Abstract
Rationale
Sexual side effects of chronic treatment with selective serotonin reuptake inhibitors (SSRIs) in humans include anorgasmia and loss of sexual desire and/or arousal which interferes with treatment compliance. There are few options at present to reduce these effects. Because orgasm and desire are mediated in part by activation of sympathetic arousal, we asked whether the sympathomimetic effects of acute caffeine treatment could reverse these effects.
Objective
The present study examined whether acute treatment with caffeine (CAF; 10 or 20 mg/kg, ip) versus vehicle could ameliorate the disruption of appetitive and consummatory measures of copulatory behavior produced by chronic fluoxetine (10 mg/kg, sc) in adult, sexually active female or male rats.
Methods
Sexually experienced female or male rats received daily injections of FLU over a 24-day period and were tested for sexual behaviors five times at 4-day intervals during this period in bilevel pacing chambers. Females had been ovariectomized and given hormone replacement with estradiol benzoate and progesterone prior to each test. Males were left gonadally intact. Four days after the final FLU test, rats were randomly assigned to one of the three doses of CAF and received ip injections of CAF or the saline vehicle 60 min before testing.
Results
Chronic FLU reduced solicitations and lordosis over time in females and reduced the number of ejaculations in males. Both doses of CAF restored solicitations and lordosis in females and ejaculations in males. On their own, both doses of CAF increased females’ pacing behavior and the number of mounts and intromissions in the males.
Conclusions
Stimulation of sympathetic outflow by CAF may constitute a readily accessible on-demand treatment for the sexual side-effects of SSRIs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatments for major depressive disorder (MDD) have a long history of problematic sexual side-effects, including delayed orgasm and reductions in both sexual desire and arousal (Baldwin et al. 2015; Clayton et al. 2014; Grimsley and Jann, 1992; Hirschfeld, 1999; Lorenz et al., 2016; Rothmore, 2020; Segraves, 1995; Zajecka et al., 1991). These side effects occur in both men and women and hamper treatment compliance. This is especially true of selective serotonin reuptake inhibitors (SSRIs), which augment neurotransmission in both ascending and descending serotonin pathways from the Raphé by blocking reuptake proteins on presynaptic terminals (Kroeze et al., 2012; Murphy et al., 1998; Stahl, 1998). Increased activation of ascending serotonin pathways that innervate hypothalamic, limbic, and especially cortical structures is part of the general mechanism for behavioral inhibition and executive function (Bari and Robbins, 2013; Logue and Gould, 2014), and can inhibit sexual arousal and desire in the presence of competent sexual cues (Pfaus, 2009). At the same time, increased activation of descending serotonin pathways from the brainstem to spinal cord inhibits genital erection, ejaculation, and orgasm (McKenna, 1998; Normandin and Murphy, 2008; Normandin and Murphy, 2011a,b; Pfaus, 2009), providing feedback that reinforces the central inhibitory state (Pfaus et al., 2012). In fact, the ability of certain SSRIs like fluoxetine (FLU) to engage these mechanisms has led to their use as short-term treatments for premature ejaculation (Jenkins et al., 2019; Waldinger, 2006, 2018).
Relatively successful off-label treatments that reverse the sexual side-effects of SSRIs have been reported, including long-term coadministration of the broad-spectrum monoamine reuptake blocker bupropion (Clayton et al., 2004; Demyttenaere and Jaspers, 2008; Modell et al., 1997) or the mixed serotonin reuptake inhibitor/5-HT2 antagonist trazodone (Stryjer et al., 2009). Another approach has been to switch therapies to other SSRIs or mixed SSRIs that also act as selective noradrenergic reuptake inhibitors (e.g., escitalopram or vortioxetine; Ashton et al., 2005; Jacobsen et al., 2015; Thase et al., 2017), or the novel antidepressant mirtazapine, which acts as an antagonist at noradrenergic α2 autoreceptors and 5-HT2 and 5-HT3 receptors (Gelenberg et al., 2000; Ozmenler et al., 2008). A recent recommendation by Montejo et al. (2019) suggested targeting different sexual dysfunctions selectively: “… for low sexual desire, switching to a non-serotoninergic drug, lowering the dose, or associating bupropion or aripiprazole; for unwanted orgasm delayal or anorgasmia, dose reduction,”weekend holiday“, or switching to a non-serotoninergic drug or fluvoxamine; for erectile dysfunction, switching to a non-serotoninergic drug or the addition of an antidote such as phosphodiesterase 5 inhibitors (PDE-5Is); and for lubrication difficulties, switching to a non-serotoninergic drug, dose reduction, or using vaginal lubricants.” (P. 1640). Although these co-treatment or substitution approaches are generally effective in some individuals, they require chronic usage and present with their own side-effects that may also hamper treatment compliance.
Another way to circumvent sexual side effects of antidepressants has been to introduce an acute drug treatment or other counteractive measure on demand. As with men using PDE-5Is, an acute combination of sublingual testosterone and either the PDE-5I sildenafil or the 5-HT1A agonist buspirone was found to reverse the effects of SSRIs on orgasm, sexual desire, and sexual satisfaction (van Rooij et al., 2015). Ephedrine was also reported to increase qualitative measures of sexual desire and orgasm intensity and pleasure in women with SSRI-induced sexual dysfunction during a randomized, placebo-controlled crossover study (Meston, 2004). What these acute and chronic treatments have in common is the activation of autonomic arousal. Consistent with this, a regimen of regular cardiovascular exercise immediately before sexual activity significantly improved sexual desire and global sexual functioning in women that experienced sexual side effects of various antidepressants (Lorenz and Meston, 2014). Lorenz and Meston also found that having sex regularly improved orgasm ratings, suggesting that tolerance may accrue to the delayed orgasm effects.
