Abstract
Rationale
Synthetic cathinones (“bath salts”) are β-ketone analogs of amphetamines, yet few studies have examined their potential neurotoxic effects.
Objective
In the current study, we assessed the persistent behavioral and neurochemical effects of exposure to the second-generation synthetic cathinone α-pyrrolidinopropiophenone (α-PPP).
Methods
Male, Swiss-Webster mice were exposed to α-PPP (80 mg/kg) using a binge-like dosing regimen (QID, q2h). Behavior was assessed 4–5 days after the dosing regimen, and neurochemistry was assessed the following day. Behavior was studied using the elevated plus maze, Y-maze, and novel object recognition tests. Regional levels of dopamine, serotonin, norepinephrine, and the major dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) were determined in the prefrontal cortex and striatum using high-pressure liquid chromatography. Additional experiments assessed the time courses of the effects of α-PPP on locomotor activity and core temperature using telemetry.
Results
Exposure to α-PPP significantly impaired performance in the Y-maze, decreased overall exploratory activity in the novel object recognition test, and resulted in regionally specific depletions in monoamine neurochemistry. In contrast, it had no significant effect on elevated plus maze performance or object discrimination in the novel object recognition test. The locomotor-stimulant effects of α-PPP were comparable to cocaine (30 mg/kg), and α-PPP (80 mg/kg) did not induce hyperthermia.
Conclusions
α-PPP exposure results in persistent changes in exploratory behavior, spatial working memory, and monoamine neurochemistry. This research highlights potential dangers of α-PPP, including potential neurotoxicity, and suggests that the mechanisms underlying the persistent untoward effects of the cathinones may be distinct from those of the amphetamines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amphetamine derivatives such as methamphetamine (METH) and 3,4-methylenedioxymethamphetamine (MDMA) have high abuse liabilities and have been associated with loss of brain content of monoamine neurotransmitters and catabolic enzymes (Kish et al. 2000; Wilson et al. 1996) and loss of monoamine neurotransmitter regulating proteins (McCann et al. 1998; Reneman et al. 2001). Amphetamines can also induce changes in learning and memory and behavior. For example, significant reductions in spontaneous locomotor activity (Timar et al. 2003) and deficits in appetitive and aversive Pavlovian learning (Achat-Mendes et al. 2005) have been reported in animals exposed to amphetamines. Our laboratory has reported that dosing regimens of METH and para-chloroamphetamine (PCA) decreased tissue content of monoamines and impaired long-term memory in an inhibitory avoidance task (Murnane et al. 2012). Such studies have informed our understanding of the potential dangers of amphetamine derivatives.
Synthetic cathinones are β-ketone analogs of amphetamine. Their misuse is prevalent, and they carry serious abuse liability and physiological risks. These agents are often marketed as “not for human consumption” and sold as “bath salts” or “research chemicals.” Previous studies of synthetic cathinones have primarily examined the “first-generation” compounds methylone, mephedrone, and methylenedioxypyrovalerone (MDPV), which were among the first cathinones showing widespread abuse (Wood et al. 2012) and were initially placed in schedule 1 of the Controlled Substances Act in 2011. A recent literature has begun to document their potential neurotoxic effects. For example, binge-like self-administration of MDPV resulted in neurodegeneration and deficits in the novel object recognition (NOR) test (Sewalia et al. 2018). Likewise, adolescent rats exposed to methylone exhibited persistent depletions of serotonin and deficits in reference memory (Lopez-Arnau et al. 2014). However, mephedrone and methylone have also been reported to not persistently deplete brain monoamine content (Baumann et al. 2012). These studies warrant further research into the effects of exposure to synthetic cathinones.
