Abstract
Rationale
Ethanol-induced behavioural sensitization (EBS) does not occur uniformly in mice exposed to the sensitization paradigm. This suggests innate differential responses to ethanol (EtOH) in the reward circuitry of individual animals.
Objectives
To better characterize the adaptive differences between low-sensitized (LS) and high-sensitized (HS) mice, we examined excitatory amino acid (EAA) and inhibitory amino acid (IAA) neurotransmitter levels in the nucleus accumbens (NAc) during EBS expression.
Methods
Male DBA/2J mice received five ethanol (EtOH) (2.2 g/kg) or saline injections, and locomotor activity (LMA) was assessed during EBS induction. EtOH mice were classified as LS or HS on the basis of final LMA scores. Following an EtOH challenge (1.8 g/kg) 2 weeks later, LMA was re-evaluated and in vivo microdialysis samples were collected from the NAc.
Results
Most differences in amino acid levels were observed within the first 20 min after EtOH challenge. LS mice exhibited similar glutamate levels compared with acutely treated (previously EtOH naïve) mice, and generally increased levels of the IAAs GABA, glycine, and taurine. By contrast, HS mice exhibited increased glutamate and attenuated levels of GABA, glycine, and taurine.
Conclusion
These data suggest that the profile of amino acid neurotransmitters in the NAc of LS and HS mice significantly differs. Elucidating these adaptive differences contributes to our understanding of factors that confer susceptibility/resilience to alcohol use disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite the widespread use of alcohol, only a subpopulation of consumers develops diagnosable alcohol use disorder (AUD). Interestingly, a similar phenomenon is observed in laboratory mice exposed to ethanol-induced behavioural sensitization (EBS) paradigms (Abrahao et al. 2014; Abrahao et al. 2012; Dahchour et al. 2000; Juarez et al. 2017; Nona et al. 2013). EBS describes the progressive and persistent increases in psychomotor response to repeated exposure to a constant dose of psychostimulants (Robinson and Berridge 1993; Segal and Mandell 1974). The observation of EBS in laboratory animals supports the incentive sensitization theory of addiction proposed by Robinson and Berridge in 1993, which posits that sensitization to drugs of abuse is a core contributor to the “wanting” behaviour observed in addiction (Robinson and Berridge 1993; Robinson and Berridge 2008). Locomotor activity is used as an indirect proxy for incentive sensitization, and constitutes a robust aspect of the psychomotor response associated with EBS. However, it is important to note that behavioural sensitization in rodent models may be dissociated from voluntary oral consumption. DBA/2J mice (used in the present study) show a robust psychomotor sensitization response to repeated intermittent EtOH injections, despite low levels of voluntary EtOH consumption owing to a genetically linked aversion to the taste of EtOH (McCool and Chappell 2014). Despite the taste aversion, DBA/2J mice self-administer larger bouts of intragastric EtOH compared with C57/6J mice that do not exhibit the same taste aversion to EtOH (Fidler et al. 2011).
In humans, data on the phenomenon of sensitization to stimulants has been relatively sparse. However, the preponderance of evidence suggests that, in humans, repeated exposure to stimulants is associated with behavioural and neural sensitization, particularly when coupled to conditioned cues (Vezina and Leyton 2009). For instance, subjects without substance dependence that receive repeated intermittent administration of relatively high doses of d-amphetamine demonstrate progressive increases in vigour and eyeblink responses, as well as increased ventral striatum dopamine release (reviewed in (Leyton 2007)).
Although a direct link between incentive sensitization and later development of addiction behaviours has been elusive, there is some evidence to suggest a causal association. For instance, pre-treatment with drugs that produce psychomotor sensitization, such as amphetamine, facilitate the later development of addiction-like behaviours such as drug self-administration (Ferrario and Robinson 2007; Robinson and Berridge 2008). Therefore, characterizing the neuroadaptive changes that confer susceptibility and resilience in EBS paradigms may be valuable in gaining a better understanding of factors that contribute to AUD development.
The role of dopaminergic neurotransmission in behavioural sensitization has been well-explored, highlighting the role of ventral tegmental area (VTA) activation in the induction/development phase of sensitization, and dopamine (DA) transmission in axon terminal fields of the nucleus accumbens (NAc) in the expression phase of sensitization (Abrahao et al. 2011; Camarini et al. 2011; Kai et al. 2015; Kalivas and Stewart 1991; Robinson and Berridge 1993). Considerable evidence also suggests an important role of glutamatergic neurotransmission in EBS. Studies have shown that N-methyl-D-aspartate receptor (NMDAR) antagonism blocks both the induction of EBS (Broadbent and Weitemier 1999; Camarini et al. 2000), as well as the expression of EBS (Broadbent et al. 2003; Nona and Nobrega 2018). Additionally, the accumbal glutamate level is elevated during the expression of EBS, particularly in mice sensitized to EtOH during adolescence (Carrara-Nascimento et al. 2011; Nona and Nobrega 2018). While underexplored, γ-aminobutyric acid (GABA)-ergic neurotransmission has also been implicated as playing a role in EBS. GABAB-positive modulation has been demonstrated to block the induction of EBS (Broadbent and Harless 1999), although more recent evidence has contradicted these findings (Kruse et al. 2012). Additionally, altered expression of GABAA subunits in the NAc has been demonstrated in sensitized mice (Linsenbardt and Boehm 2010).
Outside of sensitization paradigms, the effect of EtOH on amino acid neurotransmission has been extensively described. Acute EtOH administration has been demonstrated to cause inhibition of NMDARs (Lovinger et al. 1989), whereas increased extracellular glutamate and NMDAR expression/activity is observed with chronic EtOH exposure (Holmes et al. 2013; Hu and Ticku 1995; Krystal et al. 2003) and EtOH withdrawal (Rossetti and Carboni 1995)—an effect that is also observed in clinical populations (Tsai et al. 1998; Umhau et al. 2010). EtOH has also been shown to allosterically potentiate the activity of the GABA receptor complex (Koob 2004; Mihic et al. 1997), and chronic EtOH exposure increases accumbal levels of GABA (Dahchour et al. 1996; Dahchour et al. 1994). Furthermore, antagonism of GABAA and GABAB receptors in the NAc attenuates intracranial self-infusion of EtOH, suggesting involvement of GABAergic neurotransmission in the reinforcing effects of EtOH (Ding et al. 2015). Acute administration of EtOH also causes increased levels of the inhibitory neurotransmitter taurine in the NAc, amygdala, hippocampus, and frontal cortex. Furthermore, evidence suggests that dopaminergic neurotransmission in the VTA is partly controlled by strychnine-sensitive glycine receptors (GlyRs), which are activated by both glycine and taurine (Adermark et al. 2011; Vengeliene et al. 2010). Antagonism of accumbal GlyRs prevents increased DA levels following EtOH administration, while glycine perfusion into the NAc has the opposite effect (Molander and Soderpalm 2005). There remains a stark lack of behavioural sensitization studies that take into consideration individual responses to psychostimulants, which may be essential in delineating mechanisms of resilience to sensitization. In one early study, rats were selectively bred into high-alcohol sensitive (HAS) and low-alcohol sensitive (LAS) lines, and microdialysis was used to assess levels of amino acids following an acute EtOH challenge (Dahchour et al. 2000). This study reported a decrease in alanine, arginine, and glutamate in HAS, and an increase in alanine, glutamate, and taurine in LAS following an EtOH challenge. However, the design of this study does not account for the induction phase of behavioural sensitization, and therefore does not include a psychomotor component. Rather, the authors focus on neurotransmitter assessment in a selective breeding model. To address the neuroadaptive changes over repeated EtOH exposure, we have previously utilized an EBS paradigm in DBA/2J mice, which differentiates between high-sensitized (HS) and low-sensitized (LS) mice following repeated EtOH exposure. DBA/2J inbred mice are commonly used for studies investigating behavioural sensitization to EtOH as they show a robust locomotor response to the stimulant effect of EtOH and are more susceptible to the development of EBS than other strains (Hitzemann and Hitzemann 1997). Using this paradigm, we demonstrated that glutamatergic adaptations in the NAc are involved in the expression of behavioural sensitization (Nona et al. 2014; Nona and Nobrega 2018).