In male rats, acute FLU dose-dependently delays ejaculation and increases the total number of mounts before ejaculation and lengthens the post-ejaculatory refractory period (Vega Matuszcyk et al., 1998b; Yells et al., 1994). Furthermore, male rats that were chronically administered FLU at 10 or 20 mg/kg showed fewer ejaculations and a decrease in measures of sexual motivation relative to baseline (Cantor et al. 1998; Yells et al., 1994). In female rats, FLU decreased the motivation to interact with a sexual partner (quantified by the number of nose-pokes performed to gain access to a male counterpart; Uphouse et al., 2015). Also, FLU-treated females showed a decrease in the magnitude of the lordosis reflex and the number of hops and darts (Vega Matuszcyk, Larsson, and Eriksson, 1998a). Because the effect of SSRIs in rats successfully mimics two of the most prominent symptoms of sexual dysfunction observed among humans (anorgasmia and decreased sexual desire), rats have been used as preclinical models to delineate both the basic neuropharmacology of SSRI effects on sexual behavior, and as proof of concept in testing potential treatments (Dulawa et al. 2004). Lesions of the nucleus paragigantocellularis in males (which contains serotonin neurons that project from the brainstem to spinal cord) reversed the effects of FLU on ejaculation (Yang et al., 2013; Yells et al., 1994).
As in humans, activation of the sympathetic nervous system has been shown to reverse FLU-associated sexual inhibition in rats, for instance via oxytocin administration. Acute treatment with oxytocin (200 ng/kg) to male rats that had been administered FLU (10 mg/kg) daily for several weeks completely reversed the decreases in ejaculation and appetitive level changes (a measure of sexual motivation) in a bilevel chamber (Cantor et al. 1999). A similar amelioration of treatment-resistant anorgasmia in a human male has been reported with intranasal oxytocin (IsHak et al., 2008). Oxytocin plays an important role in ejaculation and orgasm (Courtois et al., 2013), and facilitates ejaculation at both central hypothalamic (Clément et al., 2008) and peripheral genital (Gupta et al., 2008) sites. Oxytocin also increases systolic blood pressure (Carmichael et al., 1994), an action that is consistent with the facilitation of sympathetic arousal and its important role in ejaculation and orgasm.
It would appear that a common action of all treatments that reverse SSRI-induced sexual dysfunction is the activation of sympathetic and central arousal. Therefore, the present study asked whether an acute dose of caffeine (CAF) could reverse the effects of chronic FLU on the sexual behavior of female and male rats. Caffeine (1,3,7-trimethylxanthine) is arguably the most widely used central nervous system (CNS) stimulant in the world (Nehlig et al., 1992). It exerts its actions through the antagonism of adenosine receptors, the inhibition of phosphodiesterase, the release of calcium from intracellular stores, and antagonism of benzodiazepine receptors (see Fisone et al., 2004 for review). Adenosine acting at both A1 and A2a receptors inhibits sympathetic outflow; thus, blockade of adenosine receptors by CAF leads to increases in heart rate and both central and peripheral noradrenaline and dopamine transmission. On its own, low doses of CAF (10–30 mg/kg) facilitate copulatory behavior in sexually naïve (Soulairac and Coppin-Monthillaud, 1951) and sexually experienced (Pfaus et al., 2010; Zimbardo and Barry 3rd, 1958) male rats. This facilitation consists of increases in the proportion of males that copulate, increases in appetitive sexual responses, decreases in the mount, intromission, and ejaculation latencies, and increases in the mount rate prior to ejaculation. Caffeine administered sub-chronically at doses of 50 mg kg/day for 15 days increased both anogenital investigations and mounting performance in male rats but left erectile and ejaculatory responses unaffected (Taha et al., 1995). Zimbardo and Barry 3rd (1958) demonstrated that 20 mg/kg of caffeine administered intraperitoneally (ip) significantly “decrease[d] latencies to mount, copulate, and ejaculate and increase[d] the frequency and rate of copulation” in sexually experienced males. In ovariectomized, hormone-primed female rats, acute administration of CAF (7.5, 10, or 15 mg/kg) reduced the latency to return to a male in a unilevel pacing chamber following ejaculation, and increased the number of visits that females made to males in a partner preference paradigm, both indicators of increased female sexual motivation (Guarraci and Benson, 2005; Guarraci and Bolton, 2014).
In the present study, sexually experienced female or male rats were administered FLU (10 mg/kg, sc) daily for 31 days, with sexual behavior tested at 4-day intervals as in Cantor et al. (1999). Rats were then assigned randomly to one of three groups that received acute CAF (10 or 20 mg/kg, ip) or vehicle 30 min prior to the final test.
Materials and methods
Animals, surgery, and hormone treatments
Male (N = 30) and female (N = 30) Long-Evans rats were obtained from Charles River Inc. (Kingston, NY). Males were housed in groups of 2 to 4 whereas females were housed in groups of 2 in a colony room on a reverse 12 h:12 h light/dark cycle (lights go off at 8 h) at 21 °C with unlimited tap water and Purina® rat chow available in each cage.
To prevent pregnancy and allow common hormone levels, bilateral ovariectomies were performed on the females via lumbar incision approximately 2 weeks before the beginning of the experiment. General anesthesia was induced with ketamine (50 mg/ml, CDMV, ID UN7919, Wyeth)/xylazine (4 mg/ml, Rompun, DIN 02169592, Bayer) mixed in a 4:3 ratio, respectively, and administered ip at a dose of 1 ml/kg. After surgery, females were administered 0.1 ml of Penicillin G/Procaine Injectable Suspension as an antibiotic.
Sexual receptivity was induced in each female by sc injections of 10 μg estradiol benzoate (EB, 17β-diol 3-benzoate, ID E0970–000, Steraloids) 48 h prior each training session, and 500 μg of progesterone (P, 4-Pregnen-3, 20-dione, ID Q2600–000, Steraloids) 4 h prior to each training and test session. Steroids were dissolved in reagent grade sesame oil and injected in a volume of 0.1 ml.
Drugs
Fluoxetine hydrochloride was purchased from Sigma (St. Louis, Mo.). The dose of 10 mg/kg/ml was selected given its effectiveness in previous studies (e.g., Cantor, Binik, & Pfaus, 1999; Damjanoska et al., 2003; Dulawa et al., 2004; Vega Matuszcyk, et al., 1998a; Yells et al., 1993). Fluoxetine solutions were made fresh daily by dissolving in distilled water and 0.9 M Tris Buffered Saline (v:v 50:50) with sonication for at least 10 min to assure it was completely dissolved. FLU was injected SC once daily prior to the rat’s sleep cycle (between 19 h and 20 h) to avoid FLU-induced taste aversions (Prendergast, Hendricks, Yells, & Balogh, 1996). Accordingly, each animal’s weight was monitored on a daily basis prior to injection.
