Abstract
Rationale
Substance use disorders are characterized by a loss of executive control over reward-based decision-making, and disruption of fronto-striatal connectivity has been implicated in this process. Sub-anesthetic ketamine has recently been shown to bolster fronto-striatal connectivity in drug-naïve subjects.
Objectives
The influence of ketamine treatment was examined on the disruptive effects of cocaine on functional connectivity (FC) and on cocaine-seeking behavior in female rhesus monkeys.
Methods
Three female rhesus were trained for unanesthetized MRI scanning. Each received three drug-naïve/abstinent pharmacological MRI scans with acute injections of saline, cocaine (0.3 mg/kg i.v.), and cocaine (0.3 mg/kg i.v.) 48-h after a ketamine treatment (low dose = 0.345 mg/kg bolus + 0.256 mg/kg/h for 1 h; i.v.), and a fourth scan with saline injection following 2 months of daily cocaine self-administration. A separate cohort of five rhesus (4 female), all with extensive histories of cocaine exposure, underwent reinstatement testing 48 h after ketamine (or vehicle) treatment. Two sub-anesthetic doses were tested: low dose and high dose = 0.69 mg/kg + 0.512 mg/kg/h for 1 h.
Results
Ketamine treatment attenuated the effects of cocaine on both global and fronto-striatal FC in drug-naïve/abstinent subjects. Two months of daily cocaine self-administration led to prolonged disruption of both global and fronto-striatal FC. Cocaine-seeking behavior during reinstatement was reduced following ketamine treatment at the low dose, but not high dose.
Conclusion
These findings illustrate the disruptive effects of cocaine on functional connectivity and provide evidence for the potential efficacy of ketamine as a treatment for stimulant use disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The global burden of psychostimulant abuse is substantial, with estimates of prevalence numbering in the tens of millions (Degenhardt et al. 2014). Substance use disorders are chronic illnesses (McLellan et al. 2000), characterized by repeated relapse and an inability to control use despite negative consequences (Hasin et al. 2013). The transition from initial use to compulsive use (Everitt and Robbins 2005; Kalivas and O’Brien 2008) is related to a loss of control over drug-seeking and intake (George and Koob 2010; Jentsch and Taylor 1999) that has been attributed, in part, to the dysfunction of the prefrontal cortex (PFC) (Goldstein and Volkow 2011) and fronto-striatal circuitry (Jentsch and Taylor 1999; Koob and Volkow 2010). The pathways linking prefrontal regions to the reward processing regions of the striatum are well characterized (Haber and Knutson 2010), and aberrant processing specifically by glutamatergic neurons connecting the PFC to the nucleus accumbens (NAcc) has been implicated in substance use disorders (Kalivas 2009; Kalivas et al. 2005).
Translational research in animal models provides further evidence for the importance of fronto-striatal circuitry in regulating substance use behaviors. A recent functional magnetic resonance imaging (fMRI) study in unanesthetized rhesus monkeys demonstrated that an acute i.v. dose of cocaine robustly decreased functional connectivity (FC) between the dorsolateral PFC (dlPFC) and the NAcc (Murnane et al. 2015). Furthermore, cocaine intake during self-administration was negatively correlated with the baseline FC between dlPFC and NAcc (Murnane et al. 2015), indicating dlPFC-NAcc FC as a possible biomarker for vulnerability to substance use disorders. Behavioral studies in rodents further suggest that neuroplasticity within this circuitry plays a key role in drug dependence, as the transition from initial drug exposure to compulsive drug seeking is associated with deficits in synaptic plasticity in both the rodent PFC (DePoy and Gourley 2015; Pitts et al. 2016) and NAcc (Kasanetz et al. 2010; Martin et al. 2006). These findings have led to the hypothesis that new medications affecting neuroplasticity in PFC-NAcc circuitry could be beneficial for treating substance use disorders (Kalivas and Volkow 2011). One potential candidate drug is ketamine.
Sub-anesthetic ketamine produces rapid antidepressant effects in clinical studies (Zarate et al. 2006), and there are corresponding antidepressant-like effects in rodent behavioral models that are mediated by neuroplastic changes in the PFC (Kavalali and Monteggia 2012; Li et al. 2010). Ketamine has been shown to induce changes in functional brain networks (Maltbie et al. 2017), including increases to FC between dlPFC and NAcc in rhesus monkeys (Gopinath et al. 2016). This effect is in direct opposition to robust decreases to FC between dlPFC and accumbens observed during acute administration of cocaine (Murnane et al. 2015). Two recent pilot studies in cocaine-dependent (human) subjects indicate that ketamine treatment reduces cue-induced cocaine craving while increasing motivation to quit (Dakwar et al. 2014) and also decreases cocaine choice and self-reported cocaine intake (Dakwar et al. 2016). Thus, further investigation of ketamine as a potential treatment for cocaine addiction is warranted (see Ivan Ezquerra-Romano et al. (2018) for further review).
The present study investigates the use of ketamine as a treatment for cocaine dependence utilizing a combination of neuroimaging and behavioral pharmacology in a translational nonhuman primate model. Drug-naïve (or abstinent) rhesus monkey subjects were extensively trained to undergo unanesthetized (awake and unsedated) fMRI, enabling investigation of the interaction of sub-anesthetic ketamine infusion with the effects of cocaine on functional brain networks. The effects of cocaine on FC were examined with and without ketamine treatment 2 days prior. Following these initial scanning experiments, the subjects were trained to perform cocaine self-administration to evaluate the effects of chronic cocaine exposure on FC.
The effects of ketamine treatment on cocaine self-administration behavior were further examined in a separate cohort of rhesus monkeys, each with an extensive history of exposure to psychostimulant drugs of abuse. A second-order schedule of cocaine reinforcement and reinstatement procedures were used to investigate the effects of ketamine on drug-seeking behavior. The second-order schedule of reinforcement was utilized to emphasize drug-associated conditioned stimuli and to produce high behavioral output from a limited amount of drug reinforcement, minimizing any indirect effects of the drug and ensuring a direct correspondence between the response rate and the reinforcing effects of the drug (Howell and Fantegrossi 2009). Reinstatement was used as a behavioral model for relapse. While the translational validity as a model for drug relapse in humans has yet to be firmly established (Epstein et al. 2006; Katz and Higgins 2003), reinstatement procedures are useful for measuring drug-seeking behaviors in the absence of active drug reinforcement.
