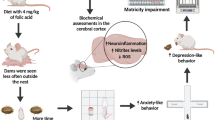
Abstract
Rationale
B vitamins play essential roles in brain development and functionality; however, the effects of their deficiency during early life on mental health are not thoroughly understood.
Objectives
The objective of this study is to investigate the effects of a maternal deficiency of vitamin B6, B9 (folate), and B12 on behavioral changes in adult offspring.
Methods
Female C57BL/6 J mice were put on a diet lacking vitamin B6, B9, B12, or the above three vitamins from pregnancy to weaning. The growth and developmental characteristics of both the pregnant mothers and offspring were collected. In the adult offspring, the serum levels of neuroactive substances were measured using an enzyme-linked immunosorbent assay. The level of BDNF and dimethylated lysine 9 on histone H3 (H3K9me2) was detected by immunohistochemical staining. In addition, their depressive-like behaviors, anxiety-like behaviors, and sociability were recorded using sucrose preference, a forced swim, social interaction, tail suspension, and open field tests.
Results
The maternal deficiency of the three B vitamins delayed offspring development. Compared to the controls, all of the groups showed decreased serum levels of 5-HT and neuropeptide Y. In the groups with deficiency of B9 or the three B vitamins, there were significant changes in sociability and social novelty preference. In groups with deficiencies in B9, B12, or all three B vitamins, the expression levels of BDNF and H3K9me2 in the hippocampus were significantly decreased.
Conclusions
Maternal deficiencies of the major B vitamins caused changes in social behaviors in adult mice accompanied with epigenetic alterations in the brain and changes in the serum levels of neuroactive substances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Human diet has a direct effect on brain development and functionality, with studies pointing toward the efficacy of vitamin and mineral supplementation in preventing and alleviating nervous system diseases (Reynolds 2006; Smithells et al. 1976). In healthy humans, multivitamin supplementation has been shown to improve cognitive performance and reduce negative mood states, including depression, anxiety, and stress (Haskell et al. 2008; Stough et al. 2011; White et al. 2015). In the clinic, folate fortification during early pregnancy is recommended to reduce the risk of neural tube and other congenital malformations in developing fetuses (Devakumar et al. 2016; van Gool et al. 2018).
B vitamins are required for various cortical processes involved in metabolism, such as in the methylation of homocysteine to methionine, which is essential for DNA synthesis, repair, and methylation, and the synthesis of numerous neurochemicals and signaling molecules in the central nervous system (Miller 2003). B6, B9, and B12 are the most prominent B vitamins involved in homocysteine metabolism that provides a substrate for genomic and non-genomic methylation (Kennedy 2016). Recent animal studies have suggested that maternal folate deficiency is associated with abnormalities in neurobehavioral development (Henzel et al. 2017; Wang et al. 2017). In a human cohort study, child emotional problems were reported to be associated with maternal folate status during early pregnancy (Steenweg-de Graaff et al. 2012). In addition, long-term treatment with folic acid, B6, and B12 was associated with a reduction in the hazard for major depression (Almeida et al. 2010). However, given their pivotal physiological significance, our understanding of the role of B vitamins on brain development and functionality is still limited.
The pathophysiological changes of mental diseases primarily occur in the central nervous system, and epigenetic mechanisms, including DNA methylation and histone modification, are involved in neurodevelopmental processes (Maze et al. 2010). Although direct analysis of these epigenetic alterations in the human brains is difficult, investigations of blood biomarkers for psychological diseases have found that brain-derived neurotrophic factor (BDNF), dopamine (DA), serotonin (5-HT), norepinephrine (NE), and neuropeptide Y (NPY) levels are generally decreased in the serum of patients with depression, schizophrenia, or anxiety disorders (Alexopoulos et al. 2009; Kupfer et al. 2011; Mellios et al. 2009; Wolkowitz et al. 2011). Therefore, the circulating levels of these factors in serum may help to identify alterations in brain development.
To investigate the relationship between maternal deficiencies in major B vitamins and behavioral changes in adult offspring, in this study, a mouse model and a diet with deficiencies in the major B vitamins were used. The results demonstrated that maternal deficiencies in the major B vitamins might result in abnormal levels of neuroactive substances and behavioral changes in the sociability of adult offspring. This was found to be accompanied with alterations of BDNF and epigenetics in the brain. Therefore, this study provides new evidence for the critical roles of B vitamins in brain development during early life.