Caffeine was purchased from Sigma (St. Louis, Mo). Doses of 10 or 20 mg/kg/ml were made in distilled water and prepared fresh immediately before injection. Injections of CAF were made 30 min before testing. Caffeine’s modulation of sexual behavior can be described as an inverted “U”-shaped curve (Pfaus et al., 2010). The optimal for sexual behavior among males is 20 mg/kg, and 10 mg/kg for females (Guarraci & Benson, 2005; Pfaus et al., 2010; Zimbardo & Hebert, 1958). At these doses, caffeine has been found to promote sexual behavior by decreasing the latency to engage in sexual behavior (Guarraci & Benson, 2005; Pfaus et al., 2010; Zimbardo & Hebert, 1958). Particularly for females, it has been observed that the lower dose has no locomotor effects (Guarraci & Benson, 2005).
Procedure
For both females and males, the procedure had 3 phases: (1) training; (2) FLU dosing; and (3) the final test with CAF. Male rats were given training sessions of sexual behavior at 4-day intervals prior to fluoxetine dosing until all males achieved 3 ejaculations per session. This occurred by the sixth training test, so female rats were given the same number of sessions prior to drug testing. All training sessions and tests took place at 4-day intervals in bilevel chambers (Pfaus, Mendelson, and Phillips, 1990; Pfaus, Smith and Coopersmith, 1999). During each test, a single male was placed into the bilevel chamber for 5 min prior to the introduction of a sexually receptive female for a 30-min test. The last 3 training tests were recorded, and the last test taken as baseline prior to FLU dosing in the second phase. On the evening of the final training test, female or male subjects were given their first dose of FLU. Daily dosing continued for 24 days prior to the final CAF test. Five tests were conducted at 4-day intervals during this period. The effects of CAF were tested 4 days after the fifth FLU alone test. Female and male rats were assigned randomly to one of 3 groups and received either 0 (vehicle), 10, or 20 mg/kg of CAF 30 min prior to the final test.
Behavioral measures
In addition to bodyweight, the following measures of sexual response were recorded for females and males during each test:
-
Females. The number of full solicitations (headwise orientation to the male and a runaway to another level), partial solicitations (hops and darts on the same level as the male), pacing (level changes/mount, LC/M), and measures of lordosis quotients (LQs) and reflex magnitudes (LMs, scored as 1–3 for low, medium, and high, as in Hardy and DeBold, 1971), rejection responses, and the number of ejaculations received during the 30-min test were taken as in Pfaus, Smith, and Coopersmith (1999).
-
Males. The number of appetitive level changes made during the 5-min period prior to the introduction of the female; mount, intromission, and ejaculation latencies, the duration of the first post-ejaculatory interval (time from the first ejaculation to the next intromission), and the total number of mounts, intromissions, and ejaculations made during the 30-min test were taken, as in Pfaus, Mendelson, and Phillips (1990). All sexual behavior trials were recorded on video during the test and scored subsequently using the Behavioral Observation Program (Cabilio, 1996).
Statistical analyses
Bodyweight was analyzed using a 3 × 7 between-within, mixed design analysis of variance (ANOVA) with the 3 CAF Treatment Groups (0, 10, and 20 mg/kg) as the between-group condition, and the baseline (B), five FLU tests, and the final FLU-CAF test as the within condition. For each sexual behavioral measure, mixed-design, between-within 3 × 3 analyses of variance (ANOVAs) were conducted with the CAF Treatment Group as the between variable and Test (B, last FLU test, and FLU-CAF test) as the within variable. Post hoc Tukey HSD tests were conducted for all significant main effects and interactions, p < 0.05. Estimates of effect size were made using partial eta squared (\({\eta}_p^2\)). Although significance was set two-tailed at p < 0.05, it is justified to report effects with p values between 0.05 and 0.10 if the \({\eta}_p^2\) effect size estimates are moderate (0.09 to 0.25) or strong (0.25+) (Kline, 2004; Lakens, 2013).
Results
Bodyweight
Figure 1 shows the weights of females and males taken on the last day of baseline (B), the 5 FLU-alone tests, and the final FLU-CAF test.
-
Females. A 3 × 7 between-within, mixed-design ANOVA did not detect any significant overall differences between the groups summed over test periods, F(2.27) = 0.79, NS. However, there was a significant within effect over time, F(6.162) = 19.41,, \({\eta}_p^2\) = 0.41, p < 0.0001. Post hoc analyses revealed that female weights at baseline were significantly higher overall (by approximately 10 g) relative to the weights during subsequent tests. Although the daily injections overall appeared to reduce female weights slightly but significantly relative to the baseline test, weights were also reduced in the control group suggesting that FLU treatment alone could not account for the slight but consistent weight loss. None of the other test days differed significantly from one another. Accordingly, no significant interaction of groups by test days was found, F(12.162) = 0.43, NS.
-
Males. Likewise, for males, there was no significant overall difference between the groups summed over test period, F(2.27) = 1.72, NS. However, there was a significant within effect over time, F(6.162) = 19.78,, \({\eta}_p^2\) = 0.42, p < 0.0001. Post hoc analyses revealed that overall weights at baseline and the first FLU alone test were significantly lower than the weights on subsequent tests. There was no significant interaction with test days. F(12.162) = 1.42, NS. Thus, the daily injections did not lead to a loss of bodyweight for the males.
Female sexual behaviors
Figure 2 shows the mean number of full solicitations, pacing behaviors, lordosis reflex magnitudes, and the number of high (3) lordosis reflex scores taken from the last day of baseline (B), the last FLU-alone test (FLU), and the final FLU-CAF test.
