Abstract
Rationale
Synthetic cannabinoid receptor agonists (SCRAs) are found in illicit smoking products, such as “K2” or “Spice.” Convulsions are commonly reported adverse effects of SCRAs but are poorly understood.
Objectives
We determined convulsant effects of SCRAs AB-PINACA, and 5F-ADB-PINACA in adult male NIH Swiss mice, and then determined if convulsant effects of AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, and JWH-018 elicited seizure-like effects using EEG.
Methods
Mice were administered SCRAs or pentylenetetrazole (PTZ) and placed in observation chambers where convulsant effects were scored. The capacity of the CB1R antagonist rimonabant, the benzodiazepine diazepam, or the non-specific CYP450 inhibitor 1-aminobenzotriazole (1-ABT) to attenuate convulsant effects was determined. Other mice were prepared with EEG headmounts to ascertain whether observed convulsions occurred concurrently with seizure-like effects by assessing root-mean-square (RMS) power, high amplitude EEG spike analysis, and videography.
Results
Mice receiving AB-PINACA or 5F-ADB-PINACA exhibited dose-dependent convulsant effects that were blocked by 10 mg/kg rimonabant pretreatment but not by pretreatment with 10 mg/kg diazepam; these convulsant effects were not altered in the presence of 100 mg/kg 1-ABT. Repeated administration of 10 mg/kg AB-PINACA and 3 mg/kg 5F-ADB-PINACA produced partial tolerance to convulsant effects but did not lead to cross-tolerance to PTZ-induced convulsions. In EEG studies, convulsant doses of AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, and JWH-018 did not produce seizures concomitantly with convulsions.
Conclusions
These data extend previous findings of convulsant effects of SCRAs and suggest that convulsant effects of AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, and JWH-018 are CB1R-mediated but are not associated with electroencephalographic seizures. These results further suggest that benzodiazepines may not effectively treat convulsions elicited by SCRA use in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seizures and convulsions have been reported following abuse of illegal “K2” or “Spice” herbal products in humans (Wolfe et al. 2019; de Havenon et al. 2011; Andreias et al. 2020; Schneir and Maumbacher 2012; Lapoint et al. 2011). K2/Spice products contain diverse synthetic cannabinoid receptor agonists (SCRAs) which are associated with numerous adverse effects in humans which are more severe than those reported for plant cannabis (Schwartz et al. 2015; Tatusov et al. 2019; Zaurova et al. 2016; Srisung et al. 2015). SCRAs typically bind with high affinity to both cannabinoid type 1 and 2 receptors (CB1R and CB2R) where they elicit full agonist efficacy (Rajasekaran et al. 2013; Aung et al. 2000). The resulting psychoactive effects of SCRAs occur with greater in vivo potency and effectiveness than those of Δ9-tetrahydrocannabinol (THC) (Ginsburg et al. 2012; Canazza et al. 2016; Canazza et al. 2017; Hruba and McMahon 2017).
Reports of SCRA-elicited seizure and convulsion in human users are relatively common in the clinical literature (Tait et al. 2016). One particularly interesting case report described convulsions elicited by the SCRA PB-22 in a human user and his dog (Gugelmann et al. 2014), demonstrating the cross-species generalizability of these effects. Laboratory studies using electroencephalography (EEG) have reported that administration of SCRAs such as JWH-018 and its fluorinated analogue AM-2201 cause seizure activity in rodents (Malyshevskaya et al. 2017; Funada and Takebayashi-Ohsawa 2018). Convulsant effects of SCRAs including CUMYL-4CN-BINACA (Kevin et al. 2019), JWH-073, AM-2201, and HU-210 (Breivogel et al. 2020) have also been reported. Our previous work determined the pharmacology underlying observable convulsant effects of SCRAs JWH-018 and 5F-AB-PINACA in mice (Wilson et al. 2019). Results from all of the studies above demonstrate that CB1Rs mediate both observed convulsions and EEG seizures across the SCRAs tested, but there is a lack of methodological standardization in these studies to evaluate seizures or convulsions induced by SCRAs. This may present future challenges in analysis of EEG data interpretation, especially due to the continual emergence of newer SCRAs on the illicit market. Use of root-mean-square (RMS) power analysis may mitigate this inconsistency. RMS is a validated, quantitative method to measure the intensity of electrical activity in the processing of biological signals produced in EEG (Mann et al. 1993; Krauss et al. 2018), electromyography (EMG) (Arabadzhiev et al. 2010; Farfan et al. 2010), and electrocardiography (ECG) (Lux et al. 2014; Hermans et al. 2017) and has proven its utility in the processing of EEG signals in pilocarpine- and pentylenetetrazole (PTZ)-induced mouse models of epilepsy (Naydenov et al. 2014; Phelan et al. 2015, 2017; Cozart et al. 2020). Here, we investigated the convulsant effects of structurally related SCRAs AB-PINACA and 5F-ADB-PINACA using an observational rating scale. As an extension of our previous work studying SCRA-elicited convulsions, we also sought to determine if convulsant doses of AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, and JWH-018 also produced concomitant seizure-like events in mice via EEG.
Materials and methods
Drugs
AB-PINACA (N-[(1S)-1-(Aminocarbonyl)-2-methylpropyl]-1-pentyl-1H-indazole-3-carboxamide) and 5F-AB-PINACA (N-[(2S)-1-Amino-3-methyl-1-oxobutan-2-yl]-1-(5-fluoropentyl) indazole-3-carboxamide) were purchased from Cayman Chemical (Ann Arbor, MI). 5F-ADB-PINACA (N-(1-Amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(5-fluoropentyl)-1H-indazole-3-carboxamide) was obtained from the Drug Enforcement Administration Special Research and Testing Laboratory (Springfield, VA). JWH-018 ((Naphthalen-1-yl)(1-pentyl-1H-indol-3-yl)methanone) and diazepam were obtained from the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD). Rimonabant was synthesized in the laboratory of Thomas E. Prisinzano, Ph.D., at the University of Kentucky School of Pharmacy, Department of Medicinal Chemistry (Lexington, KY). Pentylenetetrazol (PTZ) and 1-aminobenzotriazole (1-ABT) were purchased from Sigma-Aldrich (St. Louis, MO). AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, JWH-018, and rimonabant were all dissolved in a vehicle containing ethanol, Tween-80, and 0.9% physiologic saline at a ratio of 1:1:18. 1-ABT, PTZ, and diazepam were dissolved in 0.9% physiological saline. All injections were administered intraperitoneally (IP) at a constant volume of 0.01 cc/g.
