Abstract
Rationale and objective
In this study, we hypothesized that the chronic use of clozapine affects cytokine expression and has a greater effect on female patients than on male patients. The aims of this study were to detect (1) whether serum cytokine levels were altered in patients with chronic schizophrenia after clozapine treatment compared with age- and sex-matched healthy controls, (2) whether there was a gender difference in serum cytokine levels after clozapine treatment, and (3) whether there was a correlation between serum cytokine levels and clozapine daily dosage in patients with schizophrenia.
Methods
Forty-nine inpatients with schizophrenia treated with clozapine and fifty-three sex- and age-matched healthy controls were recruited. The patients’ psychiatric symptoms were measured by the Positive and Negative Syndrome Scale (PANSS). Blood samples from both patients and healthy controls were collected. Serum IL-1β, IL-2, IL-6, IL-17, IFN-γ, and TNF-α levels were measured in duplicate by sandwich enzyme-linked immunosorbent assay.
Results
We found that chronic clozapine treatment in patients with schizophrenia resulted in the abnormal expression of serum cytokines, such as IL-2, IL-6, IL-17, and TNF-α, compared with the healthy controls. In addition, there was a gender difference in the abnormal expression of cytokines between male and female patients with schizophrenia. In the female group, IL-2 serum levels were lower than those in the male group. Interestingly, there was a positive correlation between serum IL-2 levels and the daily clozapine dosage in female patients with schizophrenia.
Conclusion
Findings from our study have shown clear evidence that clozapine had a greater effect on immune function in female patients with schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a common, severe, and chronic psychiatric disorder in China. According to the latest national epidemiological survey, the prevalence of schizophrenia is 0.7% during the lifetime of survey participants in China. In other words, more than 9.8 million Chinese people suffer from schizophrenia (Huang et al. 2019). Different countries have different treatment strategies for schizophrenia.
Clozapine was introduced in China in the 1970s. Since then, Chinese psychiatrists have used clozapine as the first-line treatment for schizophrenia for several years (Nielsen et al. 2016; Tang et al. 2008). Thus far, clozapine has remained the first choice for patients with schizophrenia in some parts of China throughout the 1990s (Xiang et al. 2007; Wang and Li 2012; Hou et al. 2015).
Clozapine is an effective antipsychotic drug for treating chronic schizophrenia, particularly for treatment-resistant schizophrenia. Previous clinical trials have demonstrated the superiority of clozapine for treatment-resistant schizophrenia (Wimberley et al. 2017; Üçok et al. 2015; Kane et al. 2016; Mortimer et al. 2010). Patients who did not respond to typical antipsychotic drugs had a more than 60% chance of responding to clozapine. In addition, clozapine is the most economical antipsychotic drug. Clozapine costs approximately USD 6.34 a month if given 300 mg orally daily in China. More importantly, clozapine is cost-efficient in decreasing the number of hospitalizations and the length of stay in hospitals. Given these advantages, the use of clozapine in patients with schizophrenia is as high as 60% in some parts of China, such as in Anhui Province (Si et al. 2012; Xu et al. 2020).
Clozapine possesses immunomodulatory properties, which may downregulate or upregulate cytokine levels (Klemettilä et al. 2014; Fang et al. 2020; Usta et al. 2021). A previous study reported an increase in the plasma levels of tumor necrosis factor (TNF)-α after clozapine treatment (Zhao et al. 2021). Clozapine-induced fever is followed by the upregulation of TNF-α and interleukin (IL)-6 in the plasma (Hung et al. 2017).
In patients with schizophrenia, a meta-analysis study showed that IL-1β, IL-6, and transforming growth factor-β functioned as state cytokine markers, but IL-12, interferon (IFN)-γ, and TNF functioned as trait cytokine markers (Potvin et al. 2008). The state cytokine markers were elevated in first-episode patients and normalized after antipsychotic drug treatment. Conversely, the trait markers were elevated during acute exacerbation and remained elevated even after treatment with antipsychotic drugs. These cytokines have been linked to mental illnesses. IL-2 regulates neurotransmitter metabolism, including dopamine metabolism, and IL-6 acts as a neurotrophic factor in the central nervous system.