-
Appetitive sexual behaviors. A significant within-subjects main effect of test was found for full solicitations, F(2.52) = 9.70, \({\eta}_p^2\) = 0.27, p < 0.0003. Post hoc analyses revealed that the overall number of full solicitations was higher at baseline than after FLU treatment or during the FLU-CAF test. A trend toward significance was detected for the interaction of CAF Treatment Group and Test, F(4.52) = 2.35, \({\eta}_p^2\) = 0.16, p < 0.06. Post hoc analyses revealed that the number of full solicitations went down significantly in the control group from B to the fifth FLU test and decreased further during the FLU-CAF test. Females that received the 20-mg/kg dose had a significantly lower number of solicitations after FLU treatment, but this number increased significantly during the FLU-CAF test. There were no significant effects of FLU or FLU-CAF treatment on the number of hops and darts.
-
Pacing behavior. A significant within-subjects main effect of test was found for pacing (LC/M), F(2.52) = 5.19, \({\eta}_p^2\) = 0.17, p < 0.009. Post hoc analyses revealed that the number of LC/M had dropped significantly from B to the last FLU test but increased significantly during the FLU-CAF test. A trend toward significance was detected for the interaction of Dose Treatment Group and Test, F(4.52) = 2.11, \({\eta}_p^2\) = 0.14, p < 0.09. Post hoc analyses revealed that the number of LC/M had increased significantly during the FLU-CAF test in both the 10- and 20-mg/kg dose groups relative to the last FLU test. No significant differences were detected across the tests for the control group.
-
Lordosis. Although lordosis quotients were not altered significantly by any treatment, lordosis reflex magnitudes (LMs) were. For overall LMs, there was a significant within-subjects effect for test, F(2.52) = 3.63, \({\eta}_p^2\)= 0.12, p < 0.04. Post hoc analyses revealed that the LMs during the last FLU test were significantly lower than during B or the FLU-CAF test. A trend toward significance was also found for the interaction of CAF Treatment Group and Test, F(4.52) = 2.47, \({\eta}_p^2\) = 0.16, p < 0.06. Post hoc analyses revealed that the control group’s LM was significantly lower than the 10- and 20-mg/kg CAF group’s LMs during the FLU-CAF test. Individual LMs also changed. The ANOVA detected a significant within-subjects effect for test for both LM3 and LM1, F(2.52) = 8.41,\({\eta}_p^2\)= 0.25, p < 0.0007, and F(2.52) = 5.22, \({\eta}_p^2\)= 0.17, p < 0.009, respectively. Post hoc analyses revealed that the mean number of LM3s was significantly lower during the final FLU test compared to B. Although the number went up during the FLU-CAF test, it was still significantly lower than B. In contrast, post hoc analyses revealed the opposite pattern for LM1s, with a significant increase from baseline during the final FLU test, and a significant decrease back to B levels during the FLU-CAF test.
-
Ejaculations received. The ANOVA analysis showed a significant within-subjects effect of test for the number of ejaculations received, F(2.52) = 7.75, \({\eta}_p^2\) = 0.23, p < 0.002. Post hoc analyses revealed a pattern similar to the LM3s in which the mean number was significantly lower during the final FLU test compared to B. However, the number received did not go up further during the final FLU-CAF test.
Mean number of full solicitations, pacing behaviors, lordosis reflex magnitudes and the number of high (3) lordosis reflex scores of female rats in the three CAF dose groups (0 (control), 10, or 20 mg/kg) taken from the last day of baseline (B), the last FLU-alone test (FLU) and the final FLU-CAF test. *p < 0.05 from baseline unless otherwise noted. See Results for details
There were no significant effects on other measures of female sexual behavior.
Male sexual behaviors
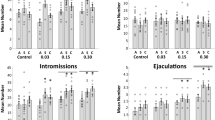
Figure 3 shows the mean number of appetitive level changes, number of intromissions, number of ejaculations, and the mean ejaculation latencies taken from the last day of baseline (B), the last FLU-alone test (FLU), and the final FLU-CAF test.
-
Appetitive level changes. A significant within-subjects main effect of test was found, F(2.54) = 3.23, \({\eta}_p^2\) = 0.11, p < 0.05. Post hoc analyses revealed that, overall, the number of level changes was higher during the FLU-CAF test relative to B or the last FLU test. The ANOVA also found a significant interaction of CAF Treatment Group and Test, F(4.54) = 3.83, \({\eta}_p^2\) = 0.23, p < 0.009. Post hoc analyses revealed that the increase during the FLU-CAF test was significantly higher in the 10-mg/kg dose group relative to the other groups, and the control group was significantly lower than both the 10- and 20-mg.kg groups. No significant effect was found for the latency to level change in any of the groups or tests.
-
Mounts and intromissions. The ANOVA detected a significant between-subjects effect for the number of mounts, F(2.27) = 3.59, \({\eta}_p^2\) = 0.21, p < 0.05. Post hoc analyses revealed that there were more mounts overall in the 10-mg/kg group compared to the other groups. The ANOVA also detected a trend toward a significant within-subjects effect, F(2.54) = 2.81, \({\eta}_p^2\) = 0.10, p < 0.07. Post hoc analyses revealed a higher number of mounts during the FLU-CAF test relative to the other tests. No significant effect was found for the mount latency in any of the groups or tests. A similar pattern was observed for intromissions. The ANOVA detected a significant between-subjects effect for the number of intromissions, F(2.27) = 3.73, \({\eta}_p^2\) = 0.22, p < 0.04. Post hoc analyses revealed a higher overall number of intromissions in the 10-mg/kg group relative to the other groups. The ANOVA also detected a trend toward a significant within-subjects effect, F(2.54) = 3.14, \({\eta}_p^2\) = 0.10, p < 0.06. Post hoc analyses revealed that there were more intromissions during the FLU-CAF test than during the last FLU alone test. Finally, the ANOVA detected a significant within-subjects main effect for the intromission latency, F(2.54) = 4.71, \({\eta}_p^2\)= 0.15, p < 0.02. Post hoc analyses revealed that the intromission latency was significantly higher during the last FLU test than during either baseline or the FLU-CAFS tests.