Methods
Experimental animals and design
This animal study was approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University (No. 2017[092]). The experiments were conducted according to the National Institutes of Health guide for the care and use of Laboratory animals, and all efforts were made to minimize animal suffering. In brief, weaned C57BL/6 J mice weighing 15–18 g were obtained from Guangdong Medical Laboratory Animal Center (Foshan, Guangdong, China) and maintained there in a specific pathogen-free animal facility (temperature: 23 °C ± 2; illumination: 12-h light and 12-h dark cycle) with free access to food and water.
The 8-week-old female mice were randomized into five groups (eight mice per group) with the following diets before mating: (i) control diet (0.07% vitamin B6, 0.02% folate, 0.25% vitamin B12, with an energy content of 17.9% protein, 7.0% fat, and 64.4% carbohydrate) (NOR); (ii) vitamin B6 deficient diet (without vitamin B6) (DB6); (iii) folate-deficient diet (without folate) (DB9); (iv) vitamin B12–deficient diet (without vitamin B12) (DB12); and (v) vitamin B6–, B9–, and B12–deficient diet (without vitamin B6, folate, and vitamin B12) (DB6912). All of the diets were purchased from the Keao Xieli Feed Company (Beijing, China). After confirmation of pregnancy, the female mice were kept on the respective diet as described above in individual cages with the offspring until 4 weeks after weaning. Their offspring (F1 generation) were fed a normal diet until they were 8 weeks old (adulthood).
There were eight female pregnant mice in each group, and their weights were measured on the 1st and 17th day of fertilization, and that of the offspring mice at birth and at 4 and 8 weeks of age. When the offspring were 8 weeks old, 16 mice per group were randomly selected for biochemical assays, and eight mice per group were randomly selected for behavioral tests. For the biochemical assay and immunohistochemistry analysis, approximately 500 uL of blood was collected using eyeball dissection, followed by brain dissection on ice. The collected blood samples were set at room temperature for 1 h and centrifuged at 775 g for 15 min to separate the serum. All of the tissue and serum samples were stored at − 80 °C for later use. For the behavioral test, the animals were sent to the commercial test center at Guangdong Medical Laboratory Animal Center (Foshan, Guangdong, China). After the behavioral test at 8 weeks of age, all of the offspring mice were euthanized using CO2.
Measurement of neuroactive substances
Individual offspring serum was collected at adulthood, and levels of NPY, DA, BDNF, NE, and 5-HT were measured using an enzyme-linked immunosorbent assay (ELISA) kit (Arigo biolaboratories, Hsinchu, China) according to the manufacturer’s instructions. The absorbance was measured at 450 nm using a microplate reader (Bioteck, USA).
Immunohistochemistry of the mouse brain
The mice were euthanized using CO2 and perfused with chilled 0.03% heparin in a phosphate buffering solution (PBS). The whole brains were drop-fixed in 4% paraformaldehyde (PFA) in PBS overnight at 4 °C and embedded using paraffin. The regions of the prefrontal cortex (PFC), nucleus accumbens (NAc), striatum (CPU), CA3 area of the hippocampus (Hip), and ventral tegmental area (VTA) in whole brains were cut into 5-μm slices and mounted onto polylysine-charged glass slides. Antigen retrieval was performed in a citrate buffer (pH 6.0) at 120 °C for 10 min, and the endogenous peroxidase activity was blocked by exposure to 3% H2O2 for 15 min. For immunohistochemistry (IHC) analysis of BDNF and dimethylated lysine 9 on histone H3 (H3K9me2) in the mice, the sections were incubated with biotinylated antibody (1:500, Abcam, USA) overnight at 4 °C. The sections were washed and incubated with Lab Vision™ UltraVision™ Large Volume Detection System: anti-Polyvalent, HRP (ThermoFisher Scientific, USA). The sections were then imaged using an Olympus microscope (Olympus, Germany). Briefly, the regions of interest (Hip, PFC, NAc, CPu, and VTA) were outlined, and BDNF and H3K9me2 were analyzed according to the brightness threshold. The area covered with HRP staining was reported as a percentage of the total area analyzed.
Sucrose preference test
Briefly, the mice were individually housed and acclimated to cages with water from two 30-mL sipper tubes containing tap water for 3 days prior to the test. All of the mice were then exposed to two 30-mL sipper tubes, one with tap water and the other with 1% sucrose solution. Measurements of the consumed tap water and sucrose solution were taken daily. To prevent any bias, tube placement was switched every 12 h. The ratio of sucrose preference was calculated using the intake of sucrose solution and total fluid.