However, to our knowledge, no study has reported gender differences in serum cytokine levels between Chinese male and female patients with schizophrenia after clozapine treatment, particularly the relationship between these cytokines and the daily dosage of clozapine. In this study, we hypothesized that the chronic use of clozapine affects cytokine expression and has a greater effect on female patients than on male patients. The aims of this study were to detect (1) whether serum cytokine levels were altered in patients with chronic schizophrenia after clozapine treatment compared with age- and sex-matched healthy controls, (2) whether there was a gender difference in serum cytokine levels after clozapine treatment, and (3) whether there was a correlation between serum cytokine levels and clozapine daily dosage in patients with schizophrenia.
Materials and methods
Subjects
Forty-nine inpatients with schizophrenia were recruited from Chaohu Hospital of Anhui Medical University. All patients with schizophrenia must meet the following inclusion criteria: (1) is 35–75 years old and is Han Chinese, (2) has confirmed DSM-IV diagnosis of schizophrenia based on the Structured Clinical Interview for DSM-IV from the consensus of two psychiatrists, (3) had at least 5 years of illness, (4) had been receiving stable doses of oral clozapine (Shanghai Xinyi Pharmaceutical Co. LTD, 25 mg) for at least 12 months before entry into the study, and (5) had not received any immunomodulators or anti-oxidants in the past 12 weeks. Patients who met the following criteria were excluded from our study: (1) received an antipsychotic drug other than clozapine at the same time and (2) were unable to understand and sign informed consent.
Fifty-three sex- and age-matched healthy controls were recruited from the Physical Examination Center of Chaohu Hospital of Anhui Medical University. None of the patients had received any immunomodulators or anti-oxidants in the past 12 weeks. The current mental status and personal or family history of any mental disorder were assessed using unstructured interviews. None of the healthy control subjects presented with a personal or family history of psychiatric disorders.
A complete medical history, physical examination results, and laboratory test results were obtained from the patients and healthy controls. Subjects with major medical illnesses were excluded. None of the subjects met the criteria for drug or alcohol abuse or dependence.
This study was approved by the Human Research and Ethics Committee of Chaohu Hospital of Anhui Medical University (Approval No. 201805-kyxm-03), and all study procedures were performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants after a detailed description of the study.
Clinical measurement
Two senior psychiatrists measured the patients’ psychiatric symptoms by using the Positive and Negative Syndrome Scale (PANSS). Furthermore, the psychiatrists simultaneously attended a training session using the PANSS. Repeated assessments of the PANSS total score showed that an inter-rater correlation coefficient greater than 0.8 was maintained. Daily doses of clozapine were also recorded and converted to chlorpromazine equivalents.
Blood collection and serum cytokine level measurement
Blood samples from both patients and healthy controls were collected between 7 and 9 a.m., following our previous study (Liu et al. 2020; Yang et al. 2021). The serum was separated, aliquoted, and stored at − 80 °C before use. Serum IL-1β, IL-2, IL-6, IL-17, IFN-γ, and TNF-α levels were measured in duplicate by sandwich enzyme-linked immunosorbent assay using a commercially available kit (Sangon Biotech, Shanghai, China). The kits were directly purchased from Sangon Biotech. Duplicate assays were performed for both sample and standard testing. All samples were assayed by the same investigator, who was blinded to the clinical situation. The inter-assay coefficients were 7%, 7%, and 4%, respectively. The intra-assay variation coefficients were 5%, 9%, and 8%, respectively.