-
Ejaculations. The ANOVA detected a significant within-subjects main effect for the number of ejaculations, F(2.54) = 6.69, \({\eta}_p^2\) = 0.20, p < 0.003. Post hoc analyses revealed that the number of ejaculations was significantly lower during the last FLU test compared to baseline and the FLU-CAF test. Accordingly, the ejaculation latency was also affected by FLU treatment. The ANOVA detected a significant within-subjects main effect, F(2.54) = 5.86, \({\eta}_p^2\) = 0.18, p < 0.005. Post hoc analyses revealed that the latencies were significantly higher during the last FLU test compared to the B or the FLU-CAF test. The ANOVA also detected a trend toward a significant interaction, F(4.54) = 2.37, \({\eta}_p^2\)= 0.15, p < 0.06. Post hoc analyses revealed that the overall within-subjects effect was due largely to differences in latencies within the 10-mg/kg dose group.
Mean number of appetitive level changes, number of intromissions, number of ejaculations, and the mean ejaculation latencies of male rats in the three CAF dose groups (0 (control), 10, or 20 mg/kg) taken from the last day of baseline (B), the last FLU-alone test (FLU) and the final FLU-CAF test. *p < 0.05 from baseline unless otherwise noted. See the “Results” section for details
There were no significant effects on other measures of male sexual behavior.
Discussion
The present study examined whether an acute dose of CAF could reverse the disruptive effects of chronic FLU on measures of appetitive and consummatory sexual behavior in female and male rats. As in previous studies, daily FLU treatment (10 mg/kg) for 24 days reduced lordosis reflex magnitudes (Miryala et al., 2013; Uphouse et al., 2015; Vega Matuszcyk et al., 1998a) and measures of solicitation and pacing in female rats. In male rats, this treatment reduced the number of ejaculations (Cantor et al., 1999; Vega Matuszcyk, Larsson, and Erickson, 1998b; Yells et al., 1994). Acute treatment with CAF 30 min before testing reversed these effects in females and males, with the most effective doses being 20 mg/kg in females and 10 mg/kg in males. The effects of FLU and CAF were not secondary to changes in bodyweight.
Some previously reported effects were not replicated in the present study. For example, Cantor et al. (1999) found a significant decrease in appetitive level changes after chronic treatment with FLU whereas this was not observed in the present study, despite the ability of both doses of CAF to increase this measure. However, we note that the criterion reduction of ejaculations to approximately 1 or less was reached after 5 FLU tests (20 days) in the present study, whereas it was not reached until 11 tests (44 days) in the Cantor et al. study. The males in the present study had 6 training trials of sexual behavior before FLU dosing whereas the males in the Cantor et al. study had 10 training trials, potentially strengthening the ejaculatory responses and making them more resistant to disruption by FLU. Appetitive responses in males may thus require longer exposure to FLU to become disrupted, especially if appetitive responses are related to the degree of sexual reward (Pfaus et al., 2012).
The idea for testing acute CAF came from studies in which activation of sympathetic and/or central arousal with ephedrine, oxytocin, or cardiovascular exercise was able to reverse different sexual side effects of SSRI treatment (e.g., Cantor et al., 1999; IsHak et al., 2008; Lorenz and Meston, 2014; Meston, 2004). Indeed, acute CAF facilitates the initiation of copulatory behavior in sexually naïve male rats (Soulairac and Coppin-Monthillaud, 1951) and increases appetitive sexual behaviors, intromissions, and ejaculations in sexually experienced male rats (Pfaus et al., 2010; Zimbardo and Barry 3rd, 1958). Similarly, intake of 2–3 daily cups of coffee (170–375 mg/day) significantly reduces the likelihood of erectile dysfunction in men (Lopez et al., 2015). Acute CAF reduces the latency of OVX, EB + P-primed females to return to a male in a unilevel pacing chamber following ejaculation and increases the number of visits that females made to males in a partner preference paradigm (Guarraci and Benson, 2005; Guarraci and Bolton, 2014). Therefore, it is likely that the effects observed in the present study are downstream of the increase in serotonin transmission induced by SSRIs.
FLU increases serotonin transmission in the lateral aspect of the anterior hypothalamus (LHAA; Hull & Dominguez, 2015). Infusions of SSRIs to the LHAA increase the mount and intromission latencies and delay ejaculation in male rats (Hull and Dominguez, 2015; Pfaus, 2009). Furthermore, the release of serotonin in the LHAA before ejaculation is associated with a decreased release of dopamine in the striatum immediately after ejaculation (Carmichael et al., 1994; Pfaus, 2009). Thus, the increase of serotonin produced by SSRIs could delay copulatory behaviors by inhibiting dopamine transmission in the mesolimbic and/or nigrostriatal dopamine pathways.
Both CAF and its primary metabolite, paraxanthine, bind competitively as antagonists to A1 and A2a adenosine receptors and counteract the endogenous effects of adenosine. Binding of adenosine to A1 receptors induces CNS depression, inhibition of dopamine and noradrenaline release, increases cardiac vagal tone, and reduces sympathetic efferent nerve activities in both rats and humans (Biaggioni, 1992; O’Neill et al., 2007; Wood et al., 1989). Accordingly, antagonism by CAF would be expected to prevent or reverse these effects. Of particular interest for the present study is the effect of CAF on central dopamine transmission. Acute antagonism of A2a receptors in the ventral striatum (including the NAc) and putamen upregulates D2/D3 receptor populations (Volkow et al., 2015), thereby enhancing arousal, alertness, and sexual motivation in the presence of competent sexual stimuli that increase dopamine transmission (Hull and Dominguez, 2015; Pfaus, 2009; Pfaus et al., 2015). A related region critical for sexual desire is the medial preoptic area (mPOA). The state of proestrus in rats, in which estradiol, testosterone, and progesterone set up an array of protein synthetic changes that underlie solicitation, pacing, and lordosis in females also correlates with a downregulation of the A2a receptor gene in this region (Vastagh and Liposits, 2017) and an increase in dopamine transmission (Pfaus, 2009; Pfaus et al., 1995). Estradiol actions in the mPOA also enhance the response to psychomotor stimulants like cocaine in the NAc (Tobiansky et al., 2016) via projections to the ventral tegmental area that disinhibit dopamine cell firing.