In the present study, we expand on our previous work by assessing a comprehensive NAc amino acid neurotransmitter profile of HS and LS mice during the expression phase of behavioural sensitization. We also assess whether inhibition of strychnine-sensitive glycine receptors (GlyRs) during the induction phase of sensitization is effective in blocking the expression of EBS.
Methods
Subjects
Male 4-week-old DBA/2J mice (N = 94) were obtained from Charles River (Quebec, Canada) and group-housed (4 per cage) in polycarbonate cages (32 × 14 × 12 cm). Mice were allowed to acclimatize for 1 week, and were handled for 1 week before the sensitization protocol began at 6 weeks of age. Mice were housed on a 12-h light cycle (lights on at 7 a.m. and off at 7 p.m.) in a room controlled for temperature and humidity (21.2 °C, 30% humidity). Standard mouse chow and water were provided ad libitum. All procedures were approved by the Animal Care Committee at the Centre for Addiction and Mental Health and were in accordance with the guidelines and practices outlined by the Canadian Council on Animal Care.
Drugs
Anhydrous ethyl alcohol (Commercial Alcohols, Brampton, ON) was diluted with physiological saline (0.9% NaCl) to a concentration of 15% w/v. Mice received a dose of 2.2 g/kg of EtOH i.p. (15 mL/kg), or an equivalent volume of saline (SAL) during the induction phase of sensitization. Mice were challenged with a lower EtOH dose of 1.8 g/kg, i.p. for the expression phase of sensitization. These doses have been demonstrated to elicit a robust locomotor stimulant response in mice (rather than a sedative effect), and have been repeatedly shown to produce behavioural sensitization (Harrison and Nobrega 2009; Nona et al. 2014; Nona and Nobrega 2018). Brucine sulphate salt hydrate (Sigma-Aldrich, St Louis, MO, USA) was dissolved in 0.9% saline and injected subcutaneously (s.c.) at a dose of 15 mg/kg.
EtOH sensitization procedure
The EBS protocol was performed as previously reported (Nona et al. 2016; Nona et al. 2014; Nona and Nobrega 2018). Briefly, mice were habituated to the test apparatus, comprising 40 × 40 × 35 cm Plexiglas activity monitor chambers that automatically detect activity by horizontal beam breaks (MED Associates, St. Albans, VT), over three 15-min sessions across three consecutive days. Mice were counterbalanced for baseline LMA and assigned to receive five injections of either saline or EtOH (2.2 g/kg, i.p.) twice per week, with each injection being 2–3 days apart. For all injection sessions, mice were transported in their home cage to the testing room and allowed to acclimatize for at least 30 min. Immediately following injections 1, 3, and 5, LMA was assessed for 15 min to capture the stimulant phase of EtOH while avoiding the sedative phase (Crabbe et al. 1982). Following injections 2 and 4, mice were returned to their home cage and remained in the testing room for 15 min before being transported back to the colony room. All injections and testing were performed during the light cycle between 11 a.m. and 3 p.m. LMA scores corresponding to the final injection (injection 5) were rank-ordered for the EtOH-treated mice. Mice in the lowest 33% of the LMA distribution were classified as low-sensitized, and those in the upper 33% were classified as high-sensitized (Nona and Nobrega 2018; Souza-Formigoni et al. 1999). After a 14-day drug-free period, cohort mice were challenged with EtOH (1.8 g/kg, i.p.; HS and LS groups) or an equivalent volume of saline (SAL group) and LMA was measured for 15 min to verify the expression of sensitization. One to 2 days following injection no. 5 of the induction phase of sensitization, a separate cohort of mice was implanted with cannulae targeting the NAc, and challenged with EtOH (1.8 g/kg, i.p.) 14 to 20 days after surgery. As this study focuses on the relative differences between groups, a separate saline injection group was not included during the microdialysis portion of this study. Therefore, any impact of injection stress on amino acid levels was not directly assessed. However, any amino acid level changes that may be attributed to injection stress would be equal between groups, and the relative differences that emerge may be attributed to treatment differences. Microdialysis sampling was performed for 1 h before and 1 h and 45 min following the EtOH challenge to assess the effect of EtOH on accumbal amino acid neurotransmitter levels during the expression phase of sensitization in HS and LS mice in comparison to the effects of EtOH on previously EtOH-naïve mice.
Effect of brucine on EtOH sensitization
In a separate cohort of mice, the effect of brucine (BRU) on EtOH sensitization was investigated. BRU is a GlyRs antagonist that is related to strychnine, but with significantly lower toxicity. BRU was administered s.c. during the induction phase of sensitization to assess whether inhibition of GlyRs during EBS induction would effectively block EBS expression. The EBS protocol was performed as previously mentioned with n = 7–8 mice per group. However, for this experiment, interfering with EBS induction necessitated that mice in each group were analyzed collectively and could not be retrospectively separated into HS and LS. Mice received a s.c. injection of either vehicle or BRU (15 mg/kg). After 1 h, mice received an i.p. injection of either saline or EtOH (2.2 g/kg as before). Habituation and injection days followed the same schedule outlined in the previous section. Two weeks following the last induction phase test day, mice were challenged with 1.8 g/kg EtOH and LMA was re-assessed.
Microdialysis procedure
Under surgical isoflurane anaesthesia, mice were secured onto a stereotaxic frame using ear bars. A heat pad was used to maintain body temperature, and depth of anaesthesia was monitored using the toe pinch reflex and palpebral reflex, as well as breathing depth and rate. An incision was made along the scalp, and a burr hole was drilled above the NAc. The cannula was implanted into the left hemisphere NAc (A-P + 1.4, M-L 1.3, D-V − 4.7 mm), using the Franklin and Paxinos atlas for placement reference (Franklin and Paxinos 1997). The surgical opening was then covered with dental cement to secure the cannula in place, and mice were closely monitored for 1 week postoperatively.
Fourteen to 20 days after surgery, mice underwent microdialysis sampling concurrent with an EtOH challenge injection (2 mice per day). Mice were lightly anesthetized with isoflurane, and a microdialysis probe (MAB 10.8.2–4 mm, outer diameter of application 0.3 mm, membrane length 0.6 mm, PES membrane, Scientific Products) was inserted into the guide cannula. Mice were individually placed in 40 × 40 × 35 cm Plexiglas boxes with standard bedding, and allowed to explore freely for the duration of the experiment. The NAc was perfused with Ringer’s solution at a constant flow rate of 0.8 μL/min using a Hamilton microinjection pump. A 2-h equilibration period was allowed, followed by the collection of three baseline samples, 20 min apart. Mice were then injected with the EtOH challenge dose (1.8 g/kg, i.p.), and dialysate was collected every 5 min for the first 15 min to capture rapid changes that occur during the stimulant phase of ethanol with high temporal resolution. After the first 15 min, six samples were collected every 15 min, for a total of 13 dialysate samples. Brains were harvested following sampling and stored at − 80 °C until histological processing. Dialysate samples were stored at − 80 °C until HPLC analysis.
Verification of cannula placements
After the final dialysate collection, mice were sacrificed by cervical dislocation and brains were harvested to visualize microdialysis probe tracts. Brains were sectioned coronally (20 μm) on a Leica CM-3000 cryostat microtome (Richmond Hill, ON, Canada) at − 20 °C, thaw-mounted onto Fisher Scientific Positive Charge glass microscope slides (Whitby, ON, Canada), and stored at − 30 °C. Slides were postfixed in 10% formalin vapour, stained with cresyl violet, and then examined at ×10 magnification under a Nikon E600 microscope (Mississauga, Ontario, Canada). Cannula placement determinations were done without knowledge of the group membership of the animals.