Animals
Male NIH Swiss mice (Charles River Laboratories, Wilmington, MA), approximately 9 weeks of age upon arrival, were housed three animals per cage (15.24 × 25.40 × 12.70 cm) with ad libitum access to food and water. Rooms were maintained at 22 ± 2 °C at 45–50% humidity on a 12-h light/dark cycle. The animal facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All studies were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences and followed the National Institutes of Health Guide for Care and Use of Laboratory Animals. All mice were drug-naïve prior to the start of experiments and were randomly assigned to experimental groups.
Observational rating of convulsions
Convulsion intensity was scored as previously described22. Briefly, mice were injected and placed in cylindrical glass containers sealed with ventilated covers. Each mouse received an intensity score (“0”– “3”) at 15- and 30-min time points post drug administrations. These two scores were summed to provide a total convulsion intensity score and then averaged for the group. A score of “0” represents typical mouse behavior for the entire 15-min interval; “1” represents body rigidity, leg splay, and full body twitches; “2” represents all the criteria of a score of “1” as well as handling-induced convulsions at the 15- or 30-min time point; and a score of “3” was assigned when an undisturbed mouse exhibited at least one full-body convulsion within the 15-min scoring interval. Scores progressed from “0” to “1” following drug administration and then typically increased from “1” to either “2” or “3.”
Electroencephalogram (EEG) methodology
Mice were anesthetized with isoflurane and immobilized in a stereotaxic device using non-rupture ear bars. An incision was completely exposed the skull and then four small holes were drilled into the left and right frontoparietal regions. Four small stainless steel mouse EEG screws (#8403, Pinnacle Technology Inc., Lawrence, KS, USA) were placed into the holes and were used as electrodes, sited over the motor cortex (approximately 2 mm anterior to bregma and 1.2 mm lateral to the midline) and over the parietal cortex (approximately 2 mm posterior to bregma and 1.2 mm lateral to the midline) in both hemispheres. A prefabricated EEG headmount (#8402, Pinnacle Technology Inc.) was installed by soldering the wire leads of the three EEG recording screws to the head mount; the fourth EEG screw that was placed over the right frontal cortex served as the ground and, thus, had the wire removed via scissors. Dental acrylic was applied to secure the headmount to the skull. Mice were singly housed following surgery for 4–7 days and then were placed individually into a plastic arena where they could move freely upon being connected to a preamplifier tethered to the Pinnacle 8200 system. EEG signals and synchronized video were recorded. The signal was amplified with a high-pass filter of 1 Hz with epoch lengths of 10 s. The sampling rate was 400 Hz, and the video frame rate was 30 frames/s. One of the EEG channels was randomly selected for RMS power analysis. The full frequency was set at 0 to 1000 Hz, and high amplitude EEG spikes were observed by manually scrolling through 1-min windows of EEG tracings via offline Sirenia Seizure Pro software version 2.1.3 (Pinnacle Technology Inc., Lawrence, KS, USA). A seizure was defined as high-amplitude EEG spikes (≥ 240 µV) that were rhythmically distinct spike-wave discharges lasting for at least 5 s, which is based on criteria defined provided by other research labs (Luttjohann et al. 2009; Van Erum et al. 2019). To determine convulsion latency, animal behavior from the video was assessed using the convulsion rating scale. Only spontaneous convulsions (an intensity score of “3”) were used.
Experimental design
Dose–effect studies
Mice (n = 5/drug dose; total of 60 animals) were injected IP with AB-PINACA (0.3, 1, 3, and 10 mg/kg), 5F-ADB-PINACA (0.3, 1, and 3 mg/kg), cannabinoid vehicle, saline, or PTZ (30, 40, and 50 mg/kg). Following drug administration, mice were placed into observation chambers for 30 min. Convulsion intensity was scored according to the observational rating scale previously described.
Acute pretreatment studies
Mice (n = 5/drug/pretreatment; total of 60 animals) received a pretreatment of saline, 10 mg/kg rimonabant, or 10 mg/kg diazepam 30 min prior to IP administration of cannabinoid vehicle or convulsant doses of AB-PINACA (10 mg/kg), 5F-ADB-PINACA (3 mg/kg), or PTZ (50 mg/kg). In a separate study, mice (n = 5/drug/pretreatment; total of 30 animals) were pretreated IP with either 100 mg/kg 1-ABT or the vehicle for 1-ABT (saline) 120 min prior to administration of 10 mg/kg AB-PINACA, 3 mg/kg 5F-ADB-PINACA, or 50 mg/kg PTZ. Following administration of AB-PINACA, 5F-ADB-PINACA, or PTZ, mice in either study were then placed into observation chambers for 30 min to be scored according to the observational convulsion rating scale.
Cross-tolerance studies
Mice (n = 5/group; total of 10 animals) received daily IP injections of either 10 mg/kg AB-PINACA or 3 mg/kg 5F-ADB-PINACA every 24 h for 5 days. The day following the final SCRA injection, mice were challenged with a single IP injection of PTZ (50 mg/kg). Immediately following each injection (daily SCRA treatment or PTZ test), mice were placed into observation chambers and scored for 30 min according to the observational convulsion rating scale.
EEG studies
Mice (n = 4/group; total of 24 animals) were injected IP with convulsant doses of AB-PINACA (10 mg/kg), 5F-AB-PINACA (10 mg/kg), 5F-ADB-PINACA (3 mg/kg), JWH-018 (10 mg/kg), or PTZ (50 mg/kg) or with the cannabinoid vehicle or saline to assess seizure-like effects. An 8-h baseline EEG was recorded prior to the experiment. At least 10 min of baseline EEG was recorded on the day of the test, followed by 4 h of EEG recording after drug administration. Because convulsions were observed during the first 30 min post drug injection, only the first 30 min of EEG recording following convulsant drug administration is reported here.
Statistical analyses
In dose–effect convulsion studies, a Kruskal–Wallis one-way analysis of variance (ANOVA) was performed followed by a Dunn’s multiple comparisons test for each drug dose compared to its corresponding control (cannabinoid vehicle vs. AB-PINACA and 5F-ADB-PINACA; saline vs. PTZ). In the rimonabant and diazepam pretreatment studies, a one-way ANOVA followed by a Newman-Keuls multiple comparisons test was performed. In the 1-ABT pretreatment studies, Kruskal–Wallis one-way ANOVA followed by Dunn’s multiple comparisons test was performed within drug treatment group. In cross-tolerance convulsion studies, a repeated two-way (day and drug) ANOVA followed by Tukey’s multiple comparisons test was used. In the EEG studies, a one-way ANOVA followed by a Tukey’s multiple comparisons test was performed on the RMS values, number of high amplitude EEG spikes, and convulsion latency. All Figures were drawn with, and statistical analyses were performed using GraphPad Prism software version 8.4.3 (San Diego, CA, USA). Statistical significance was defined as P < 0.05. Data are presented as group means ± standard error of the mean (S.E.M.). When indicators of variability are not shown, this demonstrates that the variance is contained within the point or bars.
