Abstract
Background
Numerous studies based on voxel-based morphometry (VBM) have revealed gray matter (GM) alterations in multiple brain regions for addiction. However, findings are poorly replicated, and it remains elusive whether distinct diagnoses of addiction are underpinned by shared abnormalities. Our aim was to conduct a quantitative meta-analysis of structural neuroimaging studies investigating GM abnormalities in two main categories of addiction: substance use disorders (SUD) and behavioral addictions (BA).
Method
A systematic database search was conducted in several databases from Jan 1, 2010, to Oct 23, 2020, to identify eligible VBM studies. Meta-analysis was performed with the seed-based d mapping software package to compare alternations between individuals with addiction-related disorders and healthy controls (HC).
Results
A total of 59 VBM studies including 2096 individuals with addiction-related disorders and 2637 HC met the inclusion criteria. Individuals with addiction-related disorders showed shared GM volume decrease in bilateral prefrontal cortex, bilateral insula, bilateral rolandic operculum, left superior temporal gyrus, and right Heschl gyrus and GM increase in right lingual gyrus and right fusiform gyrus comparing with HC (p < 0.005). Subgroup analysis found heterogeneity between SUD and BA mainly in left inferior occipital gyrus and right striatum (p < 0.005). Meta-regression revealed that GM atrophy in right anterior cingulate (r = 0.541, p = 0.03 (uncorrected)) and left inferior frontal gyrus (r = 0.595, p = 0.015) were positively correlated with higher impulsivity.
Conclusions
This meta-analysis identified a concordance across subtypes of addiction in terms of the brain structural changes in prefrontal and insula areas, which may relate to higher impulsivity observed across addiction diagnoses. This concordance provides an organizing model that emphasizes the importance of shared neural substrates in addiction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Addiction is a growing mental health issue, though no consensus exists on whether behavioral addictive disorders (BA) fall into the same category as substance use disorders (SUD) (Potenza 2014). SUD are defined as a chronic relapsing disorder characterized by diminished control over substance intake related to neurobiological changes (Unterrainer et al. 2019). Lifetime prevalence of SUD has been estimated at up to 3% for the general population in the European Union (Alonso et al. 2004; Rehm et al. 2005), imposing a significant burden on society and healthcare systems. BA, on the other hand, known as non-substance addiction, are characterized by distress or interference with personal functions that develop as a result of repetitive rewarding behaviors other than the use of dependence-producing substances (Alabdulsalam et al. 2014). The estimated 12-month prevalence of BA in US adults is between 2% (Internet addiction) and 10% (work addiction), causing disruptions to daily functioning (Sussman et al. 2011). More evidence is thus needed to understand the structural and functional abnormalities of BA, especially in relation to SUD.
Addiction-related disorders share common characteristics in clinical and epidemiological samples and frequently co-occur with two or more disorders. Recently, DSM-V classifies gambling disorders as a separate non-substance-related disorder from other substance-related disorders (although they fall under the same category). This is due to the absence of the potent pharmacological effects of drugs of abuse in behavioral addictions, although similar neurobiological mechanisms have been proposed to underlie BA and SUD (Fauth-Bühler et al. 2017). In fact, the reinforcers present in behavioral addictions like gambling have been proposed to act on the dopaminergic system similarly to the SUD (Linnet et al. 2011; Joutsa et al. 2011), which may illustrate the common pathway in BA and SUD. Moreover, both disorders have deficits in impulse control which is based on impulsivity, poor control, and compulsion of addiction (Fineberg et al. 2014). Impulsivity and compulsivity are multidimensional constructs. They cover a variety of disruption within a wide range of neural processes, involving coordination of motor or cognitive responses (Robbins and Roberts 2007; Brewer and Potenza 2008). Several strands of evidence suggest that impulsivity may be an endophenotypic marker for addiction risk (Wit 2009). Nevertheless, it remains unknown whether this similarity is mediated by shared or distinct neurobiological substrate because few structural magnetic resonance imaging (MRI) studies have directly compared the two disorders.
With the quick development of high-resolution MRI and the technology of voxel-based morphometry (VBM), subtle GM alterations can also be detected, which has been widely applied in mental disorders to find evidence of gray matter (GM) changes between patients and healthy controls (Ashburner and Friston 2001). In SUD, meta-analyses of different substances using conducted by voxel-based morphometry (VBM) studies of gray matter volume (GMV) have been reported almost consistent GMV reduction relative to healthy controls in the bilateral insula, dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), superior frontal gyrus, middle frontal gyrus, and superior temporal gyrus (Yang et al. 2020; Klaming et al. 2019; Hall et al. 2018). Moreover, a meta-analysis of region of interest (ROI) found that a decrease GMV in the insula was correlated with duration of substances using (Yang et al. 2020; Hall et al. 2018). In BA, VBM studies comparing with healthy controls reported GMV reduction in the left ACC extending to the left medial superior frontal gyrus (mSFG) and bilateral orbitofrontal gyrus (OFG), right putamen, and right supplementary motor area (SMA) (Qin et al. 2020), including internet gaming disorder (IGD), pathological gambling (PG), problematic hypersexual behavior (PHB), and mobile phone dependence (MPD). In addition, Qin’s results also showed that higher BIS-11(Barratt Impulsiveness Scale-11) scores in BA were positively associated with lower GMV in the ACC (Qin et al. 2020). But there has been few comprehensive meta-analysis investigating brain structural changes in distinct subtypes of addiction including both SUD and BA.
As well as the distinct clinical features in different addictions, important confounding factors such as comorbidity and medication can no doubt contribute to the inconsistency. Moreover, the lack of statistical power is also a major problem, resulting from the typically small sample size in single study. The aim of our study was to conduct a voxel-based meta-analytic comparison of all published whole-brain structural MRI studies of GMV abnormalities to explore (a) shared GM abnormalities and common neurobiological substrate for individuals with addictions, (b) heterogeneity of these findings in subgroup analysis, and (c) the association between some common addiction-related variants and GM alterations by meta-regression. We focused on studies of tobacco use disorder (TUD), alcohol use disorder (AUD), cocaine dependence (CD), pathological gambling (PG), and internet gaming disorder (IGD). Tobacco, alcohol, and cocaine use disorders were part of the SUD group, while PG and IGD formed the BA group. For the VBM meta-analysis, we hypothesized that patients with addictions would show disorder-specific GMV decrease primarily in the prefrontal and insula regions, while we expected that addiction-related variants would be associated with GMV in the prefrontal areas.