HPLC analyses of microdialysis samples
HPLC analyses for the amino acids in dialysate samples were carried out using a BASi 460 Microbore HPLC system with electrochemical detection (Bioanalytical Systems Inc., West Lafayette, IN, USA) together with a Uniget C-18 reverse phase microbore column (BASi, Cat no. 8912; analytical—1 × 150 mm, 5 μm ODS) as the stationary phase, as previously described (Chatterjee et al. 2014; Nona and Nobrega 2018). Five microlitres of dialysate sample was used for O-phthalaldehyde (OPA) derivatization, and 5 μl of homo-serine (125 pmol in ACSF) was added as the internal standard to all samples during OPA derivatization. After OPA derivatization, 10 μl of derivatized solution was injected into the column. The mobile phase consisted of 0.15 M sodium acetate buffer, 1 mM EDTA, pH 5.4, and 50% acetonitrile. The flow rate was 0.8 ml/min. The working electrode (Uniget 3-mm glassy carbon, BAS P/N MF-1003) was set at 750 mV vs the Ag/Ag/Cl reference electrode. Detection gain was 1.0 nA, the filter was 0.2 Hz, and the detection limit was set at 100 nA. Standard amino acids (Sigma Chemicals) were used to quantify and identify the peaks on the chromatographs.
Statistical analyses
LMA data for sensitization induction, LMA data for sensitization expression following EtOH challenge (1 min bins), and microdialysis data were analyzed with mixed-model ANOVAs, with drug treatment as the between-subject factor and time (injection day, time bin, or sample collection time) as repeated measures factors. LMA data for sensitization expression following EtOH challenge (total distance) were analyzed by one-way ANOVA. Significant ANOVAs were followed by Bonferroni-adjusted post hocs. In cases where variances were unequal between groups, Welch-corrected or Greenhouse-Geisser-corrected ANOVAs were used, with Games-Howell post hoc tests when necessary. Data were analyzed using SPSS 20.0 (IBM Corp.) software.
Results
EBS induction and expression
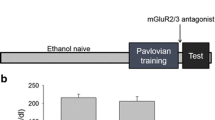
Figure 1 illustrates the induction and expression of EBS as measured by LMA following EtOH injections. For the induction data (Fig. 1a), a repeated measures ANOVA with a Greenhouse-Geisser correction revealed significant main effects of treatment (F2,21 = 30.63, P < 0.001), test day (F2.18,45.80 = 23.85, P < 0.001), and test day by treatment interaction (F4.36,45.80 = 10.23, P < 0.001). Pairwise comparisons revealed significantly higher LMA for HS mice compared with SAL and LS mice at injection 1 (P = 0.001 and P = 0.041, respectively), injection 3 (P < 0.001 and P < 0.001, respectively), and injection 5 (P < 0.001 and P < 0.001, respectively).
Behavioural sensitization induction and expression. a Induction of sensitization. EtOH-treated mice were retrospectively classified as high- (HS) or low-sensitized (LS) on the basis of their locomotor activity (LMA) scores on the last EtOH injection. SAL = saline controls. b, c Expression of sensitization. Total LMA (b) and 1-min bins of LMA (c) were analyzed. LMA was measured for 15 min. Values are means ± SEM. Group Ns are indicated in parentheses. Significant pairwise differences between SAL vs HS (*P < 0.05, **P < 0.01, ***P < 0.001), SAL vs LS (#P < 0.05, ##P < 0.01, ###P < 0.001), and LS vs HS (‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001) are indicated
For the challenge data (Fig. 1b), a one-way ANOVA revealed a significant main effect of treatment (F2,21 = 14.48, P < 0.001). Pairwise comparisons revealed significantly higher LMA for HS mice compared with SAL and LS mice (P < 0.001 and P = 0.005, respectively). Expanding this data into 1-min time bins allowed for a more detailed assessment of locomotor fluctuations during the 15-min test (Fig. 1c). A repeated measures ANOVA with a Greenhouse-Geisser correction of this data revealed significant main effects of treatment (F2,21 = 22.37, P < 0.001), time bin (F3.97,83.32 = 7.09, P < 0.001), and time bin by treatment interaction (F7.94,83.32 = 2.96, P = 0.006). Pairwise comparisons revealed significantly higher LMA for HS mice compared with SAL at nearly every time point (with the exception of 12–13 min) (P < 0.05). LMA for HS was also significantly higher than LS values at most time points between 1 and 9 min (with the exception of 7–8 min) (P < 0.05). It is also noteworthy that the magnitude of LMA elevation was most pronounced during the first 5 min and, while remaining relatively elevated, gradually tapered during the latter part of the test. Indeed, within the HS group, LMA values at the 1–2-, 2–3-, and 3–4-min time points were significantly higher than various time points between 6 and 15 min (not depicted in Fig. 1) (P < 0.05). The LMA of LS mice was slightly elevated above SAL mice throughout the trial, but only reached a statistically significant difference at 3–4 min (P = 0.004), and there were no significant differences within the group across time bins.
Taken together, these data confirm the induction and expression of behavioural sensitization to EtOH in the HS group.
EBS for microdialysis mice
Figure 2 shows the microdialysis probe placements. Only mice with probes placed in the NAc were included in the final analyses. Figure 3 shows behavioural verification of EtOH sensitization for the cohort of mice that underwent microdialysis. For the induction data (Fig. 3a), a repeated measures ANOVA revealed significant main effects of treatment (F2,21 = 22.33, P < 0.001), test day (F3,63 = 44.29, P < 0.001), and test day by treatment interaction (F6,63 = 9.73, P < 0.001). Pairwise comparisons revealed significantly higher LMA for HS mice compared with SAL mice at baseline (Hab3; P = 0.046), injection 1 (P = 0.047), injection 3 (P < 0.001), and injection 5 (P < 0.001). HS mice also had significantly higher LMA compared with LS mice at baseline (P = 0.004), injection 3 (P < 0.001), and injection 5 (P < 0.001).
Microdialysis probe placement. In panel a, lines represent microdialysis probes. Only animals with probes placed in the NAc were included in the final analyses. The number at the top of each panel corresponds to the distance from bregma in millimetres according to the Franklin and Paxinos 2008 atlas. b Illustrative photomicrograph at ×10 showing the tip of a microdialysis probe in the NAc (arrow). The anterior commissure (aca) is labelled for reference
Behavioural data for mice that underwent microdialysis. a Induction of sensitization. HS = high-sensitized mice; LS = low-sensitized mice; SAL = saline controls. b, c Expression of sensitization. Total LMA (b) and 1-min bins of LMA (c) were analyzed. LMA was measured for 15 min. Values are means ± SEM. Group Ns are indicated in parentheses. Significant pairwise differences between SAL vs HS (*P < 0.05, **P < 0.01, ***P < 0.001), SAL vs LS (#P < 0.05, ##P < 0.01, ###P < 0.001), and LS vs HS (‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001) are indicated
Figure 3 b shows the expression of sensitization following a challenge dose of EtOH in half of the mice. The remaining mice in this cohort underwent cannulation and were only challenged during microdialysis sampling. One-way ANOVA did not reach statistical significance (F2,7 = 2.80, P = 0.128), despite HS LMA scores more than doubling SAL and LS LMA scores following EtOH challenge. For the expanded 1-min time bin assessment (Fig. 1c), a repeated measures ANOVA did not reveal significant main effects of treatment (F2,7 = 2.81, P = 0.13) or time bin by treatment interaction (F28,98 = 0.48, P = 0.99), but did reveal a significant main effect of time bin (F14,98 = 4.35, P < 0.001). Pairwise comparisons revealed significantly higher LMA for HS mice compared with SAL and LS mice at 6–7 min (P = 0.042 and P = 0.027, respectively) and 13–14 min (P = 0.018 and P = 0.029, respectively). Although the number of animals in this trial was low due to splitting of the cohort, overall, these behavioural data were consistent with the cohort of mice that was exclusively used to establish sensitization induction and expression. Behavioural data for all cohorts were also consistent with previous work from our group (Nona and Nobrega 2018).
Extracellular amino acid levels in the nucleus accumbens following an EtOH challenge
Aspartate
Extracellular aspartate levels after the EtOH challenge are shown in Fig. 4a. A repeated measures ANOVA revealed main effects of time (F12,108 = 13.39, P < 0.001), but not treatment (F2,9 = 0.32, P = 0.738) or the time by treatment interaction (F24,108 = 0.89, P = 0.610). Within-group comparisons indicated that the main effect of time was attributable to differences within acute and HS mice following EtOH injections. That is, aspartate levels from 0 to 5 min following EtOH injections (0-min time point) were significantly higher than levels from 45 min onward for both groups (P < 0.05; not shown in Fig. 4). However, levels of aspartate following EtOH injections did not significantly differ from any of the baseline collection values for any group.
