigated whether 2-BFI would maintain self-administration in rats treated with CFA, which models a chronic pain-like condition. Even in these rats, 2-BFI did not maintain self-administration whereas cocaine did. In a crossover experiment, we also demonstrated that the CFA-treated rats that previously had failed to self-administer 2-BFI reliably increased drug-taking behavior when the drug was changed to cocaine, suggesting that the lack of 2-BFI intake had not arisen due to some other anomaly with the experimental subjects. Thus, 2-BFI does not appear to have reinforcing effects indicative of abuse liability.
In contrast, morphine maintained robust self-administration, producing a classical inverted U-shape dose-effect curve. Since we were interested in whether 2-BFI would increase the abuse liability of morphine, we administered non-contingent pretreatments of 2-BFI at doses previously shown to produce analgesia (Siemian et al. 2016a). If 2-BFI did enhance morphine abuse liability, we would expect leftward or upward shifts in the morphine dose-effect curve. In fact, 2-BFI dose-dependently decreased morphine intake, producing downward shifts of the morphine dose-effect curve. This reduction in responding seems somewhat specific to morphine intake, as these 2-BFI doses decreased schedule-controlled responding for food rewards to a lesser degree (An et al. 2012; Siemian et al. 2016b). In any case, the most conservative conclusion to be drawn from this finding is that 2-BFI does not appear to increase the reinforcing properties of morphine at analgesic doses.
Lastly, we investigated whether I2R agonists affect the discriminative stimulus properties of morphine. Drug discrimination paradigms are used to assess the “subjective effects” of drugs, which are often closely related to their abuse liability. In rats trained to discriminate morphine from saline, neither 2-BFI nor BU224 alone induced significant responding on the morphine-associated lever when administered up to doses that began to affect responding rate. It is worth noting that both compounds do not significantly affect the general locomotor activity in rats up to a dose of 10 mg/kg (Thorn et al. 2012). These results first showed that generalization between the discriminative stimulus effects of I2R ligands and opioids is asymmetrical, since morphine partially or fully generalized to the drug cue in rats trained to discriminate an I2R ligand from saline (Qiu et al. 2014, 2015). However, pretreatments of either drug produced significant rightward shifts of the morphine dose-effect curve. This suggests that I2R agonists attenuate the discriminative stimulus effects of morphine, which again indicate that they may reduce opioid abuse liability.
A variety of factors can contribute to a reduction in behavioral indices of abuse-related effects by an intervention compound. Thus, it is difficult to propose a mechanistic explanation for the present results, and doubly difficult to do so for I2R ligands due to a lack of genetic and mechanistic information about I2Rs to begin with. Still, it is apparent that this function of I2R agonists is not due to orthosteric antagonism at µ opioid receptors. In vitro receptor binding studies showed that I2R agonists do not exhibit selectivity for µ opioid receptors (Qiu et al. 2015). If this was different in vivo, we would not have previously found enhancement of opioid analgesia, and the present study would have observed parallel rightward shifts in the morphine dose-effect curve for both self-administration and drug discrimination (Li et al. 2008; Tanda et al. 2016). Instead, I2R agonists may affect some downstream µ opioid receptor-mediated signaling mechanisms to produce the unique profile of enhanced analgesia but reduce abuse liability. Although speculative, one interesting hypothesis that may explain these results is biased agonism (DeWire et al. 2013; Soergel et al. 2014). In regard to the µ opioid receptor, biased agonism hypothesizes that analgesia is produced by mechanisms that engage G protein signaling, whereas side effects (e.g., respiration, tolerance) are produced by mechanisms that engage β-arrestins. Since the pattern of opioid receptor-I2R interactions appears to be to increase analgesia while decreasing side effects, it may be that I2R ligands directly or indirectly bias the activation of the µ opioid receptor towards G protein signaling and/or inhibit the engagement of the β-arrestin-dependent mechanisms. Further behavioral investigation into how I2R ligands alter the physiological side effects of opioids, such as respiratory and gastrointestinal dysfunction, will further clarify this hypothesis. Alternatively, effects of I2R agonists on central nervous system monoamine levels may also account for this interaction profile. In both preclinical and clinical studies, drugs that increase serotonin and norepinephrine levels have been shown to increase opioid analgesia while decreasing opioid consumption (Bedin et al. 2016; Higgins et al. 1994; Shen et al. 2013; Wang et al. 1995). Since I2R agonists are putative monoamine oxidase inhibitors and have been demonstrated to increase serotonin and norepinephrine in the CNS (Ferrari et al. 2011; Nutt et al. 1995; Ugedo et al. 1999), this may also explain their interactions with opioids. Indeed, our previous study has demonstrated that I2R agonist-induced antinociception was mediated by serotonergic and noradrenergic mechanisms with 5-HT1A, 5-HT2A and α1-adrenoceptor being particularly important (Siemian et al. 2018). Future studies using selective norepinephrine and serotonin receptor antagonists may help to confirm the involvement of these monoamines.
