Abstract
Rationale
Stress disorders cause abnormal regulation of fear-related behaviors. In most rodent models of these effects, stress was administered before fear conditioning, thereby assessing its impact on both the formation and extinction of fear memories, not the latter alone. Here, we dissociated the two processes by also administering stress after fear conditioning, and then compared how pre-conditioning versus post-conditioning exposure to chronic stress affects subsequent acquisition and recall of fear extinction.
Methods
Male Wistar rats were subjected to chronic immobilization stress (2 h/day, 10 days); the morphological effects of which were analyzed using modified Golgi-Cox staining across brain areas mediating the formation and extinction of fear memories. Separate groups of rats underwent fear conditioning followed by acquisition and recall of extinction, wherein stress was administered either before or after fear conditioning.
Results
When fear memories were formed after chronic stress, both acquisition and retrieval of extinction was impaired. Strikingly, these deficits were absent when fear memories were formed before the same stress. Chronic stress also reduced dendritic spine density in the infralimbic prefrontal cortex, but enhanced it in the basolateral amygdala.
Conclusion
Chronic stress, administered either before or after fear learning, had distinct effects on the acquisition and recall of fear extinction memories. Stress also strengthened the structural basis of synaptic connectivity in the amygdala, but weakened it in the prefrontal cortex. Thus, despite eliciting a specific pattern of brain region-specific morphological changes, the timing of the same stress gave rise to strikingly different behavioral effects on the extinction of fear.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Auditory fear conditioning is an associative learning paradigm wherein subjects rapidly encode memories associating a neutral tone (the conditioned stimulus, or CS) with a coincident noxious stimulus (unconditioned stimulus, US), such as a foot shock. Subsequent presentation of the CS alone elicits a conditioned response in the form of ‘freezing,’ or cessation of locomotor activity, in rodents. The level of freezing elicited by the CS provides a behavioral measure of the learned fear response. Further, repeated unreinforced presentations of the CS cause a gradual reduction in the conditioned fear response, a form of learning referred to as fear extinction. Three functionally interconnected brain regions play a pivotal role in different phases of the acquisition and retrieval of fear and extinction memories. Neurons in the basolateral nucleus of the amygdala (BLA) are essential for the acquisition and storage of fear memories (Ledoux 2000), as well as the initial retrieval of this memory during fear extinction (Sierra-Mercado et al. 2011). Extinction of fear memories also involves the hippocampus and medial prefrontal cortex (mPFC). For instance, the ventral hippocampus is important for fear expression (Kjelstrup et al. 2002; Trivedi and Coover 2004), the contextual modulation of fear extinction (Sierra-Mercado et al. 2011), and also for maintaining low levels of freezing after the fear is extinguished (Sotres-Bayon et al. 2012). Within the mPFC, the prelimbic cortex (PL) is involved in the expression of fear (Sierra-Mercado et al. 2011) and stimulating PL increases fear expression (Vidal-Gonzalez et al. 2006). On the other hand, multiple lines of evidence including lesion (Morgan and LeDoux 1995; Chang and Maren 2010), inactivation (Sierra-Mercado et al. 2011), and optogenetic manipulations (Do-Monte et al. 2015) have established a role for the infralimbic cortex (IL) in the formation and storage of fear extinction memory. Together, interactions between circuits spanning these three brain regions coordinate distinct behavioral responses during acquisition and extinction of fear memories.
These same brain areas have also been the focus of many studies on stress, not only because of their disparate roles in regulating the stress response via the hypothalamic–pituitary–adrenal (HPA) axis, but also because of their susceptibility to stress-induced changes in structure and function. While the mPFC and hippocampus play a pivotal role in the negative-feedback regulation of the stress response, the amygdala has an opposing effect on the HPA axis. Moreover, exposure to prolonged stress elicits divergent patterns of structural and functional changes in these brain regions (Chattarji et al. 2015). For instance, failure to extinguish fear is one of the key impairments reported in patients of post-traumatic stress disorder (PTSD) (Wessa and Flor 2007; Wicking et al. 2016). Neuroimaging studies in PTSD patients also show enhanced activity in the amygdala (Rauch et al. 2000, 2006; Koenigs and Grafmann 2009), but reduced activity in both the hippocampus (Bremner 2002; Rauch et al. 2006) and mPFC (Shin et al. 2005; Koenigs and Grafmann 2009). Findings from various rodent models are in broad agreement with these clinical observations (Rao et al. 2009). For example, repeated stress causes dendritic growth and spinogenesis in principal neurons of the BLA (Vyas et al. 2002; Mitra et al. 2005, 2009; Mitra and Sapolsky 2008), while causing the opposite effects in both the mPFC (Radley et al. 2005, 2006, 2008; Miracle et al. 2006; Goldwater et al. 2009) and hippocampus (McEwen 1999; Silva-Gomez et al. 2003; Pawlak et al. 2005; McLaughlin et al. 2007; Silva-Gómez et al. 2013). Furthermore, molecular and physiological mediators of synaptic plasticity also exhibit contrasting features across these brain areas (Roozendaal et al. 2009; Arnsten 2015; Chattarji et al. 2015; McEwen et al. 2015).
Consistent with these findings on stress-induced plasticity, enhanced fear learning and impaired fear extinction has been reported using multiple stress paradigms, including restraint (Miracle et al. 2006; Baran et al. 2009; Wilber et al. 2011; Farrell et al. 2013; Hoffman et al. 2014), immobilization (Mitra and Sapolsky 2009), swim stress (Izquierdo et al. 2006), and single prolonged stress (Yamamoto et al. 2008; Knox et al. 2012). In a majority of these studies, however, rats were first exposed to stress and then subjected to fear conditioning and extinction. Given that stress enhances fear memories (Suvrathan et al. 2014), the deficits in fear extinction might reflect a failure to extinguish stronger fear memories created by prior exposure to stress. Although a few studies have subjected animals to post-conditioning stress (Maroun et al. 2013; Moench et al. 2016), these used acute stress paradigms. Unlike repeated or chronic stress, acute stressors are not known to elicit the divergent and robust patterns of structural plasticity in dendrites and spines (Mitra et al. 2005; Moench et al. 2016) across the three brain regions that together encode various facets of fear learning and extinction in rodents.