Data analysis
The demographic variables of the patients and healthy controls were compared using t-tests or analysis of variance for continuous variables and chi-square tests for categorical variables. Considering that all the cytokine markers were normally distributed in patients and healthy controls (Shapiro–Wilk test, all P values > 0.05), independent-samples t-tests were used for the comparison between patients and healthy controls. Independent-samples t-tests were used to compare the cytokine expression levels between male and female patients. Relationships between clinical ratings and cytokine expression levels were examined using Spearman’s correlation. SPSS version 13 (SPSS Inc., Chicago, IL, USA) was used to conduct the statistical tests. Statistical significance was set at P < 0.05.
Results
Demographic data of healthy controls and patients with schizophrenia
Table 1 shows the demographic data of all participants in this study. Forty-nine patients with schizophrenia and fifty-three healthy controls were enrolled in this study. There were no significant differences in age, sex, and education between patients with schizophrenia and healthy controls (all P values > 0.05). The body mass index (BMI) of patients with schizophrenia was higher than that of the healthy controls, but the difference between the two groups was not significant (P = 0.16).
Age, education, duration of treatment, and BMI were not significantly different between male and female patients with schizophrenia (all P values > 0.05; Table 2). In addition, there were no significant differences in the severity of psychotic symptoms (PANSS total score) and chlorpromazine equivalent between male and female patients (all P values > 0.05).
Serum IL-1β, IL-2, IL-6, IL-17, IFN-γ, and TNF-α levels in patients with schizophrenia versus healthy controls
To demonstrate altered immune function in schizophrenia, the levels of six cytokines, namely, IL-1β, IL-2, IL-6, IL-17, IFN-γ, and TNF-α, were examined in patients with schizophrenia who were treated with clozapine and were compared with those of the healthy controls.
In this study, we found that patients with schizophrenia had significantly lower IL-2 levels than the healthy controls (8.56 ± 0.90 vs. 8.91 ± 0.76 pg/mL, t = 2.05, P = 0.04; Table 1). Compared with the healthy controls, patients with schizophrenia also showed higher IL-1β levels in this study (0.67 ± 0.31 vs. 0.79 ± 0.51 pg/mL). However, there were no significant differences between the two groups for this immune cytokine marker (t = 1.41, P = 0.16; Table 1).
In addition, we found that patients with schizophrenia had significantly higher IL-6, IL-17, and TNF-α levels than the healthy controls (1.35 ± 1.05 vs. 0.65 ± 0.27 pg/mL, t = − 4.57, and P = 0.00; 0.71 ± 0.74 vs. 0.26 ± 0.41 pg/mL, t = − 3.76, and P = 0.00; and 0.36 ± 0.21 vs. 0.13 ± 0.16 pg/mL, t = − 6.39, and P = 0.00, respectively). These differences remained even after we controlled for age, sex, years of education, and BMI (Table 1). However, there were no significant differences in the serum levels of IFN-γ between the two groups (P = 0.54).
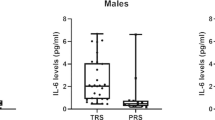
Serum IL-1β, IL-2, IL-6, IL-17, IFN-γ, and TNF-α levels in male and female patients with schizophrenia
The IL-1β level was 0.77 ± 0.31 ng/mL in the male schizophrenia patient group and 0.52 ± 0.26 ng/mL in the female schizophrenia patient group (Fig. 1). The serum IL-1β levels in female patients with schizophrenia were lower than those in male patients with schizophrenia (t = 3107, P = 0.00; Fig. 1).
The mean serum IL-2 level was 8.79 ± 0.80 ng/mL among male patients with schizophrenia and 8.24 ± 0.96 ng/mL in the male schizophrenia patient group (Fig. 1). Serum IL-2 levels in female patients with schizophrenia were lower than those in male patients with schizophrenia (t = − 2116, P = 0.04; Fig. 1).
The IL-6, IL-17, IFN-γ, and TNF-α levels between female and male patients with schizophrenia were compared. However, there was no significant difference between the two groups (all P values > 0.05; Fig. 1).