In summary, this study found that I2R agonists did not increase but in fact decreased abuse-related behavioral effects of morphine. 2-BFI was not found to produce reinforcing effects in pain-free or CFA-treated rats and also produced significant downward shifts of the morphine dose-effect curve in rats trained to self-administer morphine. Lastly, both 2-BFI and BU224 produced significant rightward shifts of the morphine dose-effect curve in rats discriminating morphine from saline. When considered with previous studies, these results further support that I2R agonists are useful in combination therapy with opioids, in that they enhance opioid analgesia while suppressing opioid side effects such as tolerance, dependence, and abuse liability.
Abbreviations
- 2-BFI:
-
2-(2-Benzofuranyl)-2-imidazoline
- ANOVA:
-
Analysis of variance
- BU224:
-
2-(4,5-Dihydroimidazol-2-yl)quinoline hydrochloride
- CFA:
-
Complete Freund’s adjuvant
- FR:
-
Fixed ratio
- PWT:
-
Paw withdrawal threshold
References
An XF, Zhang Y, Winter JC, Li JX (2012) Effects of imidazoline I(2) receptor agonists and morphine on schedule-controlled responding in rats. Pharmacol Biochem Behav 101:354–359
Bedin A, CaldartBedin RA, Vieira JE, Ashmawi HA (2016) Duloxetine as an analgesic reduces opioid consumption after spine surgery: a randomized, double-blind, controlled study. Clin J Pain. https://doi.org/10.1097/AJP.0000000000000471
Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, Dana T, Bougatsos C, Deyo RA (2015) The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med 162:276–286
DeWire SM, Yamashita DS, Rominger DH, Liu G, Cowan CL, Graczyk TM, Chen X-T, Pitis PM, Gotchev D, Yuan C, Koblish M, Lark MW, Violin JD (2013) A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther 344:708–717
Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, Giordani A, Lanza M, Caselli G (2011) Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res 4:111–125
Higgins GA, Wang Y, Corrigall WA, Sellers EM (1994) Influence of 5-HT3 receptor antagonists and the indirect 5-HT agonist, dexfenfluramine, on heroin self-administration in rats. Psychopharmacology 114:611–619
Ishihara M, Togo H (2007) Direct oxidative conversion of aldehydes and alcohols to 2-imidazolines and 2-oxazolines using molecular iodine. Tetrahedron 63:1474–1480
Kissin I (2010) The development of new analgesics over the past 50 years: a lack of real breakthrough drugs. Anesth Analg 110:780–789
Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G (2014) Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol 171:3693–3701
Li JX, McMahon LR, France CP (2008) Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltrexol in morphine-dependent and in nondependent rhesus monkeys. Psychopharmacology 195:479–486
Li JX, Zhang Y, Winter JC (2011) Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I(2) receptor ligands. Eur J Pharmacol 669:59–65
Li JX, Shah AP, Patel SK, Rice KC, France CP (2013) Modification of the behavioral effects of morphine in rats by serotonin 5-HT(1)A and 5-HT(2)A receptor agonists: antinociception, drug discrimination, and locomotor activity. Psychopharmacology 225:791–801
Li J-X, Thorn DA, Qiu Y, Peng B-W, Zhang Y (2014) Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Brit J Pharmacol 171:1580–1590
Liu JF, Thorn DA, Zhang Y, Li JX (2016) Effects of trace amine-associated receptor 1 agonists on the expression, reconsolidation, and extinction of cocaine reward memory. Int J Neuropsychopharmacol 19(7):pyw009
NIH (2013) Pain in America NINDS Chronic Pain Information Page. Bethesda, MD
Nutt DJ, French N, Handley S, Hudson A, Husbands S, Jackson H, Jordan S, Lalies MD, Lewis J, Lione L et al (1995) Functional studies of specific imidazoline-2 receptor ligands. Ann N Y Acad Sci 763:125–139
Qiu Y, He XH, Zhang Y, Li JX (2014) Discriminative stimulus effects of the novel imidazoline I(2) receptor ligand CR4056 in rats. Sci Rep 4:6605
Qiu Y, Zhang Y, Li JX (2015) Discriminative stimulus effects of the imidazoline I2 receptor ligands BU224 and phenyzoline in rats. Eur J Pharmacol 749:133–141