Extracellular amino acid levels after EtOH challenge. “Acute” EtOH refers to mice treated with SAL during the induction phase and given EtOH for the first time during the challenge test. HS = high-sensitized mice; LS = low-sensitized mice. Arrow labelled “EtOH” represents the time point corresponding with EtOH injections. The X-axis represents the time point (in minutes) relative to EtOH injection time point. Values are means ± SEM. Group Ns are indicated in parentheses. Significant pairwise differences between SAL vs HS (*P < 0.05, **P < 0.01, ***P < 0.001), SAL vs LS (#P < 0.05, ##P < 0.01, ###P < 0.001), and LS vs HS (‡P < 0.05, ‡‡P < 0.01, ‡‡‡P < 0.001) are indicated
Glutamate
Extracellular glutamate levels after the EtOH challenge are shown in Fig. 4b. A repeated measures ANOVA revealed main effects of time (F12,108 = 30.39, P < 0.001) and the time by treatment interaction (F24,108 = 1.73, P = 0.030), but not treatment (F2,9 = 0.99, P = 0.407). HS mice had higher levels of accumbal glutamate than mice in the acute and LS groups at 0 min (P = 0.014, P = 0.008, respectively), and 5 min (P = 0.048, P = 0.047, respectively). There were no significant differences between LS mice and those acutely treated with EtOH at any time point. Within-group comparisons indicated that in acute and LS mice, EtOH induced an increase in glutamate immediately following injections (0 min) in comparison baseline (P < 0.05; not shown in Fig. 4). In HS mice, EtOH induced increases in glutamate from 0 to 10 min in comparison baseline levels (P < 0.05; not shown in Fig. 4).
Glycine
Extracellular glycine levels after the EtOH challenge are shown in Fig. 4c. A repeated measures ANOVA revealed main effects of time (F12,108 = 24.25, P < 0.001), treatment (F2,9 = 5.36, P = 0.029), and the time by treatment interaction (F24,108 = 11.53, P < 0.001). LS mice had higher levels of accumbal glycine than mice in the acute group at 5 min (P = 0.004), and a higher level of glycine than mice in the HS group at 5 min (P = 0.001) and 10 min (P = 0.010). At 30 min, glycine levels increased in the acute group relative to HS (P = 0.004) and LS (P = 0.009) mice, and remained elevated compared with HS mice at 45 min (P = 0.008) and 60 min (P = 0.030), and compared with LS mice at 45 min (P = 0.027). Within-group comparisons indicated that in acute mice, EtOH induced an increase in glycine at 0 min in comparison baseline, and this increase was significantly reversed at 15 min before increasing again at 30 min (P < 0.05; not shown in Fig. 4). In LS mice, EtOH induced increases in glycine from 0 to 10 min compared with baseline, and this increase was significantly reversed from 15 min onward (P < 0.05; not shown in Fig. 4). In HS mice, there were no significant changes in glycine levels throughout collection.
Taurine
Extracellular taurine levels after the EtOH challenge are shown in Fig. 4d. A repeated measures ANOVA revealed main effects of time (F12,108 = 26.93, P < 0.001) and the time by treatment interaction (F24,108 = 5.04, P < 0.001), but not treatment (F2,9 = 2.54, P = 0.133). Taurine levels in LS mice were significantly higher than in HS mice after EtOH challenge. Specifically, LS mice had higher levels of accumbal taurine than mice in the HS groups at 5 min (P = 0.015). There were no significant differences between HS mice and those acutely treated with EtOH at any time point. Within-group comparisons indicated that in acute mice, EtOH induced an increase in taurine at 5 min in comparison with values at 15 and 30 min (P < 0.05; not shown in Fig. 4), but not in comparison to baseline. In LS mice, EtOH induced a spike in taurine at 5 min that was significantly higher than baseline levels and all subsequent time points (P < 0.05; not shown in Fig. 4). In HS mice, there were no significant changes in glycine levels throughout collection.
GABA
Extracellular GABA levels after the EtOH challenge are shown in Fig. 4e. A repeated measures ANOVA revealed main effects of time (F12,108 = 21.98, P < 0.001) and the time by treatment interaction (F24,108 = 4.83, P < 0.001), but not treatment (F2,9 = 1.26, P = 0.329). Acute mice had higher levels of accumbal GABA compared with mice in the HS group at 0 min (P = 0.009), 5 min (P = 0.028), 15 min (P = 0.009), and 30 min (P = 0.023), and compared with mice in the LS group at 15 min (P = 0.004) and 30 min (P = 0.041). There were no significant differences between LS and HS mice at any time point. Within-group comparisons indicated that in acute mice, EtOH induced increases in GABA from 0 to 5 min in comparison baseline, and this increase was significantly reversed from 15 min onward (P < 0.05; not shown in Fig. 4). In LS and HS mice, EtOH induced increases in GABA at 15 min compared with baseline (P < 0.05; not shown in Fig. 4).
Cystine
Extracellular cystine levels after the EtOH challenge are shown in Fig. 4f. A repeated measures ANOVA revealed main effects of time (F12,108 = 3.20, P = 0.001), but not treatment (F2,9 = 0.27, P = 0.770) or the time by treatment interaction (F24,108 = 1.07, P = 0.387). Within-group comparisons did not detect significant differences for any group.
Effect of brucine on EtOH sensitization
Figure 5 illustrates the effects of brucine injections on the induction and expression of EBS as measured by LMA following EtOH injections. For the induction data (Fig. 5a), a repeated measures ANOVA with a Greenhouse-Geisser correction revealed significant main effects of treatment (F3,21 = 7.27, P = 0.002), test day (F2.45,51.49 = 14.75, P < 0.001), and test day by treatment interaction (F7.36,51.49 = 4.21, P = 0.001). Pairwise comparisons revealed significantly higher LMA for mice that received EtOH injections without brucine pre-treatment (VEH + EtOH) compared with mice that received brucine pre-treatment without EtOH injections (BRU + SAL) throughout the induction phase (P < 0.01). During injection 3, mice that received EtOH injections without brucine pre-treatment also had higher LMA compared with mice that did not receive either brucine pre-treatment or EtOH (VEH + SAL) (P = 0.04). It is noteworthy that results from injection 1 are not presented. During this trial, a higher dose of BRU (30 mg/kg) was used, which overtly depressed nearly all locomotion in mice despite previous reports in rats showing that 30 mg/kg of BRU does not affect LMA (Li et al. 2014). It was, therefore, decided that the dose of BRU would be halved to 15 mg/kg starting with injection 2.
Effect of brucine injections on behavioural sensitization induction and expression. a Induction of sensitization. b Expression of sensitization. The matrix below the bar graph clarifies the groups by indicating the presence (+) or absence (−) of vehicle, brucine, saline, and ethanol during the induction phase and expression (challenge) phase. VEH = vehicle, BRU = brucine, SAL = saline, EtOH = ethanol. Values are means ± SEM. Group Ns are indicated in parentheses. Significant pairwise differences between VEH + EtOH vs BRU + SAL (**P < 0.01), and VEH + EtOH vs VEH + SAL (#P < 0.05) are indicated
For the challenge data (Fig. 5b), a one-way ANOVA did not reveal a significant main effect of treatment (F3,2 = 1.98, P = 0.14).
