Abstract
Rationale
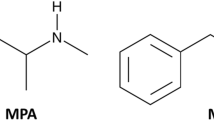
Methamnetamine (MNA; PAL-1046) is a new psychoactive substance that acts as a full biogenic amine transporter (BAT) substrate. BAT substrates promote neurotransmitter release from the nerve terminal and can be abused as stimulants. However, scientific information on the abuse potential of methamnetamine is lacking.
Objective
We evaluated the abuse liability of methamnetamine.
Methods
The effective dose range of methamnetamine was determined using a climbing behavior test. The rewarding effect and reinforcing effect of the test compound were evaluated in mice by conditioned place preference (CPP) testing and self-administration (SA) testing at the selected doses. Dopamine level changes were analyzed using synaptosomes and in vivo microdialysis to investigate the effects of methamnetamine on the central nervous system. Drug discrimination experiments were used to examine the potential similarity of the interoceptive effects of methamnetamine and cocaine.
Results
A significant response was observed in the climbing behavior test with 10 and 40 mg/kg intraperitoneally administered methamnetamine. In the CPP test, mice intraperitoneally administered methamnetamine (10 and 20 mg/kg) showed a significant preference for the drug-paired compartment. In the SA test, mice that intravenously received 1 mg/kg/infusion showed significant active-lever responses. Dopamine was significantly increased in synaptosomes and in in vivo microdialysis tests. Furthermore, methamnetamine showed cross-generalization with cocaine in a dose-dependent manner.
Conclusions
Methamnetamine exhibits interceptive stimulus properties similar to those of cocaine and induces rewarding and reinforcing effects, suggesting its dependence liability potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
New psychoactive substances (NPSs) are a range of new uncontrolled drugs or psychotropic drugs, as defined by the United Nations Office on Drugs and Crime (1961, New York) or the United Nations Office on Drugs and Crime (1971, Vienna; Merz 2018). The frequency of NPS use is steadily increasing among younger generations (Spiller et al. 2011), and NPS abuse continues to increase globally. The sale and purchase of NPSs over the internet is fast, unrestricted, and easy. To control the sale of drugs, legal regulations are required. For MNA, an NPS with an amphetamine structure, there is currently insufficient scientific evidence to prove its abuse potential.
NPSs are mostly produced by changing the chemical structure of existing psychoactive substances. MNA, also known as PAL-1046, is such an analog of an existing drug that was developed from amphetamine. Amphetamine-based drugs such as methamphetamine (METH) and (+)-amphetamine increase dopamine levels in the brain. MNA was originally developed as a medical drug, but has also been used for recreational purposes. Although the exact mechanism of action of MNA is unknown, it can cause symptoms such as anxiety, sweating, depression, emotional change, and dehydration (Hunt 1997). The first use of MNA in Denmark was reported by the United Nations Office on Drugs and Crime, and the drug was listed as an NPS by the European Union in 2015 (EMCDDA-Europol 2015).
Biogenic amine transporters such as dopamine, serotonin, and norepinephrine transporters regulate the neurotransmission of biogenic amine neurotransmitters such as dopamine, serotonin, and norepinephrine. Many stimulant drugs interact with biogenic amine transporters and predominantly increase dopamine release by targeting the dopamine transporter. Amphetamines, including METH and 3,4-methylenedioxymethamphetamine (MDMA), act as not only uptake inhibitors but also releasers of dopamine via the dopamine transporter. Similar to (+)-amphetamine, MNA promotes dopamine activity by affecting the dopamine transporter (Kahlig et al. 2005). It has been reported to increase dopamine and serotonin (5-HT) in a microdialysis study (Rothman et al. 2012).
Physical dependence on a particular drug leads to an uncomfortable feeling when drug use is discontinued. Unlike physical dependence, which disappears within a few days after drug use is stopped, psychological dependence lasts longer and is one of the main causes of relapse. MNA likely induces dependence via dopamine activity, but there is currently no accurate information on the dependence mechanism (Volkow et al. 2004).
The dependence potential of MNA has been studied in accordance with guidelines for drug dependence in two-stage abuse studies by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) (US FDA 2017; EMA 2006). First, in vivo receptor-binding and microdialysis assay results are assessed based on pharmacological data related to neurotransmitters. Second, drug dependence potential is evaluated in two representative experimental animal models through self-administration (SA) and conditioned place preference (CPP) studies in behavioral pharmacology research (Gorelick et al. 2004; Mucha et al. 1982). Drug discrimination (DD) determines the similarity between existing and new substances of abuse.
This is the first study to investigate the reinforcing and reward effects of MNA. Dopamine signaling can be upregulated as a rewarding and reinforcement response to a drug of abuse. In this study, we analyzed dopamine release by isolated synaptosomes in response to MNA and MNA-induced dopamine levels in vivo using microdialysis experiments. Before conducting the behavioral pharmacological study, the drug dose range was determined based on a climbing test, as mice are known to exhibit climbing behavior upon stimulation of the dopamine receptor in the striatum (Protais et al. 1976). In addition, the similarity to cocaine was evaluated using the DD test. Based on these various experiments, the abuse potential of MNA was evaluated.