Open field test
The open field test (Flydi Biotechnology, Guangzhou, China) consisted of a plastic cage (L25 × W25 × H35 cm), a digital camera, and a computer with video recording ability. The cage was divided into the center area (12 × 12 cm square) and the thigmotaxis area, which included the peripheral region of the arena (12 × 6 cm square). The animal leaning against the wall at a high position and their position entering the wall area were defined as a “high-leaning behavior.” During the test session, the time engaged in high-leaning behavior, number of activities, the total distance traveled, and the time in center area were measured and analyzed. All of the animals were subjected to the open field test for 5 min each time for a total of three times, and they were placed in the testing room for 3 h before adapting to the environment test. The apparatus was cleaned with 75% ethanol before each animal was tested.
Forced swim test
Mice were individually forced to swim in glass beakers (height 30 cm, diameter 20 cm) containing 10 cm of water maintained at a temperature of 25 °C. During a test period of 4 min, the time that mice remained immobile or made only small limb movements necessary for floating was recorded by another experimenter using a timer, and the researcher did not know whether the mice had been treated. The immobility time was measured manually, and the researchers were blinded to the treatment. The mice were then lightly dried before being returned to their cages, and the water was changed for each mouse.
Tail suspension test
The total duration of immobility induced by tail suspension was measured according to a previously reported method (Hainmueller and Bartos 2018). The mice were suspended 58 cm above the floor by adhesive tape that was attached approximately 1 cm from the tip of the tail. During a test period of 5 min, the time during which the mice remained immobile was quantified by another researcher using a timer, and the researcher did not know whether the mice had been treated. The mice were considered immobile only when they hung passively and completely motionless. The immobility time was measured manually, and the researchers were blinded to the treatment.
Social interaction test
The social interaction test systems (Jiliang Software Technology, Shanghai, China) consisted of a three-chambered box with a lid, a digital camera, and a computer with video recording ability. The subject mice were placed in the middle chamber and allowed to freely explore all three chambers for 10 min after social isolation for 6 h and before this test. In the sociability test for 10 min, the subject mouse was placed in the middle chamber. The stranger mouse (stranger mouse “A” in social novelty preference test), coming from a different cage but belonging to the same group, was enclosed in a small and round wire cage that allowed nose contact between the bars with the mouse in the right chamber, but it prevented fighting. In the social novelty preference test for 10 min, which was based on the sociability test, the other stranger mouse (“B”) was enclosed in a small and round wire cage of the left chamber. During this period, the latency, number of contacts between the subject mouse and stranger mouse B, and the total interaction time the mice remained together were measured.
Statistical analysis
All of the data analysis and graphing was conducted using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) and SPSS 20.0 software (SPSS IBM, Armonk, New York, USA). One-way ANOVA estimated the comparisons among multiple groups. Student’s t test was used to analyze the differences between two groups. P values < 0.05 were considered to be statistically significant.
Results
The growth and development of pregnant mice and their offspring
There were eight female pregnant mice in each group, and the total number of offspring in the NOR, DB6, DB9, DB12, and DB6912 groups was 56 (female vs female: 29:27), 53 (25:28), 60 (30:30), 52 (26:27), and 48 (25:23), respectively. Compared to the NOR group, the pregnancy duration was significantly longer and the maternal weight gain was less in the DB6912 group (Table 1). In addition, the litter number in the DB12 group and DB6912 group was significantly lower than in the DB9 group (Table 1).
The offspring weight at birth in the DB6912 group was significantly lower than that in the NOR group (Table 1). At 4 weeks of age, the offspring weight of the DB12 group and DB6912 group was significantly lower than the NOR group, and the weight of the DB6912 group was also significantly lower than the DB6 group and DB9 group (Table 1). However, at the age of 8 weeks, there was no statistical difference in the offspring weight among all of the groups (Table 1).
Behavioral assessment of adult offspring mice
The sucrose preference was tested in the offspring mice to assess attenuation in the response to positive affective stimuli as a measurable sign for depression. A reduction in sucrose preference was thought to be an indication of reduction in positive affective stimuli. The sugar water preference of the DB6 group was significantly higher, and the DB9 group was significantly lower than that of the NOR group (Fig. 1 A).