Discussion
In the first part of the present study, we recapitulated our previous model of differential EBS by showing that subsets of EtOH-treated mice respond to the sensitization protocol with varying degrees of sensitivity (Nona and Nobrega 2018). As with earlier studies, the sensitization protocol began at 6 weeks of age during adolescence. This time course has previously been demonstrated to maximize the differential response to EtOH exposure since drug-induced neuroadaptive changes in reward circuitry is more pronounced during adolescence, with effects lasting into adulthood (Carrara-Nascimento et al. 2011). To this end, we showed that LS mice exhibit lower levels of LMA, which are not significantly different from saline controls, during the induction and expression phases of EBS compared with HS mice. Behavioural results from a cohort of mice that underwent microdialysis were consistent with these observations. In this cohort, we examined the profile of amino acid neurotransmitter activity in the NAc during the expression phase of sensitization. Longstanding observations have suggested an important role for the NAc during the expression phase of sensitization to EtOH and psychostimulants (Abrahao et al. 2011; Carrara-Nascimento et al. 2011; Kalivas 1995; Nona et al. 2015; Tzschentke and Schmidt 2003), whereas dopaminergic activity in the VTA appears to play a more pertinent role in sensitization induction (Vanderschuren and Kalivas 2000). In addition to accumbal dopamine transmission, the expression of sensitization to psychostimulants is dependent on amino acid transmission in the NAc, particularly glutamatergic (Kalivas 1995). In line with observations from other groups (Carrara-Nascimento et al. 2011), we have previously shown that accumbal glutamate is increased in HS mice during the expression, but not induction, phase of sensitization, and that blocking striatal synaptic glutamate release with LY354740 prevents the expression of behavioural sensitization in HS mice (Nona and Nobrega 2018). Additionally, NMDAR antagonism has been demonstrated to block the expression of sensitization to EtOH (Broadbent et al. 2003; Nona and Nobrega 2018).
Excitatory amino acid neurotransmitters
In line with the aforementioned evidence, our results showed a peak 184% increase in HS accumbal glutamate (compared with baseline) during the first 10 min after EtOH challenge, corresponding to the stimulant phase of EtOH. Interestingly, this pattern of glutamate elevation being most pronounced in the first few minutes following EtOH injections closely mirrored the behavioural data observed for this group (Fig. 1c). Although acutely treated and LS mice also showed an increase in glutamate immediately following EtOH injections (approximately 140%), the increase was not as persistent and was significantly lower than that seen in the HS group. Accumbal aspartate also showed a 140–170% increase in all groups. However, these increases did not reach statistical significance for any group, and groups did not differ from each other. This may be explained by the tenuous role of aspartate as a neurotransmitter and, by extension, the likely importance of aspartate in the adaptive changes leading to EBS. Indeed, it was recently demonstrated that glutamate alone was sufficient to account for all NMDA receptor activity and excitatory synaptic transmission in hippocampal CA1 pyramidal neurons (Herring et al. 2015), excluding a role for aspartate as a neurotransmitter in the CA1. Based on the results from the current study, further investigation into the possible role of aspartate may be warranted and we cannot rule out a contribution of aspartate signalling in the development of EBS.
Inhibitory amino acid neurotransmitters
During the stimulant phase EtOH, IAA neurotransmitters were generally elevated in LS mice compared with HS mice. In acutely treated mice, GABA showed a peak 413% increase compared with baseline immediately following EtOH injections. LS and HS mice exhibited peaks at 10 min, with LS mice showing a 423% increase above baseline and HS mice showing a 277% increase. During the first 10 min after injections, GABA levels differed between acutely treated mice and HS mice, but not LS mice, with acute values then dropping below both LS and HS mice at 15 to 30 min. Results from the acute group are consistent with the preponderance of evidence spanning decades of transgenic, in vitro, and electrophysiological research, which suggests that acute administration of EtOH potentiates GABAergic transmission, particularly through GABAA receptors (Lobo and Harris 2008; Weiner and Valenzuela 2006). However, it is noteworthy that in vivo microdialysis data examining acute EtOH-induced GABA release is sparse, with some data suggesting increased GABA release in the central amygdala (Roberto et al. 2004), and other data suggesting no change in accumbal GABA levels (Dahchour et al. 1994). To our knowledge, this is the first study to demonstrate a difference in accumbal GABA release between LS and HS mice in an EBS paradigm. EtOH self-administration in alcohol preferring rats has been shown to be attenuated with the GABAA antagonist bicuculline and the GABAB antagonist SCH 50911 (Ding et al. 2015). While this data suggests an important role for GABAergic transmission in the EtOH-seeking behaviour of alcohol-preferring rats, it is not directly applicable to the expression of EBS as these rats are selectively bred for alcohol preference rather than undergoing sensitization. Therefore, the selective breeding model may be more useful for examining innate difference that lead to differential alcohol preference, while our model is more useful in elucidating the adaptive differences that develop with repeated exposure.
Acutely treated mice showed a peak 354% increase in accumbal glycine immediately after EtOH injections, and a peak 395% increase in accumbal taurine at 5 min, although the latter did not reach statistical significance. LS mice showed a peak 552% increase in glycine and 676% increase in taurine at 5 min. By contrast, HS mouse levels of glycine did not significantly differ throughout collection for either glycine or taurine. HS glycine levels were lower than acute and LS levels during the first 10 min after EtOH injections, and HS taurine levels were lower than LS levels at 5 min. Similar to GABA, this is the first study to our knowledge that demonstrates a difference in accumbal glycine and taurine release between LS and HS mice in an EBS paradigm. Glycine and taurine, both of which act on strychnine-sensitive GlyRs, have been shown to be increased in the NAc in acute and chronic paradigms of EtOH injections and self-administration (Dahchour and De Witte 2000; Dahchour et al. 2000; Dahchour et al. 1994; Ericson et al. 2017; Li et al. 2008; Smith et al. 2004). Specifically, taurine elevation in the NAc appears to be a product of EtOH per se, while elevation in glycine may be related to anticipation of reward as demonstrated by a conditioned operant setting (Li et al. 2008). GlyRs in particular have been extensively studied for their role in alcohol dependence. These receptors are highly expressed by GABAergic medium spiny neurons (MSNs) in the NAc. The NAc and VTA project bidirectionally to form a NAc-VTA-NAc circuitry, with MSN GlyRs playing a critical regulatory role in dopaminergic feedback to the NAc (Adermark et al. 2011; Molander et al. 2005; Molander and Soderpalm 2005). EtOH-induced swelling of astrocytes in the NAc appears to be a primary mechanism of EtOH-induced taurine release (Adermark et al. 2011), while glycine is primarily released by glycinergic neurons and the level of glycine at excitatory and inhibitory synapses is controlled by glycine transporters (GlyTs) on neighbouring glial cells and pre-synaptic terminals (Harvey and Yee 2013). Interestingly, GlyTs have been successfully targeted in reducing relapse-like EtOH drinking in rats (Vengeliene et al. 2010). Inhibition of GlyRs by chronic BRU injections (30 mg/kg) has also been shown to reduce EtOH intake in EtOH-preferring rats (Li et al. 2014).
Effect of GlyR inhibition during EBS induction
To further investigate the role of GlyRs on the induction and expression of EBS, we conducted an experiment in a separate cohort of mice (Fig. 5). During the induction phase of EBS, mice were pre-treated with BRU prior to EtOH exposure, and their LMA scores were compared with mice that did not receive BRU pre-treatment. The effects of BRU alone on LMA were also assessed. Although mean LMA scores for BRU + EtOH mice were lower than those for VEH + EtOH mice throughout induction, these results did not reach statistical significance. This may be attributable to the large variations in LMA scores observed in the VEH + EtOH group, which for the purpose of this experiment did not distinguish between mice that variably sensitized to EtOH. The induction results were further complicated by the observation that BRU alone may acutely depress LMA, although no significant differences were observed between the VEH + SAL group and the BRU + SAL group.
During the expression phase of this experiment, all mice received a challenge dose of EtOH in the absence of BRU to test whether GlyR inhibition during the induction phase alone was sufficient to block the expression of EBS. Mice that were pre-treated with BRU (both with and without EtOH injections) as well as mice that did not receive BRU or EtOH during induction showed comparable LMA scores following a challenge dose of EtOH. Although the EtOH group that was not pre-treated with BRU (VEH + EtOH) showed nearly 40% higher LMA compared with the BRU + EtOH group, these results also did not reach statistical significance. Therefore, while GlyR antagonism during EBS induction may be a viable strategy for blocking the expression of EBS, we are unable to make strong conclusions based on the results of this experiment, and would strongly encourage further investigation with lower doses of BRU and larger numbers of animals.