Correlation between serum IL-1β and IL-2 levels and daily clozapine dosage in patients with schizophrenia
Analysis was conducted to determine whether the daily clozapine dosage had an effect on the immune response. The mean daily clozapine dosage was 232.76 ± 95.46 mg in male patients and 237.50 ± 100.82 mg in female patients (Table 2). There was a positive correlation between IL-2 levels and daily clozapine dosage in female patients with schizophrenia (Fig. 2d). Unfortunately, no significant correlation was observed in male patients. Moreover, we found no significant correlation between IL-1β levels and daily clozapine dosage in either the male or female patient groups (Fig. 2a and c).
Discussion
We found that chronic clozapine treatment in patients with schizophrenia resulted in the abnormal expression of serum cytokines, such as IL-2, IL-6, IL-17, and TNF-α, compared with the healthy controls. In addition, there was a gender difference in the abnormal expression of cytokines between male and female patients with schizophrenia. In the female group, IL-2 serum levels were lower than those in the male group. Interestingly, there was a positive correlation between serum IL-2 levels and the daily clozapine dosage in female patients with schizophrenia.
Growing evidence from preclinical and clinical studies demonstrates that the etiology of schizophrenia is associated with immunological abnormalities. The upregulation of pro-inflammatory cytokine levels in human samples, such as serum or plasma, has been reported in several studies. The systematic review of Upthegrove found that the cytokine profile at the onset of psychosis was not confounded by medication (Upthegrove et al. 2014). There was a significant elevation in the levels of pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, in the serum of patients with medication-naive first-episode psychosis. Another study showed that baseline levels of IL-6 and TNF-α were elevated during acute psychotic episodes (Simşek et al. 2016).
However, the downregulation of anti-inflammatory cytokines is also altered in schizophrenia. Compared with the healthy controls, patients with schizophrenia showed significantly higher levels of IL-5, IL-13, and TNF-α and significantly lower levels of IL-4, IL-12, and IFN-γ (Yeh et al. 2019). Unfortunately, the cytokine levels of patients with schizophrenia are inconsistent. In addition, psychotropic drugs also affect the expression of immune cytokines in patients.
Previous studies have shown that antipsychotic treatment modulates cytokine levels. Marcinowicz et al. (2021) analyzed the expression of IL-1β, IL-2, IL-4, IL-6, IL-10, IL-17, TNF-α, and IFN-γ levels before and after antipsychotic treatment via a meta-analysis. In first-episode psychosis patients, antipsychotic treatment is related to decreased concentrations of pro-inflammatory cytokines IL-1β, IL-6, IFN-γ, TNF-α, and anti-inflammatory cytokines IL-4 and IL-10. By contrast, the levels of pro-inflammatory cytokines IL-2 and IL-17 remain unaffected. Only two studies detected the effect of clozapine on the expression of cytokines in patients with schizophrenia (Klemettilä et al. 2014; Fang et al. 2019). The chronic use of clozapine further aggravates the abnormal immune function in patients with schizophrenia. Giridharan et al. (2020) indicated that clozapine exhibited anti-inflammatory effects. Clozapine reduced the IL-1α, IL-1β, IL-2, and IL-17 levels. Similarly, IL-2 and IL-6 levels showed a significant decrease after four weeks of antipsychotic treatment.
Clozapine had a greater effect on the immune function of female patients with schizophrenia than that of male patients with schizophrenia. O’Connell et al. (2014) compared the levels of IL-1β, IL-6, IL-8, IL-17, IL-23, and TNF-α in female patients with schizophrenia who were treated with clozapine and those of the healthy controls. The levels of IL-1β, IL-8, IL-17, and IL-23 were elevated in female patients with schizophrenia who were treated with clozapine. Clozapine also increased the secretion of IL-6 in vitro (Hinze-Selch et al. 1998). Our group previously focused on the expression of pro-inflammatory cytokines in male patients before and after clozapine treatment. Clozapine-treated patients had higher IL-1β, IL-6, and TNF-α levels. In the current study, we measured the expression of immune cytokines after clozapine treatment not only in male patients with schizophrenia but also in female patients with schizophrenia.