Therefore, the present study combines behavioral and morphometric analyses to address two questions. First, we examined if and how stress specifically affects expression of fear during and after extinction, when the fear memories are acquired before exposure to chronic immobilization stress. We also used the conventional experimental design wherein animals were first subjected to the same chronic stress, followed by fear conditioning and extinction. This allowed us to directly compare the effects of the same chronic stress administered either before or after the same auditory fear conditioning paradigm. Together, this offered an opportunity to dissociate the impact of chronic stress on the acquisition versus extinction of fear memories. Second, although various rodent models of stress are known to elicit divergent patterns of morphological plasticity across the BLA, mPFC, and the hippocampus—earlier studies used a range of stress protocols that varied in their modality, duration, and intensity. Further, each of these studies often used different morphological assays and focused primarily on one brain area at a time. Hence, our aim was to confirm, and add to, earlier findings by generating a unified framework for quantifying the morphological impact of the same chronic stress paradigm on all three areas in the same brain.
Materials and methods
Animals
Male Wistar rats from Charles River laboratories, 50–60 days old at the beginning of the experiment, were used for this study. Animals were housed in groups of two animals per cage with ad libitum access to food and water, and maintained on a 14 h:10 h light:dark cycle in a temperature-controlled environment. All maintenance and experimental procedures were approved by the Institutional Ethics Committee, National Centre for Biological Sciences, India.
Stress procedure
Prior to stress, animals were handled for a period of 3 days, and then randomly assigned to the chronic immobilization stress (chronic stress) and control groups. Stress was done as previously described (Vyas et al. 2002; Mitra et al. 2005; Suvrathan et al. 2014). Briefly, animals were immobilized in plastic immobilization cones with no access to food or water for 2 h/day, for a period of 10 consecutive days. All stress protocols were carried out between 10 am and 12 pm. Control rats were not subjected to any stress, and were housed in a room different from the stressed animals. Different batches of animals were used for morphology and behavior experiments.
Golgi-Cox staining
Twenty-four hours after the final stress episode, stressed and control animals were decapitated after halothane anesthesia, and brains were removed for modified Golgi-Cox staining, as described previously (Patel et al. 2017). Following staining, 120-μm thick coronal sections were collected on gelatin-chrome alum-coated slides using a Leica vibratome (VT-1200S), and developed in 5% sodium carbonate. Finally, sections were dehydrated in grades of alcohol and mounted with DPX (Nice Chemicals, India).
Dendritic spine analysis
Prepared slides were coded and dendritic spine analysis was done blind. Across all regions, apical dendrites of pyramidal neurons were analyzed. For this study, the dendrite originating directly from the cell body was considered as a main shaft, and dendrites branching off from the main shaft were considered as primary dendrites (Fig. 1). Neurons were chosen for analysis based on criteria described earlier (Mitra et al. 2005; Rao et al. 2012). Each primary dendrite chosen was analyzed for a length of 70 μm from its origin. When multiple dendrites were analyzed from the same neuron, an average of the dendrites was taken while plotting segmental analysis per neuron. All protrusions from the primary dendrite were considered as spines, irrespective of their morphological characteristics. Analysis was performed using Neurolucida image analysis software from Micro-BrightField, Williston, VT, USA, attached to an Olympus BX61 microscope (100 ×, 0.95 Numerical Aperture, Olympus BX61) from Olympus, Shinjuku-Ku, Tokyo, Japan.
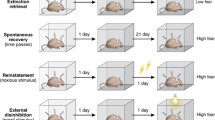
Experimental design. One cohort of rats was used to characterize the morphological effects of stress. Following 10 days of chronic immobilization stress (2 h/day), rats were sacrificed on day 11 and brain was collected for modified Golgi-Cox staining. Image shows a representative neuron at low magnification alongside a high-magnification image of a primary dendrite showing dendritic spines (scale bar 20 μm). In two separate cohorts of rats, the behavioral effects of stress were analyzed by adopting two different approaches. In the post-stress conditioning experiment, after 10 days of chronic immobilization stress, rats underwent fear conditioning in context A. Fear extinction acquisition and fear extinction recall took place in context B 24 h and 48 h later respectively, on days 12 and 13. In the pre-stress conditioning experiment, rats first underwent fear conditioning in context A, following which they underwent chronic immobilization stress. 10 days later, they underwent fear extinction acquisition in context B on day 11. Fear extinction recall took place 24 h later in the same context, on day 12
Auditory fear conditioning
Animals underwent auditory fear conditioning either 24 h after the last episode of stress, on day 11 (post-stress conditioning), or 24 h before they were stressed, on day 0 (pre-stress conditioning) (Fig. 1). For both experiments, two distinct chambers were used for conditioning and extinction/extinction recall. Context habituation and fear conditioning chamber (context A, model E10-11R, Coulbourn Instruments, USA) was dimly illuminated by a single house light and had a metal grid floor on which the rats were placed. Extinction acquisition and recall were done in a home cage-like chamber (context B) brightly illuminated with white light along with transparent Plexiglas walls. Additionally, foam with peppermint odor was kept on the floor of context B during both these sessions. A sound-attenuating cubicle (model E10-24, Coulbourn Instruments) housed both the contexts, and a video camera was used to record behavior. On the day of conditioning, following a 3-min acclimation period to context A, rats received five presentations of a 20-s tone conditioned stimulus (CS) (5 kHz, 70 ± 5 dB) alone. This was followed by seven presentations of CS that co-terminated with a foot-shock unconditioned stimulus (US) (0.5 s, 0.7 mA). The average pseudo-random inter-trial interval (ITI) was 100 s. Following conditioning, all rats were returned to their home cages. For acquisition of fear extinction, rats were placed in context B and presented with 15 tones (20 s, 5 kHz, 70 ± 5 dB) with an average ITI of 100 s, following which they were returned to their home cages. They were reintroduced to the same context (context B) 24 h later and again presented with 15 tones (20 s, 5 kHz, 70 ± 5 dB) to undergo test for fear extinction memory. The apparatus was cleaned with 70% ethyl alcohol between sessions.