The effects of a maternal deficiency of vitamin B6, B9 (folate), and B12 on behavioral changes in adult offspring. (A) The ratio of sucrose preference in 1-h sucrose preference test. The time in high-leaning (B) and number of activity (C) in the open field test. The distance (D) and time (E) in the center area in the open field test. (F) The immobile time of 4 min in the forced swim test. (G) The immobile time of 5 min in tail suspension test. (H) The number spent with stranger mice in the social interaction test. (I) The time spent with stranger mice in the social interaction test. *P < 0.05 compared to NOR group, data are shown as mean ± SEM. NOR, group with control diet; DB6, group with vitamin B6–deficient diet; DB9, group with B9-deficient diet; DB12, group with vitamin B12–deficient diet; DB6912, group with vitamin B6–, B9–, and B12–deficient diet. N = 8 mice per group
The open field test was assessed for anxiety-like behavior. The results demonstrated that there were no statistically significant differences between these groups (Fig. 1 B, C, D, and E).
The forced swimming test and tail suspension were also assessed for depression evaluation. The results showed that, compared to the NOR group, the fixed time in the forced swimming test of all of the groups was not significantly different (Fig. 1 F). However, the fixed time in the tail suspension test of the offspring mice in the DB9, DB12, and DB6912 groups all increased, and that in the DB6912 group was statistically significant (Fig. 1 G).
Based on these data, there was a tendency of changes in depressive-like behaviors (sucrose preference and tail suspension) in mice with a maternal deficiency of B vitamins, but the measured results were inconsistent. This discrepancy may have resulted from the limited number of tested animals, or the changes in depressive-like behaviors were too subtle to be consistently detected.
In addition, social behavior was assessed using the social interaction test, and it was found that the exploration time and numbers with stranger mice in the DB12 group and DB6912 group were significantly lower than in the NOR group, and the difference was statistically significant and consistent (Fig. 1 H, I).
Expression level of serum neuroactive substances in adult offspring mice
The serum concentrations of 5-HT and NPY of mice from the DB6, DB9, DB12, and DB6912 groups were significantly decreased compared with the NOR group (Fig. 2 A, B). The concentration of BDNF in the DB9, DB12, and DB6912 also decreased compared to the NOR group, but it did not reach statistical significance yet (Fig. 2 C). However, there was no significant difference in DA and NE between these groups (Fig. 2 D, E).
The effects of a maternal deficiency of vitamin B6, B9 (folate), and B12 on serum levels of neuroactive substances in the adult offspring mice. (A) 5-HT; (B) NPY; (C) BDNF; (D) DA; (E) NE. *P < 0.05 compared to NOR group, data are shown as mean ± SEM. NOR, group with control diet; DB6, group with vitamin B6 deficient diet; DB9, group with B9 deficient diet; DB12, group with vitamin B12 deficient diet; DB6912, group with vitamin B6–, B9–, and B12–deficient diet. N = 16 mice per group
Expression level of BDNF and H3K9me2 in offspring mice brains
In the semiquantitative analysis of tissue, the grayscale value in the Image J software is inversely proportional to the expression of the measured protein. The IHC analysis results showed that the positive gray value of BDNF expression in the CA3 area of the hippocampus in the DB9 group, DB12 group, and DB6912 group was significantly higher than in the NOR group. This indicated that the expression level of BDNF in the CA3 area of the hippocampus in the three groups was statistically significantly lower than in the NOR group (Fig. 3 A; Fig. S1 A). However, the difference in the other four areas (mPFC, NAc, CPu, and VTA) of the brain was not significant (Fig. 3 B, C, D, and E; Fig. S1 B, C, D, and E).
The expression level of BDNF detected by immunohistochemical staining in five areas of the brain in adult offspring mice. (A) Mean gray value in the CA3 area from five groups; (B) mean gray value in the mPFC area from five groups; (C) mean gray value in the NAc area from five groups; (D) mean gray value in the CPu area from five groups; (E) mean gray value in the VTA area from five groups. *P < 0.05 compared to the NOR group, data are shown as mean ± SD. NOR, group with control diet; DB6, group with vitamin B6 deficient diet; DB9, group with B9 deficient diet; DB12, group with vitamin B12 deficient diet; DB6912, group with vitamin B6–, B9–, and B12–deficient diet. N = 16 mice per group
The number of H3K9me2 positive cells in the CA3 area of the hippocampus in the DB9, DB12, and DB6912 groups was significantly lower than that in the NOR group (Fig. 4 A; Fig. S2 A). However, the differences in the other four areas (mPFC, NAc, CPu, and VTA) of the brain were not significant (Fig. 4 B, C, D, and E; Fig. S2 B, C, D, and E).