We operationally define “second-generation” cathinones as the compounds, such as pentylone and alpha-pyrrolidinopentiophenone (α-PVP), which have shown increased abuse more recently and were placed into the Controlled Substances Act in 2014 or remain unscheduled. In recent years, numerous second-generation synthetic cathinones have emerged that contain a pyrrolidine ring, similar to MDPV and α-PVP, in place of the secondary amine of methamphetamine-like synthetic cathinones. There is much reason for concern regarding this second generation of synthetic cathinones as paranoia and psychosis elicited by pyrrolidine synthetic cathinones have been reported in the clinical literature (John et al. 2014; Murphy et al. 2013; Penders et al. 2012; Prosser and Nelson 2012; Ross et al. 2012; Stoica and Felthous 2013). A prototypical second-generation pyrrolidine synthetic cathinone is the compound alpha-pyrrolidinopropiophenone (α-PPP). Its molecular pharmacology was recently characterized, demonstrating that α-PPP has high affinity for the dopamine and norepinephrine transporters (approximately 1–2 μM), where it functions as a reuptake inhibitor. In contrast, α-PPP did not have any substrate-based releasing properties and substantially lower affinity for the serotonin transporter (approximately 163 μM) (Eshleman et al. 2017). Likewise, it was recently demonstrated that α-PPP maintains responding in a self-administration paradigm where it was available under a fixed ratio 5 schedule of reinforcement, and its rank order of potency for self-administration in comparison to other second-generation cathinones was consistent with its affinity for the dopamine transporter (Gannon et al. 2018). Furthermore, it was recently demonstrated that α-PPP is a locomotor stimulant and has cocaine-like and methamphetamine-like discriminative stimulus effects (Gatch et al. 2017), and its locomotor-stimulant effects can be blocked by the selective dopamine D1-like receptor antagonist SCH-23390 (Marusich et al. 2014). As such, α-PPP is an emerging second-generation pyrrolidine derivative of MDPV with clear reinforcing and stimulant effects, and individuals who consume α-PPP may be at risk of persistent untoward behavioral and neurobiological changes consistent with neurotoxicity.
In the present study, to elucidate the persistent effects of exposure to α-PPP, we examined the effects of administration of α-PPP on neurochemistry and behavior. Four days after the dosing regimen, we examined working memory, recognition memory, and anxiety. The following day, we performed an extensive neurochemical profiling across the striatum and prefrontal cortex for monoamine neurochemistry. We hypothesized that exposure to α-PPP would deplete brain levels of dopamine and norepinephrine as well as induce memory deficits and increase anxiety.
Materials and methods
Animals
The test subjects were male Swiss Webster mice (Charles River Laboratories; Wilmington, MA) that weighed 27–33 g and ranged in age from 2 to 3 months. For the experiments examining the persistent effects of α-PPP on neurochemistry and behavior, 18 mice treated with α-PPP and 16 mice treated with saline were included in the final analyses, divided between two groups. The mice were housed 3 or 4 per cage in a temperature-regulated room. For the locomotor activity and core temperature experiments, an additional six mice were prepared with telemetry probes (see surgery section). These mice weighed 25–30 g and ranged in age from 2 to 3 months at the time of the surgery and were kept singly housed for the duration of the study. All mice had ad libitum access to food and water. Mice were housed in rooms maintained in a 12-h light/dark cycle. All experiments were conducted during the light phase and at typical ambient temperatures (approximately 18–21 °C). All experiments were conducted using protocols approved by the Mercer University Institutional Animal Care and Use Committee.
Drugs and chemicals
Perchloric acid (HClO4, 3752) was purchased from GFS Chemicals (Powell, OH). Hydrochloric acid (HCl, CAS 7647-01-0) was purchased from Carolina Biological Supply Company (Burlington, NC). 3,4-dihydroxyphenylacetic acid (850217), dopamine hydrochloride (H8502), serotonin hydrocloride (H9523), and norepinephrine bitartrate (A0937) were purchased from Sigma-Aldrich (St. Louis, MO). All injections were administered intraperitoneally at a volume of 0.01 ml physiological (0.9%) saline (vehicle) or drug solution (dissolved in vehicle) per gram body weight of each mouse. All doses are reported in the salt form.
Dosing regimen
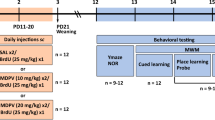
The outline for this study is presented in Fig. 1. All drugs were administered 4 times with 2 h separating each administration. Amphetamine derivatives induce persistent neurochemical depletions as well as disruption of learning and memory under this dosing regimen (Murnane et al. 2012). All treatments are described as the unit dose per administration. We used an effect-scaling procedure, wherein the unit dose was increased until greater than 10% lethality was observed, to achieve near maximal toxicity (Fantegrossi et al. 2008; Wang et al. 2004). As outlined in Fig. 1, experiments were conducted in two groups. Group 1 underwent dosing with α-PPP (80 mg/kg, QID, q2h, IP) and was assessed in the Y-maze and elevated plus maze (EPM) 4 days later, with EPM assessments occurring at least 3 h after the Y-maze assessments. Group 2 underwent dosing with α-PPP and was assessed in the NOR test 4 days later and sacrificed for neurochemical analysis the following day.