Materials and methods
Animals
Male Sprague–Dawley (SD) rats (7–8 weeks old, weighing 180–220 g) and C57BL/6 mice (7 weeks of age, weighing 15–20 g) were used in this study. All animals were supplied by Orientbio Animal Laboratory (Seongnam, Korea). The animals were allowed to acclimatize for 1 week before the experiments. They were maintained under a 12-h light/dark cycle and controlled temperature (23 °C ± 1 °C) and humidity (55% ± 5%), and were given ad libitum access to laboratory animal food and water. Before the experiments, the animals were randomly assigned to treatment groups. On the last day of each experiment, the animals were euthanized according to animal ethics guidelines. All procedures pertaining to animal care, maintenance, and use in the present study were approved by the National Institute of Food and Drug Safety Evaluation/Ministry of Food and Drug Safety Animal Ethics Board (approval number: MFDS-19-007/009/010).
Chemical reagents
Racemic MNA (≥98.95% purity), synthesized at and purchased from Kyunghee University (Seoul, Korea), was dissolved in vehicle (saline:DMSO:Tween-80 = 18:1:1). The nuclear magnetic resonance (NMR) data (for structural confirmation) and high-resolution mass spectrometry data (for accurate mass check) are provided as Supplements 1 and 2, respectively. (+)-methamphetamine hydrochloride (≥ 98% pure; METH), obtained from Kyung Hee University (Seoul, Korea), was dissolved in the vehicle. DMSO and polyoxyethylene sorbitan mono-oleate (Tween-80) were purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). Heparin was purchased from JW-Pharmaceutical (Seoul, Korea).
Equipment
For the climbing action test, a stainless steel cylinder with numerous vertical bars that the mice could use to go up was used. The bottom diameter was 12 cm and the length of the vertical bars was 24 cm. The locomotion test apparatus (ENV-520; Med Associates, Inc., St. Albans, VT, USA) consisted of a plastic box (43 cm × 43 cm floor area and 31 cm height), with a sensor on the floor to measure movements. The CPP apparatus (MED-CPP-3013AT; Med Associates) comprised a black room and white room, which were separated by a guillotine door. The white room had a mesh grid and the black room had a bar grid. Both rooms were 16 cm × 13 cm × 12 cm (W × D × H). Between the two rooms, there is a small gray room measuring 6 cm × 13 cm × 12 cm. The rooms are illuminated with low-intensity lighting (12 lx). The amount of time spent in each room by mice was recorded with an infrared sensor control. The SA test equipment (MED-307A-CT-B1) was also purchased from Med Associates. The operant chamber was 43 cm × 43 cm × 31 cm. Each chamber had two levers (an active lever and an inactive lever) connected to an infusion pump. The infusion pump, connected to a 1-mL syringe, was placed outside the operator chamber. A computerized system (Med-PC) connected to the SA test equipment (Med Associates) was used for data recording.
Climbing behavior test procedure
Climbing behavior experiments were conducted based on a previous report (Protais et al. 1976) in a dark environment, which is the optimal activity environment for experimental animals. The mice were allowed to adapt to the climbing device for 30 min before the experiment, and were divided into nine treatment groups: a negative control (vehicle, intraperitoneal (i.p.)) group, a positive control (METH, 1 mg/kg) group, and seven groups treated with different concentrations of MNA (1, 10, 20, 40, 60, 80, and 100 mg/kg). To assess climbing behavior, the time spent on the vertical bars with all four feet was measured for 20 min after the drug treatment.
CPP test procedure
The CPP test was conducted using male mice (n = 7–8), as reported previously (de la Peña et al. 2012). The test was conducted in five steps. (1) In the first 2 days (days 1 and 2), the animals were allowed free movement in the two chambers (white or black) for 30 min per day for acclimation. (2) After measuring pre-conditioning for 15 min on day 3, the mice with more than 10% difference in the time spent in one specific chamber were excluded, and the remaining mice (vehicle group n = 7, other groups n = 8) were randomly assigned to treatment groups. (3) On days 4–13, vehicle or MNA (1, 10, 20 mg/kg, i.p.) was administered every other day, and the mice were placed in a specific chamber for 40 min after the administration. (4) Post-conditioning was measured for 15 min. (5) The time difference between the test values (post-conditioning) and baseline values (pre-conditioning) was calculated as the CPP score. METH (1 mg/kg, i.p.) was used and validated as a positive control for the CPP test.
SA test procedure
To train the lever-pressing behavior in the experimental animals, 45 mg of food pellets was set to fall on the plate of the operator chamber when the animal pressed the active one of the two levers. During the 2 days of this food training, food outside the training time was restricted to 15 g of basal diet per day. The animals were trained for 2 h per day until the test criterion was achieved (100 food pellets were collected daily for 3 days). Only mice that successfully achieved the test criterion underwent canulation surgery. Mice (n = 8) were anesthetized with sodium pentobarbital (50 mg/kg; Entobar R, Hanlim Pharmaceuticals, Seoul, Korea) after which a catheter was inserted into the jugular vein. After surgery, all mice were given a 7-day recovery period in a controlled cage. After the canulation surgery, the mice were allowed to self-administer MNA (i.v.) or vehicle for 2 h according to the fixed-ratio 1 (FR1) reinforcement schedule, and the timeout time (i.e., the period when the lever was pressed and the drug was not administered after drug administration) was set to 20 s. The SA test was validated using METH (0.1 mg/kg/infusion, i.v.) as a positive control.