The experimental designs for the two experiments were as follows:
-
1.
Post-stress conditioning: (Fig. 1) following handling, rats were randomly divided into the chronic stress or the control group. Chronic stress animals underwent immobilization stress (as described above) from days 1–10, while controls were handled every day. On days 9–10, all animals were habituated to the fear conditioning context A. On day 11, both groups underwent auditory fear conditioning. Twenty-four hours later (on day 12), all animals underwent fear extinction training in context B. Finally, on day 13, all animals were tested for fear extinction memory in the same context B.
-
2.
Pre-stress conditioning: (Fig. 1) all animals were handled for 3 days following which they underwent 2 days of context habituation for 30 min each day, in context A. Next, all animals underwent fear conditioning as described above on day 0. Animals were then randomly assigned into the chronic stress or the control group. Chronic stress animals underwent 10 days of immobilization while control animals were handled during this period (days 1–10). Twenty-four hours after the last immobilization (on day 11), both groups underwent fear extinction training in context B. Finally on day 12, all animals were tested for fear extinction memory in the same context B.
Behavioral analysis
Behavioral analysis was done blind using the video recordings from all the experimental sessions. Response to the auditory stimuli was evaluated in the form of freezing response during the 20 s of tone presentation. Freezing response was defined as the absence of movement except due to respiration (Blanchard and Blanchard 1988), and was then converted into percentage. Each trial block is an average of two tones. The percentage freezing was also measured in every context for 20 s in the absence of any auditory stimulus, and was defined as the pre-tone. The tone habituation represents the mean freezing during all the five CS presented. Subsequently, animals were classified as learners if freezing in the first trial block of extinction acquisition was (a) at least three times higher than freezing during pre-tone of the same session and (b) higher than freezing during tone habituation. For the post-stress conditioning experiment, there were nine non-learners in the control group and seven non-learners in the chronic stress group. For the pre-stress conditioning experiment, there was one non-learner in the control group and two non-learners in the chronic stress group. Across both the fear extinction experiments, the same criteria of selection were used to classify the non-learners and only learners were included in the final analysis. Freezing to context was measured during a 20-s time window immediately preceding the onset of every CS. The inter-trial interval being pseudo-random (100 s, on average), this ensured randomized sampling of contextual freezing throughout the whole session. For the purpose of graphical representation, tones and pre-tones 1–14 were averaged in pairs (tones/pre-tones 1 and 2, tones/pre-tones 3 and 4, and so on), and each pair was plotted as either a ‘trial block’ (for the tone) or a ‘pre-trial block’ (for the pre-tone).Tone and pre-tone 15 has not been shown. This method of representation has been adapted from a number of previous reports including Santini et al. 2008, Burgos-Robles et al. 2007, Sierra-Mercado et al. 2011, and Do-Monte et al. 2015.
Statistical analysis
All values are reported as mean ± SEM unless mentioned otherwise. Each data set was evaluated for outliers, which was defined as greater than twice the standard deviation away from the mean. The total number of dendritic spines was compared by using Student’s unpaired two-tailed t test. For comparison of spine number across 10-μm segments, two-way repeated measures ANOVA was used, followed by post-hoc Sidak’s test. Likewise, for the behavior experiments, two-way repeated measures ANOVA was used, followed by post-hoc Sidak’s test. For comparison of the first and last trial blocks of fear extinction as well as for contextual freezing across sessions, unpaired t tests were used, with corrections for multiple comparisons using Holm-Sidak’s method. All statistical analyses and plots were done using GraphPad Prism software (GraphPad software Inc., La Jolla, California, USA, version 6). The figure panels were made with Adobe Creative Design Suite, version 5.
Results
Spine plasticity across brain areas involved in fear learning and extinction
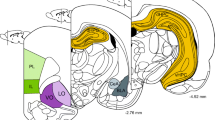
We first examined the impact of chronic immobilization stress (2 h/day, for 10 days) on dendritic spines across the four brain regions—the infralimbic (IL) and prelimbic (PL) areas of the mPFC, ventral CA1 (vCA1) area of the hippocampus, and the basolateral amygdala (BLA). Analysis of spine densities was carried out on Golgi-Cox stained brains 24 h after the end of the 10th day of chronic stress. Within each region, spine density was quantified along a 70-μm long segment of primary apical dendrite of pyramidal neurons. Chronic stress significantly reduced spine density on apical dendrites of layer II/III pyramidal neurons in IL (t6 = 4.8, p < 0.01) (Fig. 2a). Upon detailed analysis of spine numbers in steps of 10-μm segments along the length of the dendrite, we found that there was a main effect of stress (F(1,6) = 23.68, p < 0.01) in a two-way repeated measures ANOVA. Further, post-hoc analysis revealed that this decrease was evident throughout the dendrite, in both proximal as well as distal dendritic segments (Fig. 2b).
Chronic stress has divergent effects on dendritic spine density across the fear circuit. a Chronic stress decreases spine density in layer II/III pyramidal neurons of IL but not in c layer V/VI pyramidal neurons of IL. e Chronic stress does not affect spine density in both layer II/III pyramidal neurons of PL, as well as in g layer V/VI pyramidal neurons of PL. i Chronic stress does not affect spine density in vCA1 pyramidal neurons. k Chronic stress increases spine density of BLA pyramidal neurons (control, N = 4 animals; chronic stress, N = 4 animals). Segmental analysis of mean numbers of spines in each successive 10-μm segment of the primary dendrite as a function of the distance of that segment from the origin of the branch in b layer II/III of IL, d layer V/VI of IL, f layer II/III of PL, h layer V/VI of PL, j vCA1, and l BLA neurons (control, N = 4 animals; chronic stress, N = 4 animals). Representative images show primary dendrites with dendritic spines across all regions analyzed (scale bar 20 μm). * indicates p < 0.05 and ** indicates p < 0.01 in Student’s unpaired two-tailed t test. Ψ indicates p < 0.05 and Ψ Ψ indicates p < 0.01 in main effect of stress in two-way repeated measures ANOVA. # indicates p < 0.05 and ## indicates p < 0.01 in post-hoc Sidak’s test
In layer V/VI pyramidal neurons of the IL, stress did not affect the overall density of dendritic spines (t6 = 2.22, p = 0.07) (Fig. 2c), and segmental analysis revealed no effect of stress (F(1,6) = 5.09, p= 0.07). However, post-hoc analysis showed a significant decrease in spines only in the initial, proximal dendritic segment (Fig. 2d). In both layers of IL, there was also a significant effect of the distance of dendritic segment from the origin of the dendrite (layer II/III F(6,36) = 4.92, p < 0.0001; layer V/VI F(6,36) = 4.46, p < 0.01), but there was no interaction between the two factors in either layer (layer II/III F(6,36) = 0.61, p = 0.72; layer V/VI F(6,36) = 1.71, p = 0.15).