The number of H3K9me2 positive cells detected by immunohistochemical staining in five areas of the brain in adult offspring mice. (A) Number of positive cells in the CA3 area from five groups; (B) number of positive cells in the mPFC area from five groups; (C) number of positive cells in the NAc area from five groups; (D) number of positive cells in the CPu area from five groups; (E) number of positive cells in the VTA area from five groups. *P < 0.05 compared to the NOR group, data are shown as mean ± SD. NOR, group with control diet; DB6, group with vitamin B6–deficient diet; DB9, group with B9 deficient diet; DB12, group with vitamin B12–deficient diet; DB6912, group with vitamin B6–, B9–, and B12–deficient diet. N = 16 mice per group
Discussion
According to the theory of the developmental origins of health and disease, many diseases may originate in the early stages of life development (Gillman 2005). The maternal diet during pregnancy is an important factor that affects embryos and offspring development, eventually resulting in changes in their body structure and functionality. In this study, using animal models of dietary interventions, it was demonstrated that maternal deficiencies of the major B vitamins decreased the body weight of offspring on the day of birth and at 4 weeks old, but not at the age of 8 weeks, with a significant growth catch-up effect. This result indicated that after weaning, a normal intake of vitamin B can be restored in offspring mice and meet their growth needs.
However, the data demonstrated that mice with a maternal deficiency of B6, B9, and B12 are more prone to display significant behavioral changes in sociability and social novelty preference, which cannot be corrected by dietary supplements after weaning. Because B vitamins act as co-enzymes in a vast array of catabolic and anabolic enzymatic reactions, vitamins B6, B9, and B12 are the most prominent B vitamins involved in homocysteine metabolism. Therefore, B vitamin deficiency in the maternal diet may result in permanent alterations in offspring brain development and function (Kennedy 2016).
Serum analysis found that levels of neuroactive substances such as NPY, 5-HT, and possibly BDNF decreased in the offspring with observed mental diseases. NPY, a polypeptide composed of 36 amino acid residues belonging to the NPY family of neuroendocrine peptides, signals the central nervous system for a prerequisite of energy in the hypothalamus by mediating the appetite, and it shows an orexigenic effect (Li et al. 2019; Shende and Desai 2020). In addition, 5-HT, a vital neuromodulatory transmitter with distinctive neuroplastic capabilities, is a well-known key mechanism for learning, memory, and mood (Kraus et al. 2017). Unequivocal studies have shown that low levels of 5-HT can result in abnormal behavioral changes due to a dysfunction of synaptic plasticity, which explains the pathophysiology of depression (Li 2020; Liu et al. 2019). In this study, the findings suggested that social behavioral changes in adulthood may also be related to altered serum neuroactive substances that may be caused by a maternal deficiency of vitamin B during their early life. Importantly, reserves of vitamin B in the normal diet after weaning cannot supply the need in the offspring with maternal deficiencies during the neurodevelopmental phase, as biochemical indexes in these animals failed to reach normal levels.
In addition, a histochemical analysis of the brains demonstrated that the mice displaying abnormal behaviors had low levels of BDNF in the CA3 region of the hippocampus. In CA3, many pyramidal cells can receive the signal from mossy fibers and excite other pyramidal cells and interneurons as their axons spread throughout most of the region to form an associative network (Hainmueller and Bartos 2018; Le Duigou et al. 2014). Additionally, the BDNF can induce a lasting potentiation of synaptic efficacy at the mossy fibers projection accompanied by a structural reorganization at the CA3 area (Martinez-Moreno et al. 2020). Previous research on BDNF revealed that this neurotrophin was highly expressed in the central nervous system (particularly in the hippocampus), which has essential functions in neuronal development and neuroplasticity that are related to social behavior disorders (Brondino et al. 2018; Das 2013; Hing et al. 2018). Preclinical studies have shown that levels and expression of BDNF are altered in the brain due to an unbalanced maternal diet low in micronutrients (Sable et al. 2014). Therefore, the BDNF decrease in the CA3 area observed in this study may have been directly associated with a lack of B vitamins during early life.