Results
Dose-dependent convulsant effects of AB-PINACA, 5F-ADB-PINACA, and PTZ
No mice administered either saline (Fig. 1, open square) or cannabinoid vehicle (Fig. 1, filled square) exhibited any convulsion-associated signs during either 15-min scoring interval, resulting in intensity scores of zero for both groups. In contrast, significant main effects of drug were detected [H(7) = 37.31, P < 0.05]. For PTZ (Fig. 1, open circles), dose-dependent increases in convulsion intensity were elicited, resulting in a significantly different convulsion score at 50 mg/kg than that of the saline control (Z = 3.436, P < 0.05). Similarly, AB-PINACA (Fig. 1, filled diamonds) dose-dependently increased convulsion intensity, and convulsion scores significantly different from that of the cannabinoid vehicle were obtained following doses of 3 and 10 mg/kg (Z = 3.102 and 3.623, respectively; P < 0.05 for both comparisons). Although the 3 and 10 mg/kg doses of AB-PINACA were not significantly different from each other (Z = 0.5208, P > 0.05), the 10 mg/kg dose was used in subsequent studies because this dose elicited maximal convulsant effects. 5F-ADB-PINACA (Fig. 1, open triangles) also elicited dose-dependent convulsant effects, with intensity significantly greater than vehicle at 3 mg/kg (Z = 3.608, P < 0.05). AB-PINACA and 5F-ADB-PINACA were both more potent convulsants than PTZ.
Convulsant effects of saline (“SAL”; open square), cannabinoid vehicle solution (“VEH”; filled square), synthetic cannabinoid receptor agonists (SCRAs) AB-PINACA (filled diamonds) and 5F-ADB-PINACA (open triangles), and pentylenetetrazol (“PTZ”; open circles) in mice (n = 5/treatment group). Abscissa: dose of drug in milligram per kilogram on a logarithmic scale. Ordinate: convulsion score using an observational scale. Points represent group means, while error bars indicate ± S.E.M. Lack of error bars indicates that the variability is contained within the point. The single asterisk indicates significant differences from the VEH for the SCRAs. The number sign indicates significant differences from SAL for PTZ treatment group. Statistical significance is defined as P < 0.05
Pharmacological dissociation of convulsant effects of AB-PINACA and 5F-ADB-PINACA from those of PTZ
Consistent with the data reported above, administration of AB-PINACA, 5F-ADB-PINACA, or PTZ 30 min after saline pretreatment (Fig. 2, filled bars) elicited significant convulsions in mice [F(11,48) = 26.35, P < 0.05]. Mice pretreated with 10 mg/kg of rimonabant (Fig. 2, gray bars) were significantly protected from the convulsant effects of AB-PINACA (q = 7.940, P < 0.05) and 5F-ADB-PINACA (q = 7.940, P < 0.05) but not from convulsions elicited by PTZ. Conversely, pretreatment with 10 mg/kg diazepam (Fig. 2, open bars) significantly attenuated the convulsant effects of PTZ (q = 5.068, P < 0.05) but did not alter the convulsant effects of AB-PINACA or 5F-ADB-PINACA.
Convulsant effects of vehicle, 10 mg/kg AB-PINACA, 3 mg/kg 5F-ADB-PINACA, or 50 mg/kg PTZ administered 30 min after treatment with saline (filled bars), 10 mg/kg rimonabant (gray bars), or 10 mg/kg diazepam (open bars) in mice (n = 5/treatment group). Abscissa: dose of drugs in milligram per kilogram. Ordinate: convulsion score using an observational scale. Bars represent group means, while error bars indicate ± S.E.M. The single asterisk indicates significant differences from the saline pretreatment control within drug. Statistical significance is defined as P < 0.05
Lack of involvement of cytochrome P450-mediated phase I metabolism in convulsant effects of AB-PINACA and 5F-ADB-PINACA
Replicating the previous findings, administration of AB-PINACA, 5F-ADB-PINACA, or PTZ (Fig. 3, filled bars) 2 h after saline injection induced significant convulsant effects in mice [H(5) = 13.43, P < 0.05]. Inhibition of cytochrome P450-mediated phase I metabolism did not impact the convulsant effects of any of the drugs studied as similarly intense convulsions were observed in mice administered AB-PINACA (Z = 1.862, P > 0.05), 5F-ADB-PINACA (Z = 1.426, P > 0.05), or PTZ (Z = 0.4358, P > 0.05) 2 h after pretreatment with 100 mg/kg 1-ABT (Fig. 3, open bars).
Convulsant effects of 10 mg/kg AB-PINACA, 3 mg/kg 5F-ADB-PINACA, or 50 mg/kg PTZ administered 120 min after saline (filled bars) or 100 mg/kg 1-ABT injection (open bars) in mice (n = 5/treatment group). Abscissa, ordinate, and all other graph properties as described in Fig. 2
Repeated administration of convulsant doses of AB-PINACA and 5F-ADB-PINACA do not elicit cross-tolerance to PTZ-induced convulsant effects
In these studies, significant main effects of drug [F(2,12) = 77.61, P < 0.05] and of day [F(5.60) = 8.828, P < 0.05] were detected, and there was a significant drug × day interaction [F(10,60) = 2.688, P < 0.05]. As expected, 10 mg/kg AB-PINACA (Fig. 4, filled diamonds) and 3 mg/kg 5F-ADB-PINACA (Fig. 4, open triangles) elicited intense convulsant effects upon initial administration. Repeated daily administration of either AB-PINACA or 5F-ADB-PINACA elicited a progressive but partial tolerance to convulsant effects. Mice administered 10 mg/kg AB-PINACA exhibited significantly less intense convulsions on the third (q = 5.683; P < 0.05), fourth (q = 5.683, P < 0.05), and fifth (q = 4.736; P < 0.05) days compared to the first day of drug administration, and the convulsant effects observed on day 2 were significantly different from the third (q = 4.736; P < 0.05) and fourth (q = 4.736; P < 0.05) days. In mice administered 3 mg/kg 5F-ADB-PINACA, convulsions observed on day 1 were significantly greater than those observed on the second (q = 4.736; P < 0.05), third (q = 7.104; P < 0.05), fourth (q = 6.157; P < 0.05), and fifth days (q = 5.683; P < 0.05). On the day 50 mg/kg PTZ (Fig. 4, gray area) was administered, convulsion intensity scores for mice previously treated with AB-PINACA (closed circles) and 5F-ADB-PINACA (open circles) increased from the day before, although the effect was not significant due to large variability within each group.