Rovati LC, Brambilla N, Blicharski T, Connell J, Vitalini C, Bonazzi A, Giacovelli G, Girolami F, D’Amato M (2020) Efficacy and safety of the first-in-class imidazoline-2 receptor ligand CR4056 in pain from knee osteoarthritis and disease phenotypes: a randomized, double-blind, placebo-controlled phase 2 trial. Osteoarthr Cartil 28:22–30
Shen F, Tsuruda PR, Smith JA, Obedencio GP, Martin WJ (2013) Relative contributions of norepinephrine and serotonin transporters to antinociceptive synergy between monoamine reuptake inhibitors and morphine in the rat formalin model. PLoS ONE 8:e74891
Siemian JN, Li J, Zhang Y, Li JX (2016a) Interactions between imidazoline I2 receptor ligands and acetaminophen in adult male rats: antinociception and schedule-controlled responding. Psychopharmacology 233:873–882
Siemian JN, Obeng S, Zhang Y, Zhang Y, Li JX (2016b) Antinociceptive interactions between the imidazoline I2 receptor agonist 2-BFI and opioids in rats: role of efficacy at the mu-opioid receptor. J Pharmacol Exp Ther 357:509–519
Siemian JN, Wang K, Zhang Y, Li JX (2018) Mechanisms of imidazoline I(2) receptor agonist-induced antinociception in rats: involvement of monoaminergic neurotransmission. Br J Pharmacol 175:1519–1534
Soergel DG, Subach RA, Burnham N, Lark MW, James IE, Sadler BM, Skobieranda F, Violin JD, Webster LR (2014) Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. PAIN® 155:1829–1835
Sullivan MD, Howe CQ (2013) Opioid therapy for chronic pain in the United States: promises and perils. Pain 154(Suppl 1):S94-100
Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL (2016) Lack of specific involvement of (+)-naloxone and (+)-naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 41:2772–2781
Thorn DA, Zhang Y, Peng BW, Winter JC, Li JX (2011) Effects of imidazoline I(2) receptor ligands on morphine- and tramadol-induced antinociception in rats. Eur J Pharmacol 670:435–440
Thorn DA, An X-F, Zhang Y, Pigini M, Li J-X (2012) Characterization of the hypothermic effects of imidazoline I2 receptor agonists in rats. Br J Pharmacol 166:1936–1945
Thorn DA, Jing L, Qiu Y, Gancarz-Kausch AM, Galuska CM, Dietz DM, Zhang Y, Li JX (2014) Effects of the trace amine-associated receptor 1 agonist RO5263397 on abuse-related effects of cocaine in rats. Neuropsychopharmacol: Off Publ Am Coll Neuropsychopharmacol 39:2309–2316
Thorn DA, Siemian JN, Zhang Y, Li JX (2015) Anti-hyperalgesic effects of imidazoline I2 receptor ligands in a rat model of inflammatory pain: interactions with oxycodone. Psychopharmacology 232:3309–3318
Thorn DA, Zhang Y, Li JX (2016) Effects of the imidazoline I receptor agonist 2-BFI on the development of tolerance and behavioral/physical dependence to morphine in rats. Br J Pharmacol 173:1363–1372
Turk DC, Wilson HD, Cahana A (2011) Treatment of chronic non-cancer pain. Lancet 377:2226–2235
Ugedo L, Pineda J, Martin-Ruiz R, Ruiz-Ortega JA, Artigas F (1999) Imidazoline-induced inhibition of firing rate of 5-HT neurons in rat dorsal raphe by modulation of extracellular 5-HT levels. Ann N Y Acad Sci 881:365–368
Wang Y, Joharchi N, Fletcher PJ, Sellers EM, Higgins GA (1995) Further studies to examine the nature of dexfenfluramine-induced suppression of heroin self-administration. Psychopharmacology 120:134–141
Zimmermann M (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110
Funding
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Award no. R01DA034806). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
The main idea of this study was from JS and J-XL. JS and J-XL designed the study, conducted the data analysis, and wrote the first draft of the manuscript. KW performed the pain tests. JS and DHL performed the self-administration experiments. KW performed the drug discrimination experiments. YZ provided 2-BFI and BU224. All authors contributed to and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siemian, J.N., Woodhouse, K., Liu, D.H. et al. The imidazoline I2 receptor agonist 2-BFI reduces abuse-related effects of morphine: self-administration and drug discrimination. Psychopharmacology 241, 479–487 (2024). https://doi.org/10.1007/s00213-023-06524-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-023-06524-2