Measurement of the dopamine level in synaptosomes
The dopamine level in synaptosomes was measured using the sucrose gradient method reported in previous studies (Birch and Fillenz 1985; Kamat et al. 2014; McKenna et al. 1991). Briefly, non-treated male SD rats were sacrificed with CO2, and the striatum region of the brain (n = 3) was dissected quickly. The striatum was homogenized in 0.32 M sucrose solution and the lysate supernatant was diluted with Krebs–HEPES buffer (1:1; 117 mM NaCl, 4.8 mM KCl, 2.5 mM MgCl2, 25 mM HEPES; pH 7.4). The mixture was centrifuged at 10,000×g, 4 °C for 20 min. The pellet was mixed with 20 nM standard dopamine, incubated at 37 °C for 15 min, and centrifuged at 10,000×g for 10 min to obtain a pellet. Thereafter, 300 μL of METH (0.01, 0.05, 0.1, 1, and 10 μM) or MNA (0.01, 0.05, 0.1, 1, 10, and 100 μM) was added to the pelleted synaptosomes, followed by incubation at 37 °C for 15 min. The solution was then centrifuged at 4 °C 10,000×g for 10 min and the supernatant was collected. Dopamine release level in the supernatant was measured by high-performance liquid chromatography (HPLC). Vehicle (Krebs–HEPES buffer) was used as a negative control. After filtration through a PVDF syringe filter (0.22 μm), the supernatant was analyzed using the Agilent Infinity 1260 HPLC system (Agilent Technologies, Santa Clara, CA, USA), Agilent 6460 Triple Quadrupole Mass Spectrometer (Agilent Technologies, Santa Clara, CA, USA), and a liquid chromatography mass spectrometer (LC-MS/MS). The HPLC mobile phase consisted of tertiary distilled water with 2 mM ammonium formate, 0.1% formic acid and acetonitrile with 2 mM ammonium formate, and 0.1% formic acid. The flow rate was set to 300 μL/min and the injection volume was 5 μL. A reversed-phase Poroshell 120 EC-C18 column (100 mm × 2.1 mm, 2.7 μm, Agilent) was used for separation. Sample detection was conducted for 10 min. The LC-MS/MS conditions were ionized in the ESI+ mode, and the multiple reaction monitoring (MRM) mode was selected for sensitive quantitative measurement.
In vivo microdialysis procedure
After adaptation to cannula implantation for 24 h, dialysis experiments were conducted while the SD rats (n = 3) were free to move. Ringer’s solution (NaCl 145 mM, KCl 2.7 mM, CaCl2 0.9 mM, MgCl2 0.1 mM, and Na2HPO4 2 mM; pH 7.4) was perfused at a constant flow rate of 2 μL/min. Perfusion solution was injected into the liquid chromatography system every 20 min using an autoinjector. The dialyzed dopamine was separated using a reversed-phase Poroshell 120 EC-C18 column (2.1 mm × 100 mm, 2.7 μm, Agilent) and detected with a graphite electrode. The mobile phase consisted of 0.1 M phosphate buffer (pH 5.5) containing 500 mg/L sodium decanesulfonate, 50 mg/L EDTA, and 1% methanol. Eighty minutes before sample collection, the perfusion was started for acclimation. Samples were collected for 40 min before drug administration and for 120 min after drug administration.
Locomotor activity
For acclimation, the mice (n = 6) were allowed to move freely for 120 min in a small-animal activity monitor device (ENV520; Med Associates) for 3 days, without interference. The travel distance and number of jumps were automatically measured using a laser sensor installed on the floor, and all values were recorded using an SW Activity Monitor SOF-812 (Med Associates). On day 4 after adaption, migration was measured every 5 min after i.p. administration of MNA (0, 10, and 20 mg/kg) for 60 min. It was verified by treatment with METH (1 mg/kg i.p.) as a positive control for measuring exercise activity.
DD test procedure
For fasted rats to react to the food, they were trained to press the active lever for 45 mg of food pellets, according to the FR1 reinforcement schedule, for 1 h every day. Each daily session started with the lighting of a cue lamp located above the lever of the operant chamber. The food pellets (45 mg) were delivered after each lever press. When the rat ate 30 pellets, one lever was deactivated and the other was activated. All rats were trained to FR10, in which each 10th response produced a pellet. Whenever food pellet was delivered, the lever was deactivated for a 5-s timeout period during which the cue indicator lamp was turned off. Lever-press training was conducted until the criterion of acquiring 60 pellets for 3 consecutive days within one session according to the FR10 reinforcement schedule was achieved.
DD training was conducted to differentiate between cocaine (10 mg/kg) and vehicle. Rats received an i.p. administration of drugs or saline before they were placed in the chamber. Thereafter, the cue light was turned on, the two levers were activated in the chamber, and the 15-min training sessions were started. Food pellets were delivered when cocaine was administered after the FR10 requirement was met on one of the levers. Subsequently, there was a 5-s timeout period during which the chamber light was turned on and the levers were retracted. When the vehicle was administered during the training session, the food pellets were delivered in the same way if the FR10 requirement was achieved on the other lever. When the wrong lever was pressed, no food pellets were provided, and only the number of presses was recorded. Cocaine or vehicle was administered randomly every other day. In the training phase, the number of days of drug (cocaine) and vehicle training was shifted for three consecutive days. Training was terminated 15 min after the start of the daily session or after the rats had obtained 20 food pellets. Training was continued until more than 85% of the responses occurred on the correct lever for 3 consecutive sessions.