Methods
Selection of studies for meta-analysis
We searched PubMed, Web of Science, and Medline for all studies from Jan 1, 2010, to Oct 23, 2020. Studies include patients with PG, AUD, CD, IGD, and TUD. The several search terms were as follows: “internet addiction,” “internet gaming disorder,” “video game addiction,” “cocaine-related disorders,” “cocaine addiction,” “alcohol addiction,” “Alcohol Dependence,” “Alcohol Use Disorder,” “smoking,” “nicotine,” “tobacco,” and “cigarette” coupled with “VBM,” “gray matter,” “voxel based morphometry,” and “voxel-wise.” The reference lists of studies and some details can be found in Table 1.
Studies were selected if (1) diagnoses of each study were based on DSM/ICD, quantitative assessment tools, or both; (2) they used VBM to analyze gray matter; (3) they included healthy control participants; (4) they performed a whole-brain analysis; (5) they reported coordinates in a defined stereotaxic space (e.g., Talairach space or Montreal Neurological Institute space); (6) they used consistent thresholds in different regions; and (7) studies were peer reviewed and published in English as an article. Studies were excluded if (1) the patient group included other diseases such as Parkinson’s disease (PD), multiple sclerosis (MS), and mental illness; (2) they did not use VBM; (3) peak coordinates were not reported; (4) only region of interest results were available; and (5) inconsistent thresholds were applied in different regions. Two authors (Zhang and Gao) independently searched, selected, and cross-checked in order to insure our study reliable. Any difference was discussed and settled by consensus.
Meta-analysis across all studies
The anisotropic seed-based d mapping (AES-SDM) meta-analytic software (www.sdmproject.com) are employed widely in recent meta-analysis of addiction disorders or some psychiatric diseases (Yang et al. 2020; Lukito et al. 2020). ASE-SDM can use peak coordinates and effect sizes (t-scores) data to calculate signed (positive/negative) effect sizes and variance maps of brain regional differences between patient and control groups by convolving an anisotropic non-normalized Gaussian kernel with Hedges effect size of each peak.
AES-SDM in neuroimaging meta-analysis, maps are combined across studies based on random-effect model, taking sample size, within-study variability, and between-study heterogeneity into account. Correlated datasets (e.g., when the same group of participants completed several cognitive tasks) were included in the meta-analysis as a single set (Norman et al. 2016). Then, modulate it according to shared variance of brain activation or structure across datasets. Here are the following steps: (1) p value or z value in some studies needed to convert into t value online (http://www.sdmproject.com/utilities/?show=Statistics); (2) convert peak coordinates into standardized MNI space; (3) set the full width at half maximum (FWHM) to 20 mm because this will keep balance among sensitivity and specificity and other parameters including voxel p < 0.005, peak height threshold > 1, and cluster extent threshold > 10 voxels (Radua and Mataix-Cols 2009); (4) jack-knife sensitivity analysis performed to verify the stability and reliability of the findings by repeating the meta-analysis after excluding one study at a time. If one brain region survives in the most of the repeats, we can get the conclusion that this abnormality is stable (Radua and Mataix-Cols 2009).
Subgroup meta-analysis
To verify the stability and reliability of the findings, we divided individuals into two different subtypes for next subgroup analysis: BA subjects (including PG and IGD) and SUD subjects (including TUD, CD, and AUD), using a random effects model under the same threshold as before. To rule out medication or abstinence effects, studies excluding individuals who received clinical intervention within 6 months prior to scanning formed an intervention-free subgroup. This subgroup meta-analysis can also examine potential confounding factors.
Meta-regression analysis
Meta-regression analysis was conducted to explore the association between GM alterations and clinical features such as BIS-11 score and BDI score which were more typical to be the regressor than other indices. The clinical relevance of impulsivity is frequently highlighted because it impacts many mental and behavioral disorders and BIS-11 assesses core impulsive trait in addictions (Fineberg et al. 2014). The correlation between BDI and addiction-related disorders has been reported in the previous study (Paljarvi et al. 2009). Furthermore, we used a more conservative threshold (p < 0.0005) in order to decrease the false-positive rate in this meta-regression analysis (Radua and Mataix-Cols 2009). Egger’s test and funnel plots constructing by ASE-SDM assessed potential publication bias in the disorder differences and shared findings (Egger et al. 1997).
Results
Search results and sample features
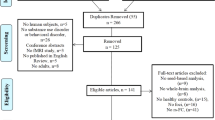
Searching in various databases, based on the eligible criteria, finally we identified 59 VBM articles in addiction-related studies for meta-analysis comprising 2096 individuals with addiction-related disorders and 2637 healthy controls (Fig. 1). Of these 59 studies, 15 were IGD, 10 were CD, 11 were AUD, 15 were TUD, and 8 were PG. Most of the participants aged between 20 and 45. See Table 1 for more demographic, clinical, and other characteristics.
Regional GM differences by pooled meta-analysis
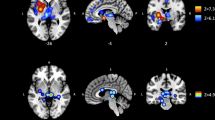
As demonstrated in Table 2 and Fig. 2, the pooled meta-analysis showed that individuals with addition-related disorders are associated with lower GM volume in bilateral anterior cingulate cortex (ACC) extending to the corresponding medial superior frontal gyrus (mSFG), bilateral superior frontal gyrus medial orbital (OFG), bilateral insula, bilateral rolandic operculum, left superior temporal gyrus, and right Heschl gyrus compared with HC. In contrast, the results also showed increased GM volume in right lingual gyrus and right fusiform gyrus.
GM reductions for 2096 individuals with addiction-related disorders compared with 2637 HC. Clusters were shown in the sagittal, axial, and coronal planes. Regions with GM enlargement were shown in red and GM reductions were displayed in blue. a GM reduction in the R ACC; b,c GM reduction in bilateral insula and L superior temporal gyrus; d GM increased in the R lingual gyrus. Abbreviations: ACC, anterior cingulate cortex; GM, gray matter; HC, healthy controls
Systematic whole-brain jack-knife sensitivity analysis demonstrated that there is a significant GM decrease in bilateral ACC (55/59), bilateral OFG (55/59), left insula (54/59), right insula (55/59), left rolandic operculum (54/59), right rolandic operculum (55/59), left superior temporal gyrus (54/59), and right Heschl gyrus (55/59). Besides, the increased brain region in lingual gyrus and right fusiform gyrus could be replicable in 58 datasets. Neither the funnel plot nor Egger’s test showed significant publication bias (p > 0.05).
Subgroup analysis
First, we divided individuals into BA group and SUD group, two independent subgroups. The results demonstrated that findings in BA (23 datasets) were substantially consistent with the pooled analysis, apart from the additional significant GM decrease in the left inferior occipital gyrus and right striatum (Table 3). In addition, no significant GM increase regions were found in BA group. The SUD group (36 datasets) consolidated the results of pooled meta-analysis further (Table 4). In order to investigate the heterogeneity of the findings above further, we next performed two group comparation: (i) individuals with BA, relative to SUD, showed lower GM volume in left inferior occipital gyrus, right striatum, and right sMFG, (ii) while, comparing with BA, individuals with SUD had consistently reduced GM volume in left superior temporal gyrus (Table 5 and Fig. 3). The results of the intervention-free subgroup (51 datasets) were substantially consistent with the pooled analysis (Table S1 and Figure S1 in Online Resource 1).