Materials and methods
Neuroimaging experiment
Subjects
The subjects were three adult female rhesus monkeys (Macaca mulatta). Two of the subjects (Ry7 and Mm7) had no previous history of exposure to cocaine or sub-anesthetic ketamine. The third subject (Jz6) had been trained to undergo daily i.v. cocaine self-administration for a previous study but had no exposure to cocaine during the 3 years prior to the start of the present study and no prior history of exposure to sub-anesthetic ketamine infusion. All protocols and animal care and handling strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, revised 2011) (National Research Council (USA). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. et al. 2011) and the recommendations of the American Association for Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee of Emory University.
Surgery and habituation to MRI
A complete description of the surgery and habituation protocol employed in this study has been described in full detail previously (Murnane and Howell 2010). Briefly, each subject was surgically implanted with a chronic indwelling venous catheter attached to a subcutaneous vascular access port prior to being habituated to MRI for the present study. Following surgery, all subjects were gradually habituated to the scanning apparatus, environment, and all procedures necessary for these experiments over a period of greater than 1 year.
Ketamine treatment
Ketamine treatments were performed 2 days (~ 48 h) prior to MRI scanning and occurred with each subject seated in a standard primate chair (primate products) while in an open laboratory environment under the supervision of the experimenter. The dosing regimen was identical to what was used for previously published imaging experiments (Gopinath et al. 2016; Maltbie et al. 2016) and consisted of a 1-min i.v. bolus of 0.345 mg/kg followed by a 1-h constant i.v. infusion of 0.256 mg/kg/h.
MRI data acquisition
The MRI imaging methods employed were described in full detail previously (Maltbie et al. 2016). Briefly, the monkeys lay prone in a custom-built restraint cradle (Murnane and Howell 2010) attached to a head coil designed specifically for rhesus monkeys. In each scanning session, BOLD MRI images were collected utilizing a whole-brain gradient echo single-shot echo planar imaging (EPI) sequence (TR/TE/FA = 2400 ms/27 ms/90°; 1.5 × 1.5 × 1.5 mm; resolution; 1200 measurements). A low-resolution T1-weighted (T1w) anatomic scan was acquired using a 3D MPRAGE sequence (TR/TE/TI/FA = 2300 ms/2.7 ms/800 ms/8°; 1.5 × 1.5 × 1.5 mm resolution) to assist in spatial normalization. Further, for each subject, a high-resolution (0.5 × 0.5 × 0.5 mm) T1w 3D MPRAGE anatomic scan (with 6 averages) was acquired in a separate scanning session to provide a high-quality anatomic image for anatomic reference and spatial normalization.
Drug infusion protocol
EPI scans were 48 min in duration and consisted of an 8-min baseline period prior to an i.v. infusion of 0.3 mg/kg of cocaine (or saline control) and followed by 40-min of continued scanning. Cocaine scans were performed alone first, then following a sub-anesthetic ketamine treatment 48 h prior to the cocaine scans. The dose of cocaine has been shown to produce significant effects on FC previously (Murnane et al. 2015) and the 40-min time-course was chosen because this duration is sufficient to capture the onset, peak, and offset of the neuropharmacological effects of cocaine in rhesus monkeys (Banks et al. 2009). Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (Bethesda, MD, USA) and dissolved in physiological saline.
fMRI data quality control
The fMRI image time-series data were examined for large motions defined as more than 0.5 mm frame-to-frame displacement. Exclusion criteria were defined as motion above this threshold in more than 10% of the fMRI volumes. None of the scans acquired for these experiments exceeded this threshold, and thus no scanning sessions were discarded or repeated.
fMRI preprocessing and spatial normalization
Methods for fMRI data analysis in nonhuman primates have been described in depth previously (Gopinath et al. 2016). For the present study, all analysis was conducted with AFNI (Cox 1996) and FSL (Smith et al. 2004) software packages as well as in-house Matlab™ (Natick, MA) scripts. The fMRI time-series images were corrected for field inhomogeneities, temporally shifted, and registered to a base volume. The fMRI time-series were then aligned (Gopinath et al. 2016) to the INIA19 rhesus monkey template atlas (Rohlfing et al. 2012). After registration to the atlas, sudden large jumps (spikes) in the voxel fMRI signal time-series were removed using the AFNI 3dDespike tool. Finally, the denoised EPI time-series were spatially smoothed with a full-width at half-maximum (FWHM) = 3 mm isotropic Gaussian filter.
FC analysis
The 48-min fMRI time-series were segmented into 8-min blocks, consisting of one baseline block and five post-infusion blocks. Functional connectivity (FC) was then calculated independently for each block resulting in an FC time-course for each individual scan. Subject-level data were averaged together at each separate block to generate group-level FC time-course data.
Global brain connectivity (GBC) was used to examine drug effects on FC at the whole-brain level (Cole et al. 2010). GBC was calculated for each voxel by averaging z-transformed cross-correlations with all other voxel time-courses.
Seed-based cross-correlation analysis (CCA) was employed to assess the strength of FC between the dlPFC seed region and NAcc target region (bilateral). The left hemisphere dlPFC was chosen to be consistent with previous experiments (Gopinath et al. 2016; Murnane et al. 2015). The regions were demarcated on the INIA19 NHP atlas based on associated NeuroMaps labels (Rohlfing et al. 2012). To reduce sensitivity to motion artifacts (Power et al. 2012) and global signal (Saad et al. 2012), the left dlPFC was subdivided into 3 × 3 × 3 mm non-overlapping sub-regions. The EPI voxel time-series within each sub-region was averaged to construct sub-region reference vectors. Within each voxel of the NAcc, the z-transformed cross-correlation coefficients of all constituent sub-regions of the left dlPFC were averaged to construct subject-level dlPFC-NAcc FC.
Cocaine self-administration with imaging subjects
Following the first set of scans, the subjects were exposed to 40 daily sessions of cocaine self-administration (SA) before undergoing additional saline control scans to test the effects of chronic cocaine exposure on FC. Subjects were trained to self-administer cocaine on a fixed ratio (FR) 20 response schedule of i.v. drug administration in an operant test chamber (Wilcox et al. 2005) using a computer-controlled operant panel equipped with stimulus lights and a response lever (MedPC, MedAssociates, St Albans, VT, USA). Drug infusions of 0.03 mg/kg were delivered by an automated pump and paired with the brief illumination of a red light and followed by a 30-s timeout. Each session lasted until 20 infusions were earned or 1 h had elapsed, whichever occurred first. Response rates for each subject served as a measure of the reinforcing effects of cocaine.