Outline of the study design. The dosing consisted of a regimen of 4 unit doses of 80 mg/kg of α-PPP or saline administered over an 8-h period with each dose separated by 2 h. The dosing regimen was administered on day 1, and its persistent effects on anxiety, memory, and tissue monoamine neurochemistry were assessed as presented. Two separate groups of mice were assessed. Group 1 underwent dosing with α-PPP (80 mg/kg, QID, q2h, IP) and was assessed in the Y-maze and elevated plus maze (EPM) 4 days later, with EPM assessments occurring at least 3 h after the Y-maze assessments. Group 2 underwent dosing with α-PPP (80 mg/kg, QID, q2h, IP) and was assessed in the NOR test 4 days later and sacrificed for neurochemical analysis the following day
Body weight and rectal temperature
Body weight and rectal temperature were recorded at baseline, immediately prior to each injection, and 2 h after the last injection. Body weight was recorded by placing the mouse on a calibrated scale. Temperature was measured by inserting a lubricated probe 1.5 cm into the rectum and recording the readout from a connected TH-8 Thermalert temperature monitor (Physitemp Instruments; Clifton, NJ, USA) after the signal reached steady state.
Y-maze
Spontaneous alternation in the Y-maze has been proposed to measure hippocampus-dependent spatial working memory (Walker and Gold 1994). Each Y-maze session lasted for 10 min. Performance in the Y-maze was assessed by both automated quantitation (Maze Engineers; Cambridge, MA) and manual observation. Spontaneous alternations were calculated as the percentage of the total arm entries minus two composed of triads containing entries into all arms.
EPM
The EPM has been proposed to measure anxiety (Nic Dhonnchadha et al. 2003) and risk-taking behavior (Laviola et al. 2003). At the beginning of each session, animals were individually placed in the central platform of the maze facing a closed arm. Each EPM session lasted for 10 min. Performance in the EPM was assessed by both automated quantitation (Maze Engineers) and manual observation. The primary dependent measures of anxiety were time spent on the open arms, number of open arm entries, and the number of open arm entries as a proportion of total entries (open arm entries: total arm entries).
NOR test
The NOR test has been proposed to measure recognition memory by allowing mice to explore novel and familiar objects (Antunes and Biala 2012). Each animal had a familiarization (training) session with a pair of identical objects (~ 5 cm long × 5 cm wide × 10 cm high) placed 5 cm away from the walls but adjacent in the open field. Each mouse was placed in the open field, its head facing the wall opposite the objects. Six hours after familiarization (Leger et al. 2013), each mouse was tested in the same box with a novel object replacing one of the familiar objects. Familiarization and testing sessions lasted 10 min, were video recorded, and were assessed offline. Exploration was defined as sniffing the object or orienting the head towards the object while the subject was within 1 cm of distance from the object. Total object exploration time was calculated as the sum of time exploring either object. The percentage of the total exploration time spent exploring the novel object was calculated to reflect recognition memory.
Brain dissection
To assess neurochemistry, mice were euthanized by cervical dislocation and decapitation. Brains were rapidly removed on ice and stored at − 80 °C for subsequent analysis. Brains were subsequently thawed at 4 °C and placed in an ice-cold mouse brain matrix. Brains were sliced into 1-mm-thick coronal sections, and these slices were placed flat on a cold plate over ice. Using a 1.5-mm diameter tissue biopsy-punch, regions of interest were taken from individual slices, as we have described previously (Murnane et al. 2012).
Neurochemical measurements
Frozen tissues were weighed, sonically disrupted in 200 μl of 0.3 N HClO4, and centrifuged for 10 min at 4 °C to remove cellular debris. A 100-μl aliquot of the supernatant was placed in an WPS-3000TBSL autosampler maintained at 10 °C, and 10 μl was injected onto a Thermo Scientific (Waltham, MA) Hypersil BDS C18 column (35 °C) with Thermo Scientific Dionex Test Phase running at a flow rate of 0.5 ml/min. Coulometric detection was accomplished with a Thermo Scientific Dionex 6011RS electrode cell, and the signal analyzed on a Thermo Scientific Dionex Chromeleon CDS processing platform. Absolute tissue concentrations (ng/mg) for the monoamine neurotransmitters dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), serotonin, and norepinephrine were determined by comparison with external standard curves and corrected for tissue weight, as we have described previously (Murnane et al. 2012).