The test session proceeded in the same way as the training session, except that the food pellets were delivered when the lever was pressed 10 times on either lever. After a 3-day training period (drug [cocaine] and vehicle), the reaction to the test drug was evaluated. Training periods and tests were repeated until all doses were tested. Graphs for percent drug-appropriate responding were plotted as a function of test compound dose (log scale).
Statistical analysis
All data are expressed as mean and standard error of the mean (± SEM) and were analyzed using Prism 6.0 software (GraphPad Software, Inc., San Diego, CA, USA). Data from the climbing behavior, CPP, SA, and locomotion tests and LC/MS/MS and microdialysis were analyzed using one- or two-way analysis of variance (ANOVA) followed by the relevant post hoc tests. The climbing behavior and CPP test results were analyzed using Dunnett’s post hoc test, and the SA, microdialysis, and locomotor test results were analyzed using Tukey’s post hoc test. Statistical significance was set at *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared with the vehicle-treated group.
Results
MNA promotes climbing behavior
The climbing behavior test was conducted to predict the range of effective doses of drugs for dopamine pathway activation. In the group that received i.p. MNA (40 mg/kg), the time spent on the apparatus significantly increased. The one-way ANOVA revealed significant differences between the experimental groups [F (7,56) = 10.20, p < 0.001]. Dunnett’s post hoc tests revealed that MNA at 10 mg/kg (q = 2.825, p < 0.05) and 40 mg/kg (q = 3.047, p < 0.05) increased the time spent on the apparatus (Fig. 1a). The movement of the mice sharply decreased by MNA at a dose of 60 mg/kg or higher, and it completely stopped at 100 mg/kg dose. At 40 mg/kg, a high climbing behavior response was observed, but convulsions and abnormal behavior were also observed. METH (1 mg/kg) used as a positive control also induced a significant increase in climbing behavior (Fig. 1b).
Changes in climbing behavior time after the administration of MNA and METH. Mice were placed in the apparatus and their climbing behavior time was recorded for 20 min after the injection of a MNA and b METH. Data are presented as mean ± SEM (n = 8 for each group). *p < 0.05 versus vehicle (one-way ANOVA followed by Dunnett’s post hoc test). METH, methamphetamine treatment group; MNA, methamnetamine treatment group
MNA promotes the CPP
Pre-recording was checked, and the mice that showed bias (when the time spent in a specific room was more than 75% of the total measurement time) were excluded. The CPP scores and times measured in the drug-paired period are indicated in Fig. 2. The system was validated using METH (1 mg/kg, i.p.) as the positive control, before the drug study. Various doses of MNA (1, 10, and 20 mg/kg) were administered to the mice. In the pre-conditioning phase, there was no significant difference in the time spent in the drug-paired room between the groups (Fig. 2a). The group administered 10 or 20 mg/kg MNA showed a significant increase in the time spent in the drug-paired room compared with that during the pre-conditioning phase of each group. The group that received METH (1 mg/kg) as the positive control also showed a significant increase in the time spent in the drug-paired room compared with the vehicle group. The CPP scores were significantly increased in the 10 and 20 mg/kg MNA treatment groups compared with those in the vehicle group. One-way ANOVA revealed significant differences between the experimental groups [F (4,34) = 13.89, p < 0.0001]. Dunnett’s post hoc tests revealed that MNA 10 mg/kg (q = 4.437, p < 0.001), MNA 20 mg/kg (q = 4.063, p < 0.01), and METH 1 mg/kg (q = 5.995, p < 0.0001) induced significant effects (Fig. 2b). However, the CPP scores in the 10 mg/kg and 20 mg/kg MNA-treated groups did not show differences. MNA (10 mg/kg) increased the CPP scores to approximately 65% of the scores induced by METH.
Effects of MNA and METH on CPP in mice. a Bars indicate the mean time spent in the drug-paired compartment during pre- and post-conditioning phases. Data are presented as mean ± SEM (n = 7–8 for each group). ***p < 0.001 versus vehicle (two-way ANOVA followed by Tukey’s post hoc test). b Difference in time spent in drug-paired compartment between the drug treatment group and the negative control group during pre- and post-conditioning phases. Data are presented as mean ± SEM (n = 7–8 for each group). **p < 0.01; ***p < 0.001 versus vehicle (Dunnett’s post hoc test). METH, methamphetamine treatment group; MNA, methamnetamine treatment group; vehicle, saline treatment group
MNA promotes the SA
The SA test was conducted for 2 h per session per day with an FR1 schedule, and MNA (1 mg/kg/infusion, i.v.) was self-administered for 7 days. Figure 3a and d presents the average number of drug injections per day during the SA period. The two-way ANOVA followed by Tukey post hoc test was used to analyze the statistical significance of the differences between the experimental groups. For MNA, the two-way ANOVA revealed significant effects of day [F (6,70) = 3.482, p < 0.01] and treatment [F (1,70) = 69.02, p < 0.001]. However, there were no interactions between the two factors [F (6,70) = 1.589, p > 0.05]. A post hoc analysis revealed that significant differences were apparent from session 2 onwards (days 2 and 5, p < 0.001; days 3 and 7, p < 0.01; days 4 and 6, p < 0.05) (Fig. 3a). For METH (0.1 mg/kg/infusion, i.v.), the two-way ANOVA revealed a significant effect of day [F (6,70) = 4.688, p < 0.001] and interaction [F (6,70) = 5.641, p < 0.001]. However, there was no significant effect of treatment [F (1,70) = 2.652, p > 0.05]. The post hoc analysis revealed that the number of injections increased on the day 6 (p < 0.05) and day 7 (p < 0.01) (Fig. 3d).