Convulsant effects of single daily injections of 10 mg/kg AB-PINACA (filled diamonds) and 3 mg/kg 5F-ADB-PINACA (open triangles) in mice (n = 5/treatment group) over 5 consecutive days. On the “Test Day” (shaded region), mice previously treated with either 10 mg/kg AB-PINACA (filled circle) or 3 mg/kg 5F-ADB-PINCA (open circle) were challenged with 50 mg/kg PTZ 24 h after the final SCRA injection. Abscissa: day of injection. Ordinate: convulsion score using an observational scale. Points represent group means, while error bars indicate ± S.E.M. The single asterisk indicates significant differences from day 1, and “ ⊗ ” indicates significant differences from day 2 within AB-PINACA drug group. The number sign indicates significant differences from day 1 within 5F-ADB-PINACA drug group. Statistical significance is defined as P < 0.05
No electroencephalographic seizures observed during convulsions elicited by AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, or JWH-018
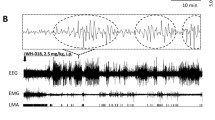
Representative EEG traces from mice receiving each of the experimental treatments are presented in Fig. 5. These raw waveforms are shaded to indicate occurrence of observable convulsions, allowing assessment of seizure-like activity at the time drug-elicited convulsions were scored. RMS power values for the full frequency band were calculated from these EEG waveforms in 1-min intervals immediately following administration of cannabinoid vehicle, 50 mg/kg PTZ, 10 mg/kg JWH-018, 10 mg/kg AB-PINACA, 10 mg/kg 5F-AB-PINACA, or 3 mg/kg 5F-ADB-PINACA by averaging the RMS values of 6 10-s epochs per drug group (Fig. 6). Because one mouse in the PTZ group died prematurely, an n = 3 for the PTZ-treated mice was used for calculations performed in Figs. 6, 7, and 8. For the convulsion latency data (Fig. 9), all mice from the PTZ group were used. Drug administration significantly impacted RMS power [F(5,180) = 13.78, P < 0.05] (Fig. 7). Analysis of RMS data up to 30 min post drug injection revealed that neuronal activity in the brain of PTZ-treated mice was significantly different from mice treated with AB-PINACA (q = 8.318; P < 0.05), 5F-AB-PINACA (q = 8.326; P < 0.05), 5F-ADB-PINACA (q = 9.659; P < 0.05), JWH-018 (q = 8.779; P < 0.05), and cannabinoid vehicle (q = 4.299; P < 0.05). Additionally, EEG activity following either 5F-ADB-PINACA (q = 5.360; P < 0.05) or JWH-018 (q = 4.480; P < 0.05) was significantly different from the cannabinoid vehicle group. Drug administration also significantly altered the number of EEG spikes recorded [F(4,14) = 30.41, P < 0.05] (Fig. 8). No high-intensity EEG spikes were recorded following administration of cannabinoid vehicle, but the number of spikes in the first 30-min following injection of PTZ was significantly greater than observed after injection of AB-PINACA (q = 12.75; P < 0.05), 5F-AB-PINACA (q = 12.87; P < 0.05), 5F-ADB-PINACA (q = 12.89; P < 0.05), and JWH-018 (q = 12.87; P < 0.05) (Fig. 8). Analysis of synchronized videography was evaluated according to the convulsion severity criteria of an intensity score of “3” previously described. Although convulsions were observed in mice treated with all the SCRAs tested, seizures did not occur simultaneously with the convulsions. There was a main effect of drug administration on convulsion latency [F(4,15) = 4.271, P < 0.05) (Fig. 9). The convulsion latency for animals treated with JWH-018 was significantly longer compared to mice treated with PTZ (q = 4.816; P < 0.05) or with AB-PINACA (q = 5.150; P < 0.05) (Fig. 9). One mouse from the PTZ treatment group expired less than 10 min after drug administration; however, no mouse from any of the SCRA treatment groups died during the recording period.
Representative EEG traces from mice administered 50 mg/kg PTZ (A), cannabinoid vehicle (B), 10 mg/kg JWH-018 (C), 10 mg/kg AB-PINACA (D), 10 mg/kg 5F-AB-PINACA (E), or 3 mg/kg 5F-ADB-PINACA (F) recorded from 0 to 15 min following injection. Shaded regions indicate times that convulsions were observed
Seizure-like effects 0 to 30 min following administration of 50 mg/kg PTZ (A), cannabinoid vehicle (B), 10 mg/kg JWH-018 (C), 10 mg/kg AB-PINACA (D), 10 mg/kg 5F-AB-PINACA (E), or 3 mg/kg 5F-ADB-PINACA (F) in mice (n = 3–4/group). Abscissae: time after injection. Ordinates: mean RMS power values in microvolts squared. Points represent the mean RMS power values in the full frequency bandwidth. Error bars indicate ± S.E.M., and lack of error bars indicates that the variability is contained within the point
RMS power values 0 to 30 min following administration of 50 mg/kg PTZ, cannabinoid vehicle (VEH), 10 mg/kg JWH-018, 10 mg/kg AB-PINACA, 10 mg/kg 5F-AB-PINACA, or 3 mg/kg 5F-ADB-PINACA in mice (n = 3–4/group). Abscissa: drug administered. Ordinate: mean RMS power values in microvolts squared. Bars represent group means, and error bars indicate ± S.E.M. The single asterisk indicates significant difference from the PTZ group. The number sign indicates significant differences from the VEH group. Statistical significance is defined as P < 0.05
EEG spikes following administration of 50 mg/kg PTZ, 10 mg/kg JWH-018, 10 mg/kg AB-PINACA, 10 mg/kg 5F-AB-PINACA, or 3 mg/kg 5F-ADB-PINACA in mice (n = 3–4/group). Abscissa: drug administered. Ordinate: averaged high-amplitude EEG spikes recorded from 0 to 30 min after drug injection. Bars represent group means, and error bars indicate ± S.E.M. The single asterisk indicates significant difference from the PTZ group. Statistical significance is defined as P < 0.05
Convulsion latency following administration of 50 mg/kg PTZ, 10 mg/kg JWH-018, 10 mg/kg AB-PINACA, 10 mg/kg 5F-AB-PINACA, or 3 mg/kg 5F-ADB-PINACA in mice (n = 4/group). Abscissa: drug administered. Ordinate: mean latency to exhibit the first spontaneous, whole body convulsion, in seconds. Bars represent group means, and error bars indicate ± S.E.M. The single asterisk indicates a significant difference from the PTZ group. The number sign indicates a significant difference from the AB-PINACA group. Statistical significance is defined as P < 0.05
Discussion