Telemetry probe surgery
To examine the locomotor-stimulant effects of α-PPP, its effects on core body temperature, and its pharmacokinetic profile, an additional six mice were prepared with Starr Life Sciences (Oakmont, PA) G2 E-Mitters using similar procedures to those described previously (Gannon et al. 2016). After achievement of an appropriate level of anesthesia (inhaled isoflurane 1–3% induction, maintenance to effect), abdominal hair was removed and the surgical area cleansed with soap and water. Subjects were placed in a supine position, the surgical site was disinfected with isopropyl alcohol, and at least 3 min later, a midline abdominal incision of less than 2 cm was made, approximately 1 cm caudal to the diaphragm. The probes were sterilized using Benz-All (benzalkonium chloride 12.9%, diluted 1:50; Moore Medical, Farmington, CT) according to the manufacturer’s instructions. The probe was implanted sagittally, ventral to the caudal arteries and veins, and dorsal to the digestive organs, and the incision was closed with interrupted mattress absorbable sutures (5-0 Vicryl). Cephazolin (10 mg/kg, IP) was given at the end of surgery for antibiotic prophylaxis, and ketoprofen (2 mg/kg, IP) was at the beginning of the surgery as well as once daily for 3 days to relieve any pain/inflammation.
Data analysis
All graphical data presentations were created using GraphPad Prism 7.0 (GraphPad Software Inc.; La Jolla, CA). The time courses of the telemetry data (locomotor activity and core temperature) comparing saline versus α-PPP were integrated by standard area under the curve analysis in GraphPad, and then analyzed by repeated measures one-way analysis of variance, with post hoc comparisons by Tukey’s test. All other data comparing saline versus α-PPP were analyzed by unpaired t test, and corrected for multiple comparisons by the Bonferroni method to maintain the probability of making a type 1 error at 5%.
Results
Body weight and rectal temperature
Over the course of the dosing regimen, body weight and rectal temperature were recorded at baseline, immediately prior to each injection, and 2 h after the last injection. The time course of the effects of α-PPP is presented in Fig. 2. Consistent with our previous studies with stimulants, α-PPP significantly reduced body weight (t = 4.15; p < 0.001) over the course of the dosing regimen. Surprisingly, there was no significant change in rectal temperature during α-PPP dosing (t = 1.96; p = 0.06).
The effects of α-PPP (N = 18) on body weight and rectal temperature in comparison to saline (N = 16) over the course of the dosing regimen. The time courses for body weight (A) and rectal temperature (B) change are presented in the top row. The peak changes over the entire time course for body weight (C) and rectal temperature (D) are presented in the bottom row. All values are the mean ± SEM. ***p < 0.001 as assessed by unpaired t test
Effects on behavior
The persistent effects of α-PPP exposure on behavior in the EPM and Y-maze were examined 4 days after dosing (Fig. 1). There was no significant effect α-PPP, at the tested dosing regimen, in comparison to saline on closed arm time, closed-arm entries, or open-arm entries in the EPM (Fig. 3). Exposure to α-PPP increased open-arm time, (t = 1.85; p = 0.04) in a manner consistent with increased risk-taking behavior, but this effect was not accepted as significant because of Bonferroni correction. There was a significant decrease in spontaneous alternations in the Y-maze in the animals exposed to α-PPP (t = 2.21; p < 0.04) in comparison to the animals exposed to saline (Fig. 4). The persistent effects of α-PPP exposure on behavior in the NOR test were examined 4 days after dosing (Fig. 1). There was a significant decrease in exploratory activity in the animals exposed to α-PPP (t = 5.40; p < 0.001) in comparison to the animals exposed to saline (Fig. 5). In contrast, there was no significant group difference in the percentage of time directed to the novel object.