Interaction of amino acid neurotransmitters in reward circuitry
Figure 6 summarizes pathways of the mesolimbic reward circuit that are relevant to the present NAc microdialysis results. Previous studies have suggested that acute EtOH treatment non-competitively inhibits NMDAR activation, while chronic treatment produces an over-compensatory effect leading to increased NMDAR expression and activity (Clapp et al. 2018). Furthermore, our previous work has suggested that chronic EtOH-induced increases in NMDAR expression across several brain regions is more strongly associated with low-sensitizers, compared with high-sensitizers, during the induction phase of EBS (Nona et al. 2014). Differences in NMDAR gene expression were largely absent between low- and high-sensitizers 2 weeks after the last induction phase injection, consistent with the observation that such changes appear to be short-lived, lasting only 24–48 h (Gulya et al. 1991). Despite this, protein-level surface expressions of NR1 subunits have been reported to be decreased in the NAc of HS mice 2 weeks following the last induction phase injection (Abrahao et al. 2013). In the present study, as with our previous observations, accumbal glutamate levels in HS mice were higher than in acutely treated and LS mice following an EtOH challenge during the expression phase of EBS. Taken together, these data suggest that the increased glutamate release in HS mice may represent a neuroadaptive response to decreased NMDAR surface expression and reduced glutamate responsivity. Furthermore, the lack of any differences in accumbal cystine levels between groups suggests that local glutamate transport to the extracellular space likely does not contribute to the observed changes in glutamate levels. This transport is regulated by the system xc− antiporter on neighbouring glial cells, which releases glutamate in exchange for cystine uptake in a 1:1 ratio (Miladinovic et al. 2015). Therefore, if system xc− glutamate export contributed to our observed changes in EtOH-induced glutamate levels, we would expect to observe decreased extracellular cystine levels corresponding with increased glutamate release, which is not evident. These data are consistent with recent evidence, which more directly demonstrated that system xc− does not contribute to the observed glutamate elevation in the NAc of EtOH-dependent mice (Griffin et al. 2015). However, we cannot rule out the possibility that altered expression or activity of astrocytic excitatory amino acid transporters (EAATs), which regulate the uptake of extracellular glutamate from the synapse, contributed to our observed changes in glutamate levels. While some evidence suggests that EAATs do not contribute to this change (Griffin et al. 2015), conflicting data suggests that deficits in glutamate re-uptake may contribute to EtOH-induced increases in accumbal glutamate levels (Melendez et al. 2005). Therefore, while hyperexcitability of corticostriatal pathways is well documented in behavioural sensitization to ethanol and other drugs (Vanderschuren and Kalivas 2000; Wolf 1998), further exploration into local accumbal changes may be warranted.
Summary of reward circuitry relevant to EtOH sensitization. This figure illustrates a simplified schematic of the major systems and connections involved in reward as it relates to EtOH sensitization. D1- and D2-type medium spiny neurons (MSNs) in the nucleus accumbens (NAc) receive direct glutamatergic innervation from the prefrontal cortex (PFC), as well as other regions including the hippocampus and amygdala (not shown). Within the NAc, various types of GABAergic interneurons innervate MSNs. MSNs also receive input from glycinergic neurons and from taurine released by glial cells. MSNs projections innervate dopaminergic neurons in the ventral tegmental area (VTA), which also receive input from PFC glutamatergic projections and local GABAergic interneurons. These dopaminergic neurons innervate several brain regions, including MSNs of the NAc, creating bi-directional interaction between the NAc and VTA. In the figure, boxes with dashed borders summarize the NAc microdialysis results for mice acutely treated with EtOH, low- and high-sensitized mice (LS and HS, respectively). Note: this schematic does not exhaustively represent all sources of the depicted amino acids (e.g. astrocyte regulation of synaptic amino acids is not depicted). This figure is meant to visually highlight major reward circuitry projections and contextualize the current findings within this circuitry
EBS is associated with increased expression of α1, β2, and γ2 subunits of GABAA receptors in the NAc of sensitized mice (Linsenbardt and Boehm 2010). These changes are not observed in the VTA, suggesting that these adaptations may be particularly relevant to the expression phase of EBS. Although these data do not differentiate between low- and high-sensitizers, our data demonstrated attenuation of accumbal GABA levels in the NAc of HS mice during EBS expression. Since dopaminergic regulation of the NAc via VTA projections plays a critical role in the locomotor response to rewarding stimuli (Tran et al. 2005), we suspect that accumbal GABAergic input into the VTA would be reduced in HS mice relative to LS mice, leading to increased locomotor response to EtOH. That is, accumbal MSN activity would likely be attenuated in HS mice. Therefore, our observed relative attenuation of GABA release in HS mice may be in response increased surface expression and/or responsiveness of GABAA receptor subunits in these mice. Further investigation into the differential expression of GABA receptor subunits in LS versus HS mice is warranted to elucidate these adaptive changes.
Similar to GABA, the inhibitory neurotransmitters glycine and taurine were attenuated in HS mice following EtOH challenge. Although other models have failed to demonstrate a direct effect of ethanol on accumbal glycine levels (Adermark et al. 2011), or suggested a role for glycine in the anticipation of reward rather than as a direct result of rewarding stimuli (Li et al. 2008), our microdialysis results suggest a direct and differential response in accumbal glycine for high- versus low-sensitizers. Similar results were observed with taurine, which unlike glycine, has previously been shown to respond directly to EtOH administration (Adermark et al. 2011; Dahchour et al. 1996; Dahchour et al. 1994). These results are particularly noteworthy as a growing body of evidence is suggesting that glycinergic regulation of accumbal MSNs is critical to the dopaminergic feedback by the VTA (Molander and Soderpalm 2005). It would, therefore, be instructive to further investigate changes in GlyR subunit expression in the NAc. In line with our rationale for GABA, we would suspect that the observed relative attenuation in glycine and taurine levels in HS mice is in response to increased expression and/or responsiveness of GlyRs on accumbal MSNs.
Study Limitations
For the microdialysis portion of the present study, 4 mice per group were assessed. Caution is, therefore, advised when interpreting the microdialysis results. In particular, some of the subtler differences we observed, which in this study did not reach statistical significance (e.g. aspartate), would benefit from further investigation using larger group numbers. It should be noted, however, that we have previously demonstrated statistically significant differences in accumbal glutamate after EtOH challenge using only 2–3 mice per group (Nona and Nobrega 2018). Microdialysis is highly sensitive to detecting small neurochemical changes and is often performed with 3–6 animals per group (DeVos et al. 2013; Ulrich et al. 2013; Xie et al. 1999). It should also be noted that during sensitization, mice were placed in their home cages after EtOH injections (in the testing room) on days when they were not being tested for locomotor activity. Although this protocol provided robust LMA and neurochemical results, we would encourage placing mice in the LMA test boxes after all injections in future experiments to avoid possible influences of different conditioning contexts. Future experiments interested in absolute levels of amino acids, rather than percent group differences, would further benefit from the inclusion of a saline-only group during the expression phase of sensitization to account for any effects of injection stress on amino acid levels. In the current experiment, any effect of injection stress was assumed to be similar between groups, and therefore, any remaining differences between groups were attributed to treatment and sensitization variance. Additionally, performing behavioural and neurochemical measures during the dark phase would mitigate any possible influence of EtOH injections on the circadian rhythm during the light cycle.
Another possible limitation of the present study stems from practical considerations of the microdialysis sampling schedule. Specifically, the 12 mice included in this experiment underwent EtOH challenge and microdialysis over a 6-day span. However, we do not anticipate that this would have substantially impacted the results. We have previously seen that the sensitization behaviour is robust and persists for months following the last sensitization injection (unpublished data). Since the full sampling protocol requires approximately 5 h and our microdialysis system accommodates 2 mice at a time, opting to sample from 2 mice per day over 6 days prioritizes the elimination of possible confounding effects of circadian fluctuation in neurotransmitters, versus condensing the span of the sampling period. Furthermore, sampling was randomized such that groups were equated across the 6-day sampling period as much as possible.
Summary
We have reproduced our previous results demonstrating a differential glutamatergic response in the accumbens of high- versus low-sensitized mice. We have also built on these results by investigating other excitatory and inhibitory amino acid neurotransmitter response in the accumbens of these mice. Our results suggest that EtOH challenge induces greater excitatory, and attenuated inhibitory neurotransmitter levels in the accumbens of high-sensitizers. We suspect that these changes are compensatory responses to decreased expression and/or responsiveness of NMDAR, and increased expression and/or responsiveness of GABAA and GlyRs on the surface of accumbal MSNs. Therefore, neuroadaptations that increase excitation or attenuate inhibition of MSNs in the presence of an EtOH challenge may code for the low-sensitizing phenotype and illuminate novel targets to treat AUD.