In sharp contrast to IL-mPFC, stress did not affect the total dendritic spine density in PL-mPFC, either on pyramidal neurons of layer II/III (t6 = 0.597, p = 0.57) (Fig. 2e) or layer V/VI (t6 = 2.17, p = 0.07) (Fig. 2g). Segmental analysis (Fig. 2f, h) did not reveal any effect of stress (layer II/III F(1,6) = 0.34, p = 0.58; layer V/VI F(1,6) = 4.72, p = 0.07), although there was a main effect of distance along the dendrite in layer II/III (F(6,36) = 2.61, p < 0.05) but not in Layer V/VI (F(6,36) = 2.28, p = 0.06). There was no interaction of the main factors in either layer (layer II/III F(6,36) = 0.36, p = 0.90; layer V/VI F(6,36) = 0.66, p = 0.68).
In the vCA1, chronic stress caused no change in the total spine density on pyramidal neurons (t6 = 1.54, p = 0.18) (Fig. 2i). Segmental analysis revealed only a significant effect of distance (F(6,36) = 3.82, p < 0.01) but no effect of stress (F(1,6) = 2.36, p = 0.18), and there was no interaction between the two factors (F(6,36) = 1.496, p = 0.21). Although there was a visible reduction in spine density in a proximal dendritic segment, this did not reach statistical significance upon post-hoc analysis (p = 0.08) (Fig. 2j).
Finally, chronic stress increased spine density on pyramidal neurons of the BLA (t6 = 2.913, p < 0.05) (Fig. 2k). ANOVA showed significant effects of both stress (F(1,6) = 8.48, p < 0.05) as well as distance of individual dendritic segments from the origin of the dendrite (F(6,36) = 7.90, p < 0.0001), without any interaction between the two (F(6,36) = 0.74, p = 0.62). Further, post-hoc analysis revealed that this increase in spines was robust, and seen throughout the length of the dendrite analyzed, in both proximal and distal segments (Fig. 2l).
While the above segmental analysis was done by subject number, we also carried out this analysis by number of neurons, as had been reported in several earlier studies that focused on changes in dendritic spine density (Hayashi et al. 2004, 2007; Mitra et al. 2005; Rao et al. 2012; Do-Monte et al. 2015) (Online Resource 1). Notably, this reanalysis confirmed the significant changes in spine numbers revealed in the layer II/III of IL (F(1,64) = 23.43, p < 0.0001) (Online Resource 1a) as well as in the BLA (F(1,55) = 22.88, p < 0.0001) (Online Resource 1f). Further, a main effect of stress was revealed in both layer V/VI of IL (F(1,54) = 5.53, p < 0.05) as well as vCA1 (F(1,50) = 6.438, p < 0.05) while post-hoc differences between specific segments emerged in both layers of IL (Online Resource 1a,b) in addition to the vCA1 (Online Resource 1f) and BLA (Online Resource 1f). The additional details of these statistical analyses by number of neurons are summarized in Online Resource 2.
Together, these results show that the same chronic stress elicits specific and contrasting patterns of changes in dendritic spine numbers across the three brain areas, which is in agreement with a growing body of earlier work. Moreover, these differences were found not just between brain areas, but also between two sub-regions (IL versus PL) within the same structure (mPFC). Importantly, accumulating evidence has implicated each of these brain areas in the encoding and recall of specific aspects of the acquisition and extinction of fear memories in rodents. Thus, these morphological data provide the basis for examining specific behavioral consequences of chronic immobilization stress.
Extinction of fear memories formed after stress
As mentioned earlier, a majority of previous studies subjected animals to fear conditioning and extinction after exposure to stress (Izquierdo et al. 2006; Maren and Holmes 2016; Miracle et al. 2006). Thus, we first repeated this experimental design by administering our 10-day chronic immobilization stress protocol before an auditory fear conditioning paradigm (Fig. 3).
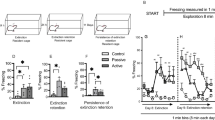
Chronic stress impairs extinction of fear memories formed after stress exposure. a Stressed animals learn faster than controls during fear conditioning. b Stress impairs acquisition of fear extinction. c Stressed animals do not show any decrease in freezing between the first and the last trial blocks during fear extinction acquisition, unlike controls. d Chronic stress impairs recall of fear extinction (control, N = 11; chronic stress, N = 15). Ψ indicates p < 0.05, Ψ Ψ indicates p < 0.01, and Ψ Ψ Ψ Ψ indicates p < 0.0001 indicate a main effect of stress in two-way repeated measures ANOVA. # indicates p < 0.05, ## indicates p < 0.01, and ### indicates p < 0.001 in post-hoc Sidak’s test. * indicates p < 0.05 in Student’s unpaired two-tailed t test. Each trial block is an average of two trials
Prior to conditioning, both stressed and control animals showed low-baseline freezing to the tone alone during tone habituation (Fig. 3a). During fear conditioning, however, freezing response to successive presentations of tone-shock pairings was affected by stress (F(1,24) = 6.45, p < 0.05) (Fig. 3a). Two-way repeated measures ANOVA also showed a main effect of trials (F(7,168) = 26.38, p < 0.05), as well as a significant interaction between the two factors (F(7,168) = 3.37, p < 0.001), indicating effects of chronic stress on freezing behavior over successive trials. Post-hoc analysis revealed that stressed animals indeed showed significantly higher levels of freezing compared to controls in the third and fourth trials, although both groups eventually reached similar levels of freezing by the last trial of fear conditioning (Fig. 3a).