The persistent effects of α-PPP (N = 9) on anxiety in comparison to saline (N = 8) as assessed using the elevated plus maze 4 days after dosing. Abscissae: distribution of behavior on the closed or open arms of the maze. Ordinates: time spent on each arm (a) or the number of entries onto each kind of arm (b) over the 10-min session. All values are the mean ± SEM. There was no significant difference between the treatments on either measure
The persistent effects of α-PPP (N = 9) on working memory in comparison to saline (N = 8) as assessed using the Y-Maze 4 days after dosing. Ordinates: spontaneous alternations expressed as the percentage of arm entries that were a part of triads of three unique arm entries. All values are the mean ± SEM. *p < 0.05 as assessed by unpaired t test
The persistent effects of α-PPP (N = 9) on recognition memory in comparison to saline (N = 8) as assessed using the novel object recognition test 4 days after dosing. Ordinates: time spent exploring both objects (a) or the percentage of exploration time that was directed at the novel object (b) over the 10-min retention session. All values are the mean ± SEM. *p < 0.05 as assessed by unpaired t test
Effects on neurochemistry
The persistent effects of α-PPP exposure on tissue monoamine neurochemistry were assessed by HPLC analysis of brain tissue punches 5 days after dosing (Fig. 1). Relative to saline-treated animals, there was a significant depletion of tissue dopamine levels in the striatum (t = 4.35; p < 0.001) in the animals exposed to α-PPP (Fig. 6), but no significant change in dopamine levels measured in the prefrontal cortex. Similarly, there was a significant depletion of tissue DOPAC levels in the striatum (t = 2.60; p = 0.02), but not in the prefrontal cortex (t = 2.13; p = 0.06), in the animals exposed to α-PPP. Furthermore, there was no significant change in dopamine turnover in either the striatum or the prefrontal cortex. Relative to saline-treated animals, serotonin levels in the striatum were significantly reduced (t = 5.35; p < 0.001) in the animals exposed to α-PPP; however, no changes in serotonin were detected in the prefrontal cortex (t = 2.04; p = 0.07). Norepinephrine levels were significantly reduced in the striatum (t = 3.25; p = 0.01) and the prefrontal cortex (t = 4.52; p = 0.01) in the animals exposed to α-PPP.
The persistent effects of α-PPP (N = 7) on tissue concentration of dopamine (a), DOPAC (b), turnover of dopamine into DOPAC (c), tissue concentration of serotonin (d), and tissue concentration of norepinephrine (e) in the prefrontal cortex and the striatum in comparison to saline (N = 8). Abscissae: the brain region that was assessed. Ordinates: tissue concentration expressed in nanograms of neurochemical per milligram of tissue weight, with the exception of dopamine turnover, which is expressed as the ratio of DOPAC to dopamine. All values are the mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001 as assessed by unpaired t test
Effects on locomotor activity and Core temperature
The second-generation synthetic cathinone α-PPP did not induce hyperthermia during the dosing regimen. This is somewhat surprising as we observed robust neurochemical depletions and behavioral impairments, and hyperthermia has been previously tied to the neurotoxic effects of amphetamines (Miller and O'Callaghan 1995) and we have previously observed hyperthermia with MDMA, METH, and PCA (Murnane et al. 2012). We therefore used continuous telemetry assessments to verify that α-PPP functioned as a locomotor stimulant in our laboratory and to assess its effects on core temperature in real-time. The time course of the effects of α-PPP on locomotor activity and core temperature is presented in Fig. 7. For statistical analysis, we determined the area under the curve of the change in locomotor activity and core temperature in each subject. One-way repeated measures analysis of variance revealed a significant main effect of 40 and 80 mg/kg of α-PPP (F3,23 = 7.35; p < 0.01) on locomotor activity in comparison to saline and a positive control dose of cocaine (30 mg/kg) that we have previously determined to robustly increase locomotor activity. Tukey’s post hoc analysis revealed a significant difference between saline and 40 mg/kg of α-PPP (p < 0.05), 80 mg/kg α-PPP (p < 0.01), and cocaine (p < 0.05), but no significant difference between either dose of α-PPP and cocaine. In contrast, one-way repeated measures analysis of variance revealed no significant main effect of cocaine and α-PPP on core temperature in comparison to saline.
The effects of α-PPP on locomotor activity and core temperature (N = 6). a Time course of the locomotor-stimulant effects of α-PPP in comparison to cocaine and to saline. b Time course of the effects of α-PPP on core temperature in comparison to saline. c Area under the curve of the locomotor-stimulant effects of α-PPP in comparison to cocaine and to saline. d Area under the curve of the effects of α-PPP on core temperature in comparison to saline. All values are the means + SEM. *p < 0.05; **p < 0.01 as assessed by one-way analysis of variance and Tukey’s post hoc test
Discussion
In this study, we document persistent effects of the second-generation pyrrolidine synthetic cathinone α-PPP on behavior and neurochemistry. We document that, consistent with previous studies of amphetamine derivatives (Murnane et al. 2012), α-PPP acutely decreases body weight over the course of a standard 6-h dosing regimen. Consistent with previous studies of α-PPP (Gatch et al. 2017; Marusich et al. 2014), we report that it has robust locomotor-stimulant effects in our laboratory that are comparable to cocaine. We have found that α-PPP persistently depletes the levels of monoamine neurotransmitters in the striatum and frontal cortex and induces significant memory impairments, as measured in the Y-maze assay, and decreased exploratory activity in the NOR test. However, these deficits were not matched by similar changes in the EPM test or deficits in recognition memory in the NOR test.