Intravenous SA of MNA and METH. Data are presented as mean number of drug injections (a, d), active lever presses (b, e), and inactive lever presses (c, f) following the administration of MNA (1 mg/kg) and METH (0.1 mg/kg). Responses were measured for 7 sessions (2 h/day) according to a fixed-rate 1 (FR = 1) schedule. Data are presented as mean ± SEM of eight independent measurements. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle (two-way ANOVA followed by Tukey’s post hoc test). METH, methamphetamine treatment group; MNA, methamnetamine treatment group; vehicle, saline treatment group
Figure 3b and e shows the average number of active lever presses during the 2-h SA session. There was no significant difference in the number of operations of the inactive lever between the MNA group and the vehicle group. For MNA, the two-way ANOVA revealed a significant effect of day [F (6,70) = 6.147, p > 0.001] and treatment [F (1,70) = 22.13, p < 0.001]. However, there were no interactions between the two factors [F (6,70) = 1.034, p > 0.05]. The post hoc analysis revealed that no significant differences were apparent (Fig. 3b). For METH, the two-way ANOVA revealed a significant effect of day [F (6,70) = 7.151, p < 0.001], treatment [F (1,70) = 7.844, p < 0.01], and interaction [F (6,70) = 5.388, p < 0.001]. The post hoc analysis revealed that no significant differences were apparent (Fig. 3e).
Figure 3c and f shows the average number of inactive lever presses during the 2-h SA session. There was no significant difference in the number of operations of the inactive lever between the MNA group and vehicle group. For MNA, the two-way ANOVA revealed a significant effect of treatment [F (1,70) = 6.396, p < 0.05]. However, there was no significant effect of day [F (6,70) = 1.242, p > 0.05] and interaction [F (6,70) = 0.8238, p > 0.05]. The post hoc analysis revealed that no significant differences were apparent (Fig. 3c). For METH, the two-way ANOVA revealed a significant effect of treatment [F (1,70) = 29.99, p < 0.001]. However, there was no significant effect of day [F (6,70) = 0.7271, p > 0.05] and interaction [F (6,70) = 0.9464, p > 0.05]. The post hoc analysis revealed that significant differences were apparent from session 2 (p < 0.01) and session 3 (p < 0.05) (Fig. 3f).
In vitro dopamine release from synaptosomes
After the treatment of striatal synaptosomes isolated from the brains of SD rats with MNA, the changes in dopamine release were evaluated. To determine the presence of synaptosomes, N-methyl d-aspartate receptor subunit 2A (NMDAR-2A) was detected by western blotting. The isolated synaptosomes showed complete differences from the cytoplasm in the presence of NMDAR-2A (Fig. 4a). MNA treatment at doses of 0.05–100 μM increased dopamine release levels in a dose-dependent manner; METH treatment at doses of 0.05–10 μM also increased dopamine release levels. The one-way ANOVA revealed significant differences between the experimental groups [F (6,14) = 553.2, p < 0.0001]. According to Dunnett’s post hoc tests, MNA (0.05, 0.1, 1, 10, and 100 μM) induced statistically significant effects (p < 0.0001 for all concentrations). METH also induced significant effects [F (5,12) = 47.67, p < 0.0001] at 0.05 (p < 0.05), 0.1 (p < 0.01), 1 (p < 0.0001), and 10 μM (p < 0.0001) (Fig. 4b and c). MNA more strongly induced dopamine release than METH at the same concentration.
Changes in the dopamine level induced by MNA and METH. Protein expression of NMDA2A in the cytosol and synaptosome of rat neurons was analyzed by western blotting (a). Changes in the dopamine level induced by various concentrations of MNA (0.01, 0.05, 0.1, 1, 10, and 100 μm) and METH (0.01, 0.05, 0.1, 1, and 10 μm) (b, c). Changes in the dopamine level in the synaptosome as measured by HPLC. Data are presented as mean ± SEM of more than 10 independent measurements. HPLC analysis was conducted after culturing synaptosomes at 37 °C for 15 min using an HPLC-MS/MS detector (flow rate: 300 μL/min, injection amount: 5 μL). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 versus vehicle (one-way ANOVA followed by Dunnett’s post hoc test). METH, methamphetamine treatment group; MNA, methamnetamine treatment group; vehicle, 5% dimethyl sulfoxide-treated group
Dopamine changes in the microdialysis study in vivo
The dopamine level peaked 40 min after the administration of MNA (20 mg/kg, i.p.) and was approximately 32.67 times the level in the control group (p < 0.001) at this point. The two-way ANOVA revealed significant differences in response time [F (8,36) = 3.663, p < 0.01] and drug [F (1,36) = 33.25, p < 0.0001] among the experimental groups (Fig. 5a). METH (5 mg/kg, i.p.) also induced a peak in dopamine at 40 min (p < 0.001), with an approximately 95.56-fold increase (Fig. 5b). The MNA-induced dopamine peak was approximately 54.7% of the peak level induced by METH. The dopamine levels gradually decreased over time. The areas under the curves (AUCs) for dopamine level changes induced by the drugs were measured, and they are shown in Fig. 5c. The one-way ANOVA revealed significant differences between the experimental groups [F (1,6) = 13.64, p < 0.01]. Dunnett’s post hoc tests revealed that 20 mg/kg MNA (q = 2.997, p < 0.05) and 5 mg/kg METH (q = 5.203, p < 0.01) induced significant effects. The drug response time and drug decay were similar between MNA and METH (Fig. 5).