Meta-regression analysis
The variables explored by meta-regression analysis included BIS-11 score and BDI score. The results showed that higher BIS-11 scores (16 datasets) in addictions were positively associated with GM reduction in the right ACC (MNI coordinate, 6, 30, 12; SDM-Z, 3.067; p = 0.00001; 67 voxels; r = 0.541, p = 0.03) (uncorrected), and left inferior frontal gyrus (MNI coordinate, -58, 4, 14; SDM-Z, 3.114; p = 0.00001; 253 voxels; r = 0.595, p = 0.015) (Fig. 4). No significant linear correlations were found with BDI score (12 datasets).
Results of the meta-regression analysis showing the positive correlation between BIS-11 and regional GM reduction in left inferior frontal gyrus. In this plot, each study is marked as a dot and the size of each dot depends on its sample size. The regression line is presented as a straight line. The SDM values (effect sizes) were extracted from the peak of maximum slope significance. Note that the meta-regression SDM value is derived from the proportion of studies that reported gray matter changes near the voxel, so it is expected that some values are at 0 or near ± 1. Abbreviations: BIS-11, Barratt Impulsiveness Scale-11; SDM, signed differential mapping
Discussion
To our knowledge, there has been few comprehensive meta-analysis investigating shared structural changes in distinct addictions including both SUD and BA. Our study integrated the findings from 59 VBM studies on five different subtypes of addiction, using the anisotropic seed-based d mapping (AES-SDM) method to explore brain GM alterations compared with HC. Shared GM reduction were found in the prefrontal cortex (PFC), bilateral insula, bilateral rolandic operculum, left superior temporal gyrus, and right Heschl gyrus, stable and replicable under jack-knife sensitivity analysis. In addition, we found heterogeneity between SUD and BA mainly in left inferior occipital gyrus and right striatum. Moreover, meta-regression analysis revealed that higher BIS-11 score was correlated with decreased GMV in the right ACC and left inferior frontal gyrus. These findings reveal potential mechanisms of overlap in SUD and BA and emphasize the importance of shared neural structural biomarker across addiction-related disorders.
Shared GM abnormalities in BA and SUD
Consistent with previous findings, robust GM decrease in the ACC and insula was also observed in the pooled meta-analysis across addiction-related disorders. A lot of single addiction disorders have revealed the abnormalities of ACC and insula such as tobacco, alcohol, and PG (Goldstein and Volkow 2011; Moccia et al. 2017). Researchers found that addiction was caused by an imbalance between unconscious impulsive system and conditional reflex system controlled by consciousness and cognition (Claus et al. 2013). When the balance broken, the inhibitory function would not work effectively, which lead to people could not help to use drugs (David et al. 2005). Cingulate gyrus and insula, the structural basis of the reflex system, which are responsible for impulsive control, decision-making, and emotional regulation (Jastreboff et al. 2015). Moreover, ACC and insula are critical components of salience network (SN). The SN is implicated in cognition and motivation and affects processing and is hypothesized to dynamically allocate cognitive and attentional resources between the default mode (DMN) and executive control (ECN) networks to realize the transformation of different status of the body (Menon 2011; Beissner et al. 2013). Disruptions of the SN and dysregulated connectivity with other networks, like the DMN, are observed across multiple neuropsychiatric diseases, including SUD (Goodkind et al. 2015). From this meta-analysis, we found GM atrophy in the ACC and insula across addiction diagnoses, which may suggest that such abnormalities lead to poor impulsive control and make people indulge in a certain status, presented with constantly seeking drugs and repetitive behaviors.
Individuals with addiction-related disorders also demonstrated significant GM decrease in the PFC, specifically in the OFC and superior frontal gyrus compared with HC. Within the prefrontal cortex, the OFC, by its connections with limbic areas, is uniquely positioned to use associative information to project into the future and to use the value of perceived or expected outcomes to guide decisions (Schoenbaum et al. 2006). GM atrophy in the OFC leads to the loss of integrated information, which could account for the propensity of seeking drugs and repetitive behaviors, despite the almost inevitable negative consequences of such behavior. Consistent with our findings, this pattern of deficits in decision-making also existed in addiction animal models (Miles et al. 2003). As the result of the brain structure changes, it might suggest the character of addiction—maladaptive decision-making.
GM atrophy in the temporal lobe and rolandic operculum observed in our meta-analysis is a novel structural alteration in addiction. According with our results, previous researches on addiction have demonstrated that the temporal cortex has been observed abnormalities in both SUD (Robbins et al. 2008) and BA (Weinstein and Lejoyeux 2010; Hahn and Kim 2014). Moreover, superior temporal gyrus is associated with urges and craving (Ko et al. 2009) and participated in regulatory control over reward-seeking behavior (Chiamulera 2005), which is an important part of the addiction process. As for rolandic operculum, it has been deeply discussed in previous studies of Parkinson’s disease(Bayram et al. 2020) and epilepsy (Dong et al. 2016). Rolandic operculum has been found associated with execution of finger movements (Engel et al. 2012), involved in sensory motor integration in movement (Ciccarelli et al. 2005), and integrated exteroceptive-interoceptive signals related to self-consciousness cooperating with left temporal superior gyrus and supramarginal gyrus (Blefari et al. 2017). However, the exact mechanism of rolandic operculum abnormalities in addiction-related disorders remains to be fully explored, and it should be paid more attention in the future research.
In contrast, this study also observed that addiction-related disorders are linked to higher GM volume in right lingual gyrus and right fusiform gyrus. The fusiform gyrus is regarded as a component part of the temporal and occipital lobes, involved in processing color information, face and body recognition, and word recognition, as well as the perception of emotions in facial stimuli (Parvizi et al. 2012; Weiner et al. 2014). As for right lingual gyrus, it is an important section of the occipital lobe, linked to visual association cortex (Yang et al. 2020). Recent studies found that individuals with major depressive disorder showed increased GMV in lingual gyrus comparing with HC (Du et al. 2014) and the brain activity was also increased during emotion recognition (Scheuerecker et al. 2010). This indicated lingual gyrus may be associated with emotion-related abnormalities. At present, the two of the brain regions have too little discussion to get the conclusion that the lingual gyrus and the fusiform gyrus are the common biomarker in addiction, though there are two researches supporting our results (Yang et al. 2020; Sun et al. 2014). We believe that this finding warrants further investigation to clarify its significance.