Twenty-four hours later, when tested for recall of fear memory in a different context (context B), both groups showed comparable levels of freezing in the first trial block (Fig. 3b). Control animals showed a gradual acquisition of fear extinction over successive trial blocks (Fig. 3b), with a significant reduction in freezing in the last trial block as compared to the first (t20 = 2.51, p < 0.05) (Fig. 3c). Stressed animals did not exhibit this gradual reduction but showed comparable levels of freezing between the first and last trial block (t28 = 0.77, p = 0.45), indicating an impairment in acquisition of fear extinction (Fig. 3b, c). There was a significant effect of stress (F(1,24) = 12.99, p < 0.01) on extinction acquisition (Fig. 3b), as well as an effect of trial blocks (F(6,144) = 4.19, p < 0.001), but no interaction between trial blocks and stress (F(6,144) = 1.373, p = 0.23).
When the recall of fear extinction memory was tested 24 h later, stressed animals showed significantly higher levels of freezing compared to controls, indicating impaired extinction memory (F(1,24) = 28.06, p < 0.0001) (Fig. 3d). Strikingly, this impairment persisted throughout the subsequent presentations of CS such that the stressed animals were unable to reduce their freezing to the levels exhibited by their unstressed counterparts (Fig. 3d). There was neither any effect of trial blocks (F(6,144) = 1.99, p = 0.07) nor an interaction between the two factors (F(6,144) = 0.19, p = 0.98).
In addition to quantifying freezing during CS, we also measured freezing to the context during all three sessions (Fig. 4). This was done by measuring freezing in the absence of any tone during a 20-s ‘pre-tone’ duration that preceded each presentation of the CS. As the intervals between the tones were pseudo-random, our method of analysis ensured contextual freezing was randomly sampled throughout the entire duration of the session. During fear conditioning, freezing to the context was not affected by stress (F(1,24) = 1.832, p = 0.18). Freezing, however, changed over time (F(7,168) = 47.61, p < 0.0001) for both groups, with stressed animals showing a higher freezing to the context before trial 3 (Fig. 4a). Similar to fear conditioning, stress also did not affect contextual freezing during acquisition or recall of fear extinction (extinction acquisition F(1,24) = 3.09, p = 0.09; extinction recall F(1,24) = 1.57, p = 0.23) (Fig. 4b, c). Although there was a main effect of time during extinction recall (extinction acquisition F(6,144) = 1.35, p = 0.23; extinction recall F(6,144) = 3.15, p < 0.01), there was no interaction between the factors in any of the sessions (fear conditioning F(7,168) = 1.67, p = 0.12; extinction acquisition F(6,144) = 1.35, p = 0.23; extinction recall F(6,144) = 3.15, p < 0.01). Finally, mean levels of contextual freezing across all three sessions showed no difference between stressed and control animals (fear conditioning t24 = 1.68, p = 0.11; extinction acquisition t24 = 1.93, p = 0.065; extinction recall t24 = 1.28, p = 0.21) (Fig. 4d). This confirmed that the differences in freezing during fear extinction were not influenced by a generalized, non-specific increase in freezing behavior but rather specific impairments in learning and memory.
Chronic stress does not affect freezing to the context, when fear memories are formed after stress exposure. Freezing to context was measured during a 20-s pre-tone window before every tone presented. a Chronic stress increased freezing to the context before trial 3 (pre-trial 3). Chronic stress did not affect freezing to the context during b extinction acquisition or c extinction recall. d Chronic stress also did not affect mean freezing to the context during any of the sessions (control, N = 11; chronic stress, N = 15). # indicates p < 0.05 in post-hoc Sidak’s test
Extinction of fear memory formed before stress
Next, we wanted to examine the effects of the same chronic stress specifically on the acquisition and recall of fear extinction alone. Hence, animals, all unstressed at this point, were first subjected to the same auditory fear conditioning paradigm used in the experiments described above. However, in the interest of greater clarity, freezing levels in animals which were subsequently assigned to either stress or control groups after fear conditioning are depicted separately during fear conditioning as well. This is to ensure there were no inherent baseline differences between the two groups prior to subjecting them to stress (Fig. 5a). In addition, both groups have been combined and plotted as a single control group in Online Resource 3.
Chronic stress does not affect extinction of fear memories formed before stress exposure. a Animals that were subsequently assigned to control or chronic stress groups show no differences in fear learning. b Chronic stress does not affect the acquisition of fear extinction. c Stressed and control animals both show a significant reduction in freezing by the end of extinction acquisition. d Chronic stress does not impair recall of fear extinction, of fear memory formed before stress (control, N = 11; chronic stress, N = 12). ** indicates p < 0.01 in Student’s unpaired two-tailed t test. Each trial block is an average of two trials
On the day of conditioning, both groups of rats showed low-baseline freezing to the tone alone (Fig. 5a, tone habituation). Next, they showed a gradual increase in freezing over seven successive presentations of tone-shock pairings (Fig. 5a) as evidenced by a significant effect of trials (F(7,147) = 11.58, p < 0.0001). In striking contrast to the previous experiment, however, there was no effect of stress (F(1,21) = 0.18, p = 0.68) in this case. There was also no interaction between the two factors (F(7,147) = 1.6, p = 0.14).