Neuroimaging group-level analysis
Non-parametric statistics were used for hypothesis testing on the FC metrics (z-transformed CC or GBC) to minimize assumptions about the underlying distribution. The Friedman rank sum test was used to test for significant group-level (N = 3; all female) differences in drug condition (saline, cocaine, and cocaine after ketamine) across time-blocks (the first 4 blocks post-infusion were used), and Conover’s test was used for post hoc pairwise comparisons with a false discovery rate correction for multiple comparisons. Statistical testing was performed using RStudio (RStudio 2016) with a significance threshold of alpha = 0.05. The first four post-infusion time-blocks (representing 0–32 min) were chosen for comparisons as this time period corresponds to the strongest effects of cocaine (illustrated clearly in Fig. 2c).
Reinstatement and reacquisition experiment
Subjects
Five adult (four female) individually housed rhesus monkeys (Macaca mulatta) weighing 8–15 kg served as subjects in this study. All subjects had a history of exposure to psychostimulants (including cocaine) and were well-trained at performing cocaine self-administration. The animals were fed Purina monkey chow (Ralston Purina, St. Louis, MO), supplemented with fruit and vegetables daily, and water was continuously available. Housing consisted of stainless-steel home cages with environmental enrichment provided on a regular basis. An ambient temperature of 22 ± 2 °C at 45–50% humidity was maintained throughout the colony, and the lights were set to a 12-h light/dark cycle (lights on at 7 h; lights off at 19 h). All protocols and animal care and handling strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, revised 2011) and the recommendations of the American Association for Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee of Emory University. Each subject was surgically implanted with a chronic indwelling venous catheter attached to a subcutaneous vascular access port as described by Howell and Fantegrossi (2009).
Cocaine self-administration with a second-order schedule of reinforcement
Self-administration sessions lasting approximately 1 h were conducted in an operant test chamber with a controlled environment (Wilcox et al. 2005) and consisted of a second-order schedule of cocaine reinforcement, described previously (Berro et al. 2017). Briefly, following a 5-min start-delay, a red light above the lever on the operant panel is illuminated to act as the discriminative stimulus. The second-order schedule proceeded such that after a fixed interval of 10 min (FI10) elapsed, completion of a fixed ratio of 20 lever presses (FR 20) within a 60-s limited hold resulted in delivery of a cocaine infusion (1 mL over 6 s) and the illumination of white light for 15 s, acting as the conditioned stimulus. A 1-min timeout followed each component during which no lights were illuminated and responding on the lever had no programmed consequences. Completing 20 lever presses prior to the end of the FI10 resulted in a brief, 2 s illumination of the conditioned stimulus light. Each operant session consisted of five components, and thus a maximum of five infusions could be earned during a single session. A unit dose of 0.1 mg/kg of cocaine was used for each infusion, allowing for a maximum cocaine intake of 0.5 mg/kg per session. This dose was chosen because it maintained high rates of responding in all subjects and was previously shown to produce the peak dose-response in three of the five subjects (Berro et al. 2017). Response rates were calculated as the total number of lever presses during the active period divided by the duration of active time throughout the session.
Reinstatement procedure
Subjects were initially required to maintain stable cocaine self-administration behavior, defined as response rates that varied by < 30% over 3 consecutive days. Following a stable maintenance period, behavior was extinguished by replacing cocaine with an infusion of saline. The extinction criterion was operationally defined as two consecutive sessions with response rates < 20% of the 3-day mean response rate for the prior maintenance period. The extinction sessions were identical to the self-administration maintenance sessions except that the conditioned stimulus (white light) was never illuminated. The day after extinction criteria were met, a baseline reinstatement test occurred. For the reinstatement test session, an experimenter-administered intravenous cocaine prime was delivered 5 min before the start of the session and the conditioned stimulus light was illuminated upon completion of the FR20, but only saline infusions could be earned. Thus, we describe these sessions as drug + cue-induced reinstatement tests. No experiments occurred the day after the baseline reinstatement test, and the animals were not moved from their home cage. On the second day following the baseline reinstatement test, the subjects received a ketamine treatment (vehicle, low dose, or high dose). Two days later, a post-treatment reinstatement test session occurred that was identical to the baseline reinstatement session. Two days after the post-treatment reinstatement session, the subjects were returned to maintenance sessions of cocaine self-administration. A cocaine-priming dose of 0.1 mg/kg was used for all reinstatement sessions as this dose was previously shown to engender peak reinstatement response rates in four of the five subjects (Berro et al. 2017). The combination of drug prime and cue, rather than drug or cue alone, was utilized to ensure robust reinstatement responding in all subjects.
Reacquisition procedure
Further experiments were performed to test the effects of ketamine treatment on reacquisition of cocaine self-administration. Maintenance and extinction sessions were identical to those for reinstatement (described above). Following a stable maintenance period (defined above), behavior was extinguished. Two days after meeting extinction criteria (defined above), subjects received a ketamine treatment (vehicle, low dose, or high dose). Subjects then underwent a maintenance session of cocaine self-administration to test response rates during reacquisition. In order to test the effects of repeated ketamine treatments on reacquisition an extra treatment was added 3 days after the first. Reacquisition of maintenance responding was then tested following the repeated treatments.
Ketamine treatment prior to reinstatement and reacquisition
Two sub-anesthetic treatment doses were used in addition to vehicle (saline) treatment. The low dose consisted of a 1-min bolus of 0.345 mg/kg followed by a 1-h constant infusion of 0.256 mg/kg/h. The high dose consisted of a 1-min bolus of 0.69 mg/kg followed by a 1-h constant infusion of 0.512 mg/kg/h. The low dose is identical to what was used in the imaging experiments as well as previous imaging studies (Gopinath et al. 2016; Maltbie et al. 2016). The high dose was chosen to further investigate the ketamine dose-response and produced plasma ketamine levels approximately twice that of the low dose while remaining sub-anesthetic. The treatments occurred in a separate room from self-administration and in the presence of the experimenter. The order of vehicle and low-dose treatments were counterbalanced across subjects, while all subjects received the high-dose treatment last. There was a minimum time of 2 weeks between vehicle and low-dose treatments and a minimum of 3 weeks between low-dose and high-dose treatments.
Behavioral data analysis
To evaluate the effects of treatment on reinstatement responding, the post-treatment response rates are presented as a percentage of the response rate measured for the baseline reinstatement session prior to treatment for each individual subject. Group data indicate the average of the normalized change in response rate following treatment and are collapsed across sex (N = 5; one male). One-way repeated-measures analysis of variance (ANOVA) and post hoc Dunnett’s tests were used to assess the statistical significance of the effect of treatment on both reinstatement and reacquisition responding. Statistical analysis was performed using Prism 5 (GraphPad Software) with a significance threshold of alpha = 0.05.