Distinct GM abnormalities in BA and SUD
Analyzing by subgroups of addiction, results of SUD group were broadly consistent with the pooled analysis. Evidence from a recent research of polysubstance users has suggested similar prefrontal cortex dysfunction in SUD (Kaag et al. 2018). In contrast to SUD, results in BA subgroup showed more significant GM decrease in the left inferior occipital gyrus, right striatum, and right superior frontal gyrus. Such abnormalities derived from behavior itself without the neurotoxic effect of drugs are inexplicable, indicating subtle dissimilarities between the neural mechanism of BA and SUD (Robbins and Clark 2015). The striatum is anatomically subdivided into the ventral striatum and the dorsal striatum, involved in processing inputs and outputs from numerous brain regions such as prefrontal cortex, ventral tegmental area, and thalamus (Yager et al. 2015). Zhou’s study proposed a functional ventral-dorsal shift theory: Ventral striatal reward system was associated with excessive drug using at an early stage while dorsal part dominated after the formation of habitual behaviors (Zhou et al. 2019). Recent studies found that individuals with behavioral addiction showed decreased GMV in putamen comparing with HC (Qin et al. 2020). Moreover, excessive drug using like CD leads to down-regulation of post-synaptic dopamine receptors (Volkow et al. 2014). It may result in putaminal hypertrophy as a compensatory process to produce more dopamine to maintain dopaminergic transmission (Volkow et al. 2014). Therefore, the difference between SUD and BA in the striatum is consistent with such a shift theory and compensatory mechanism of SUD, and the intervention strategies targeting ventral-dorsal shift may prevent high-risk individuals from both SUD and BA.
To eliminate possible medication and abstinence effects, we excluded studies recruiting participants with current intervention therapy. The results were replicable despite the evidence that pharmacotherapy can alter GM volume in other psychiatric conditions (Vita et al. 2015). Moreover, lack of clarity on the confounding effects made it premature to rule out structural effects of medication and abstinence in addictions. As robust our result seems to be, it should be interpreted with caution.
Clinical association with impulsivity
Meta-regression analysis found that the BIS-11 score was positively associated with GM decrease in the right ACC and left inferior frontal gyrus for addiction-related disorders, with smaller ACC in more impulsive individuals. Our results are in line with previous findings of a relationship between ACC volume and impulsivity in IGD (Lee et al. 2018), CD (Meade et al. 2020), and AUD (Crunelle et al. 2014). Such a relationship also existed in animal models (Winstanley 2011), which may suggest that the positive association between impulsivity and lower GMV variation was not related to diagnostic groups. In addition, inhibitory control is the ability to overcome impulsive behavior, and impulse-control deficits represent a risk factor for addiction. As mentioned earlier, dysfunction of the ACC is involved in impaired response inhibition (Holst et al. 2012b), which is the core cognitive function of addiction. Our study demonstrated the homogeneity cross diagnostic and further benefit clinical assessment and treatment of addiction-related disorder.
Limitation
This meta-analysis sheds new light on shared structural abnormalities in distinct addictions including both SUD and BA. However, we need to point out several limitations in our study. First, the causality between GM decrease and development of addiction is still inexplicable by means of the integration of cross-sectional studies. According to causal network of structural covariance (Zhang et al. 2017), we will go a step further to investigate the causation in the future. Second, a number of other types of addiction were lacking; we therefore could only select five typical addiction-related disorders in our meta-analysis. Third, we can hardly get rid of the inter-study heterogeneity in methodology (including software, thresholds, diagnostic criteria, magnetic field strength), which may influence our results. Future research should focus on these aspects.
Conclusion
In summary, evidence in our pooled meta-analysis was able to support the idea that prefrontal and insula regions might be a common neurobiological substrate for addiction-related disorders. Besides, subgroup and meta-regression analysis further explored brain structural changes in SUD and BA as well as association with clinical information, which could pave a way for future treatment. Nevertheless, the fact that common brain structural alterations are seen despite potentially differing etiologies raises the possibility that these brain regions could be used as potential therapeutic neuro-target across various addictions. Future large-scale and longitudinal studies using multimodal methods would further prove and supplement our findings.
References
Alabdulsalam A, Zaidi SZ, Tailor I, Orz Y, Al-Dandan S (2014) Primary burkitt lymphoma of the fourth ventricle in an immunocompetent young patient. Case Rep Pathol 2014:630954. https://doi.org/10.1155/2014/630954
Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ (2011) Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch Gen Psychiatry 68(3):283–294. https://doi.org/10.1001/archgenpsychiatry.2011.10
Alonso J, Angermeyer MC, Lépine JP, European Study of the Epidemiology of Mental Disorders (ESEMeD) Project (2004) The European Study of the Epidemiology of Mental Disorders (ESEMeD) project: an epidemiological basis for informing mental health policies in Europe. Acta Psychiatr Scand Suppl 420:5–7. https://doi.org/10.1111/j.1600-0047.2004.00325.x
Ashburner J, Friston KJ (2001) Why voxel-based morphometry should be used. Neuroimage 14(6):1238–1243. https://doi.org/10.1006/nimg.2001.0961
Barros-Loscertales A, Garavan H, Bustamante JC, Ventura-Campos N, Llopis JJ, Belloch V, Parcet MA, Avila C (2011) Reduced striatal volume in cocaine-dependent patients. Neuroimage 56(3):1021–1026. https://doi.org/10.1016/j.neuroimage.2011.02.035