Another potential mechanism of action for both FLU and CAF is on benzodiazepine binding sites on the GABA A receptor (Tunnicliff et al., 1999; Jain, Hirani and Chopde, 2005). On one hand, FLU interaction with GABA A receptors increases the synthesis of allopregnanolone, a neurosteroid with anxiolytic properties (Norman, 2015). However, acute administration of FLU results in anxiogenic-like behavior in certain animal models (Norman, 2015), although the data are not consistent regarding chronic FLU administration, with reports of both anxiogenic- and anxiolytic-like effects (Norman, 2015). On the other hand, CAF is a known anxiolytic that interacts with benzodiazepine receptors during acute administration (Ribeiro & Sebastião, 2010). However, it is commonly agreed that the anxiogenic effects during chronic administration are A2-mediated (Ribeiro & Sebastião, 2010). Because CAF was administered acutely in the present experiment, it is possible that some of its effects on sexual behavior could be mediated by interaction with GABA A receptors in certain regions of the brain.
The present study did not assess the locomotor effects of caffeine specifically. We note, however, that locomotion is embedded in the level-to-level movements that comprise certain appetite behaviors observed in bilevel chambers, such as solicitation and pacing in females and appetitive level changes in males. Although caffeine returned solicitations to baseline, it increased pacing in females and appetitive level changes in males above baseline, which could indicate an increase in locomotion. We also observed an increase in the number of mounts without intromission in males with the highest dose (data not shown). An increase in mounts without intromission could indicate an increase in locomotor activity at the expense of erection, reminiscent of the effects of amphetamine (Pfaus et al., 2010). In females, however, there was no interaction between group and pacing (level changes per mount), similar to the effects reported by Guarraci and Benson (2005). Thus, the lower dose of caffeine appeared to increase sexual behavior specifically. We note that previous studies have reported sex differences in response to the stimulant effects of caffeine (e.g., Turgeon et al., 2016). In that study, caffeine decreased amphetamine-induced rearing in adolescent male rats but had no effect in adolescent female rats. Our findings also show a more pronounced effect of the lower dose in males compared to females, consistent with previous findings.
Finally, we also note that the rats used here were caffeine naïve. This would not be expected of human patients taking SSRIs, who very likely have been exposed to caffeine for years. It would be important, therefore, to assess a range of doses of caffeine in any human clinical trial, especially given preclinical data on sex differences and potential tolerance effects. Caffeine derived from coffee, tea, kola, guaraná, and mate has a long history as a CNS stimulant and aphrodisiac in diverse human cultures (Ben Nahum, 1933/2010; Miller, 1993; Rätsch and Müller-Ebeling, 2013; Ross, 2005). Its pharmacological actions in preclinical rat models seem well-suited to override the major inhibitory effects of SSRIs on orgasm and sexual arousal and/or desire in both sexes. This is because a decrease in lordosis reflex magnitudes (especially full reflex arcs characteristic of LM3s) and solicitations are predictive of sexual nonreward in female rats tested in bilevel chambers (Coria-Avila et al., 2008), whereas decreased ejaculations and appetitive level changes are predictive of the same in male rats (Cantor et al., 1999; Ismail et al., 2009). Thus, acute CAF could be considered an on-demand treatment should appropriate randomized clinical trials in patients taking SSRIs or those with endogenous sexual arousal, desire, or orgasm difficulties, showing its efficacy.
References
Ashton AK, Mahmood A, Iqbal F (2005) Improvements in SSRI/SNRI-induced sexual dysfunction by switching to escitalopram. J Sex Marital Ther 31:257–262
Baldwin DS, Manson C, Nowak M (2015) Impact of antidepressant drugs on sexual function and satisfaction. CNS Drugs 29:905–913
Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79
Ben Nahum P (1933/2010) The Turkish art of love. Kessinger Publishing, Whitefish, MT
Biaggioni I (1992) Contrasting excitatory and inhibitory effects of adenosine in blood pressure regulation. Hypertension 20:457–465
Cabilio S (1996) Behavioral scoring program. Unpublished software, Concordia University Montréal
Cantor JM, Binik YM, Pfaus JG (1999) Chronic fluoxetine inhibits sexual behavior in the male rat: reversal with oxytocin. Psychopharmacol 144:355–362
Carmichael MS, Warburton VL, Dixen J, Davidson JM (1994) Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav 23(1):59–79
Clayton AH, Warnock JK, Kornstein SG, Pinkerton R, Sheldon-Keller A, McGarvey EL (2004) A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry 65:62–67
Clayton AH, Croft HA, Handiwala L (2014) Antidepressants and sexual dysfunction: mechanisms and clinical implications. Postgrad Med 126:91–99
Clément P, Peeters M, Bernabé J, Denys P, Alexandre L, Giuliano F (2008). Brain oxytocin receptors mediate ejaculation elicited by 7-hydroxy-2-(di-N-propylamino) tetralin (7-OH-DPAT) in anaesthetized rats. Br J Pharmacol 154:1150–1159
Coria-Avila GA, Solomon CE, Barbosa-Vargas E, Lemme I, Ryan R, Ménard S, Gavrila AS, Pfaus JG (2008) Neurochemical basis of conditioned partner preference in the female rat: 1. Disruption by naloxone. Behav Neurosci,122:385-395
Courtois F, Carrier S, Charvier K, Guertin PA, Journel NM (2013) The control of male sexual responses. Curr Pharm Des 19:4341–4356
Demyttenaere K, Jaspers L (2008) Bupropion and SSRI-induced side effects. J Psychopharmacol 22:792–804
Dulawa SC, Holick KA, Gundersen B, Hen R (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29:1321–1330