In our study, we found that IL-2 serum levels in the female group were significantly lower than those in the male group. More importantly, there was a positive correlation between serum levels of IL-2 and the daily dosage of clozapine in female patients with schizophrenia. IL-2, which is an important interleukin, plays essential roles in the functions of the immune system, as well as in tolerance and overall immunity. One study showed that the levels of IL-2 in adolescent patients with schizophrenia who were not administered drug treatments were significantly higher than those in the control group (Simşek et al. 2016). Consistent with our results, the levels of IL-2 in the patient group were significantly lower than those in the pretreatment group after 4 or 8 weeks of treatment with clozapine. The change in IL-2 was positively correlated with the change in positive syndrome and total score. However, there was a positive correlation between serum IL-2 levels and daily clozapine dosage in female patients with schizophrenia.
This study had some limitations. First, we investigated the effect of clozapine on immune function in only forty-nine patients with schizophrenia. Owing to the small sample size, the results must be considered preliminary results. To verify our results, larger samples will be recruited in a future study. Second, we did not measure the levels of cytokines in drug-naive patients with schizophrenia, and we did not compare the differences in cytokine expression levels between male and female patients when they were drug-free.
In conclusion, we found that chronic treatment with clozapine downregulated IL-1β and IL-2 levels and upregulated IL-6, IL-17, and TNF-α levels. Furthermore, the serum IL-2 levels of the female group were lower than those of the male group. The levels of IL-2 in female patients had a negative relationship with daily clozapine dosage. Schizophrenia impaired the immune function of patients compared to healthy controls. Immune function was further impaired in patients with schizophrenia after treatment with clozapine. Furthermore, the effects on immune function were more impaired in female schizophrenic patients. Therefore, after clozapine treatment, female patients with schizophrenia need to pay more attention to their immune function, especially the expression of IL-2, in order to prevent severe immune disorders.
References
Fang X, Wang Y, Chen Y, Ren J, Zhang C (2019) Association between IL-6 and metabolic syndrome in schizophrenia patients treated with second-generation antipsychotics. Neuropsychiatr Dis Treat 15:2161–2170. https://doi.org/10.2147/NDT.S202159
Fang X, Yu L, Wang D, Chen Y, Wang Y, Wu Z, Liu R, Ren J, Tang W, Zhang C (2020) Association between SIRT1, cytokines, and metabolic syndrome in schizophrenia patients with olanzapine or clozapine monotherapy. Front Psychiatry 11:602121. https://doi.org/10.3389/fpsyt.2020.602121
Giridharan VV, Scaini G, Colpo GD, Doifode T, Pinjari OF, Teixeira AL, Petronilho F, Macêdo D, Quevedo J, Barichello T (2020) Clozapine prevents poly (I: C) induced inflammation by modulating NLRP3 pathway in microglial cells. Cells 9(3):577. https://doi.org/10.3390/cells9030577
Hou CL, Cai MY, Ma XR, Zang Y, Jia FJ, Lin YQ, Chiu HF, Ungvari GS, Ng CH, Zhong BL, Cao XL, Li Y, Shinfuku N, Xiang YT (2015) Clozapine prescription and quality of life in Chinese patients with schizophrenia treated in primary care. Pharmacopsychiatry 48(6):200–204. https://doi.org/10.1055/s-0035-1555939