Results
Non-parametric statistical analysis of the group-level effects of acute drug condition on FC
The non-parametric Friedman rank sum test was used to test for significant effects of drug condition (saline, cocaine, and cocaine after ketamine treatment) on FC across the first four post-infusion time-blocks (one block = 8 min; four blocks = 32 min). There was a significant effect of drug condition on whole-brain GBC (Friedman’s Q = 6.5, df = 2, p = 0.039). Post hoc analysis indicates that cocaine induced a significant reduction to GBC compared to saline control (p = 0.01), while ketamine treatment significantly attenuated the effect of cocaine (p = 0.023). Following ketamine treatment, cocaine did not induce a significant reduction to GBC compared to saline (p = 0.23).
Identical effects were observed on dlPFC-NAcc FC over the first four post-infusion time-blocks. There was a significant effect of drug condition on dlPFC-NAcc FC (Friedman’s Q = 6.5, df = 2, p = 0.039). Post hoc analysis indicates that with no ketamine treatment, cocaine significantly (p = 0.01) reduced dlPFC-NAcc FC compared to saline. However, following ketamine treatment, there was a significant (p = 0.023) attenuation of the effect of cocaine, which no longer evoked a significant difference (p = 0.23) from saline control.
Effects of acute cocaine administration on FC
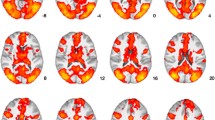
The acute effects of cocaine on FC prior to the start of self-administration protocols are illustrated in Fig. 1. Acute administration of 0.3 mg/kg of cocaine significantly reduced GBC in gray matter voxels at the whole-brain level. The distribution of voxel-wise differences in GBC between cocaine and saline control are shown in Fig. 1a. Compared to saline control, cocaine administration induced a reduction of − 1.31 standard deviations in the mean GBC for all gray matter voxels. Qualitatively, the cocaine-induced reduction in GBC was evident in nearly every region of the brain, with the notable exception of the NAcc which exhibited (non-significant) increased GBC compared to saline, as shown overlaid on a coronal section in Fig. 1b.
Effects of acute administration of cocaine on FC. a Histogram showing the distribution of voxel-wise contrast in z-score of GBC values for cocaine vs. saline for all gray matter voxels. The leftward shift indicates cocaine GBC < saline GBC for most gray matter voxels (p = 0.01). b Coronal section with voxels colored by contrast in z-score of GBC values for cocaine vs. saline. Voxels with cocaine GBC < saline GBC are blue, while voxels with cocaine GBC > saline GBC are orange-yellow. c Same coronal section shown in b, but with regions of interest highlighted. Yellow = NAcc; Red = dlPFC. d Plot of normalized group-average dlPFC-NAcc FC over time during the cocaine scan. Error bars indicate standard error of the mean (SEM)
The delineation of dlPFC and NAcc by NeuroMaps labels (Rohlfing et al. 2012) that was used for regional analysis of the effects of cocaine on FC is shown in Fig. 1c. Cocaine administration decreased FC between the dlPFC and NAcc as plotted over time in Fig. 1d. The cocaine-induced reduction in dlPFC-NAcc FC follows a “U”-shaped curve characterized by a nadir of 33 ± 5% of the average baseline connectivity between 8 and 16 min, and a recovery to baseline levels after 32 min. This time-course (Fig. 1d) closely resembles the time-course for the effects of cocaine on extracellular dopamine levels in the striatum (Banks et al. 2009).
Effects of ketamine treatment on cocaine-induced changes in FC
The effects of cocaine administration 48 h after sub-anesthetic ketamine infusion (labeled KetCoc) are shown in Fig. 2. Following ketamine treatment, the effect of cocaine on GBC at the whole-brain level (Fig. 2a) was significantly attenuated (p = 0.023). Over 32 min following drug infusion, the mean GBC among all gray matter voxels was only − 0.02 standard deviations different from saline control (non-significant) when acute cocaine administration followed ketamine treatment. Fig. 2b displays qualitative differences in GBC between KetCoc and saline control in a coronal section that shows the anterior striatum. KetCoc induced (non-significant) increases to GBC in the NAcc, but overall, the contrast map (Fig. 2b) resembles random noise with no consistent global effect compared to the saline control.
Effects of acute administration of cocaine following ketamine treatment (KetCoc) on FC. a Histogram showing the distribution of voxel-wise contrast in z-score of GBC values for KetCoc vs. saline for all gray matter voxels. The normal distribution indicates not a significant difference in KetCoc GBC vs. saline GBC at the whole-brain level. b Coronal section with voxels colored by contrast in z-score of GBC values for KetCoc vs. saline. Voxels with KetCoc GBC < saline GBC are blue, while voxels with KetCoc GBC > saline GBC are orange-yellow. c Normalized group-average dlPFC-NAcc FC over time is plotted for cocaine and KetCoc scans (error bars = SEM). d Chart comparing dlPFC-NAcc FC averaged over 0–32 min after drug infusion for saline, cocaine, and KetCoc scans (error bars = SEM). FC is reduced for cocaine compared to both saline (p = 0.01) and KetCoc (p = 0.023). FC does not significantly differ between saline and KetCoc
Ketamine treatment also attenuated the effects of acute cocaine administration on FC between the dlPFC and NAcc. The time-course of dlPFC-NAcc FC over time for the KetCoc scan (Fig. 2c) reveals a cocaine-induced reduction leading to a U-shaped curve with a nadir of 49 ± 21% of the average baseline connectivity between 8 and 16 min, qualitatively similar to the time-course for cocaine without treatment. However, following ketamine treatment, the effects of cocaine were attenuated at every time-block. These results are further summarized in Fig. 2d, which shows the average dlPFC-NAcc FC from 0 to 32 min post-infusion.
Effects of cocaine on individual subject dlPFC-NAcc FC and cocaine self-administration
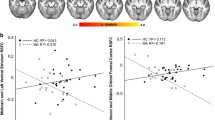
The peak cocaine-induced reduction in dlPFC-NAcc FC (difference in dlPFC-NAcc FC between the within-session baseline and time period 8–16 min post-infusion) measured for individual subjects under each of the drug conditions is displayed in Fig. 3a. Two of the subjects that underwent these experiments (Ry7 and Mm7) had no prior history of drug self-administration. The third (Jz6) did have a history of cocaine self-administration but had not had access to any psychostimulant drugs for a 3-year period prior to the initiation of the study. Thus, while the present study was not powered to examine differences caused by differing drug histories, it may be notable that (as shown in Fig. 3a) Jz6 exhibited the largest acute effect of cocaine on dlPFC-NAcc FC and also exhibited the smallest attenuation of the cocaine effect following ketamine treatment.