Following conditioning, animals were divided into two groups—half were stressed for the next 10 days while the others served as unstressed controls. Twenty-four hours after the final and tenth session of 2-h stress (day 11), both groups underwent extinction training in a different context B. During acquisition of fear extinction, freezing levels changed over trials for both groups (F(6,126) = 12.94, p < 0.0001) without any effect of stress (F(1,21) = 0.21, p = 0.65) or any interaction between both factors (F(6,126) = 0.98, p = 0.44). Stressed and control animals showed similar levels of freezing in the first trial block indicating comparable levels of fear recall (Fig. 5b). Subsequently, the freezing levels were significantly lower by the seventh trial block for both groups (control t20 = 3.39, p < 0.01; chronic stress t22 = 3.65, p < 0.01) (Fig. 5c) indicating a gradual acquisition of extinction by the end of the session. This was in striking contrast to the group that underwent post-stress conditioning, wherein stressed animals failed to show a significant decrease in freezing (Fig. 3b, c). Although stressed animals initially started from a higher level of freezing when tested for extinction recall 24 h later, their freezing decreased and came down to that of control levels upon subsequent presentations of CS, suggesting that they were able to extinguish the fear fully (Fig. 5d). As in the previous two sessions, there was only a significant effect of trial blocks (F(6,126) = 2.317, p < 0.05) without any main effect of stress (F(1,21) = 1.63, p = 0.22) or interaction between the two factors (F(6,126) = 0.241, p = 0.24) during recall of fear extinction.
To ensure that stress did not enhance freezing to the context in absence of the CS, we next quantified contextual freezing as described before (Fig. 6). Here too, stress did not affect freezing to context during fear conditioning (F(1,21) = 0.04, p < 0.84) (Fig. 6a), extinction acquisition (F(1,21) = 0.997, p = 0.33) (Fig. 6b), or extinction recall (F(1,21) = 0.05, p = 0.83) (Fig. 6c). However, contextual freezing varied over time during all three sessions (fear conditioning F(7,147) = 31.55, p < 0.0001; extinction acquisition F(6,126) = 4.35, p < 0.001; extinction recall F(6,126) = 2.39, p < 0.05). Stress affected freezing over time only during extinction acquisition, as indicated by a significant statistical interaction between both factors (F(6,126) = 2.489, p < 0.05), but post-hoc comparisons failed to find differences between the two groups. There was no interaction during either fear conditioning (F(7,147) = 0.99, p = 0.44) or extinction recall (F(7,147) = 1.25, p = 0.29). Finally, stress did not affect mean levels of contextual freezing across all three sessions (fear conditioning t21 = 0.03, p = 0.98; extinction acquisition t21 = 0.80, p = 0.43; extinction recall t21 = 0.24, p = 0.81) (Fig. 6d). Thus, in addition to extinction acquisition and recall not being affected by stress exposure after fear conditioning, freezing to the context was also not affected by chronic stress.
Chronic stress does not affect freezing to the context, when fear memories are formed before stress exposure. Freezing to context was measured during a 20-s pre-tone window before every tone presented. Chronic stress did not affect freezing to the context during a fear conditioning, b extinction acquisition, or c extinction recall. d Chronic stress also did not affect mean freezing to the context during any of the sessions (control, N = 11; chronic stress, N = 12)
Discussion
The present study examined the effects of chronic immobilization stress at two levels of neural organization. We first showed that the same chronic immobilization stress elicited divergent patterns of changes in dendritic spine density across different regions within the same brain (Fig. 7a). Stress caused a significant increase in spine density in the BLA, in agreement with previous reports (Mitra et al. 2005; Suvrathan et al. 2014). However, the same stress did not affect density of dendritic spines in ventral CA1, as has been reported with exposure to multimodal and restraint stressors (Maras et al. 2014). In addition, chronic stress significantly decreased spine density on apical dendrites of IL-mPFC principal neurons without any effect in the PL-mPFC. Within the IL region, the decrease was more pronounced in layer II/III neurons as compared to layer V/VI. Although chronic stress has previously been shown to reduce spines and dendritic arborization in both the IL (Goldwater et al. 2009) and PL regions of the mPFC (Liston et al. 2006; Radley et al. 2006, 2009b; Garrett and Wellman 2009), the divergent effect seen here might be because the PL neurons need a more prolonged exposure to stress to elicit morphological changes compared to IL neurons. In fact, the same stressor has been shown to cause a reduction in dendritic arborization in the IL, but not in PL, suggesting that the former is more susceptible to stress exposure (Izquierdo et al. 2006).
Summary of experimental results. a Summarized result of changes in spine density due to chronic stress. BLA is represented by the positive y-axis, and IL is represented by the negative y-axis. Chronic stress decreases spine density in layer II/III of IL, and increases it in the BLA. Spine density in the PL and vCA1 remains unaffected by stress. * indicates reduction in spine density seen in proximal dendritic segment in layer V/VI of IL. b Summary of behavior results. Chronic stress impairs both acquisition and recall of fear extinction when fear learning happens after, but not before stress. ‘X’ indicates no change
Interestingly, an earlier report using the same chronic immobilization stress did not report any difference in spine density in the IL (Shansky et al. 2009). This could be because shorter dendritic segments of varying lengths were analyzed in this study. In contrast, analysis of a fixed length of primary dendrite (70 μm) starting from its origin has revealed the distinct effects of stress on proximal versus distal dendritic spine distribution in our study. In the present study, these differential effects of stress on the total versus segmental spine density are most clearly visible in layer V/VI neurons of IL-mPFC. Detailed segmental analysis of spine numbers, by keeping track of the location of spines along the dendrite, is also important because in both cortical and hippocampal pyramidal neurons, different segments of dendrites are known to receive specific afferent inputs (Spruston 2008). Therefore, synapses at proximal versus distal regions of the same dendrite might have different functional implications for interactions between brain regions after stress exposure. Spines on the proximal dendritic regions of principal neurons of the BLA, for example, were found to be the best predictor of stress-induced increase in anxiety-like behavior on the elevated plus maze (Rao et al. 2012). Moreover, functional connectivity from the BLA to hippocampal area CA1 grows stronger during stress, while weakening CA3-to-CA1 connectivity at the same time (Ghosh et al. 2013), raising the possibility that specific patterns of spine changes may serve as a synaptic substrate for the stress-induced modulation of directional influence from one region to another.