Extracellular dopamine level changes in the striatum after MNA or METH administration. a Changes in dopamine after i.p. injection of vehicle or MNA (20 mg/kg). b Changes in the dopamine concentration after i.p. injection of vehicle or METH (5 mg/kg,). Arrows indicate the time of drug infusion. Data are presented as mean ± SEM of 8 independent measurements. **p < 0.01, ***p < 0.001 versus vehicle (two-way ANOVA followed by Tukey’s post hoc test). c Average area under the curve of dopamine for 180 min after i.p. injection of vehicle, MNA, or METH. Data are presented as mean ± SEM of eight independent measurements. *p < 0.05, **p < 0.01 versus vehicle (one-way ANOVA followed by Dunnett’s post hoc tests). METH, methamphetamine treatment group; MNA, methamnetamine treatment group
MNA stimulates locomotor activity in mice
The travel distance during the first 40 min was significantly longer in all drug treatment groups than in the vehicle group. The group treated with MNA at 10 mg/kg showed a significant increase in the locomotor activity over time (Fig. 6a), whereas the group treated with MNA at 20 mg/kg showed a rapid increase in the locomotor activity, which then decreased in a manner similar to that in the positive control group. Furthermore, the travel distance in this group was similar to that in the positive control group (Fig. 6b and c). In addition, a peak in the locomotor activity was measured at 20 min, and the activity at this point was 5.81-fold that in the vehicle group (Fig. 6b). After 60 min, the total travel distance in the MNA (20 mg/kg) and positive control groups was approximately 4 times that in the vehicle group (p < 0.001). The one-way ANOVA revealed significant differences between the experimental groups [F (3,20) = 23.63, p < 0.0001]. Dunnett’s post hoc tests revealed that 20 mg/kg MNA (q = 7.157, p < 0.0001) and 1 mg/kg METH (q = 6.404, p < 0.0001) induced significant effects (Fig. 6d). After drug administration, the mice did not jump at the beginning of the experiment, but jumps gradually increased over time, unlike the observation for travel distance. After 20 min, the number of jumps in the MNA (20 mg/kg) and positive control groups was significantly lower than that in the vehicle group. However, between 40 and 60 min, jumps in the MNA (20 mg/kg) group significantly increased compared to those in the vehicle group. There was no significant difference in the number of jumps between the vehicle and MNA (10 mg/kg) groups (Fig. 6e). After 60 min, there was no significant difference in the total number of jumps between the groups, although the mice in the METH group tended to jump less than mice in other groups (Fig. 6f).
Locomotor responses in mice following the administration of MNA and METH. Mice injected with vehicle, MNA, or METH were placed in a locomotor activity test chamber. a MNA (10 mg/kg), b MNA (20 mg/kg), and c METH (1 mg/kg) were administered and the migration length (in meters) was measured at 5-min intervals. Data are presented as mean ± SEM of eight independent measurements. **p < 0.01, ***p < 0.001 versus vehicle (two-way ANOVA followed by Tukey’s post hoc test). d Total migration length during 60 min. Number of jumps during 60 min are expressed as mean ± SEM of eight independent measurements (***p < 0.001 versus vehicle, one-way ANOVA followed by Dunnett’s post hoc test) e in 5-min units and f as the total number of jumps. METH, methamphetamine treatment group; MNA, methamnetamine treatment group; vehicle, saline treatment group
DD study
Figure 7 shows the drug lever responses to discriminative stimulus effects of cocaine or MNA in SD rats trained to distinguish 10 mg/kg cocaine. MNA was associated with a percent response of 20.5% to 1 mg/kg cocaine and 72.23% to 10 mg/kg cocaine. MNA induced the highest drug lever response at 20 mg/kg (79.8%), and the effect of MNA was dose dependent. The median effective dose (ED50) value of MNA (~8.14 mg/kg) in SD rats trained with cocaine was approximately 4 times higher than that of cocaine (1.9 mg/kg). MNA was nearly fully generalized as a discriminative stimulus of cocaine.