Response to cocaine in individual subjects. a Cocaine-induced reduction in dlPFC-NAcc FC, with and without ketamine treatment. The reduction is calculated as the difference between the within-scan baseline and the time-period when the peak effect of cocaine was observed (8–16 min post-infusion). b Plot of cocaine-induced reduction in dlPFC-NAcc FC during the peak time-period vs. response rate during cocaine self-administration (error bars = SEM). Subjects with greater cocaine-induced reduction in dlPFC-NAcc FC exhibit higher response rates
Following the initial fMRI experiments, each subject was trained to self-administer cocaine and given daily access to up to 0.6 mg/kg for a 2-month period. Details of self-administration for each subject are provided in Table 1. One of the three subjects (Jz6) had a prior history of self-administration and the familiarity with the behavior likely influenced the high response rate in that individual. One of the other subjects (Ry7) exhibited lower sensitivity to cocaine, and a unit dose 0.03 mg/kg did not maintain responding in that animal. Even after being switched to a unit dose of 0.1 mg/kg, Ry7 exhibited a considerably lower response rate than the other two subjects. Response rates increased over time for all subjects, as evidenced by the average response rate over the final 10 sessions being higher than the overall average response rate in each subject.
As shown in Fig. 3b, the subjects with greater peak reductions in dlPFC-NAcc FC induced by cocaine exhibited higher average response rates during cocaine self-administration. The final ten-session response rate is displayed to reduce the effect of training that may be present in the earlier sessions and thus may provide a more direct representation of the reinforcing effects of the drug than the overall rate. The sample size is insufficient for a rigorous correlation analysis; however, an association between dlPFC-NAcc FC and self-administration has been observed previously (Murnane et al. 2015).
Effects of chronic cocaine self-administration on FC
The effects of chronic cocaine self-administration on FC were evaluated, and the results are shown in Fig. 4. Saline scans obtained after self-administration (Post-SA saline) were contrasted with saline control scans performed prior to the initiation of self-administration protocols (Pre-SA). The 2-month period of cocaine self-administration led to a robust decrease in GBC at the whole-brain level (Fig. 4a) that was similar to what was observed during the acute effects of Pre-SA cocaine (Fig. 1a). Compared over the entire 48-min scanning sessions, the mean GBC across all gray matter voxels was shifted lower (mean = − 1.64 standard deviations) at all six time-blocks (including the baseline) during the Post-SA saline scan compared to the Pre-SA saline control scan. This effect was significant by Friedman rank sum test (Friedman Q = 6; df = 1; p = 0.014). The Post-SA saline scans exhibited decreased GBC in nearly all brain regions apart from the NAcc (Fig. 4b), where there were (non-significant) increases in GBC compared to Pre-SA saline control. These effects of chronic cocaine self-administration on GBC followed the same pattern observed for the acute effects of cocaine prior to the initiation of self-administration protocols (Fig. 1a, b).
Effects of chronic cocaine self-administration on FC. a Histogram showing the distribution of voxel-wise contrast in z-score of GBC values for Post-SA vs. Pre-SA saline scans for all gray matter voxels. The leftward shift indicates Post-SA saline GBC < Pre-SA saline GBC for most gray matter voxels (p = 0.014). b Coronal section with voxels colored by contrast in z-score of GBC values for Post-SA vs. Pre-SA saline scans. Voxels with Post-SA saline GBC < Pre-SA saline GBC are blue, while voxels with Post-SA saline GBC > Pre-SA saline GBC are orange-yellow. c Chart comparing Pre-SA dlPFC-NAcc FC averaged over the full 48-min scan including both baseline and saline infusion for both Pre-SA and Post-SA saline scans (error bars = SEM). FC is reduced for the Post-SA saline scan compared to Pre-SA saline scan (p = 0.014)
Following the 2-month period of self-administration, there was also a reduction in FC between the dlPFC and NAcc. Figure 4c displays the average dlPFC-NAcc FC over the entire 48-min Post-SA saline scan compared to the entire 48-min Pre-SA saline scan. There was a significant (Friedman Q = 6; df = 1; p = 0.014) reduction in dlPFC-NAcc FC following cocaine self-administration. During the Post-SA saline scan, the average dlPFC-NAcc FC was 49 ± 8% of the Pre-SA baseline. For comparison, during the Pre-SA saline scan, the average dlPFC-NAcc FC was 105 ± 13% of the Pre-SA baseline.
Effects of ketamine treatment on reinstatement of cocaine self-administration behavior
The second-order schedule of cocaine reinforcement maintained high, stable rates of responding in all subjects. Subjects earned an average of 4.7 infusions per session during maintenance with a range of 4.3 to 5.0 across the individuals. Maintenance sessions produced stable within-subject response rates, and individuals typically met stable response criteria after the first three sessions. As shown in Fig. 5a, maintenance response rates varied considerably across the individual subjects, ranging from 0.39 to 1.94 responses per second with a group-average (± standard error) of 1.0 ± 0.3 responses per second. This high variability in individual response rates between subjects was also seen during baseline reinstatement testing (Fig. 5a) and necessitated the within-subject normalization used throughout the study. During extinction, response rates declined to below 20% of the mean maintenance rate within 1–3 sessions, with no more than 4 sessions required to achieve extinction criteria. Ketamine treatments were well tolerated in all subjects. Treatments utilizing the high dose of ketamine elicited an appearance of mild sedation, drooping of the eyelids, and noticeable salivation, while low-dose ketamine treatments were not obviously distinguishable from vehicle treatments.