Our findings also reveal that the eventual impact of chronic stress depended on its timing with respect to the formation of the fear memory (i.e., pre- versus post-stress conditioning). How does the behavioral impact of the same stress differ so significantly between the two experimental designs? In agreement with several past studies, the results from our post-stress conditioning experiments suggest that the balance of activity in the BLA is tilted in favor of enhanced fear. This is in agreement with previous reports on stress-induced spinogenesis and enhanced LTP, alongside decreased inhibitory tone, in the BLA (Suvrathan et al. 2014). Together, these cellular changes contribute to the stress-induced facilitation in fear expression seen here, as well as in past studies (Conrad et al. 1999; Rodrigues et al. 2009; Suvrathan et al. 2014; Maren and Holmes 2016). On the other hand, the same stress weakens the structural basis of synaptic connectivity, as evidenced by the loss of dendritic spines, on IL pyramidal neurons. IL activity is required for within-session extinction of fear (Do-Monte et al. 2015) and consolidation of fear extinction (Burgos-Robles et al. 2007), as well as fear extinction recall (Milad and Quirk 2002; Sierra-Mercado et al. 2011). Chronic stress has also been shown to impair LTP induction (Goldwater et al. 2009) and activity of IL neurons during extinction (Wilber et al. 2011). Moreover, the BLA and IL have contrasting roles in the expression of fear (Sierra-Mercado et al. 2011). Therefore, enhanced activity in the BLA, alongside impaired IL function, is likely to tilt the balance in favor of higher fear that is resistant to subsequent extinction, as observed in the persistently high levels of freezing during the acquisition and recall of fear extinction in animals that underwent post-stress conditioning (Fig. 3b–d). These deficits could not be explained by enhanced generalized freezing to the extinction context which remained unaffected by stress (Fig. 4), suggesting that they were indeed specific deficits in acquisition of fear extinction and its subsequent recall.
Surprisingly, when fear memories were formed before the same chronic stress, this balance appears to shift in a manner that no longer impairs the acquisition and recall of fear extinction (Fig. 7b). In other words, decoupling the effects of stress on acquisition versus extinction of fear revealed that stress acts primarily on acquisition, and there is no significant deficit in fear extinction per se. How is it that stress-induced strengthening of fear memories in the BLA has no visible impact on freezing levels in the pre-stress conditioning experiments? An intriguing possibility is suggested by a recent study by Do-Monte et al. 2015, reporting that neural circuits mediating the recall of fear memories shift over time such that targeted inactivation of the BLA 6 h, but not 7 days, after fear conditioning reduced freezing. In other words, the 10-day long chronic stress paradigm may have introduced a sufficiently long delay between the formation and recall of fear memories. And this, in turn, resulted in the BLA, despite stress-induced hyperactivity, having no impact on the subsequent expression of fear. Furthermore, accumulating evidence points to an important role for the PL-mPFC in the expression of fear (Sierra-Mercado et al. 2011; Dejean et al. 2016). Notably, the chronic stress paradigm used here did not affect PL spine density. Taken together, these findings suggest that in animals that underwent conditioning before stress, the expression of fear after extinction reflects normal function of the PL-mPFC, and not stress-induced changes in either the BLA or IL-mPFC. These findings, in turn, give rise to specific predictions on how the timing of stress may have differential effects on neural activity in these brain areas during the formation and extinction of fear memories. Future studies, using in vitro and in vivo electrophysiological analyses, will be needed to address these questions in greater detail.
In conclusion, comparing the results emerging from the two different experimental designs used in this study, we find that chronic stress differentially affects the extinction of fear memories that are formed after stress, to those that are formed before—underlining the importance of timing of fear memory formation with respect to when the stressful experience occurs. A better understanding of this temporal interplay between stress, fear, and extinction and how that affects functional interactions between multiple brain regions may lead to new strategies for the treatment of stress-related psychiatric disorders.
References
Arnsten AFT (2015) Stress weakens prefrontal networks: molecular insults to higher cognition. Nat Neurosci 18(10):1376–1385
Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD (2009) Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem 91(3):323–332
Blanchard DC, Blanchard RJ (1988) Ethoexperimental approaches to the biology of emotion. Annu Rev Psychol 39(1):43–68
Bremner JD (2002) Neuroimaging studies in post-traumatic stress disorder. Curr Psychiatr Rep 4(4):254–263
Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ (2007) Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron 53(6):871–880
Chang CH, Maren S (2010) Strain difference in the effect of infralimbic cortex lesions on fear extinction in rats. Behav Neurosci 124(3):391–397
Chattarji S, Tomar A, Suvrathan A, Ghosh S, Rahman MM (2015) Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 18(10):1364–1375
Conrad CD, LeDoux JE, Magariños AM, McEwen BS (1999) Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113(5):902–913
Dejean C, Courtin J, Karalis N, Chaudun F, Wurtz H, Bienvenu TCM, Herry C (2016) Prefrontal neuronal assemblies temporally control fear behaviour. Nature 535(7612):420–424
Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ (2015) Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci 35(8):3607–3615
Farrell, M. R., Sengelaub, D. R., & Wellman, C. L. (2013). Physiology & Behavior Sex differences and chronic stress effects on the neural circuitry underlying fear conditioning and extinction. Physiol Behav, 122, 208–215
Garrett JE, Wellman CL (2009) Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience 162(1):195–207
Ghosh S, Laxmi TR, Chattarji S (2013) Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J Neurosci 33(17):7234–7244
Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH (2009) Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience 164(2):798–808
Hayashi ML, Choi SY, Shankaranarayana Rao BS et al (2004) Altered cortical synaptic morphology and impaired memory consolidation in forebrain- specific dominant-negative PAK transgenic mice. Neuron 42:773–787
Hayashi ML, Rao BSS, Seo J-S, Choi HS, Dolan BM, Choi SY, Chattarji S, Tonegawa S (2007) Inhibition of p21-activated kinase rescues symptoms of fragile X syndrome in mice. Proc Natl Acad Sci 104:11489–11494
Hoffman AN, Lorson NG, Sanabria F, Olive MF, Conrad CD (2014) Chronic stress disrupts fear extinction and enhances amygdala and hippocampal Fos expression in an animal model of post-traumatic stress disorder. Neurobiol Learn Mem 112:139–147
Izquierdo A, Wellman CL, Holmes A (2006) Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26(21):5733–5738
Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB (2002) Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci 99(16):10825–10830