Similar to what we have previously reported for JWH-018 and 5F-AB-PINACA, the structurally related synthetic cannabinoids AB-PINACA and 5F-ADB-PINACA also dose-dependently induced convulsions in male mice. Here, AB-PINACA and 5F-ADB-PINACA elicited convulsions with greater potency and similar effectiveness to the chemical convulsant PTZ. 5F-ADB-PINACA elicited significant convulsions at 3 mg/kg, a dose one-half log lower than that required to induce convulsions with every other SCRA we have tested. In the pretreatment studies, convulsant effects elicited by AB-PINACA and 5F-ADB-PINACA were pharmacologically distinct from those of PTZ. Pretreatment with the CB1R antagonist/inverse agonist rimonabant abolished convulsant effects of AB-PINACA and 5F-ADB-PINACA at a dose which did not alter convulsant effects of PTZ. In contrast, no changes in SCRA-induced convulsant effects in male mice treated with AB-PINACA or 5F-ADB-PINACA were observed in the presence of 10 mg/kg diazepam pretreatment, although this pretreatment significantly attenuated PTZ-elicited convulsant effects. This is consistent with our previous studies where diazepam did not attenuate convulsions induced by either JWH-018 or 5F-AB-PINACA22, but in the present studies, we used a diazepam dose more than threefold larger than before. The fact that rimonabant, but not diazepam, dramatically reduces convulsant effects of SCRAs suggests that CB1Rs mediate these convulsant effects. Therefore, acute administration of a CB1R antagonist, like rimonabant, might be a useful treatment for these serious effects in humans. However, adverse psychiatric effects have been reported in humans chronically treated with rimonabant (King 2010), and rimonabant was demonstrated to induce seizures in a patient with a history of epilepsy (Braakman et al. 2009). Interestingly, the non-psychotropic phytocannabinoid cannabidiol (CBD) has demonstrated anticonvulsant and antiseizure effects in humans (Devinsky et al. 2018; Laux et al. 2019; Koo et al. 2020) and in animal models (Jones et al. 2010; Vilela et al. 2017; Kaplan et al. 2017; Gu et al. 2019) and has been approved for treatment of certain pediatric epilepsies by the US Food and Drug Administration. In addition, CBD has also been shown to attenuate cocaine-induced seizures in rodents (Gobira et al. 2015), while the structurally similar phytocannabinoid cannabidivarin (CBDV) also has anticonvulsant effects in rodents (Hill et al. 2012, 2013; Huizenga et al. 2019). It may be the case that these and other phytocannabinoids might be useful in the mitigation of convulsant effects of SCRAs, but no studies in this regard have yet been performed. Effective anticonvulsant drugs for the treatment of SCRA-induced convulsions are needed.
Phase I metabolism of AB-PINACA and 5F-ADB-PINACA has been studied in human liver microsomes and hepatocytes. Oxidative metabolism of the aminoalkylindole SCRA JWH-018 produces metabolites that retain pharmacological activity in vitro and in mice (Brents et al. 2011). Moreover, SCRAs in the aminoalkylindole class were shown to produce abundant metabolites in human urine via metabolic monohydroxylation and dihydroxylation (Hutter et al. 2012). Active hydroxylated metabolites of the indazole-derived SCRA AB-PINACA have also been described (Hutchison et al. 2018). However, oxidative phase I metabolites of AB-PINACA and 5F-ADB-PINACA did not appear to contribute to the convulsant effects observed in the present study, suggesting a possible difference between aminoalkylindole and indazole-carboxamide SCRAs. This is consistent with our previous convulsion data pertaining to 5F-AB-PINACA metabolism but distinct from our findings with JWH-018 where administration of 1-ABT significantly attenuated convulsion intensity (Wilson et al. 2019). Over 20 phase I metabolites of AB-PINACA have been identified as a result of several biotransformations that included carboxamide hydrolysis, hydroxylation, ketone formation, carboxylation, and other reactions to yield AB-PINACA carboxylic acid, carbonyl-AB-PINACA, and hydroxypentyl AB-PINACA (Wohlfarth et al. 2015). Additionally, AB-PINACA was recently found to inhibit the activity of CYP2C8, CYP2C9, CYP2C19, and CYP3A4 (Park et al. 2020). In a study of 5F-ADB-PINACA metabolism, via oxidative defluorination followed by carboxylation, 12 metabolites were produced (Carlier et al. 2017). Although it was determined that certain non-carboxylic acid metabolites of 5F-ADB-PINACA retained in vitro activity at cannabinoid receptors, metabolite contribution to the cannabimimetic effects of the parent drug are possible (Longworth et al. 2017). Nevertheless, in the present study, cytochrome P450-mediated phase I metabolites of AB-PINACA and 5F-ADB-PINACA did not contribute to convulsant effects of their respective parent drugs.
Repeated administration of AB-PINACA and 5F-ADB-PINACA for 5 days failed to induce complete tolerance to their convulsant effects. In contrast, we previously demonstrated that single daily administration of convulsant doses of 5F-AB-PINACA and JWH-018 resulted in complete tolerance to convulsant effects within 5 days (Wilson et al. 2019). Tolerance to drug-elicited convulsions does not only occur with SCRAs, as tolerance to cocaine-induced convulsions has also been shown to develop in cats (Castellani et al. 1978), monkeys (Matsuzaki 1978), and mice (Shimosato et al. 1996). Even so, tolerance development to a given drug can become limited due to pharmacokinetic (e.g., drug metabolism) and pharmacodynamic (e.g., receptor downregulation and internalization) factors, and this may be especially relevant to SCRAs (Fantegrossi et al. 2014). This suggests that certain cannabinoid effects are susceptible to rapid and dramatic tolerance in laboratory animals, although this tolerance develops at different rates for different effects (Elmore and Baumann 2018; Gomez et al. 2021). Although incomplete, tolerance to convulsant effects of AB-PINACA and 5F-ADB-PINACA did not confer cross-tolerance to PTZ-induced convulsions in male mice. This is consistent with our previous cross-tolerance experiments and further suggests that convulsant effects of SCRAs are mediated by mechanisms distinct from the convulsant effects of PTZ, most likely CB1Rs.