Effects of vehicle, low dose (0.345 mg/kg + 0.256 mg/kg/h), and high dose (0.69 mg/kg + 0.512 mg/kg/h) ketamine treatment on cocaine prime (0.1 mg/kg) + cue-induced reinstatement responding. a Individual subject response rates during maintenance of cocaine-reinforced responding and baseline reinstatement testing. During maintenance, the unit dose of cocaine was 0.1 mg/kg/infusion and was paired with a cue-light. During reinstatement testing subjects earned injections of only the (saline) vehicle and response rates were maintained by the cue-light and a non-contingent cocaine-priming injection (0.1 mg/kg; i.v.) at the start of the session. The error bars (shown only for values ≥ 0.05) indicate standard error of the mean. Post-treatment reinstatement response rates as a percentage of baseline reinstatement response rate are shown at, b the group-level, where the low-dose reduced response rate compared to vehicle (p = 0.04; error bars = SEM) and c the individual subject-level. All conditions were tested in the same (N = 5) subjects; Yt8 is the only male
Each subject underwent three baseline reinstatement test sessions (one prior to each treatment). Baseline reinstatement testing produced a wide range of individual average response rates (Fig. 5a), from 0.17 to 2.15 responses per second. The response rates exhibited much lower within-subject variability than between-subject variability, and the order of individuals (from highest rate to lowest) was the same for the baseline reinstatement response rates as for the maintenance response rates (Fig. 5a). The effects of ketamine treatment on reinstatement responding at the group-level are shown in Fig. 5b. The reinstatement rate following vehicle treatment was marginally increased to an average (± standard error) of 116 ± 14% of the baseline response rate. Following treatment with the low dose of ketamine, reinstatement response rates were reduced to 79 ± 17% of the baseline response rate. The high dose of ketamine was less effective than the low dose, leading to a group-average response rate of 88 ± 9% of baseline levels. Overall, there was a significant effect of drug on reinstatement responding (F = 4.74; df = 2; p = 0.04; by one-way repeated-measures ANOVA). Compared to the vehicle treatment, reinstatement responding was significantly attenuated after treatment (p = 0.04, by Dunnett’s test) with the low dose, but not the high dose of ketamine. Figure 5c shows the effects of vehicle and low-dose ketamine treatment on reinstatement responding in the individual subjects. While there was high variability in individual response rates following vehicle treatment, reinstatement responding was reduced by a consistent fraction following treatment with the low dose of ketamine compared to vehicle in four of the five subjects. The single subject (Rh7) that did not display lower responding after low-dose ketamine than after vehicle exhibited the second largest decrease in responding after the high dose of ketamine, dropping to 76% of the baseline response rate. Thus, there may have been a difference in sensitivity to ketamine in that subject.
Effects of ketamine treatment on reacquisition of cocaine self-administration behavior
Four (three female) out of the original five subjects completed the reacquisition experiments, with a subset of three subjects (all female) receiving the high-dose ketamine treatment that was shown to be less effective in the reinstatement experiments. None of the subjects required more than a single session to reacquire response rates that were at least 70% of previous maintenance levels after any single treatment. Thus, only the first reacquisition session following treatment was used to determine the reacquisition response rate. The group-level effects of ketamine treatment on reacquisition of cocaine-maintained responding are shown in Supplemental Fig. 1a. There were no statistically significant differences in reacquisition responding following the vehicle, low-dose, or high-dose treatments. Supplemental Fig. 1b shows the changes to reacquisition response rates in individual subjects following each treatment. Only a single subject (Rh7) showed a reduction in reacquisition responding following ketamine treatment at the low dose. Of the three subjects receiving the high-dose treatment, Rh7 again showed the largest effect.
The effects of repeated ketamine treatments on reacquisition of cocaine-maintained responding are shown in Supplemental Fig. 2. The group-level effects of two repeated treatments with either vehicle or low-dose ketamine are displayed in Supplemental Fig. 2a. Following repeated vehicle treatments, the reacquisition response rate was 82 ± 7% of the average response rate over the prior stable maintenance period. After repeated low-dose ketamine treatments, the reacquisition response rate was 78 ± 13% of the average maintenance response rate over the prior stable maintenance period and did not significantly differ from rates after repeated vehicle treatments. Supplemental Fig. 2b shows the effects of repeated treatments in the individual subjects. While there was not a consistent group effect, the same subject (Rh7) that exhibited the largest reduction in reacquisition responding following the single ketamine treatment (Supplemental Fig. 1b) also exhibited the largest reduction in responding following repeated treatment with low-dose ketamine compared to vehicle.
Results summary
Global and region-specific changes to FC were characterized following acute i.v. administration of cocaine in conjunction with fMRI in unanesthetized rhesus monkeys. The results demonstrate that acute administration of cocaine induces a pronounced reduction in voxel-wise GBC at the whole-brain level as well as in FC between the dlPFC and NAcc regions specifically. Both the reduction in whole-brain GBC and the reduction in dlPFC-NAcc FC were attenuated following ketamine treatment 48 h prior. Following an ensuing 2-month period of daily cocaine self-administration dlPFC-NAcc FC and whole-brain GBC were reduced compared to control scans prior to self-administration. Finally, behavioral testing in a separate cohort of rhesus monkeys found that ketamine treatment 48 h prior significantly attenuated drug + cue-induced reinstatement of extinguished behavior previously maintained by cocaine, while reacquisition of maintenance responding was not affected by ketamine treatment.
Discussion
These findings support the use of dlPFC-NAcc FC as a biomarker for cocaine use disorder. The present study was motivated in large part by prior findings that acute sub-anesthetic ketamine infusion induced robust increases in whole-brain GBC (Maltbie et al. 2017) and functional connectivity from many regions, including the NAcc, to the dlPFC (Gopinath et al. 2016), in direct opposition to the acute effects of cocaine (Murnane et al. 2015). The Murnane et al. study (2015) was performed in a sample of three subjects, all with an extensive history of psychostimulant self-administration but following a multi-year period of drug abstinence. The results showed a significant correlation between baseline dlPFC-NAcc FC and cocaine intake during ensuing self-administration. Those findings were strongly corroborated by the results of the present study, which showed nearly identical effects of cocaine on whole-brain connectivity and dlPFC-NAcc FC. The design of the present study was intended to maintain a consistent level of cocaine intake across subjects during self-administration. Hence, the correlation of baseline dlPFC-NAcc FC to cocaine intake could not be tested. However, similar to the results reported by Murnane et al. (2015), an association was observed between the reduction in dlPFC-NAcc FC induced by acute administration of cocaine and response rates during cocaine self-administration.
The finding of cocaine-induced decreases to GBC at the whole-brain level, but not within the NAcc, could be related to the reductions in glucose utilization observed globally during acute cocaine administration (London et al. 1990) and throughout much of the frontal cortex in chronic users (Volkow et al. 1992). In a cross-sectional FC study comparing active cocaine users to matched healthy control subjects, Gu et al. (2010) found cocaine users to have decreased resting-state FC to five of six mesocorticolimbic seeds tested. These included the amygdala, hippocampus, mediodorsal thalamus, rostral anterior cingulate cortex, and ventral tegmental area. The lone exception was the NAcc, which showed no difference between cocaine users and healthy controls. Indeed, the network connectivity related to the NAcc may be a particularly important biomarker for cocaine abuse, as two human brain imaging studies in abstinent cocaine users found high resting-state FC to NAcc to be associated with relapse (Camchong et al. 2014; Contreras-Rodriguez et al. 2015).