Epigenetic alterations in the brain, such as histone methylation and acetylation, are recognized as important mechanisms that may affect psychiatric conditions (Toth 2014). For instance, in adult rats with adolescent intermittent ethanol exposure, both histone H3-K9 acetylation levels and BDNF protein in the hippocampus were decreased (Sakharkar et al. 2016). However, histone methylation alterations, especially H3K9me2, have been more widely reported. This is probably because S-adenosylhomocysteine is involved in H3K9me2, and folate and vitamin B12 are required both in the methylation of homocysteine to methionine and in the synthesis of S-adenosylhomocysteine (Bottiglieri 1996; Zhou et al. 2013). For instance, it has been reported that H3K9me2 plays an essential role in cocaine-induced structural and behavioral plasticity in the nucleus accumbens of mice (Maze et al. 2010). In addition, the decreased level of H3K9me2 can promote the expression of BDNF in the hippocampus (Zhao et al. 2020), and mice exerting more social interaction could have significantly elevated levels of H3K9me2 and BDNF in the brain (Jeyaraj et al. 2020). In this study, the data supported that mice with decreased sociality had lower levels of H3K9me2 and reduced BDNF. However, the related molecular mechanisms require further investigation to understand the interactions between the observed epigenetic alterations and behavioral changes.
Although the vitamins in this study belong to the same B vitamin family, there are still different roles and relationships that B vitamin 6, 9, and 12 deficiencies play in the behaviors and the expression of BDNF and H3K9 in the brain. It has been reported that long-term vitamin B6 restriction results in damage and/or abnormal development of the neuronal system associated with locomotor behavior (Guilarte et al. 1991), but the effects of B6 deficiency on H3K9 methylation and BDNF expression remain unclear. However, as the metabolism of B9 (folate) and vitamin B12 is intimately connected, a deficiency of either vitamin may lead to a morphologically indistinguishable anemia and result in a number of neurologic and psychiatric diseases (Bottiglieri 1996). Therefore, numerous studies have reported that B9, often accompanied with B12, can cause mental diseases and alter H3K9 methylation or BDNF alteration. For instance, B9 exhibited antidepressant-like effects in a rat model by increasing the levels of monoamine neurotransmitters and BDNF (Yan et al. 2017). A maternal deficiency of folate (B9) and vitamin B12 during pregnancy increases the risk of cognitive disorders in their offspring associated with decreased levels of BDNF (Kemse et al. 2018; Liu et al. 2020; Yang et al. 2019). In addition, the observed changes in neurotrophins during gestation may be related to their interaction with micronutrients such as vitamin B6 and vitamin B12 (Dhobale 2014). In this study, the sociability decreased in the B12 and B6/B9/B12 groups, and only the B9, B12, or B6/B9/B12 groups had significantly altered BDNF and H3K9me2. This suggests that B9 and B12 are more tightly linked, and a lack of either B9 or B12 during early life was associated with adult behavioral changes in sociability, accompanied by altered epigenetics in the brain.
In conclusion, this study indicates that a maternal deficiency of the major B vitamins leads to significant decreases in the serum NPY and 5-HT. In the groups with deficiencies of B12 and all three B vitamins, the adult offspring had significant changes in sociality and social novelty preference, accompanied by H3K9me2 and BDNF alterations in their brains. Therefore, the current research confirmed the critical roles of the major B vitamins for brain development during early life, and a clinical supply of B vitamins in pregnant women may have a long-lasting and neuroprotective effect on their children.
References
Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Glatt CE, Latoussakis V, Kelly RE Jr, Kanellopoulos D, Klimstra S, Lim KO, Young RC, Hoptman MJ (2009) Serotonin transporter polymorphisms, microstructural white matter abnormalities and remission of geriatric depression. J Affect Disord 119:132–141
Almeida OP, Marsh K, Alfonso H, Flicker L, Davis TM, Hankey GJ (2010) B-vitamins reduce the long-term risk of depression after stroke: the VITATOPS-DEP trial. Ann Neurol 68:503–510
Bottiglieri T (1996) Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev 54:382–390