Bayram E, Kaplan N, Shan G, Caldwell JZK (2020) The longitudinal associations between cognition, mood and striatal dopaminergic binding in Parkinson’s Disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 27(4):581–594. https://doi.org/10.1080/13825585.2019.1653445
Beissner F, Meissner K, Bar KJ, Napadow V (2013) The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33(25):10503–10511. https://doi.org/10.1523/JNEUROSCI.1103-13.2013
Blefari ML, Martuzzi R, Salomon R, Bello-Ruiz J, Herbelin B, Serino A, Blanke O (2017) Bilateral Rolandic operculum processing underlying heartbeat awareness reflects changes in bodily self-consciousness. Eur J Neurosci 45(10):1300–1312. https://doi.org/10.1111/ejn.13567
Brewer JA, Potenza MN (2008) The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol 75(1):63–75. https://doi.org/10.1016/j.bcp.2007.06.043
Brooks SJ, Dalvie S, Cuzen NL, Cardenas V, Fein G, Stein DJ (2014) Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: a voxel-based morphometry study. Metab Brain Dis 29(2):311–321. https://doi.org/10.1007/s11011-014-9489-4
Bu L, Yu D, Su S, Ma Y, von Deneen KM, Luo L, Zhai J, Liu B, Cheng J, Guan Y, Li Y, Bi Y, Xue T, Lu X, Yuan K (2016) Functional connectivity abnormalities of brain regions with structural deficits in young adult male smokers. Front Hum Neurosci 10:494. https://doi.org/10.3389/fnhum.2016.00494
Chiamulera C (2005) Cue reactivity in nicotine and tobacco dependence: a “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res Brain Res Rev 48(1):74–97. https://doi.org/10.1016/j.brainresrev.2004.08.005
Choi J, Cho H, Kim JY, Jung DJ, Ahn KJ, Kang HB, Choi JS, Chun JW, Kim DJ (2017) Structural alterations in the prefrontal cortex mediate the relationship between Internet gaming disorder and depressed mood. Sci Rep 7(1):1245. https://doi.org/10.1038/s41598-017-01275-5
Ciccarelli O, Toosy AT, Marsden JF, Wheeler-Kingshott CM, Sahyoun C, Matthews PM, Miller DH, Thompson AJ (2005) Identifying brain regions for integrative sensorimotor processing with ankle movements. Exp Brain Res 166(1):31–42. https://doi.org/10.1007/s00221-005-2335-5
Claus ED, Blaine SK, Filbey FM, Mayer AR, Hutchison KE (2013) Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology 38(12):2363–2372. https://doi.org/10.1038/npp.2013.134
Crunelle CL, Kaag AM, van Wingen G, van den Munkhof HE, Homberg JR, Reneman L, van den Brink W (2014) Reduced frontal brain volume in non-treatment-seeking cocaine-dependent individuals: exploring the role of impulsivity, depression, and smoking. Front Hum Neurosci 8:7. https://doi.org/10.3389/fnhum.2014.00007
Dalvie S, Stein DJ, Koenen K, Cardenas V, Cuzen NL, Ramesar R, Fein G, Brooks SJ (2014) The BDNF p.Val66Met polymorphism, childhood trauma, and brain volumes in adolescents with alcohol abuse. BMC Psychiatry 14:328. https://doi.org/10.1186/s12888-014-0328-2
David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM, Walton RT (2005) Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry 58(6):488–494. https://doi.org/10.1016/j.biopsych.2005.04.028
de Wit H (2009) Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol 14(1):22–31. https://doi.org/10.1111/j.1369-1600.2008.00129.x
Dong L, Wang P, Peng R, Jiang S, Klugah-Brown B, Luo C, Yao D (2016) Altered basal ganglia-cortical functional connections in frontal lobe epilepsy: a resting-state fMRI study. Epilepsy Res 128:12–20. https://doi.org/10.1016/j.eplepsyres.2016.10.011
Draps M, Sescousse G, Potenza MN, Marchewka A, Duda A, Lew-Starowicz M, Kopera M, Jakubczyk A, Wojnar M, Gola M (2020) Gray matter volume differences in impulse control and addictive disorders-an evidence from a sample of heterosexual males. J Sex Med 17(9):1761–1769. https://doi.org/10.1016/j.jsxm.2020.05.007
Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, Yang Y, Zhang W, Zhou D, Bi F, Kendrick KM, Gong Q (2014) Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci 39(6):397–406. https://doi.org/10.1503/jpn.130275
Du X, Qi X, Yang Y, Du G, Gao P, Zhang Y, Qin W, Li X, Zhang Q (2016) Altered structural correlates of impulsivity in adolescents with internet gaming disorder. Front Hum Neurosci 10:4. https://doi.org/10.3389/fnhum.2016.00004
Egger M, Smith GD, Phillips AN (1997) Meta-analysis: principles and procedures. BMJ 315(7121):1533–1537. https://doi.org/10.1136/bmj.315.7121.1533
Engel A, Bangert M, Horbank D, Hijmans BS, Wilkens K, Keller PE, Keysers C (2012) Learning piano melodies in visuo-motor or audio-motor training conditions and the neural correlates of their cross-modal transfer. Neuroimage 63(2):966–978. https://doi.org/10.1016/j.neuroimage.2012.03.038
Fauth-Bühler M, Mann K, Potenza MN (2017) Pathological gambling: a review of the neurobiological evidence relevant for its classification as an addictive disorder. Addict Biol 22(4):885–897. https://doi.org/10.1111/adb.12378
Fineberg NA, Chamberlain SR, Goudriaan AE, Stein DJ, Vanderschuren LJ, Gillan CM, Shekar S, Gorwood PA, Voon V, Morein-Zamir S, Denys D, Sahakian BJ, Moeller FG, Robbins TW, Potenza MN (2014) New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr 19(1):69–89. https://doi.org/10.1017/S1092852913000801
Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H, Childress AR (2014) The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS ONE 9(8):e104102. https://doi.org/10.1371/journal.pone.0104102
Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, Hosten N, Lotze M (2014) Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology 39(11):2594–2600. https://doi.org/10.1038/npp.2014.112
Galandra C, Crespi C, Basso G, Manera MR, Giorgi I, Poggi P, Canessa N (2020) Decreased information processing speed and decision-making performance in alcohol use disorder: combined neurostructural evidence from VBM and TBSS. Brain Imaging Behav. https://doi.org/10.1007/s11682-019-00248-8
Gardini S, Venneri A (2012) Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res Bull 87(2–3):205–211. https://doi.org/10.1016/j.brainresbull.2011.11.021
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12(11):652–669. https://doi.org/10.1038/nrn3119