In the EEG studies, RMS values calculated during the first 30 min following drug administration resulted in increased neuronal activity and the occurrence of seizures and convulsions in male mice administered PTZ. In contrast, male mice treated with the SCRAs exhibited dampened neuronal activity and no seizures during observed convulsions. These EEG data are intriguing given that convulsant effects of all SCRAs were similar in magnitude to those of PTZ. Therefore, it was expected that animals treated with any of the SCRAs in the EEG experiments would also display seizure-like effects similar to those of PTZ during observed convulsions. The RMS power analysis performed in the present studies revealed that neuronal activity in mice administered convulsant doses of AB-PINACA, 5F-AB-PINACA, and 5F-ADB-PINACA is distinct from the EEG activity of PTZ-treated mice. It is unclear why the convulsant doses of the tested SCRAs failed to elicit PTZ-like electroencephalographic seizures, but provoked seizures likely occur due to the neurochemical imbalance between excitatory (glutamate) and inhibitory (GABA) neurotransmission in the brain (Buck et al. 1991; Diana and Marty 2004; Barker-Haliski and White 2015). With cannabinoid drugs, neurochemical imbalances may also occur given that activation of presynaptic CB1Rs on both glutamatergic and GABAergic axon terminals leads to decreased action potential summation and the inhibition of the release of glutamate or GABA, respectively (Marsicano and Lutz 1999; Riegel and Lupica 2004; Melis et al. 2004; Hoffman et al. 2017). Interestingly, an association between SCRA-induced seizure activity and the stimulation of glutamate release from the hippocampus was observed in mice administered AM-2201 (Funada and Takebayashi-Ohsawa 2018). Therefore, the results of the present study could suggest that larger doses of AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, and JWH-18 could inhibit hippocampal glutamate release to such an extent that seizures do not occur. Indeed, a limitation of this study is that only a single convulsant dose of each drug was tested in EEG experiments, and these doses were relatively large compared to related studies in the literature. One study reported that 2.5 mg/kg JWH-018 and 10 mg/kg THC demonstrated electroencephalographic seizure effects (Malyshevskaya et al. 2017). In another study, dose-dependent seizure effects of SCRAs AM-2201 and AB-CHIMINACA were observed at doses below 3 mg/kg (Funada and Takebayashi-Ohsawa 2018). In the present studies, we used SCRA doses of 10 mg/kg, except for the unexpectedly more potent 5F-ADB-PINACA which was tested at 3 mg/kg. Methodological differences beyond the doses of the drugs tested which may also explain these discrepancies in results may also include operational definitions and identification of seizures and convulsions, EEG headmounts used, data analysis techniques, and mouse strain. Although expressed primarily in the central nervous system, CB1Rs are also distributed throughout the body in areas including the cardiovascular system (Rajesh et al. 2012), gastrointestinal tract (Storr et al. 2004), the eyes (Straiker et al. 1999), and skeletal muscle (Crespillo et al. 2011). Thus, convulsions observed in the present study may also be due to activation of peripheral CB1Rs in the skeletal muscle. To our knowledge, this is the first study to demonstrate a phenomenological separation between electroencephalographic seizure activity and convulsant effects of SCRA drugs administered at convulsant doses. The underlying mechanisms to explain such findings require further study.
Only male mice were studied in these experiments, and there are known sex differences in cannabinoid effects across species. However, the directionality of such sex differences is fairly consistent, with females generally being more sensitive to cannabinoid effects than males. For example, the acute antinociceptive effects of cannabinoid agonists are more potent in female rats than in male rats (Tseng and Craft 2001; Romero et al 2002; Craft et al 2012), and this is also the case in a rat model of persistent pain (Craft et al 2013). The same relationship appears to hold for motoric effects of cannabinoid agonists, where various drugs including THC and CP55,940 have been reported to suppress locomotor activity, disrupt operant responding, and induce catalepsy more potently in female rats as compared to males (Tseng and Craft 2001; Craft et al 2012; Weed et al 2016; Wiley et al 2017). As such, it may be the case that convulsant effects of SCRAs would be expected to similarly differ as a function of sex, with females likely to be more susceptible to these effects than males. This hypothesis should be objectively tested.
In conclusion, the present work demonstrates that the structurally related SCRA compounds, AB-PINACA and 5F-ADB-PINACA, dose-dependently elicit convulsions, which are CB1R-mediated with high potency and effectiveness in mice. These convulsant effects occur in the absence of seizure-like effects as measured by EEG. Importantly, acute treatment with a CB1R antagonist—but not a benzodiazepine—significantly protected against SCRA-elicited convulsions. Taken together, this work further supports the notion that benzodiazepines typically used to treat provoked seizures in clinical settings may not be appropriate to treat SCRA-induced convulsions.
Abbreviations
- CB1Rs:
-
CB1 cannabinoid receptors
- CB2Rs:
-
CB2 cannabinoid receptors
- THC:
-
Δ9-Tetrahydrocannabinol
- PTZ:
-
Pentylenetetrazol
- JWH-018:
-
1-Naphthalenyl(1-pentyl-1H-indol-3-yl)-methanone
- rimonabant:
-
5-(4-Chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide
- AB-PINACA:
-
(S)-N-(1-amino-3-methyl-1-oxobutan-2-yl)-1-pentyl-1H-indazole-3-carboxamide
- 5F-ADB-PINACA:
-
N-[1-(aminocarbonyl)-2,2-dimethylpropyl]-1-(5-fluoropentyl)-1H-indazole-3-carboxamide
- 5F-AB-PINACA:
-
N-[(2S)-1-amino-3-methyl-1-oxobutan-2-yl]-1-(5-fluoropentyl)indazole-3-carboxamide
- EEG:
-
Electroencephalography
- RMS:
-
Root-mean-square
References
Andreias L, Roffe E, Eng A et al (2020) Synthetic cannabinoid receptor agonists-induced recurrent seizure in elderly patient. Am J Emerg Med 38(4):850.e5-850.e6
Arabadzhiev TI, Dimitrov VG, Dimitrova NA et al (2010) Interpretation of EMG integral or RMS and estimates of “neuromuscular efficiency” can be misleading in fatiguing contraction. J Electromyogr Kinesiol 20(2):223–232
Aung MM, Griffin G, Huffman JW et al (2000) Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB (1) and CB (2) receptor binding. Drug Alcohol Depend 60(2):133–140