Hinze-Selch D, Becker EW, Stein GM, Berg PA, Mullington J, Holsboer F, Pollmächer T (1998) Effects of clozapine on in vitro immune parameters: a longitudinal study in clozapine-treated schizophrenic patients. Neuropsychopharmacol 19(2):114–122. https://doi.org/10.1016/S0893-133X(98)00006-2
Huang Y, Wang YU, Wang H, Liu Z, Yu X, Yan J, Yu Y, Kou C, Xu X, Lu J, Wang Z, He S, Xu Y, He Y, Li T, Guo W, Tian H, Xu G, Xu X, Ma Y, Wang L, Wang L, Yan Y, Wang B, Xiao S, Zhou L, Li L, Tan L, Zhang T, Ma C, Li Q, Ding H, Geng H, Jia F, Shi J, Wang S, Zhang N, Du X, Du X, Wu Y (2019) Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 6(3):211–224
Hung YP, Wang CSM, Yen CN, Chang HC, Chen PS, Lee IH, Chen KC, Yang YK, Lu RB, Wang TY (2017) Role of cytokine changes in clozapine-induced fever: a cohort prospective study. Psychiatry Clin Neurosci 71(6):395–402. https://doi.org/10.1111/pcn.12508
Kane JM, Correll CU (2016) The role of clozapine in treatment-resistant schizophrenia JAMA. Psychiatry 73(3):187–188. https://doi.org/10.1001/jamapsychiatry.2015.2966
Klemettilä JP, Kampman O, Seppälä N, Viikki M, Hämäläinen M, Moilanen E, Leinonen E (2014) Cytokine and adipokine alterations in patients with schizophrenia treated with clozapine. Psychiatry Res 218(3):277–283. https://doi.org/10.1016/j.psychres.2014.04.049
Liu Z, Zhang Y, Zhao T, Wang J, Xia L, Zhong Y, Yang Y, Ning X, Zhang Y, Ren Z, Liu H (2020) A higher body mass index in Chinese inpatients with chronic schizophrenia is associated with elevated plasma orexin-A levels and fewer negative symptoms. Nord J Psychiatry 74(7):525–532. https://doi.org/10.1080/08039488.2020.1755995
Marcinowicz P, Więdłocha M, Zborowska N, Dębowska W, Podwalski P, Misiak B, Tyburski E, Szulc A (2021) A meta-analysis of the influence of antipsychotics on cytokines levels in first episode psychosis. J Clin Med 10(11):2488. https://doi.org/10.3390/jcm10112488
Mortimer A, Singh P, Shepherd C, Puthiryackal J (2010) Clozapine for treatment-resistant schizophrenia: National Institute of Clinical Excellence (NICE) guidance in the real world. Clin Schizophr Relat Psychoses 4(1):49–55. https://doi.org/10.3371/CSRP.4.1.4
Nielsen J, Young C, Ifteni P, Kishimoto T, Xiang YT, Schulte PF, Correll CU, Taylor D (2016) Worldwide differences in regulations of clozapine use. CNS Drugs 30(2):149–161. https://doi.org/10.1007/s40263-016-0311-1
O’Connell KE, Thakore J, Dev KK (2014) Pro-inflammatory cytokine levels are raised in female schizophrenia patients treated with clozapine. Schizophr Res 156(1):1–8. https://doi.org/10.1016/j.schres.2014.03.020
Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E (2008) Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry 63(8):801–808. https://doi.org/10.1016/j.biopsych.2007.09.024
Si TM, Zhang YS, Shu L, Li KQ, Liu XH, Mei QY, Wang GH, Bai PS, Ji LP, Cheng XS, Ma C, Shi JG, Zhang HY, Ma H, Yu X (2012) Use of clozapine for the treatment of schizophrenia: findings of the 2006 research on the China psychotropic prescription studies. Clin Psychopharmacol Neurosci 10(2):99–104. https://doi.org/10.9758/cpn.2012.10.2.99
Simşek S, Yildırım V, Cim A, Kaya S (2016) Serum IL-4 and IL-10 levels correlate with the symptoms of the drug-naive adolescents with first episode, early onset schizophrenia. J Child Adolesc Psychopharmacol 26(8):721–726. https://doi.org/10.1089/cap.2015.0220
Tang YL, Mao PX, Jiang F, Chen Q, Wang CY, Cai ZJ, Mitchell PB (2008) Clozapine in China. Pharmacopsychiatry 41(1):1–9. https://doi.org/10.1055/s-2007-993224