Goodkind M, Eickhoff SB, Oathes DJ, Jiang Y, Chang A, Jones-Hagata LB, Ortega BN, Zaiko YV, Roach EL, Korgaonkar MS, Grieve SM, Galatzer-Levy I, Fox PT, Etkin A (2015) Identification of a common neurobiological substrate for mental illness. JAMA Psychiat 72(4):305–315. https://doi.org/10.1001/jamapsychiatry.2014.2206
Hahn C, Kim DJ (2014) Is there a shared neurobiology between aggression and Internet addiction disorder? J Behav Addict 3(1):12–20. https://doi.org/10.1556/JBA.3.2014.1.2
Hall MG, Hauson AO, Wollman SC, Allen KE, Connors EJ, Stern MJ, Kimmel CL, Stephan RA, Sarkissians S, Barlet BD, Grant I (2018) Neuropsychological comparisons of cocaine versus methamphetamine users: a research synthesis and meta-analysis. Am J Drug Alcohol Abuse 44(3):277–293. https://doi.org/10.1080/00952990.2017.1355919
Han DH, Lyoo IK, Renshaw PF (2012) Differential regional gray matter volumes in patients with on-line game addiction and professional gamers. J Psychiatr Res 46(4):507–515. https://doi.org/10.1016/j.jpsychires.2012.01.004
Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ (2011) Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology 218(4):681–692. https://doi.org/10.1007/s00213-011-2360-y
He Q, Turel O, Wei L, Bechara A (2020) Structural brain differences associated with extensive massively-multiplayer video gaming. Brain Imaging Behav. https://doi.org/10.1007/s11682-020-00263-0
Heikkinen N, Niskanen E, Kononen M, Tolmunen T, Kekkonen V, Kivimaki P, Tanila H, Laukkanen E, Vanninen R (2017) Alcohol consumption during adolescence is associated with reduced grey matter volumes. Addiction 112(4):604–613. https://doi.org/10.1111/add.13697
Howell NA, Worbe Y, Lange I, Tait R, Irvine M, Banca P, Harrison NA, Bullmore ET, Hutchison WD, Voon V (2013) Increased ventral striatal volume in college-aged binge drinkers. PLoS ONE 8(9):e74164. https://doi.org/10.1371/journal.pone.0074164
Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR (2014) Cerebral gray matter volumes and low-frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend 134:51–62. https://doi.org/10.1016/j.drugalcdep.2013.09.004
Jastreboff AM, Sinha R, Lacadie CM, Balodis IM, Sherwin R, Potenza MN (2015) Blunted striatal responses to favorite-food cues in smokers. Drug Alcohol Depend 146:103–106. https://doi.org/10.1016/j.drugalcdep.2014.09.006
Jin C, Zhang T, Cai C, Bi Y, Li Y, Yu D, Zhang M, Yuan K (2016) Abnormal prefrontal cortex resting state functional connectivity and severity of internet gaming disorder. Brain Imaging Behav 10(3):719–729. https://doi.org/10.1007/s11682-015-9439-8
Joutsa J, Saunavaara J, Parkkola R, Niemela S, Kaasinen V (2011) Extensive abnormality of brain white matter integrity in pathological gambling. Psychiatry Res 194(3):340–346. https://doi.org/10.1016/j.pscychresns.2011.08.001
Kaag AM, Schulte MHJ, Jansen JM, van Wingen G, Homberg J, van den Brink W, Wiers RW, Schmaal L, Goudriaan AE, Reneman L (2018) The relation between gray matter volume and the use of alcohol, tobacco, cocaine and cannabis in male polysubstance users. Drug Alcohol Depend 187:186–194. https://doi.org/10.1016/j.drugalcdep.2018.03.010
Klaming R, Harle KM, Infante MA, Bomyea J, Kim C, Spadoni AD (2019) Shared gray matter reductions across alcohol use disorder and posttraumatic stress disorder in the anterior cingulate cortex: a dual meta-analysis. Neurobiol Stress 10:100132. https://doi.org/10.1016/j.ynstr.2018.09.009
Ko CH, Liu GC, Hsiao S, Yen JY, Yang MJ, Lin WC, Yen CF, Chen CS (2009) Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res 43(7):739–747. https://doi.org/10.1016/j.jpsychires.2008.09.012
Ko CH, Hsieh TJ, Wang PW, Lin WC, Yen CF, Chen CS, Yen JY (2015) Altered gray matter density and disrupted functional connectivity of the amygdala in adults with Internet gaming disorder. Prog Neuropsychopharmacol Biol Psychiatry 57:185–192. https://doi.org/10.1016/j.pnpbp.2014.11.003
Koehler S, Hasselmann E, Wustenberg T, Heinz A, Romanczuk-Seiferth N (2015) Higher volume of ventral striatum and right prefrontal cortex in pathological gambling. Brain Struct Funct 220(1):469–477. https://doi.org/10.1007/s00429-013-0668-6
Lee D, Namkoong K, Lee J, Jung YC (2018) Abnormal gray matter volume and impulsivity in young adults with Internet gaming disorder. Addict Biol 23(5):1160–1167. https://doi.org/10.1111/adb.12552
Lee D, Namkoong K, Lee J, Jung YC (2019) Preliminary evidence of altered gray matter volume in subjects with internet gaming disorder: associations with history of childhood attention-deficit/hyperactivity disorder symptoms. Brain Imaging Behav 13(3):660–668. https://doi.org/10.1007/s11682-018-9872-6
Li J, Wang Y, Xu Z, Liu T, Zang X, Li M, Ma L (2019) Whole-brain morphometric studies in alcohol addicts by voxel-based morphometry. Ann Transl Med 7(22):635. https://doi.org/10.21037/atm.2019.10.90
Liao Y, Tang J, Liu T, Chen X, Hao W (2012) Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol 17(6):977–980. https://doi.org/10.1111/j.1369-1600.2010.00250.x
Lin X, Dong G, Wang Q, Du X (2015) Abnormal gray matter and white matter volume in “Internet gaming addicts.” Addict Behav 40:137–143. https://doi.org/10.1016/j.addbeh.2014.09.010
Linnet J, Moller A, Peterson E, Gjedde A, Doudet D (2011) Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction 106(2):383–390. https://doi.org/10.1111/j.1360-0443.2010.03126.x
Lukito S, Norman L, Carlisi C, Radua J, Hart H, Simonoff E, Rubia K (2020) Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol Med 50(6):894–919. https://doi.org/10.1017/S0033291720000574
Matuskey D, Bhagwagar Z, Planeta B, Pittman B, Gallezot JD, Chen J, Wanyiri J, Najafzadeh S, Ropchan J, Geha P, Huang Y, Potenza MN, Neumeister A, Carson RE, Malison RT (2014) Reductions in brain 5-HT1B receptor availability in primarily cocaine-dependent humans. Biol Psychiatry 76(10):816–822. https://doi.org/10.1016/j.biopsych.2013.11.022
Meade CS, Bell RP, Towe SL, Hall SA (2020) Cocaine-related alterations in fronto-parietal gray matter volume correlate with trait and behavioral impulsivity. Drug Alcohol Depend 206:107757. https://doi.org/10.1016/j.drugalcdep.2019.107757
Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15(10):483–506. https://doi.org/10.1016/j.tics.2011.08.003