Fisone G, Borgkvist A, Usiello A (2004) Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci 61:857–872
Gelenberg AJ, Delgado P, Nurnberg HG (2000) Sexual side effects of antidepressant drugs. Curr Psychiatry Rep 2:223–227
Grimsley SR, Jann MW (1992) Paroxetine, sertraline, and fluvoxamine: new selective serotonin reuptake inhibitors. Clin Pharm 11:930–957
Guarraci FA, Benson A (2005) “Coffee, tea and me”: moderate doses of caffeine affect sexual behavior in female rats. Pharmacol Biochem Behav 82:522–530
Guarraci FA, Bolton JL (2014) “Sexy stimulants”: the interaction between psychomotor stimulants and sexual behavior in the female brain. Pharmacol Biochem Behav 121:53–61
Hardy DF, DeBold JF (1971) Effects of coital stimulation upon behavior of the female rat. J Comp Physiol Psychol 78:400–408
Hirschfeld RM (1999) Management of sexual side effects of antidepressant therapy. J Clin Psychiatry 60(Suppl 14):27–30
Hull EM, Dominguez JM (2015) Male sexual behavior. In: Plant AM, Zeleznik AJ (Eds), Knobil & Neill’s physiology of reproduction, Fourth edn. Elsevier, New York, pp 2211–2285
IsHak WW, Berman D, Peters A (2008) Male anorgasmia treated with oxytocin. J Sex Med 5:1022–1024
Ismail N, Girard-Bériault F, Nakanishi S, Pfaus JG (2009) Naloxone, but not flupenthixol, disrupts the development of conditioned ejaculatory preference in the male rat. Behav Neurosci 123:992–999
Jacobsen PL, Mahableshwarkar AR, Chen Y, Chrones L, Clayton AH (2015) Effect of vortioxetine vs. escitalopram on sexual functioning in adults with well-treated major depressive disorder experiencing SSRI-induced sexual dysfunction. J Sex Med 12:2036–2044
Jain NS, Hirani K, Chopde CT (2005) Reversal of caffeine-induced anxiety by neurosteroid 3-alpha-hydroxy-5-alpha-pregnane-20-one in rats. Neuropharmacology 48:627–638
Jenkins LC, Gonzalez J, Tal R, Guhring P, Parker M, Mulhall JP (2019) Compliance with fluoxetine use in men with primary premature ejaculation. J Sex Med 16:1895–1899
Kline RB (2004) Beyond significance testing: reforming data analysis methods in behavioral research. APA Press, Washington DC
Kroeze Y, Zhou H, Homberg JR (2012) The genetics of selective serotonin reuptake inhibitors. Pharmacol Ther 136:375–400
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 26:863
Logue SF, Gould TJ (2014) The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol Biochem Behav 123:45–54
Lopez DS, Wang R, Tsilidis KK, Zhu H, Daniel CR, Sinha A, Canfield S (2015) Role of caffeine intake on erectile dysfunction in US men: results from NHANES 2001-2004. PLoS One 10:e0123547
Lorenz TA, Meston CM (2014) Exercise improves sexual function in women taking antidepressants: results from a randomized crossover trial. Depress Anxiety 31:188–195
Lorenz T, Rullo J, Faubion S (2016) Antidepressant-induced female sexual dysfunction. Mayo Clin Proc 91:1280–1286
McKenna KE (1998) Central control of penile erection. Int J Impot Res 10(Suppl 1):S25–S34
Meston CM (2004) A randomized, placebo-controlled, crossover study of ephedrine for SSRI-induced female sexual dysfunction. J Sex Marital Ther 30:57–68
Miller RA (1993) The magical and ritual use of aphrodisiacs. Destiny books, Rochester, VT
Miryala CS, Hiegel C, Uphouse L (2013) Sprague-Dawley and Fischer female rats differ in acute effects of fluoxetine on sexual behavior. J Sex Med 10:350–361
Modell JG, Katholi CR, Modell JD, DePalma RL (1997) Comparative sexual side effects of bupropion, fluoxetine, paroxetine, and sertraline. Clin Pharmacol Ther 61:476–487
Montejo AL, Prieto N, de Alarcón R, Casado-Espada N, de la Iglesia J, Montejo L (2019) Management strategies for antidepressant-related sexual dysfunction: a clinical approach. J Clin Med 8:1640
Murphy DL, Andrews AM, Wichems CH, Li Q, Tohda M, Greenberg B (1998) Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry 59 (Suppl 15):4–12
Nehlig A, Daval JL, Debry G (1992) Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev 17:139–170
Norman TR (2015) Activity of fluoxetine in animal modes of depression and anxiety. Pinna, G (Ed.), Fluoxetine: Pharmacology, mechanisms of action and potential side effects (pp. 1-25). New York: Nova Publishers
Normandin JJ, Murphy AZ (2008) Nucleus paragigantocellularis afferents in male and female rats: organization, gonadal steroid receptor expression, and activation during sexual behavior. J Comp Neurol 508:771–794
Normandin JJ, Murphy AZ (2011a) Excitotoxic lesions of the nucleus paragigantocellularis facilitate male sexual behavior but attenuate female sexual behavior in rats. Neuroscience 175:212–223
Normandin JJ, Murphy AZ (2011b) Serotonergic lesions of the periaqueductal gray, a primary source of serotonin to the nucleus paragigantocellularis, facilitate sexual behavior in male rats. Pharmacol Biochem Behav 98:369–375
O'Neill C, Nolan BJ, Macari A, O'Boyle KM, O'Connor JJ (2007) Adenosine A1 receptor-mediated inhibition of dopamine release from rat striatal slices is modulated by D1 dopamine receptors. Eur J Neurosci 26:3421–3428
Ozmenler NK, Karlidere T, Bozkurt A, Yetkin S, Doruk A, Sutcigil L, Cansever A, Uzun O, Ozgen F, Ozsahin A (2008) Mirtazapine augmentation in depressed patients with sexual dysfunction due to selective serotonin reuptake inhibitors. Hum Psychopharmacol 23:321–326
Pfaus JG (2009) Pathways of sexual desire. J Sex Med 6:1506–1533
Pfaus JG, Damsma G, Wenkstern D, Fibiger HC (1995) Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res 693:21–30
Pfaus JG, Kippin TE, Coria-Avila GE, Gelez H, Afonso VM, Ismail N, Parada M (2012) Who, what, where, when, (and maybe even why)? How the experience of sexual reward influences sexual desire, preference, and performance. Arch Sex Behav 41:31–62
Pfaus JG, Mendelson SD, Phillips AG (1990) A correlational and factor analysis of anticipatory and consummatory measures of sexual behavior in the male rat. Psychoneuroendocrinol 15:329–340