Üçok A, Çikrikçili U, Karabulut S, Salaj A, Öztürk M, Tabak Ö, Durak R (2015) Delayed initiation of clozapine may be related to poor response in treatment-resistant schizophrenia. Int Clin Psychopharmacol 30(5):290–295. https://doi.org/10.1097/YIC.0000000000000086
Upthegrove R, Manzanares-Teson N, Barnes NM (2014) Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 155(1–3):101–108. https://doi.org/10.1016/j.schres.2014.03.005
Usta A, Kılıç F, Demirdaş A, Işık Ü, Doğuç DK, Bozkurt M (2021) Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 271(4):767–773. https://doi.org/10.1007/s00406-020-01152-9
Wang C, Li L (2012) Proper use of clozapine: experiences in China. Shanghai Arch Psychiatry 24(2):108–109. https://doi.org/10.3969/j.issn.1002-0829.2012.02.006
Wimberley T, MacCabe JH, Laursen TM, Sørensen HJ, Astrup A, Horsdal HT, Gasse C, Støvring H (2017) Mortality and self-harm in association with clozapine in treatment-resistant schizophrenia. Am J Psychiatry 174(10):990–998. https://doi.org/10.1176/appi.ajp.2017.16091097
Xiang YT, Weng YZ, Leung CM, Tang WK, Ungvari GS (2007) Clinical correlates of clozapine prescription for schizophrenia in China. Hum Psychopharmacol 22(1):17–25. https://doi.org/10.1002/hup.821
Xu SW, Dong M, Zhang Q, Yang SY, Chen LY, Sim K, He YL, Chiu HF, Sartorius N, Tan CH, Chong MY, Shinfuku N, Lin SK, Ng CH, Ungvari GS, Najoan E, Kallivayalil RA, Jamaluddin R, Javed A, Iida H, Swe T, Zhang B, Xiang YT (2020) Clozapine prescription pattern in patients with schizophrenia in Asia: The REAP survey (2016). Psychiatry Res 287:112271. https://doi.org/10.1016/j.psychres.2019.02.056
Yang Y, Zhang Y, Wang J, Ning X, Zhang Y, Zhao T, Zhong Y, Liu Z, Xia L, Li W, Yao ZK, Liu H (2021) Sex differences in the association of HOMA-IR index and BDNF in Han Chinese patients with chronic schizophrenia. Front Psychiatry 12:656230. https://doi.org/10.3389/fpsyt.2021.656230.eCollection2021
Yeh TC, Chu HT, Tsai CK, Chang HA, Yang FC, Huang SY, Liang CS (2019) Distinct inflammation biomarkers in healthy individuals and patients with schizophrenia: a reliability testing of multiplex cytokine immunoassay by bland-Altman analysis. Psychiatry Investig 16(8):607–614. https://doi.org/10.30773/pi.2019.04.14
Zhao T, Zhang K, Zhang Y, Yang Y, Ning X, Hu Y, Li X, Zhang Y, Xia L, Ren Z, Liu H (2021) Do proinflammatory cytokines play a role in clozapine-associated glycometabolism disorders? Psychopharmacol 238(7):1979–1990. https://doi.org/10.1007/s00213-021-05824-9
Acknowledgements
We thank all of the participants who volunteered to participate in the study. Thanks to Chaohu Hospital of Anhui Medical University and corresponding authors for their support.
Funding
This study was supported by the National Natural Science Foundation of China (81801341), the Anhui Provincial Key R&D Programme (202004j07020030), the China International Medical Exchange Foundation (Z-2018–35-2002), and the National Clinical Key Specialty Project Foundation (CN). The funding body did not participate in the design, conduct, or writing of the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yuan, X., Wang, S., Shi, Y. et al. Pro-inflammatory cytokine levels are elevated in female patients with schizophrenia treated with clozapine. Psychopharmacology 239, 765–771 (2022). https://doi.org/10.1007/s00213-022-06067-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-022-06067-y