Prolonged cocaine use is known to induce various neurobiological changes (DePoy and Gourley 2015; Porrino et al. 2007). While previous longitudinal brain imaging studies in NHPs have reported changes associated with chronic cocaine self-administration (Henry et al. 2010; Porrino et al. 2007), this is the first to examine changes to FC. Chronic cocaine self-administration reduced both whole-brain GBC and dlPFC-NAcc FC in a pattern mirroring the effects of acute administration of cocaine. The loss of FC within fronto-striatal circuitry could be indicative of the loss of executive control of reward-based decision-making that is characteristic of substance use disorders (Volkow and Boyle 2018). Treatments that strengthen the connectivity of this pathway may show efficacy for the treatment of substance use disorders.
The finding of reduced reinstatement responding further indicates that ketamine may decrease the effectiveness of reinforcement-associated cues, which is consistent with the human literature showing that ketamine treatment reduces cue-induced cravings in cocaine-dependent subjects (Dakwar et al. 2014) as well as with recent work in rodents indicating that sub-anesthetic ketamine decreases non-reinforced “sign-tracking” behavior while not altering food-reinforced behavior (Fitzpatrick and Morrow 2017). The behavioral effects of ketamine could potentially be the result of strengthened fronto-striatal connectivity seen with neuroimaging (Gopinath et al. 2016) leading to increased top-down modulation of reward processing, which appears to be decreased by cocaine (Murnane et al. 2015; Volkow et al. 2011). The attenuation of the effects of acutely administered cocaine on both whole-brain GBC and dlPFC-NAcc FC following ketamine treatment supports this hypothesis. Together, this combination of behavioral and neuroimaging results provides further evidence for the potential efficacy of sub-anesthetic ketamine as treatment for cocaine use disorder, though further testing is needed to establish safety and efficacy as a treatment strategy.
Ever since the rapid antidepressant efficacy of sub-anesthetic ketamine was first reported by Berman et al. (2000), the clinical use of ketamine as an off-label treatment for mood disorders has been increasing (Sanacora et al. 2017). While the clinical evidence thus far suggests that ketamine treatments are safe and well tolerated (Perry et al. 2007; Wan et al. 2015) and efficacy is maintained with repeated treatments (aan het Rot et al. 2010), use of ketamine as a treatment for substance use disorders is likely to generate concerns given the abuse liability of ketamine (Morgan et al. 2012). There is at least one report of ketamine abuse extending from treatment for major depressive disorder (Schak et al. 2016). However, substance abuse is highly context dependent (Volkow and Swanson 2003; Volkow et al. 2008) and thus the medical context of ketamine treatments administered in the clinic combined with effective monitoring should mitigate the likelihood of ketamine abuse in this clinical population (Carter and Griffiths 2009).
Alternatives to ketamine may still be preferable and the literature indicates several related compounds that provide good candidates for further investigation as potential treatments for drug dependence. Ketamine is a chiral compound consisting of a pair of (R,S) enantiomers. While the racemic mixture is typically administered for ketamine treatments, there is some evidence suggesting that R-ketamine may have greater antidepressant efficacy (Zhang et al. 2014) while also producing fewer psychotomimetic effects (Yang et al. 2015). Further, there is evidence that a specific metabolite of R-ketamine is sufficient for producing rapid antidepressant-like effects in mice without several of the side effects of racemic ketamine (Zanos et al. 2016). A higher concentration of the same metabolite has been observed in human responders compared to non-responders after ketamine treatment for both major depressive and bipolar disorders (Zarate et al. 2012). Other NMDA receptor antagonists may also warrant examination, such as the NR2B sub-unit selective compound CP-101,606 that has been shown to produce rapid antidepressant effects with a reduced side effect profile (Preskorn et al. 2008).
Limitations
The primary limitation of the present study was the small sample size consisting of just three subjects in fMRI experiments. The experiments utilized repeated measurements within each subject to maximize statistical power; however, there was not sufficient power to broaden the number of regions of interest or to investigate differences between subjects. Indeed, the subject-level results suggest there may be individual differences in the efficacy of ketamine treatment, but no conclusions could be drawn from the current data. Individual differences in vulnerability to drug dependence have been highlighted previously (George and Koob 2010), and future investigation of the efficacy of ketamine as a treatment for substance use disorders would benefit from larger samples.
Another significant limitation is that nearly all of the experimental subjects were female. All three neuroimaging subjects and four out of five subjects in the reinstatement experiment were female. Hence, the results may not be generalizable to male subjects. Sex differences have been observed in the sensitivity to ketamine treatment both clinically (Saland et al. 2017) and in preclinical models (Franceschelli et al. 2015). Further, notable sex differences have also been observed in substance use disorders (Andersen et al. 2012), with female subjects generally showing greater vulnerability than males (Anker and Carroll 2011).
Conclusions
Acute i.v. cocaine administration robustly decreases FC at the whole-brain level and between the dlPFC and NAcc regions specifically. The impact of cocaine on connectivity between those regions may be associated with the reinforcing effects of cocaine, as measured by response rates during self-administration. Similar to the acute effects of cocaine, chronic cocaine self-administration reduces FC at the whole-brain level and between the dlPFC and NAcc specifically.
Sub-anesthetic i.v. ketamine infusion, administered 48 h prior to fMRI, was shown to attenuate the effects of acute cocaine administration on FC at the whole-brain level and between the dlPFC and NAcc regions specifically. Furthermore, sub-anesthetic ketamine was shown to significantly attenuate cocaine-seeking behavior in rhesus monkeys using a potentially translational model of relapse. Importantly, these behavioral effects were reported in a cohort of nonhuman primate subjects with an extensive, multi-year history of exposure to cocaine and other psychostimulants.
This combination of neuroimaging and behavioral results supports the hypothesis that sub-anesthetic ketamine strengthens executive control networks and may attenuate the disruptive effects of cocaine on decision-making, providing evidence that ketamine could potentially have therapeutic value in the treatment of substance use disorders.