References
Abrahao KP, Quadros IM, Souza-Formigoni ML (2011) Nucleus accumbens dopamine D(1) receptors regulate the expression of ethanol-induced behavioural sensitization. Int J Neuropsychopharmacol 14:175–185. https://doi.org/10.1017/S1461145710000441
Abrahao KP, Quadros IM, Andrade AL, Souza-Formigoni ML (2012) Accumbal dopamine D2 receptor function is associated with individual variability in ethanol behavioral sensitization. Neuropharmacology 62:882–889. https://doi.org/10.1016/j.neuropharm.2011.09.017
Abrahao KP, Ariwodola OJ, Butler TR, Rau AR, Skelly MJ, Carter E, Alexander NP, McCool BA, Souza-Formigoni MLO, Weiner JL (2013) Locomotor sensitization to ethanol impairs NMDA receptor-dependent synaptic plasticity in the nucleus accumbens and increases ethanol self-administration. J Neurosci 33:4834–4842. https://doi.org/10.1523/JNEUROSCI.5839-11.2013
Abrahao KP, Goeldner FO, Souza-Formigoni ML (2014) Individual differences in ethanol locomotor sensitization are associated with dopamine D1 receptor intra-cellular signaling of DARPP-32 in the nucleus accumbens. PLoS One 9:e98296. https://doi.org/10.1371/journal.pone.0098296
Adermark L, Clarke RB, Olsson T, Hansson E, Soderpalm B, Ericson M (2011) Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict Biol 16:43–54. https://doi.org/10.1111/j.1369-1600.2010.00206.x
Broadbent J, Harless WE (1999) Differential effects of GABA(A) and GABA(B) agonists on sensitization to the locomotor stimulant effects of ethanol in DBA/2. J Mice Psychopharmacology (Berl) 141:197–205
Broadbent J, Weitemier AZ (1999) Dizocilpine (MK-801) prevents the development of sensitization to ethanol in DBA/2J mice. Alcohol Alcohol 34:283–288
Broadbent J, Kampmueller KM, Koonse SA (2003) Expression of behavioral sensitization to ethanol by DBA/2J mice: the role of NMDA and non-NMDA glutamate receptors. Psychopharmacology 167:225–234. https://doi.org/10.1007/s00213-003-1404-3
Camarini R, Frussa-Filho R, Monteiro MG, Calil HM (2000) MK-801 blocks the development of behavioral sensitization to the ethanol. Alcohol Clin Exp Res 24:285–290
Camarini R, Marcourakis T, Teodorov E, Yonamine M, Calil HM (2011) Ethanol-induced sensitization depends preferentially on D1 rather than D2 dopamine receptors. Pharmacol Biochem Behav 98:173–180. https://doi.org/10.1016/j.pbb.2010.12.017
Carrara-Nascimento PF, Griffin WC 3rd, Pastrello DM, Olive MF, Camarini R (2011) Changes in extracellular levels of glutamate in the nucleus accumbens after ethanol-induced behavioral sensitization in adolescent and adult mice. Alcohol 45:451–460. https://doi.org/10.1016/j.alcohol.2011.01.002
Chatterjee D, Shams S, Gerlai R (2014) Chronic and acute alcohol administration induced neurochemical changes in the brain: comparison of distinct zebrafish populations. Amino Acids 46:921–930. https://doi.org/10.1007/s00726-013-1658-y
Clapp PF, Bhave SF, Hoffman PL (2018) How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health 31:310–339
Crabbe JC Jr, Johnson NA, Gray DK, Kosobud A, Young ER (1982) Biphasic effects of ethanol on open-field activity: sensitivity and tolerance in C57BL/6N and DBA/2N mice. J Comp Physiol Psychol 96:440–451
Dahchour A, De Witte P (2000) Ethanol and amino acids in the central nervous system: assessment of the pharmacological actions of acamprosate. Prog Neurobiol 60:343–362
Dahchour A, Quertemont E, Dewitte P (1994) Acute ethanol increases taurine but neither glutamate nor Gaba in the nucleus-accumbens of male-rats a microdialysis study. Alcohol Alcohol 29:485–487
Dahchour A, Quertemont E, De Witte P (1996) Taurine increases in the nucleus accumbens microdialysate after acute ethanol administration to naive and chronically alcoholised rats. Brain Res 735:9–19
Dahchour A, Hoffman A, Deitrich R, de Witte P (2000) Effects of ethanol on extracellular amino acid levels in high-and low-alcohol sensitive rats: a microdialysis study. Alcohol Alcohol 35:548–553
DeVos SL et al (2013) Antisense reduction of tau in adult mice protects against seizures. J Neurosci 33:12887–12897. https://doi.org/10.1523/JNEUROSCI.2107-13.2013
Ding ZM, Ingraham CM, Rodd ZA, McBride WJ (2015) The reinforcing effects of ethanol within the nucleus accumbens shell involve activation of local GABA and serotonin receptors. J Psychopharmacol 29:725–733. https://doi.org/10.1177/0269881115581982
Ericson M, Ulenius L, Adermark L, Soderpalm B (2017) Minor adaptations of ethanol-induced release of taurine following chronic ethanol intake in the rat. Adv Exp Med Biol 975(Pt 1):217–224. https://doi.org/10.1007/978-94-024-1079-2_19
Ferrario CR, Robinson TE (2007) Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol 17:352–357. https://doi.org/10.1016/j.euroneuro.2006.08.005
Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT, Cunningham CL (2011) Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav 10:264–275. https://doi.org/10.1111/j.1601-183X.2010.00664.x
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC (2015) Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol 6:27. https://doi.org/10.3389/fphar.2015.00027
Gulya K, Grant KA, Valverius P, Hoffman PL, Tabakoff B (1991) Brain regional specificity and time-course of changes in the NMDA receptor-ionophore complex during ethanol withdrawal. Brain Res 547:129–134
Harrison SJ, Nobrega JN (2009) Differential susceptibility to ethanol and amphetamine sensitization in dopamine D3 receptor-deficient mice. Psychopharmacology 204:49–59. https://doi.org/10.1007/s00213-008-1435-x
Harvey RJ, Yee BK (2013) Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat Rev Drug Discov 12:866–885. https://doi.org/10.1038/nrd3893
Herring BE, Silm K, Edwards RH, Nicoll RA (2015) Is aspartate an excitatory neurotransmitter? J Neurosci 35:10168–10171. https://doi.org/10.1523/JNEUROSCI.0524-15.2015
Hitzemann B, Hitzemann R (1997) Genetics ethanol and the Fos response: a comparison of the C57BL/6J and DBA/2J inbred mouse strains. Alcohol Clin Exp Res 21:1497–1507
Holmes A, Spanagel R, Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology 229:539–554. https://doi.org/10.1007/s00213-013-3226-2
Hu XJ, Ticku MK (1995) Chronic ethanol treatment upregulates the NMDA receptor function and binding in mammalian cortical neurons. Brain Res Mol Brain Res 30:347–356
Juarez B, Morel C, Ku SM, Liu Y, Zhang H, Montgomery S, Gregoire H, Ribeiro E, Crumiller M, Roman-Ortiz C, Walsh JJ, Jackson K, Croote DE, Zhu Y, Zhang S, Vendruscolo LF, Edwards S, Roberts A, Hodes GE, Lu Y, Calipari ES, Chaudhury D, Friedman AK, Han MH (2017) Midbrain circuit regulation of individual alcohol drinking behaviors in mice. Nat Commun 8:2220. https://doi.org/10.1038/s41467-017-02365-8
Kai N, Nishizawa K, Tsutsui Y, Ueda S, Kobayashi K (2015) Differential roles of dopamine D1 and D2 receptor-containing neurons of the nucleus accumbens shell in behavioral sensitization. J Neurochem 135:1232–1241. https://doi.org/10.1111/jnc.13380
Kalivas PW (1995) Interactions between dopamine and excitatory amino acids in behavioral sensitization to psychostimulants. Drug Alcohol Depend 37:95–100
Kalivas PW, Stewart J (1991) Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev 16:223–244
Koob GF (2004) A role for GABA mechanisms in the motivational effects of alcohol. Biochem Pharmacol 68:1515–1525. https://doi.org/10.1016/j.bcp.2004.07.031
Kruse LC, Linsenbardt DN, Boehm SL 2nd (2012) Positive allosteric modulation of the GABA(B) receptor by GS39783 attenuates the locomotor stimulant actions of ethanol and potentiates the induction of locomotor sensitization. Alcohol 46:455–462. https://doi.org/10.1016/j.alcohol.2012.03.004