Miles FJ, Everitt BJ, Dickinson A (2003) Oral cocaine seeking by rats: action or habit? Behav Neurosci 117(5):927–938. https://doi.org/10.1037/0735-7044.117.5.927
Moccia L, Pettorruso M, De Crescenzo F, De Risio L, di Nuzzo L, Martinotti G, Bifone A, Janiri L, Di Nicola M (2017) Neural correlates of cognitive control in gambling disorder: a systematic review of fMRI studies. Neurosci Biobehav Rev 78:104–116. https://doi.org/10.1016/j.neubiorev.2017.04.025
Mohammadi B, Hammer A, Miedl SF, Wiswede D, Marco-Pallares J, Herrmann M, Munte TF (2016) Intertemporal choice behavior is constrained by brain structure in healthy participants and pathological gamblers. Brain Struct Funct 221(6):3157–3170. https://doi.org/10.1007/s00429-015-1093-9
Mohammadi B, Szycik GR, Te Wildt B, Heldmann M, Samii A, Munte TF (2020) Structural brain changes in young males addicted to video-gaming. Brain Cogn 139:105518. https://doi.org/10.1016/j.bandc.2020.105518
Morales AM, Lee B, Hellemann G, O’Neill J, London ED (2012) Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend 125(3):230–238. https://doi.org/10.1016/j.drugalcdep.2012.02.017
Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, Verdejo-Garcia A (2012) Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend 125(3):208–214. https://doi.org/10.1016/j.drugalcdep.2012.02.012
Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, Rubia K (2016) Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiat 73(8):815–825. https://doi.org/10.1001/jamapsychiatry.2016.0700
Paljarvi T, Koskenvuo M, Poikolainen K, Kauhanen J, Sillanmaki L, Makela P (2009) Binge drinking and depressive symptoms: a 5-year population-based cohort study. Addiction 104(7):1168–1178. https://doi.org/10.1111/j.1360-0443.2009.02577.x
Parvizi J, Jacques C, Foster BL, Witthoft N, Rangarajan V, Weiner KS, Grill-Spector K (2012) Electrical stimulation of human fusiform face-selective regions distorts face perception. J Neurosci 32(43):14915–14920. https://doi.org/10.1523/JNEUROSCI.2609-12.2012
Peng P, Wang Z, Jiang T, Chu S, Wang S, Xiao D (2017) Brain-volume changes in young and middle-aged smokers: a DARTEL-based voxel-based morphometry study. Clin Respir J 11(5):621–631. https://doi.org/10.1111/crj.12393
Peng P, Li M, Liu H, Tian YR, Chu SL, Van Halm-Lutterodt N, Jing B, Jiang T (2018) Brain structure alterations in respect to tobacco consumption and nicotine dependence: a comparative voxel-based morphometry study. Front Neuroanat 12:43. https://doi.org/10.3389/fnana.2018.00043
Potenza MN (2014) Non-substance addictive behaviors in the context of DSM-5. Addict Behav 39(1):1–2. https://doi.org/10.1016/j.addbeh.2013.09.004
Qian W, Huang P, Shen Z, Wang C, Yang Y, Zhang M (2019) Brain gray matter volume and functional connectivity are associated with smoking cessation outcomes. Front Hum Neurosci 13:361. https://doi.org/10.3389/fnhum.2019.00361
Qin K, Zhang F, Chen T, Li L, Li W, Suo X, Lei D, Kemp GJ, Gong Q (2020) Shared gray matter alterations in individuals with diverse behavioral addictions: a voxel-wise meta-analysis. J Behav Addict 9(1):1–14. https://doi.org/10.1556/2006.2020.00006
Radua J, Mataix-Cols D (2009) Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 195(5):393–402. https://doi.org/10.1192/bjp.bp.108.055046
Rehm J, Room R, van den Brink W, Kraus L (2005) Problematic drug use and drug use disorders in EU countries and Norway: an overview of the epidemiology. Eur Neuropsychopharmacol 15(4):389–397. https://doi.org/10.1016/j.euroneuro.2005.04.004
Robbins TW, Clark L (2015) Behavioral addictions. Curr Opin Neurobiol 30:66–72. https://doi.org/10.1016/j.conb.2014.09.005
Robbins TW, Roberts AC (2007) Differential regulation of fronto-executive function by the monoamines and acetylcholine. Cereb Cortex 17(Suppl 1):i151-160. https://doi.org/10.1093/cercor/bhm066
Robbins TW, Ersche KD, Everitt BJ (2008) Drug addiction and the memory systems of the brain. Ann N Y Acad Sci 1141:1–21. https://doi.org/10.1196/annals.1441.020
Scheuerecker J, Meisenzahl EM, Koutsouleris N, Roesner M, Schopf V, Linn J, Wiesmann M, Bruckmann H, Moller HJ, Frodl T (2010) Orbitofrontal volume reductions during emotion recognition in patients with major depression. J Psychiatry Neurosci 35(5):311–320. https://doi.org/10.1503/jpn.090076
Schoenbaum G, Roesch MR, Stalnaker TA (2006) Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci 29(2):116–124. https://doi.org/10.1016/j.tins.2005.12.006
Segobin SH, Chetelat G, Le Berre AP, Lannuzel C, Boudehent C, Vabret F, Eustache F, Beaunieux H, Pitel AL (2014) Relationship between brain volumetric changes and interim drinking at six months in alcohol-dependent patients. Alcohol Clin Exp Res 38(3):739–748. https://doi.org/10.1111/acer.12300
Seok JW, Sohn JH (2018) Altered gray matter volume and resting-state connectivity in individuals with internet gaming disorder: a voxel-based morphometry and resting-state functional magnetic resonance imaging study. Front Psychiatry 9:77. https://doi.org/10.3389/fpsyt.2018.00077
Stoeckel LE, Chai XJ, Zhang J, Whitfield-Gabrieli S, Evins AE (2016) Lower gray matter density and functional connectivity in the anterior insula in smokers compared with never smokers. Addict Biol 21(4):972–981. https://doi.org/10.1111/adb.12262
Sun Y, Sun J, Zhou Y, Ding W, Chen X, Zhuang Z, Xu J, Du Y (2014) Assessment of in vivo microstructure alterations in gray matter using DKI in Internet gaming addiction. Behav Brain Funct 24(10):37. https://doi.org/10.1186/1744-9081-10-37
Sussman S, Lisha N, Griffiths M (2011) Prevalence of the addictions: a problem of the majority or the minority? Eval Health Prof 34(1):3–56. https://doi.org/10.1177/0163278710380124
Takeuchi H, Tsurumi K, Murao T, Takemura A, Kawada R, Urayama SI, Aso T, Sugihara GI, Miyata J, Murai T, Takahashi H (2017) Common and differential brain abnormalities in gambling disorder subtypes based on risk attitude. Addict Behav 69:48–54. https://doi.org/10.1016/j.addbeh.2017.01.025
Unterrainer HF, Hiebler-Ragger M, Koschutnig K, Fuchshuber J, Ragger K, Perchtold CM, Papousek I, Weiss EM, Fink A (2019) Brain structure alterations in poly-drug use: reduced cortical thickness and white matter impairments in regions associated with affective, cognitive, and motor functions. Front Psychiatry 10:667. https://doi.org/10.3389/fpsyt.2019.00667