Effects of MNA and cocaine on the percentage of responses to the cocaine lever in rats trained to discriminate cocaine from saline. Data are presented as mean for 7–8 rats; cocaine (n = 7) and MNA (n = 8) were tested at each dose. Numbers refer to the doses of drugs during substitution sessions, expressed as milligrams per kilogram on a scale. The ordinate provides the percentage of total responses of the rats on the cocaine-appropriate lever
Discussion
The pharmacological effects on NPS-related behaviors and the central nervous system are understudied. Therefore, substance abuse remains a source of social and health problems (Baumann et al. 2014; Schifano et al. 2015; Van Hout and Hearne 2017). The control of drug abuse requires pharmacological and scientific data for new substances that have the potential to cause problems. MNA was reported as an NPS used in Europe in 2017; however, its abuse potential and adverse effects have not been sufficiently researched to date (Richeval et al. 2019). Moreover, as MNA is currently sold online and sales are not controlled, it is necessary to provide a basis for its abuse control through scientific research. MNA has an amphetamine structure and strongly stimulates the release of dopamine from synaptosomes. However, the physiological characteristics of these stimuli are not well known. In addition, although no preclinical and clinical studies on toxicity have been conducted, the adverse effects of MNA can be deduced based on its structural similarity with METH. Amphetamines are highly addictive and cause euphoria, and they are also associated with adverse effects such as delusions, anxiety, insomnia, and paranoia (Murray et al. 2012). Similar to cocaine, METH exerts stimulatory effects and has detrimental sympathetic nerve-stimulatory effects, such as addiction and hypertension. Neurotransmitter release induced by amphetamine-based drugs is referred to as “the release effect” (Rothman et al. 2012). The EC50 and EMAX values of MNA, which is a full release agent with strong neurotransmitter release effects, are 10 nm and 101%, respectively (Reith et al. 2015). Therefore, it can lead to increased dopamine secretion, which may increase drug-related adverse effects and reactions.
In this study, the CPP and SA tests and microdialysis were used to evaluate rewarding and reinforcing effects, and responses to dopamine in rodents (Bardo and Bevins 2000; Carney et al. 1991). MNA was associated with a statistically significant place preference for the conditional compartment and induced a significant increase in the frequency of SA. Based on these two experiments, behavioral pharmacological preference and addiction symptoms were identified. To investigate the mechanism of this behavioral change, the levels of dopamine, a neurotransmitter in the synaptosomes, were determined. It was confirmed that MNA induced a dose-dependent increase in the dopamine level in the synaptosomes and even in the striatum, a site of dopamine action in the brain. Dopamine presumably is closely related with the behavioral alteration caused by MNA. Considering that the CPP or SA test results have not been previously reported for MNA, these data can be used as a paradigm for the rewarding and reinforcing effects of MNA. Dopamine stimulates the locomotor activity and is closely related to control, motivation, reward, and reinforcing behavior and excitement (Estakhr et al. 2017; Howe and Dombeck 2016; Pijnenburg et al. 1976). In addition, euphoria is correlated with dopamine release (Drevets et al. 2001). Reward affects behavior and emotions, but it is difficult to investigate emotions in animals. However, they can be objectively evaluated as a selection behavior associated with subjective reward preference in animal behavior experiments (Schultz 2015). It has been confirmed that MNA and METH have similar drug reaction times and induce similar activity changes as dopamine. MNA stimulates locomotor activity and dopamine release. Here, discriminative stimulus effect tests of new substances were conducted in rats trained to differentiate cocaine. The results of the DD experiment suggested that the interceptive cues of MNA were similar to cocaine signals. In addition, the ED50 value of MNA was 4-fold that of cocaine, indicating that its potency is lower than that of cocaine.
Overall, MNA was associated with dependence potential and dopamine level changes, and had similarities to cocaine. This study represents the first attempt to assess the dependence potential of MNA through in vivo study; however, there were some limitations to the study. This study was limited to animal experiments, and the effects of MNA cannot be simply generalized to humans. Finally, in the DD test, amphetamine and cocaine should have been included for comparison.
In conclusion, the data indicating the dependence potential of MNA presented in this study provide a scientific basis for the control of this NPS worldwide. Our findings will have a significant effect on drug policies and are relevant for future research on addiction.
References
Bardo MT, Bevins RA (2000) Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153:31–43. https://doi.org/10.1007/s002130000569
Baumann MH, Solis E, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL (2014) Baths salts, spice, and related designer drugs: the science behind the headlines. J Neurosci 34:15150–15158. https://doi.org/10.1523/JNEUROSCI.3223-14.2014
Birch PJ, Fillenz M (1985) Measurement of noradrenaline synthesis in rat hippocampal synaptosomes using HPLC with ECD. J Neurosci Methods 13:231–238. https://doi.org/10.1016/0165-0270(85)90071-8
Carney JM, Landrum RW, Cheng MS, Seale TW (1991) Establishment of chronic intravenous drug self-administration in the C57BL/6J mouse. Neuroreport 2:477–480. https://doi.org/10.1097/00001756-199108000-00017
de la Peña JB, Lee HC, de la Peña IC, Woo TS, Yoon SY, Lee HL, Han JS, Lee JI, Cho YJ, Shin CY, Cheong JH (2012) Rewarding and reinforcing effects of the NMDA receptor antagonist-benzodiazepine combination, Zoletil®: difference between acute and repeated exposure. Behav Brain Res 233:434–442. https://doi.org/10.1016/j.bbr.2012.05.038
Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA (2001) Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry 49:81–96. https://doi.org/10.1016/s0006-3223(00)01038-6
EMCDDA-Europol (2015) Annual report on the implementation of council decision 2005/387/JHA
Estakhr J, Abazari D, Frisby K, McIntosh JM, Nashmi R (2017) Differential control of dopaminergic excitability and locomotion by cholinergic inputs in mouse substantia nigra. Curr Biol 27:1900–1914.e4. https://doi.org/10.1016/j.cub.2017.05.084
European Medicines Agency (2006) Guideline on the non-clinical investigation of the dependence potential of medicinal products. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-investigation-dependence-potential-medicinal-products_en.pdf
Gorelick DA, Gardner EL, Xi Z (2004) Agents in development for the management of cocaine abuse. Drugs 64:1547–1573. https://doi.org/10.2165/00003495-200464140-00004
Howe MW, Dombeck DA (2016) Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535:505–510. https://doi.org/10.1038/nature18942
Hunt D (1997) Pulse check: national trends in drug abuse. Diane Pub Co.
Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci U S A 102:3495–3500. https://doi.org/10.1073/pnas.0407737102
Kamat PK, Kalani A, Tyagi N (2014) Method and validation of synaptosomal preparation for isolation of synaptic membrane proteins from rat brain. MethodsX 1:102–107. https://doi.org/10.1016/j.mex.2014.08.002
McKenna DJ, Guan XM, Shulgin AT (1991) 3,4-Methylenedioxyamphetamine (MDA) analogues exhibit differential effects on synaptosomal release of 3H-dopamine and 3H-5-hydroxytryptamine. Pharmacol Biochem Behav 38:505–512. https://doi.org/10.1016/0091-3057(91)90005-m
Merz F (2018) United Nations Office on drugs and crime: world drug report 2017. SIRIUS Z Strat Anal 2:85–86
Mucha RF, Van Der Kooy D, O’Shaughnessy M, Bucenieks P (1982) Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243:91–105. https://doi.org/10.1016/0006-8993(82)91123-4
Murray DB, Potts S, Haxton C, Jackson G, Sandilands EA, Ramsey J, Puchnarewicz M, Holt DW, Johnston A, Nicholas Bateman D, Dear JW (2012) ‘Ivory wave’ toxicity in recreational drug users; integration of clinical and poisons information services to manage legal high poisoning. Clin Toxicol (Phila) 50:108–113. https://doi.org/10.3109/15563650.2011.647992
Pijnenburg A, Honig W, Van der Heyden J, Van Rossum JM (1976) Effects of chemical stimulation of the mesolimbic dopamine system upon locomotor activity. Eur J Pharmacol 35:45–58. https://doi.org/10.1016/0014-2999(76)90299-5
Protais P, Costentin J, Schwartz JC (1976) Climbing behavior induced by apomorphine in mice: a simple test for the study of dopamine receptors in striatum. Psychopharmacology 50:1–6. https://doi.org/10.1007/BF00634146
Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH, Partilla JS, Rothman RB, Katz JL (2015) Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol Depend 147:1–19. https://doi.org/10.1016/j.drugalcdep.2014.12.005
Richeval C, Nachon-Phanithavong M, Di Fazio V, Wiart J, Humbert L, Samyn N, Wille SMR, Allorge D, Gaulier J (2019) Prevalence of new psychoactive substances in oral fluid specimens from French and Belgian drivers: comparison 2016/2017. J Anal Toxicol 43:e9–e10. https://doi.org/10.1093/jat/bky101
Rothman RB, Partilla JS, Baumann MH, Lightfoot-Siordia C, Blough BE (2012) Studies of the biogenic amine transporters. 14. Identification of low-efficacy “partial”? substrates for the biogenic amine transporters. J Pharmacol Exp Ther 341:251–262. https://doi.org/10.1124/jpet.111.188946
Schifano F, Orsolini L, Duccio Papanti G, Corkery JM (2015) Novel psychoactive substances of interest for psychiatry. World Psychiatry 14:15–26. https://doi.org/10.1002/wps.20174
Schultz W (2015) Neuronal reward and decision signals: from theories to data. Physiol Rev 95:853–951
Spiller HA, Ryan ML, Weston RG, Jansen J (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 49:499–505. https://doi.org/10.3109/15563650.2011.590812
US FDA (2017) Assessment of abuse potential of drugs; guidance for industry. https://www.fda.gov/media/116739/download
United Nations Office on Drugs and Crime (1961) Single convention on narcotic drugs. United Nations Web. https://www.unodc.org/unodc/en/treaties/single-convention.html?ref=menuside
United Nations Office on Drugs and Crime (1971) Convention on psychotropic substances. United Nations Web. https://www.unodc.org/unodc/en/treaties/psychotropics.html?ref=menuside
Van Hout MC, Hearne E (2017) New psychoactive substances (NPS) on cryptomarket fora: an exploratory study of characteristics of forum activity between NPS buyers and vendors. Int J Drug Policy 40:102–110. https://doi.org/10.1016/j.drugpo.2016.11.007
Volkow ND, Fowler JS, Wang G, Swanson JM (2004) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry 9:557–569. https://doi.org/10.1038/sj.mp.4001507
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This research was supported by a grant (MFDS-7036-306/19181MFDS401/19181MFDS402) from the National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Republic of Korea in 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 238 kb)
Rights and permissions
About this article
Cite this article
Youn, DH., Kim, J.M., Hong, Yk. et al. Assessment of the abuse potential of methamnetamine in rodents: a behavioral pharmacology study. Psychopharmacology 238, 2155–2165 (2021). https://doi.org/10.1007/s00213-021-05840-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05840-9