References
aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145
Andersen ML, Sawyer EK, Howell LL (2012) Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp Clin Psychopharmacol 20:2–15
Anker JJ, Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96
Banks ML, Andersen ML, Murnane KS, Meyer RC, Howell LL (2009) Behavioral and neurochemical effects of cocaine and diphenhydramine combinations in rhesus monkeys. Psychopharmacology 205:467–474
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Berro LF, Perez Diaz M, Maltbie E, Howell LL (2017) Effects of the serotonin 2C receptor agonist WAY163909 on the abuse-related effects and mesolimbic dopamine neurochemistry induced by abused stimulants in rhesus monkeys. Psychopharmacology (Berl)
Camchong J, Macdonald AW 3rd, Mueller BA, Nelson B, Specker S, Slaymaker V, Lim KO (2014) Changes in resting functional connectivity during abstinence in stimulant use disorder: a preliminary comparison of relapsers and abstainers. Drug Alcohol Depend 139:145–151
Carter LP, Griffiths RR (2009) Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105(Suppl 1):S14–S25
Cole MW, Pathak S, Schneider W (2010) Identifying the brain's most globally connected regions. Neuroimage 49:3132–3148
Contreras-Rodriguez O, Albein-Urios N, Perales JC, Martinez-Gonzalez JM, Vilar-Lopez R, Fernandez-Serrano MJ, Lozano-Rojas O, Verdejo-Garcia A (2015) Cocaine-specific neuroplasticity in the ventral striatum network is linked to delay discounting and drug relapse. Addiction 110:1953–1962
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Dakwar E, Hart CL, Levin FR, Nunes EV, Foltin RW (2016) Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: a randomized, crossover trial. Mol Psychiatry
Dakwar E, Levin F, Foltin RW, Nunes EV, Hart CL (2014) The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol Psychiatry 76:40–46
Degenhardt L, Baxter AJ, Lee YY, Hall W, Sara GE, Johns N, Flaxman A, Whiteford HA, Vos T (2014) The global epidemiology and burden of psychostimulant dependence: findings from the Global Burden of Disease Study 2010. Drug Alcohol Depend 137:36–47
DePoy LM, Gourley SL (2015) Synaptic cytoskeletal plasticity in the prefrontal cortex following psychostimulant exposure. Traffic 16:919–940
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology 189:1–16
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Fitzpatrick CJ, Morrow JD (2017) Subanesthetic ketamine decreases the incentive-motivational value of reward-related cues. J Psychopharmacol 31:67–74
Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60
George O, Koob GF (2010) Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev 35:232–247
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669
Gopinath K, Maltbie E, Urushino N, Kempf D, Howell L (2016) Ketamine-induced changes in connectivity of functional brain networks in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233:3673–3684
Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y (2010) Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53:593–601
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26
Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF (2013) DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 170:834–851
Henry PK, Murnane KS, Votaw JR, Howell LL (2010) Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav 4:212–219
Howell LL, Fantegrossi WE (2009) Intravenous drug self-administration in nonhuman primates. In: Buccafusco JJ (ed) Methods of Behavior Analysis in Neuroscience (Frontiers in Neuroscience), Boca Raton (FL)
Ivan Ezquerra-Romano I, Lawn W, Krupitsky E, Morgan CJA (2018) Ketamine for the treatment of addiction: evidence and potential mechanisms. Neuropharmacology 142:72–82
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146:373–390
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572
Kalivas PW, O'Brien C (2008) Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180
Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650
Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986
Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV (2010) Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328:1709–1712
Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168:21–30
Kavalali ET, Monteggia LM (2012) Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 169:1150–1156
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN Jr (1990) Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry 47:567–574
Maltbie E, Gopinath K, Urushino N, Kempf D, Howell L (2016) Ketamine-induced brain activation in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233:961–972
Maltbie EA, Kaundinya GS, Howell LL (2017) Ketamine and pharmacological imaging: use of functional magnetic resonance imaging to evaluate mechanisms of action. Behav Pharmacol 28:610–622
Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A (2006) Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci 9:868–869
McLellan AT, Lewis DC, O'Brien CP, Kleber HD (2000) Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284:1689–1695
Morgan CJ, Curran HV, Independent Scientific Committee on D (2012) Ketamine use: a review. Addiction 107:27–38
Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL (2015) Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology 232:745–754
Murnane KS, Howell LL (2010) Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods 191:11–20
National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) (2011) Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C., p xxv, 220 p
Perry EB Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, O'Donnell E, Krystal JH, D'Souza DC, Yale Ketamine Study G (2007) Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology 192:253–260
Pitts EG, Taylor JR, Gourley SL (2016) Prefrontal cortical BDNF: a regulatory key in cocaine- and food-reinforced behaviors. Neurobiol Dis 91:326–335
Porrino LJ, Smith HR, Nader MA, Beveridge TJ (2007) The effects of cocaine: a shifting target over the course of addiction. Prog Neuro-Psychopharmacol Biol Psychiatry 31:1593–1600
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637
Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A (2012) The INIA19 template and NeuroMaps atlas for primate brain image parcellation and spatial normalization. Front Neuroinformatics 6:27
RStudio T (2016) RStudio: integrated development environment for R. RStudio, Inc., Boston, MA
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32
Saland SK, Duclot F, Kabbaj M (2017) Integrative analysis of sex differences in the rapid antidepressant effects of ketamine in preclinical models for individualized clinical outcomes. Curr Opin Behav Sci 14:19–26
Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, American Psychiatric Association Council of Research Task Force on Novel B, Treatments (2017) A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74:399–405
Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, Palmer BA, Frye MA (2016) Potential risks of poorly monitored ketamine use in depression treatment. Am J Psychiatry 173:215–218
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219
Volkow ND, Boyle M (2018) Neuroscience of addiction: relevance to prevention and treatment. Am J Psychiatry 175:729–740
Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L (1992) Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11:184–190
Volkow ND, Swanson JM (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–1918
Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, Alia-Klein N, Wong C (2011) Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One 6:e16573
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C (2008) Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 39:1266–1273
Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW (2015) Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76:247–252
Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL (2005) In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse 58:220–228
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486
Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW (2012) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864
Zhang JC, Li SX, Hashimoto K (2014) R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141
Acknowledgements
Special thanks to Dr. Lais Berro and Dr. Maylen Perez-Diaz for behavioral methods training; to Juliet Brown for performing the catheter surgeries; and to technicians Marisa Olsen, Erin Siebert, Ruth Connelly, and Sudeep Patel for laboratory and imaging support.
Funding
This research was supported by P51OD11132 (Yerkes National Primate Research Center) and DA031246 (LLH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All protocols and animal care and handling strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, revised 2011) and the recommendations of the American Association for Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee of Emory University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 948 kb)
Rights and permissions
About this article
Cite this article
Maltbie, E.A., Gopinath, K.S. & Howell, L.L. Effects of ketamine treatment on cocaine-induced reinstatement and disruption of functional connectivity in unanesthetized rhesus monkeys. Psychopharmacology 236, 2105–2118 (2019). https://doi.org/10.1007/s00213-019-05204-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05204-4