Barker-Haliski M, White HS (2015) Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med 5(8):a022863
Braakman HM, van Oostenbrugge RJ, van Kranen-Mastenbroek VH et al (2009) Rimonabant induces partial seizures in a patient with a history of generalized epilepsy. Epilepsia 50(9):2171–2172
Breivogel CS, Wells JR, Jonas A et al (2020) Comparison of the neurotoxic and seizure-inducing effects of synthetic and endogenous cannabinoids with ∆9-tetrahydrocannabinol. Cannabis Cannabinoid Res 5(1):32–41
Brents LK, Reichard EE, Zimmerman SM et al (2011) Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS ONE 6(7):e21917
Buck KJ, Hahner L, Sikela J et al (1991) Chronic ethanol treatment alters brain levels of gamma-aminobutyric acidA receptor subunit mRNAs: relationship to genetic differences in ethanol withdrawal seizure severity. J Neurochem 57(4):1452–1455
Canazza I, Ossato A, Trapella C et al (2016) Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. In vitro and in vivo pharmacological studies. Psychopharmacology (Berl) 233(21–22):3685–3709
Canazza I, Ossato A, Vincenzi F et al (2017) Pharmaco-toxicological effects of the novel third-generation fluorinate synthetic cannabinoids, 5F-ADBINACA, AB-FUBINACA, and STS-135 in mice. In vitro and in vivo studies. Hum Psychopharmacol. 32(3):2601
Carlier J, Diao X, Scheidweiler KB et al (2017) Distinguishing intake of new synthetic cannabinoids ADB-PINACA and 5F-ADB-PINACA with human hepatocyte metabolites and high-resolution mass spectrometry. Clin Chem 63(5):1008–1021
Castellani SA, Ellinwood EH, Kilbey MM (1978) Tolerance to cocaine-induced convulsions in the cat. Eur J Pharmacol 47(1):57–61
Cozart MA, Phelan KD, Wu H et al (2020) Vascular smooth muscle TRPC3 channels facilitate the inverse hemodynamic response during status epilepticus. Sci Rep 10:812
Craft RM, Wakley AA, Tsutsui KT, Laggart JD (2012) Sex differences in CB1 vs. CB2 receptor-selective antagonism of antinociception produced by delta-9-THC and CP55,490 in the rat. J Pharmacol Exp Ther 340:787–800
Craft RM, Kandasamy R, Davis SM (2013) Sex differences in anti-allodynic, anti-hyperalgesic and anti-edema effects of Δ(9)-tetrahydrocannabinol in the rat. Pain 154:1709–1717
Crespillo A, Suárez J, Bermúdez-Silva FJ et al (2011) Expression of the cannabinoid system in muscle: effects of a high-fat diet and CB1 receptor blockade. Biochem J 433(1):175–185
de Havenon A, Chin B, Thomas KC et al (2011) The secret “spice”: an undetectable toxic cause of seizure. Neurohospitalist 1(4):182–186
Devinsky O, Patel AD, Thiele EA et al (2018) Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 90(14):e1204–e1211
Diana MA, Marty A (2004) Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE). Br J Pharmacol 142(1):9–19
Elmore JS, Baumann MH (2018) Repeated exposure to the “Spice” cannabinoid JWH-018 induces tolerance and enhances responsiveness to 5-HT1A receptor stimulation in male rats. Front Psychiatry 9:55
Fantegrossi WE, Moran JH, Radominska-Pandya A et al (2014) Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to ∆9-THC: mechanism underlying greater toxicity? Life Sci 97(1):45–54
Farfán FD, Politti JC, Felice CJ (2010) Evaluation of EMG processing techniques using information theory. BioMed Eng OnLine 9:72
Funada M, Takebayashi-Ohsawa M (2018) Synthetic cannabinoid AM 2201 induces seizures: involvement of cannabinoid CB1 receptors and glutamatergic transmission. Toxicol Appl Pharmacol 338:1–8
Ginsburg BC, Schulze DR, Hruba L et al (2012) JWH-018 and JWH-073: ∆9-tetrahydrocannabinol-like discriminative stimulus effects in monkey. J Pharm Exp Ther 340(1):37–45
Gobira PH, Vilela LR, Gonҫalves BD et al (2015) Cannabidiol, a cannabis sativa constituent, inhibits cocaine-induced seizures in mice: possible role of the mTOR pathway and reduction in glutamate release. Neurotoxicology 50:116–121
Gomez DM, Everett TJ, Hamilton LR et al (2021) Chronic cannabinoid exposure produces tolerance to the dopamine releasing effects of WIN 55,212–2 and heroin in adult male rats. Neuropharmacology 182:108374
Gu B, Zhu M, Glass MR et al (2019) Cannabidiol attenuates seizures and EEG abnormalities in Angelman syndrome model mice. J Clin Invest 129(12):5462–5467
Gugelmann H, Gerona R, Li C et al (2014) ‘Crazy Monkey’ poisons man and dog: human and canine seizures due to PB-22, a novel synthetic cannabinoid. Clin Toxicol 52(6):635–638
Hermans B, Vink AS, Bennis FC et al (2017) The development and validation of an easy to use automatic QT-interval algorithm. PLoS ONE 12(9):e0184352
Hill AJ, Mercier MS, Hill TD et al (2012) Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol 167(8):1629–1642
Hill TDM, Cascio MG, Romano B et al (2013) Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol 170(3):679–692
Hoffman AF, Lycas MD, Kaczmarzyk JR et al (2017) Disruption of hippocampal synaptic transmission and long-term potentiation by psychoactive synthetic cannabinoid ‘Spice’ compounds: comparison with ∆9-tetrahydrocannabinol. Addict Biol 22(2):390–399
Hruba L, McMahon LR (2017) Apparent affinity estimates and reversal of the effects of synthetic cannabinoids AM-2201, CP-47,497, JWH-122, and JWH-250 by rimonabant in rhesus monkeys. J Pharm Exp Ther 362(2):278–286
Huizenga MN, Sepulveda-Rodriguez A, Forcelli PA (2019) Preclinical safety and efficacy of cannabidivarin for early life seizures. Neuropharmacology 148:189–198
Hutchison RD, Ford BM, Frank LN et al (2018) Atypical pharmacodynamic properties and metabolic profile of the abused synthetic cannabinoid AB-PINACA: potential contribution to pronounced adverse effects relative to ∆9-THC. Front Pharmacol 9:1084
Hutter M, Broecker S, Kneisel S et al (2012) Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ‘herbal mixtures’ using LC-MS/MS techniques. J Mass Spectrom 47(1):54–65
Jones NA, Hill AJ, Smith I et al (2010) Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharm Exp Ther 332(2):569–577
Kaplan JS, Stella N, Catterall WA et al (2017) Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA 114(42):11229–11234
Kevin RC, Anderson L, McGregor IS et al (2019) CUMYL-4CN-BINACA is an efficacious and potent pro-convulsant synthetic cannabinoid receptor agonist. Front Pharmacol 10:595
King A (2010) Neuropsychiatric adverse effects signal the end of the line for rimonabant. Nat Rev Cardiol 7:602
Koo CM, Kim SH, Lee JS et al (2020) Cannabidiol for treating Lennox-Gastaut syndrome and Dravet syndrome in Korea. J Korean Med Sci 35(50):e427
Krauss P, Schilling A, Bauer J et al (2018) Analysis of multichannel EEG patterns during human sleep: a novel approach. Front Hum Neurosci 12:121
Lapoint J, James LP, Moran CL et al (2011) Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol (phila) 49(8):760–764