Knox D, George SA, Fitzpatrick CJ, Rabinak CA, Maren S, Liberzon I (2012) Single prolonged stress disrupts retention of extinguished fear in rats. Learn Mem 19(2):43–49
Koenigs M, Grafmann J (2009) Post-traumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist 15(5):540–548
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23(1):155–184
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR et al (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26(30):7870–7874
Maras PM, Molet J, Chen Y, Rice C, Ji SG, Solodkin A, Baram TZ (2014) Preferential loss of dorsal-hippocampus synapses underlies memory impairments provoked by short, multimodal stress. Mol Psychiatry 19(7):811–822
Maren S, Holmes A (2016) Stress and fear extinction. Neuropsychopharmacology 41(1):58–79
Maroun M, Ioannides PJ, Bergman KL, Kavushansky A, Holmes A, Wellman CL (2013) Fear extinction deficits following acute stress associate with increased spine density and dendritic retraction in basolateral amygdala neurons. Eur J Neurosci 38(4):2611–2620
McEwen BS (1999) Stress and hippocampal plasticity. Annu Rev Neurosci 22(1):105–122
McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C (2015) Mechanisms of stress in the brain. Nat Neurosci 18(10):1353–1363
McLaughlin, K. J., Gomez, J. L., Baran, S. E., & Conrad, C. D. (2007). The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res, 1161(1), 56–64
Milad MRR, Quirk GJJ (2002) Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420(6911):70–74
Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL (2006) Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85:213–218
Mitra R, Adamec R, Sapolsky R (2009) Resilience against predator stress and dendritic morphology of amygdala neurons. Behav Brain Res 205(2):535–543
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S (2005) Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci 102(26):9371–9376
Mitra R, Sapolsky RM (2008) Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci 105(14):5573–5578
Mitra R, Sapolsky RM (2009) Effects of enrichment predominate over those of chronic stress on fear-related behavior in male rats. Stress 12(4):305–312
Moench KM, Maroun M, Kavushansky A, Wellman C (2016) Alterations in neuronal morphology in infralimbic cortex predict resistance to fear extinction following acute stress. Neurobiol Stress 3:23–33
Morgan MA, LeDoux JE (1995) Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci 109(4):681–688
Patel D, Chattarji S, Buwalda B (2017) Repeated social stress in rats leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Eur Neuropsychopharmacol 27:S984
Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S (2005) Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci 102(50):18201–18206
Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH (2005) Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol 196(1):199–203
Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR et al (2006) Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16(3):313–320
Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS et al (2008) Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol 507(1):1141–1150
Rao RP, Anilkumar S, McEwen BS, Chattarji S (2012) Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry 72(6):466–475
Rao, R. P., Suvrathan, A., Miller, M. M., McEwen, B. S., & Chattarji, S. (2009). PTSD: From neurons to networks. In Post-traumatic stress disorder (pp. 151–184). Humana Press
Rauch SL, Shin LM, Phelps EA (2006) Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol Psychiatry 60(4):376–382
Rauch, S. L., Whalen, P. J., Shin, L. M., McInerney, S. C., MacKlin, M. L., Lasko, N. B., … Pitman, R. K. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry, 47(9), 769–776
Rodrigues SM, LeDoux JE, Sapolsky RM (2009) The influence of stress hormones on fear circuitry. Annu Rev Neurosci 32:289–313
Roozendaal B, McEwen BS, Chattarji S (2009) Stress, memory and the amygdala. Nat Rev Neurosci 10(6):423–433
Santini, E., Quirk, G. J., & Porter, J. T. (2008). Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. Journal of Neuroscience, 28(15), 4028–4036
Shansky, R. M., Hamo, C., Hof, P. R., McEwen, B. S., & Morrison, J. H. (2009). Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral cortex, 19(10), 2479-2484.
Shin, L. M., Wright, C. I., Cannistraro, P. A, Wedig, M. M., McMullin, K., Martis, B., … Rauch, S. L. (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry, 62(3), 273–281
Sierra-Mercado D, Padilla-Coreano N, Quirk GJ (2011) Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36(2):529–538
Silva-Gomez AB, Rojas D, Juarez I, Flores G (2003) Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res 983(1–2):128–136
Silva-Gómez AB, Aguilar-Salgado Y, Reyes-Hernández DO, Flores G (2013) Dexamethasone induces different morphological changes in the dorsal and ventral hippocampus of rats. J Chem Neuroanat 47:71–78
Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ (2012) Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76(4):804–812
Spruston N (2008) Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci 9(3):206–221
Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S (2014) Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond Ser B Biol Sci 369(1633):20130151
Trivedi MA, Coover GD (2004) Lesions of the ventral hippocampus, but not the dorsal hippocampus, impair conditioned fear expression and inhibitory avoidance on the elevated T-maze. Neurobiol Learn Mem 81(3):172–184
Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ (2006) Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem 13(6):728–733
Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S (2002) Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22(15):6810–6818
Wessa M, Flor H (2007) Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatr 164(11):1684–1692
Wicking M, Steiger F, Nees F, Diener SJ, Grimm O, Ruttorf M et al (2016) Deficient fear extinction memory in posttraumatic stress disorder. Neurobiol Learn Mem 136:116–126
Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL (2011) Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience 174:115–131
Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S (2008) Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology 33(9):2108–2116
Acknowledgements
The authors would like to acknowledge Dr. Siddhartha Datta for his help with analysis of the contextual freezing data.
Funding
This work was supported by funds from the Department of Atomic Energy and Department of Biotechnology, Government of India, and the Madan and Usha Sethi Fellowship.
Author information
Authors and Affiliations
Contributions
PC and SC contributed to the experimental design. PC performed the experiments and analyzed the data. PC and SC interpreted the results. PC and SC wrote the manuscript.
Corresponding author
Ethics declarations
All maintenance and experimental procedures were approved by the Institutional Ethics Committee, National Centre for Biological Sciences, India.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article belongs to a Special Issue on Psychopharmacology of Extinction
Rights and permissions
About this article
Cite this article
Chakraborty, P., Chattarji, S. Timing is everything: differential effects of chronic stress on fear extinction. Psychopharmacology 236, 73–86 (2019). https://doi.org/10.1007/s00213-018-5053-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5053-y