Pfaus JG, Smith WJ, Coopersmith CB (1999) Appetitive and consummatory sexual behaviors of female rats in bilevel chambers. I: a correlational and factor analysis, and the effects of ovarian hormones. Horm Behav 35:224–240
Pfaus JG, Wilkins MF, DiPietro N, Benibgui M, Toledano R, Rowe A, Castro Couch M (2010) Inhibitory and disinhibitory effects of psychomotor stimulants and depressants on the sexual behavior of male and female rats. Horm Behav 58:163–176
Prendergast MA, Hendricks SE, Yells DP, Balogh S (1996) Conditioned taste aversion induced by fluoxetine. Physiol Behav 60:311–315
Rätsch C, Müller-Ebeling, C (2013) The encyclopedia of aphrodisiacs: psychoactive substances for use in sexual practices. New York: Simon and Schuster
Ribeiro JA, Sebastião AM (2010) Caffeine and adenosine. J Alz Dis 20:S3–S15
Ross IA (2005) Medicinal plants of the world, vol 3: Chemical constituents, traditional and modern medicinal uses. Humana Press, Totowa, NJ
Rothmore J (2020) Antidepressant-induced sexual dysfunction. Med J Aust 212:329–334
Segraves RT (1995) Antidepressant-induced orgasm disorder. J Sex Marital Ther 21:192–201
Soulairac A, Coppin-Monthillaud M (1951) Experimental data on the neural-hormonal relations in sexual behavior of the male rat. J Physiol Paris 43:869–872
Stahl SM (1998) Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects J Affect Disord 51:215–235
Stryjer R, Spivak B, Strous RD, Shiloh R, Harary E, Polak L, Birgen M, Kotler M, Weizman A (2009) Trazodone for the treatment of sexual dysfunction induced by serotonin reuptake inhibitors: a preliminary open-label study. Clin Neuropharmacol 32:82–84
Taha SA, Ageel AM, Islam MW, Ginawi OT (1995) Effect of (-)-cathinone, a psychoactive alkaloid from khat (Catha edulis forsk.) and caffeine on sexual behaviour in rats. Pharm Res 31:299–303
Thase ME, Danchenko N, Brignone M, Florea I, Diamand F, Jacobsen PL, Vieta E (2017) Comparative evaluation of vortioxetine as a switch therapy in patients with major depressive disorder. Eur Neuropsychopharmacol 27:773–781
Tobiansky DJ, Will RG, Lominac KD, Turner JM, Hattori T, Krishnan K, Martz JR, Nutsch VL, Dominguez JM (2016) Estradiol in the preoptic area regulates the dopaminergic response to cocaine in the nucleus accumbens. Neuropsychopharmacol 41:1897–1906
Tunnicliff G, Schindler NL, Crites GJ, Goldenberg R, Yochum A, Malatynska E (1999) The GABAa receptor comple as a taret for fluoxetine action. Neurochem Res 24:1271–1276
Turgeon SM, Townsend SE, Dixon RS, Hickman ET, Lee SM (2016) Chronic caffeine produces sexually dimorphic effects on amphetamine-induced behavior, anxiety and depressive-like behavior in adolescent rats. Pharmacol Biochem Behav 143:26–33
Uphouse L, Pinkston J, Baade D, Solano C, Onaiwu B (2015) Use of an operant paradigm for the study of antidepressant-induced sexual dysfunction. Behav Pharmacol 26:697–705
van Rooij K, Poels S, Worst P, Bloemers J, Koppeschaar H, Goldstein A, Olivier B, Tuiten A (2015) Efficacy of testosterone combined with a PDE5 inhibitor and testosterone combined with a serotonin (1A) receptor agonist in women with SSRI-induced sexual dysfunction. A preliminary study. Eur J Pharmacol 753:246–251
Vastagh C, Liposits Z (2017) Impact of proestrus on gene expression in the medial preoptic area of mice. Front Cell Neurosci 11:183
Vega Matuszcyk J, Larsson K, Eriksson E (1998a) The selective serotonin reuptake inhibitor fluoxetine reduces sexual motivation in male rats. Pharmacol Biochem Behav 60:527–532
Vega Matuszcyk J, Larsson K, Eriksson E (1998b) Subchronic administration of fluoxetine impairs estrous behaviour in intact female rats. Neurospychopharmacol 19:492–498
Waldinger MD (2006) Emerging drugs for premature ejaculation. Expert Opin Emerg Drugs 11:99–109
Waldinger MD (2018) Drug treatment options for premature ejaculation. Expert Opin Pharmacother 19:1077–1085
Wood PL, Kim HS, Boyar WC, Hutchison A (1989) Inhibition of nigrostriatal release of dopamine in the rat by adenosine receptor agonists: A1 receptor mediation. Neuropharmacology 28:21–25
Yang HP, Wang L, Han L, Wang SC (2013) Nonsocial functions of hypothalamic oxytocin. ISRN Neurosci 7:179272
Yells DP, Prendergast MA, Hendricks SE, Nakamura M (1994) Fluoxetine-induced inhibition of male rat copulatory behavior: modification by lesions of the nucleus paragigantocellularis. Pharmacol Biochem Behav 49:121–127
Zajecka J, Fawcett J, Schaff M, Jeffriess H, Guy C (1991) The role of serotonin in sexual dysfunction: fluoxetine-associated orgasm dysfunction. J Clin Psychiatry 52:66–68
Zimbardo PG, Barry H 3rd. (1958) Effects of caffeine and chlorpromazine on the sexual behavior of male rats. Science 127:84–85
Acknowledgments
The authors would like to thank Katschia Germé, Maria Alcaraz, Pierina Gonzalez Cautela, and Vasillis Palliskara for help and advice, and Drs. Tierney Lorenz, Cindy Meston, and Alessandra Relini for valuable discussions.
Funding
This work was supported by a Discovery grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada (OGP-138878) to JGP and by a Group grant from Fonds de la recherche en santé du Québec (FRSQ F00757) to the Center for Studies in Behavioral Neurobiology at Concordia University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that all animal procedures conformed to the guidelines of the Canadian Council for Animal Care. All procedures were approved by the Concordia University Animal Research Ethics Committee (Protocol #30000300 to JGP).
Conflict of interest
The authors declare they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González Cautela, B.V., Quintana, G.R., Akerman, J. et al. Acute caffeine reverses the disruptive effects of chronic fluoxetine on the sexual behavior of female and male rats.. Psychopharmacology 238, 755–764 (2021). https://doi.org/10.1007/s00213-020-05728-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-020-05728-0