Laux LC, Bebin EM, Checketts D et al (2019) Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res 154:13–20
Longworth M, Connor M, Banister SD et al (2017) Synthesis and pharmacological profiling of the metabolites of synthetic cannabinoid drugs APICA, STS-135, ADB-PINACA, and 5F-ADB-PINACA. ACS Chem Neurosci 8(8):1673–1680
Lux RL, Sower CT, Allen N et al (2014) The application of root mean square electrocardiography (RMS ECG) for the detection of acquired and congenital long QT syndrome. PLoS ONE 9(1):e85689
Lϋttjohann A, Fabene PF, van Luijtelaar G (2009) A revised Racine’s scale for PTZ-induced seizures in rats. Physiol Behav 98(5):579–586
Malyshevskaya O, Aritake K, Kaushik MK et al (2017) Natural (∆9-THC) and synthetic (JWH-018) cannabinoids induce seizures by acting through the cannabinoid CB1 receptor. Sci Rep 7(1):10516
Mann K, Bäcker P, Röschke J (1993) Dynamical properties of the sleep EEG in different frequency bands. Int J Neurosci 73(3–4):161–169
Marsicano G, Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neural subpopulations in the adult mouse forebrain. Eur J Neurosci 11(12):4213–4225
Matsuzaki M (1978) Alteration in pattern of EEG activities and convulsant effect of cocaine following chronic administration in the rhesus monkey. Electroencephalogr Clin Neurophysiol 45(1):1–15
Melis M, Pistis M, Perra S et al (2004) Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci 24(1):53–62
Naydenov AV, Horne EA, Cheah CS et al (2014) ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 mice. Neuron 83(2):361–371
Park EJ, Park R, Jeon JH et al (2020) Inhibitory effect of AB-PINACA, indazole carboxamide synthetic cannabinoid, on human major drug-metabolizing enzymes and transporters. Pharmaceutics 12(11):1036
Phelan KD, Shwe UT, William DK et al (2015) Pilocarpine-induced status epilepticus mice: a comparison of spectral analysis of electroencephalogram and behavioral grading using the Racine scale. Epilepsy Res 117:90–96
Phelan KD, Shwe UT, Cozart MA et al (2017) TRPC3 channels play a critical role in the theta component of pilocarpine-induced status epilepticus in mice. Epilepsia 58(2):247–254
Rajasekaran M, Brents LK, Franks LN et al (2013) Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol 269(2):100–108
Rajesh M, Bátkai S, Kechrid M et al (2012) Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61(3):716–727
Riegel AC, Lupica CR (2004) Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci 24(49):11070–11078
Romero E, Fernández B, Sagredo O, Gomez N, Urigüen L, Guaza C et al (2002) Antinociceptive, behavioural and neuroendocrine effects of CP 55,940 in young rats. Brain Res Dev Brain Res 136:85–92
Schneir AB, Baumbacher T (2012) Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol 8(1):62–64
Schwartz MD, Trecki J, Edison LA et al (2015) A common source outbreak of severe delirium associated with exposure to the novel synthetic cannabinoid ADB-PINACA. J Emerg Med 48(5):573–580
Shimosato K, Watanabe S, Marley RJ et al (1996) One-way cross-sensitization and cross-tolerance to seizure activity from cocaine to lidocaine. Ann NY Acad Sci 801:340–352
Srisung W, Jamal F, Prabhakar S (2015) Synthetic cannabinoids and acute kidney injury. Proc (baylor Univ Med Cent) 28(4):475–477
Storr M, Sibaev A, Marsicano G et al (2004) Cannabinoid 1 receptor type-1 modulates excitatory and inhibitory neurotransmission in mouse colon. AM J Physiol Gastrointest Liver Physiol 286(1):G110–G117
Straiker AJ, Maguire G, Mackie K et al (1999) Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest Ophthalmol vis Sci 40(10):2442–2448
Tait RJ, Caldicott D, Mountain D et al (2016) A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clin Toxicol 54(1):1–13
Tatusov M, Mazer-Amirshahi M, Abbasi A et al (2019) Clinical effects of reported synthetic cannabinoid exposure in patients admitted to the intensive care unit. Am J Emerg Med 37(6):1060–1064
Tseng A, Craft R (2001) Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol 430:41–47
Van Erum J, Van Dam D, Deyn De, pp. (2019) PTZ-induced seizures in mice require a revised Racine scale. Epilepsy Behav: E&B. 95:51–55
Vilela LR, Lima IV, Kunsch EB et al (2017) Anticonvulsant effects of cannabidiol in the pentylenetetrazol model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Beh: e&b 75:29–35
Weed PF, Filipeanu CM, Ketchum MJ, Winsauer PJ (2016) Chronic Δ9-tetrahydrocannabinol during adolescence differentially modulates striatal CB1 receptor expression and the acute and chronic effects on learning in adult rats. J Pharmacol Exp Ther 356:20–31
Wiley JL, Lefever TW, Marusich JA, Craft RM (2017) Comparison of the discriminative stimulus and response rate effects of Δ9-tetrahydrocannabinol and synthetic cannabinoids in female and male rats. Drug Alcohol Depend 172:51–59
Wilson CD, Tai S, Ewing L et al (2019) Convulsant effects of abused synthetic cannabinoids JWH-018 and 5F-AB-PINACA are mediated by agonist actions at CB1 receptors in mice. J Pharm Exp Ther 368(2):146–156
Wohlfarth A, Castaneto MS, Zhu M et al (2015) Pentylindole/pentylindazole synthetic cannabinoids and their 5-fluoro analogs produce different primary metabolites: metabolite profiling for AB-PINACA and 5F-AB-PINACA. AAPS J 17(3):660–677
Wolfe CE, Wood DM, Dines A et al (2019) Seizures as a complication of recreational drug use: analysis of the Euro-DEN Plus data-set. Neurotoxicology 73:183–187
Zaurova M, Hoffman RS, Vlahov D et al (2016) Clinical effects of synthetic cannabinoid receptor agonists compared with marijuana in emergency department patients with acute drug overdose. J Med Toxicol 12(4):335–340
Acknowledgements
We thank the UAMS Division of Laboratory Animal Medicine for their expert husbandry services. We also thank Thomas Prisinzano in the Department of Medicinal Chemistry at the University of Kansas School of Pharmacy for providing rimonabant as a generous gift.
Funding
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grants DA039143 and DA022981], by the National Institute on General Medical Sciences [IDeA Program award GM110702], by the Drug Enforcement Administration [HHSF223201610079C], and by the UAMS Translational Research Institute [RR029884].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wilson, C.D., Zheng, F. & Fantegrossi, W.E. Convulsant doses of abused synthetic cannabinoid receptor agonists AB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA and JWH-018 do not elicit electroencephalographic (EEG) seizures in male mice. Psychopharmacology 239, 3237–3248 (2022). https://doi.org/10.1007/s00213-022-06205-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06205-6