Brondino N, Fusar-Poli L, Rocchetti M, Bertoglio F, Bloise N, Visai L, Politi P (2018) BDNF levels are associated with autistic traits in the general population. Psychoneuroendocrinology 89:131–133
Das UN (2013) Autism as a disorder of deficiency of brain-derived neurotrophic factor and altered metabolism of polyunsaturated fatty acids. Nutrition 29:1175–1185
Devakumar D, Fall CH, Sachdev HS, Margetts BM, Osmond C, Wells JC, Costello A, Osrin D (2016) Maternal antenatal multiple micronutrient supplementation for long-term health benefits in children: a systematic review and meta-analysis. BMC Med 14:90
Dhobale M (2014) Neurotrophins: role in adverse pregnancy outcome. Int J Dev Neurosci 37:8–14
Gillman MW (2005) Developmental origins of health and disease. N Engl J Med 353:1848–1850
Guilarte TR, Miceli RC, Moran TH (1991) Developmental effects of vitamin B-6 restriction on the locomotor behavior of rats. Brain Res Bull 26:857–861
Hainmueller T, Bartos M (2018) Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558:292–296
Haskell CF, Scholey AB, Jackson PA, Elliott JM, Defeyter MA, Greer J, Robertson BC, Buchanan T, Tiplady B, Kennedy DO (2008) Cognitive and mood effects in healthy children during 12 weeks’ supplementation with multi-vitamin/minerals. Br J Nutr 100:1086–1096
Henzel KS, Ryan DP, Schroder S, Weiergraber M, Ehninger D (2017) High-dose maternal folic acid supplementation before conception impairs reversal learning in offspring mice. Sci Rep 7:3098
Hing B, Sathyaputri L, Potash JB (2018) A comprehensive review of genetic and epigenetic mechanisms that regulate BDNF expression and function with relevance to major depressive disorder. Am J Med Genet B Neuropsychiatr Genet 177:143–167
Jeyaraj SE, Sivasangari K, Garcia-Colunga J, Rajan KE (2020) Environmental enrichment enhances sociability by regulating glutamate signaling pathway through GR by epigenetic mechanisms in amygdala of Indian field mice Mus booduga. Gen Comp Endocrinol 300:113641
Kemse N, Kale A, Chavan-Gautam P, Joshi S (2018) Increased intake of vitamin B12, folate, and omega-3 fatty acids to improve cognitive performance in offspring born to rats with induced hypertension during pregnancy. Food Funct 9:3872–3883
Kennedy DO (2016) B vitamins and the brain: mechanisms, dose and efficacy--a review. Nutrients 8:68
Kraus C, Castren E, Kasper S, Lanzenberger R (2017) Serotonin and neuroplasticity - links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev 77:317–326
Kupfer DJ, Frank E, Phillips ML (2011) Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet 379:1045–1055
Le Duigou C, Simonnet J, Telenczuk MT, Fricker D, Miles R (2014) Recurrent synapses and circuits in the CA3 region of the hippocampus: an associative network. Front Cell Neurosci 7:262
Li YF (2020) A hypothesis of monoamine (5-HT) - glutamate/GABA long neural circuit: aiming for fast-onset antidepressant discovery. Pharmacol Ther 107494
Li C, Wu X, Liu S, Zhao Y, Zhu J, Liu K (2019) Roles of neuropeptide Y in neurodegenerative and neuroimmune diseases. Front Neurosci 13:869
Liu Y, Zhao J, Fan X, Guo W (2019) Dysfunction in serotonergic and noradrenergic systems and somatic symptoms in psychiatric disorders. Frontiers in psychiatry 10:286
Liu HY, Liu SM, Zhang YZ (2020) Maternal folic acid supplementation mediates offspring health via DNA methylation. Reproductive sciences. Thousand Oaks, Calif
Martinez-Moreno A, Rivera-Olvera A, Escobar ML (2020) BDNF induces in vivo long-lasting enhancement of synaptic transmission and structural reorganization at the hippocampal mossy fibers in a transcription and translation-independent manner. Neurobiol Learn Mem 167:107125
Maze I, Covington HE 3rd, Dietz DM, LaPlant Q, Renthal W, Russo SJ, Mechanic M, Mouzon E, Neve RL, Haggarty SJ, Ren Y, Sampath SC, Hurd YL, Greengard P, Tarakhovsky A, Schaefer A, Nestler EJ (2010) Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 327:213–216
Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S (2009) Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 65:1006–1014
Miller AL (2003) The methionine-homocysteine cycle and its effects on cognitive diseases. Altern Med Rev 8:7–19
Reynolds E (2006) Vitamin B12, folic acid, and the nervous system. Lancet Neurol 5:949–960
Sable P, Kale A, Joshi A, Joshi S (2014) Maternal micronutrient imbalance alters gene expression of BDNF, NGF, TrkB and CREB in the offspring brain at an adult age. Int J Dev Neurosci 34:24–32
Sakharkar AJ, Vetreno RP, Zhang H, Kokare DM, Crews FT, Pandey SC (2016) A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct Funct 221:4691–4703