Krystal JH, Petrakis IL, Krupitsky E, Schutz C, Trevisan L, D'Souza DC (2003) NMDA receptor antagonism and the ethanol intoxication signal: from alcoholism risk to pharmacotherapy. Ann N Y Acad Sci 1003:176–184
Leyton M (2007) Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuro-Psychopharmacol Biol Psychiatry 31:1601–1613. https://doi.org/10.1016/j.pnpbp.2007.08.027
Li Z, Zharikova A, Bastian J, Esperon L, Hebert N, Mathes C, Rowland NE, Peris J (2008) High temporal resolution of amino acid levels in rat nucleus accumbens during operant ethanol self-administration: involvement of elevated glycine in anticipation. J Neurochem 106:170–181. https://doi.org/10.1111/j.1471-4159.2008.05346.x
Li YL, Liu Q, Gong Q, Li JX, Wei SP, Wang YT, Liang H, Zhang M, Jing L, Yong Z, Lawrence AJ, Liang JH (2014) Brucine suppresses ethanol intake and preference in alcohol-preferring fawn-hooded rats. Acta Pharmacol Sin 35:853–861. https://doi.org/10.1038/aps.2014.28
Linsenbardt DN, Boehm SL 2nd (2010) Ethanol-induced locomotor sensitization in DBA/2J mice is associated with alterations in GABA(A) subunit gene expression and behavioral sensitivity to GABA(A) acting drugs. Pharmacol Biochem Behav 95:359–366. https://doi.org/10.1016/j.pbb.2010.02.014
Lobo IA, Harris RA (2008) GABA(A) receptors and alcohol. Pharmacol Biochem Behav 90:90–94. https://doi.org/10.1016/j.pbb.2008.03.006
Lovinger DM, White G, Weight FF (1989) Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science (New York, NY) 243:1721–1724
McCool BA, Chappell AM (2014) Persistent enhancement of ethanol drinking following a monosodium glutamate-substitution procedure in C57BL6/J and DBA/2J mice. Alcohol 48:55–61. https://doi.org/10.1016/j.alcohol.2013.10.008
Melendez RI, HM P, CS S, Kalivas PW (2005) Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res 29:326–333
Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL (1997) Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature 389:385–389. https://doi.org/10.1038/38738
Miladinovic T, Nashed MG, Singh G (2015) Overview of glutamatergic dysregulation in central pathologies. Biomolecules 5:3112–3141. https://doi.org/10.3390/biom5043112
Molander A, Soderpalm B (2005) Accumbal strychnine-sensitive glycine receptors: an access point for ethanol to the brain reward system. Alcohol Clin Exp Res 29:27–37
Molander A, Lof E, Stomberg R, Ericson M, Soderpalm B (2005) Involvement of accumbal glycine receptors in the regulation of voluntary ethanol intake in the rat. Alcohol Clin Exp Res 29:38–45
Nona CN, Nobrega JN (2018) A role for nucleus accumbens glutamate in the expression but not the induction of behavioural sensitization to ethanol. Behav Brain Res 336:269–281. https://doi.org/10.1016/j.bbr.2017.09.024
Nona CN, Guirguis S, Nobrega JN (2013) Susceptibility to ethanol sensitization is differentially associated with changes in pCREB, trkB and BDNF mRNA expression in the mouse brain. Behav Brain Res 242:25–33. https://doi.org/10.1016/j.bbr.2012.12.035
Nona CN, Li R, Nobrega JN (2014) Altered NMDA receptor subunit gene expression in brains of mice showing high vs. low sensitization to ethanol. Behav Brain Res 260:58–66. https://doi.org/10.1016/j.bbr.2013.11.037
Nona CN, Creed MC, Hamani C, Nobrega JN (2015) Effects of high-frequency stimulation of the nucleus accumbens on the development and expression of ethanol sensitization in mice. Behav Pharmacol 26:184–192. https://doi.org/10.1097/FBP.0000000000000033
Nona CN, Lam M, Nobrega JN (2016) Localized brain differences in arc expression between mice showing low vs. high propensity to ethanol sensitization. Pharmacol Biochem Behav 142:15–22. https://doi.org/10.1016/j.pbb.2015.12.006
Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR (2004) Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci 24:10159–10166. https://doi.org/10.1523/JNEUROSCI.3004-04.2004
Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291
Robinson TE, Berridge KC (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond Ser B Biol Sci 363:3137–3146. https://doi.org/10.1098/rstb.2008.0093
Rossetti ZL, Carboni S (1995) Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. Eur J Pharmacol 283:177–183. https://doi.org/10.1016/0014-2999(95)00344-K
Segal DS, Mandell AJ (1974) Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav 2:249–255
Smith A, Watson CJ, Frantz KJ, Eppler B, Kennedy RT, Peris J (2004) Differential increase in taurine levels by low-dose ethanol in the dorsal and ventral striatum revealed by microdialysis with on-line capillary electrophoresis. Alcohol Clin Exp Res 28:1028–1038
Souza-Formigoni MLO, De Lucca EM, Hipolide DC, Enns SC, Oliveira MGM, Nobrega JN (1999) Sensitization to ethanol's stimulant effect is associated with region-specific increases in brain D2 receptor binding. Psychopharmacology 146:262–267. https://doi.org/10.1007/s002130051115
Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Ono T (2005) Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci U S A 102:2117–2122. https://doi.org/10.1073/pnas.0409726102
Tsai GE, Ragan P, Chang R, Chen S, Linnoila VM, Coyle JT (1998) Increased glutamatergic neurotransmission and oxidative stress after alcohol withdrawal. Am J Psychiatry 155:726–732. https://doi.org/10.1176/ajp.155.6.726
Tzschentke TM, Schmidt WJ (2003) Glutamatergic mechanisms in addiction. Mol Psychiatry 8:373–382. https://doi.org/10.1038/sj.mp.4001269
Ulrich JD, Burchett JM, Restivo JL, Schuler DR, Verghese PB, Mahan TE, Landreth GE, Castellano JM, Jiang H, Cirrito JR, Holtzman DM (2013) In vivo measurement of apolipoprotein E from the brain interstitial fluid using microdialysis. Mol Neurodegener 8:13. https://doi.org/10.1186/1750-1326-8-13
Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M (2010) Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry 67:1069–1077. https://doi.org/10.1001/archgenpsychiatry.2010.125
Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology 151:99–120
Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R (2010) Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry 68:704–711. https://doi.org/10.1016/j.biopsych.2010.05.029
Vezina P, Leyton M (2009) Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology 56(Suppl 1):160–168. https://doi.org/10.1016/j.neuropharm.2008.06.070
Weiner JL, Valenzuela CF (2006) Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther 111:533–554. https://doi.org/10.1016/j.pharmthera.2005.11.002
Wolf ME (1998) The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54:679–720
Xie R, Hammarlund-Udenaes M - de Boer AG, de Boer AG de Lange EC, de Lange EC (1999) The role of P-glycoprotein in blood-brain barrier transport of morphine: transcortical microdialysis studies in mdr1a (−/−) and mdr1a (+/+) mice Br J Pharmacol 128:563–568
Acknowledgements
The authors thank Dr. Christina Nona and Roger Raymond for excellent technical assistance.
Funding
This study was supported by NSERC grant RGPIN 06615.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were approved by the Animal Care Committee at the Centre for Addiction and Mental Health and were in accordance with the guidelines and practices outlined by the Canadian Council on Animal Care.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nashed, M.G., Chatterjee, D., Nguyen, D. et al. Ethanol-induced changes in synaptic amino acid neurotransmitter levels in the nucleus accumbens of differentially sensitized mice. Psychopharmacology 236, 3541–3556 (2019). https://doi.org/10.1007/s00213-019-05324-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05324-x