van Holst RJ, de Ruiter MB, van den Brink W, Veltman DJ, Goudriaan AE (2012a) A voxel-based morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug Alcohol Depend 124(1–2):142–148. https://doi.org/10.1016/j.drugalcdep.2011.12.025
van Holst RJ, van Holstein M, van den Brink W, Veltman DJ, Goudriaan AE (2012b) Response inhibition during cue reactivity in problem gamblers: an fMRI study. PLoS ONE 7(3):e30909. https://doi.org/10.1371/journal.pone.0030909
Vita A, De Peri L, Deste G, Barlati S, Sacchetti E (2015) The effect of antipsychotic treatment on cortical gray matter changes in schizophrenia: does the class matter? A Meta-analysis and Meta-regression of Longitudinal Magnetic Resonance Imaging Studies. Biol Psychiatry 78(6):403–412. https://doi.org/10.1016/j.biopsych.2015.02.008
Volkow ND, Tomasi D, Wang GJ, Logan J, Alexoff DL, Jayne M, Fowler JS, Wong C, Yin P, Du C (2014) Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol Psychiatry 19(9):1037–1043. https://doi.org/10.1038/mp.2014.58
Wang K, Yang J, Zhang S, Wei D, Hao X, Tu S, Qiu J (2014) The neural mechanisms underlying the acute effect of cigarette smoking on chronic smokers. PLoS ONE 9(7):e102828. https://doi.org/10.1371/journal.pone.0102828
Wang J, Fan Y, Dong Y, Ma M, Ma Y, Dong Y, Niu Y, Jiang Y, Wang H, Wang Z, Wu L, Sun H, Cui C (2016) Alterations in brain structure and functional connectivity in alcohol dependent patients and possible association with impulsivity. PLoS ONE 11(8):e0161956. https://doi.org/10.1371/journal.pone.0161956
Weiner KS, Golarai G, Caspers J, Chuapoco MR, Mohlberg H, Zilles K, Amunts K, Grill-Spector K (2014) The mid-fusiform sulcus: a landmark identifying both cytoarchitectonic and functional divisions of human ventral temporal cortex. Neuroimage 84:453–465. https://doi.org/10.1016/j.neuroimage.2013.08.068
Weinstein A, Lejoyeux M (2010) Internet addiction or excessive internet use. Am J Drug Alcohol Abuse 36(5):277–283. https://doi.org/10.3109/00952990.2010.491880
Weng CB, Qian RB, Fu XM, Lin B, Han XP, Niu CS, Wang YH (2013) Gray matter and white matter abnormalities in online game addiction. Eur J Radiol 82(8):1308–1312. https://doi.org/10.1016/j.ejrad.2013.01.031
Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, Franklin TR (2015) Cannabis, cigarettes, and their co-occurring use: disentangling differences in gray matter volume. Int J Neuropsychopharmacol 18(10):pyv061. https://doi.org/10.1093/ijnp/pyv061
Wiers CE, Gawron CK, Gropper S, Spengler S, Stuke H, Lindenmeyer J, Walter H, Bermpohl F (2015) Decreased gray matter volume in inferior frontal gyrus is related to stop-signal task performance in alcohol-dependent patients. Psychiatry Res 233(2):125–130. https://doi.org/10.1016/j.pscychresns.2015.05.006
Winstanley CA (2011) The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol 164(4):1301–1321. https://doi.org/10.1111/j.1476-5381.2011.01323.x
Yager LM, Garcia AF, Wunsch AM, Ferguson SM (2015) The ins and outs of the striatum: role in drug addiction. Neuroscience 301:529–541. https://doi.org/10.1016/j.neuroscience.2015.06.033
Yang Z, Zhang Y, Cheng J, Zheng R (2020) Meta-analysis of brain gray matter changes in chronic smokers. Eur J Radiol 132:109300. https://doi.org/10.1016/j.ejrad.2020.109300
Yip SW, Worhunsky PD, Xu J, Morie KP, Constable RT, Malison RT, Carroll KM, Potenza MN (2018) Gray-matter relationships to diagnostic and transdiagnostic features of drug and behavioral addictions. Addict Biol 23(1):394–402. https://doi.org/10.1111/adb.12492
Yoon EJ, Choi JS, Kim H, Sohn BK, Jung HY, Lee JY, Kim DJ, Park SW, Kim YK (2017) Altered hippocampal volume and functional connectivity in males with Internet gaming disorder comparing to those with alcohol use disorder. Sci Rep 7(1):5744. https://doi.org/10.1038/s41598-017-06057-7
Yu R, Zhao L, Lu L (2011) Regional grey and white matter changes in heavy male smokers. PLoS ONE 6(11):e27440. https://doi.org/10.1371/journal.pone.0027440
Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA (2011) Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage 54(1):42–48. https://doi.org/10.1016/j.neuroimage.2010.08.008
Zhang Z, Liao W, Xu Q, Wei W, Zhou HJ, Sun K, Yang F, Mantini D, Ji X, Lu G (2017) Hippocampus-associated causal network of structural covariance measuring structural damage progression in temporal lobe epilepsy. Hum Brain Mapp 38(2):753–766. https://doi.org/10.1002/hbm.23415
Zhou Y, Lin FC, Du YS, Qin LD, Zhao ZM, Xu JR, Lei H (2011) Gray matter abnormalities in Internet addiction: a voxel-based morphometry study. Eur J Radiol 79(1):92–95. https://doi.org/10.1016/j.ejrad.2009.10.025
Zhou X, Zimmermann K, Xin F, Zhao W, Derckx RT, Sassmannshausen A, Scheele D, Hurlemann R, Weber B, Kendrick KM, Becker B (2019) Cue reactivity in the ventral striatum characterizes heavy cannabis use, whereas reactivity in the dorsal striatum mediates dependent use. Biol Psychiatry Cogn Neurosci Neuroimaging 4(8):751–762. https://doi.org/10.1016/j.bpsc.2019.04.006
Zois E, Kiefer F, Lemenager T, Vollstadt-Klein S, Mann K, Fauth-Buhler M (2017a) Frontal cortex gray matter volume alterations in pathological gambling occur independently from substance use disorder. Addict Biol 22(3):864–872. https://doi.org/10.1111/adb.12368
Zois E, Vollstadt-Klein S, Hoffmann S, Reinhard I, Charlet K, Beck A, Jorde A, Kirsch M, Walter H, Heinz A, Kiefer F (2017b) Orbitofrontal structural markers of negative affect in alcohol dependence and their associations with heavy relapse-risk at 6 months post-treatment. Eur Psychiatry 46:16–22. https://doi.org/10.1016/j.eurpsy.2017.07.013
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to a Special Issue on CNS Imaging
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, M., Gao, X., Yang, Z. et al. Shared gray matter alterations in subtypes of addiction: a voxel-wise meta-analysis. Psychopharmacology 238, 2365–2379 (2021). https://doi.org/10.1007/s00213-021-05920-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05920-w