Shende P, Desai D (2020) Physiological and therapeutic roles of neuropeptide Y on biological functions. Adv Exp Med Biol 1237:37–47
Smithells RW, Sheppard S, Schorah CJ (1976) Vitamin deficiencies and neural tube defects. Arch Dis Child 51:944–950
Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, Tiemeier H (2012) Maternal folate status in early pregnancy and child emotional and behavioral problems: the generation R study. Am J Clin Nutr 95:1413–1421
Stough C, Scholey A, Lloyd J, Spong J, Myers S, Downey LA (2011) The effect of 90 day administration of a high dose vitamin B-complex on work stress. Human psychopharmacol 26:470–476
Toth M (2014) Mechanisms of non-genetic inheritance and psychiatric disorders. Neuropsychopharmacology 40:129–140
van Gool JD, Hirche H, Lax H, De Schaepdrijver L (2018) Folic acid and primary prevention of neural tube defects: a review. Reproductive toxicology (Elmsford, NY) 80: 73-84
Wang X, Li W, Li S, Yan J, Wilson JX, Huang G (2017) Maternal folic acid supplementation during pregnancy improves neurobehavioral development in rat offspring. Mol Neurobiol 55:2676–2684
White DJ, Cox KH, Peters R, Pipingas A, Scholey AB (2015) Effects of four-week supplementation with a multi-vitamin/mineral preparation on mood and blood biomarkers in young adults: a randomised, double-blind, placebo-controlled trial. Nutrients 7:9005–9017
Wolkowitz OM, Wolf J, Shelly W, Rosser R, Burke HM, Lerner GK, Reus VI, Nelson JC, Epel ES, Mellon SH (2011) Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35:1623–1630
Yan Z, Jiao F, Yan X, Ou H (2017) Maternal chronic folate supplementation ameliorates behavior disorders induced by prenatal high-fat diet through methylation alteration of BDNF and Grin2b in offspring hippocampus. Molecular nutrition & food research 61
Yang Y, Yang S, Liu J, Feng Y, Qi F, Zhao R (2019) DNA hypomethylation of GR promoters is associated with GR activation and BDNF/AKT/ERK1/2-induced hippocampal neurogenesis in mice derived from folic-acid-supplemented dams. Mol Nutr Food Res 63:e1801334
Zhao M, Wang W, Jiang Z, Zhu Z, Liu D, Pan F (2020) Long-term effect of post-traumatic stress in adolescence on dendrite development and H3K9me2/BDNF expression in male rat hippocampus and prefrontal cortex. Front Cell Dev Biol 8:682
Zhou HR, Zhang FF, Ma ZY, Huang HW, Jiang L, Cai T, Zhu JK, Zhang C, He XJ (2013) Folate polyglutamylation is involved in chromatin silencing by maintaining global DNA methylation and histone H3K9 dimethylation in Arabidopsis. Plant Cell 25:2545–2559
Acknowledgments
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
Funding was provided by National Key R&D Program of China (grant no. 2018YFC1004104), the Guangdong Science and Technology Department Project (grant no. 2019B030316023), and the Key Project of Guangzhou Science and Technology Innovation Committee (grant no. 201804020057).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author declares no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Figure S1
The immunohistochemical staining analysis of BDNF in five areas of the brain in adult offspring mice. (A) In CA3 area from five groups; (B) in mPFC area from five groups; (C) in NAc area from five groups; (D) in CPu area from five groups; (E) in VTA area from five groups. NOR, group with control diet; DB6, group with vitamin B6–deficient diet; DB9, group with B9-deficient diet; DB12, group with vitamin B12–deficient diet; DB6912, group with vitamin B6–, B9–, and B12–deficient diet. N = 16 mice per group. (PDF 311 kb)
Figure S2
The immunohistochemical staining analysis of H3K9me2 positive cells in five areas of the brain in adult offspring mice. (A) In CA3 area from five groups; (B) in mPFC area from five groups; (C) in NAc area from five groups; (D) in CPu area from five groups; (E) in VTA area from five groups. NOR, group with control diet; DB6, group with vitamin B6–deficient diet; DB9, group with B9–deficient diet; DB12, group with vitamin B12–deficient diet; DB6912, group with vitamin B6–, B9–, and B12–deficient diet. N = 16 mice per group. (PDF 225 kb)
Rights and permissions
About this article
Cite this article
Xu, P., Pang, D., Zhou, J. et al. Behavioral changes and brain epigenetic alterations induced by maternal deficiencies of B vitamins in a mouse model. Psychopharmacology 238, 1213–1222 (2021). https://doi.org/10.1007/s00213-021-05766-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-021-05766-2