In the present study, α-PPP induced acute weight loss but did not induce acute hyperthermia. This may be surprising as α-PPP did induce both functional and neurochemical deficits, and previous studies indicate that hyperthermia is a key component of the neurotoxic effects of amphetamine derivatives. For example, preventing the hyperthermic response to MDMA has been shown to block MDMA exposure associated neurotoxicity (Miller and O'Callaghan 1995), and reductions in serotonin content and serotonin transporter expression were exacerbated when MDMA-induced hyperthermia than when it did not (Broening et al. 1995). Similar findings have been reported with cathinones, as exposure of adolescent rats to methylone in a warm ambient environment, using a dosing regimen similar to the one used in the present study (20 mg/kg, QID, q3h, SC), induced an acute hyperthermic response and persistent depletions of serotonin and deficits in reference memory (Lopez-Arnau et al. 2014). Possible interpretations for the lack of hyperthermia induced by α-PPP are that (1) it does not function as a stimulant in our laboratory; (2) its induction of hyperthermia is so short lasting that the hyperthermia had abated prior to each measurement at 2 h post-treatment; or (3) there was a discrepancy between rectal temperature and core temperature because of some unforeseen effect of α-PPP. However, when we implanted a different group of mice with telemetry probes to provide real-time and continuous measurements of locomotor activity and core temperature, we found that α-PPP has locomotor-stimulant effects comparable to cocaine, it is not a short-acting agent, and it did not induce a change in core temperature. It is possible that α-PPP would induce hyperthermia if administered in a warm ambient environment, as has been reported with MDMA in both rodents and nonhuman primates (Banks et al. 2008; Gordon et al. 1991; Malberg and Seiden 1998; Von Huben et al. 2007) and methylone in rodents (Lopez-Arnau et al. 2014), and that this hyperthermic response would exacerbate its persistent effects on monoamine neurochemistry and behavior.
It is also notable that both α-PPP (Eshleman et al. 2017) and MDPV (Simmler et al. 2013) function as reuptake inhibitors rather than substrate-based releasers, and the mechanism of action of methylone appears to include both reuptake inhibition and substrate-based release (Simmler et al. 2013). Although α-PPP and MDPV are amphetamine derivatives, their pharmacological mechanism of action is closer to cocaine than to amphetamines. Most previous work with stimulant-induced neurotoxicity has focused on substrate-based releasers rather than reuptake inhibitors. It is believed that the formation of reactive oxygen species and other reactive intermediates from the transfer of pro-oxidant monoamine neurotransmitters from vehicular to cytosolic pools, as well as other processes, during substrate-based release is an important element of stimulant-induced neurotoxicity (Fleckenstein et al. 2007), and this effect does not occur during reuptake inhibition. Moreover, hyperthermic responses to reuptake inhibitors have been less forthcoming than with substrate-based releasers, and we did not observe a hyperthermic response to cocaine in the present study. The facts that cathinones appear to induce neurotoxic-like effects in the absence of hyperthermia, and even when they function as reuptake inhibitors rather than substrate-based releasers, suggests that the mechanism underlying their persistent effects may be distinct from those underlying the effects of the amphetamines, a possibility bolstered by recent findings of atypical actions of MDPV, and potentially other cathinones, at the transporter (Shekar et al. 2017).
There has been little study of the persistent effects of synthetic cathinone exposure on neurochemistry, despite the developed literature with amphetamine derivatives (Hirata et al. 1995; Murnane et al. 2012; O'Callaghan and Miller 1994; Renoir et al. 2008; Sanders-Bush et al. 1975; Steranka et al. 1977; Steranka and Sanders-Bush 1980; Stone et al. 1987), and some cathinones do not persistently deplete brain monoamine content (Baumann et al. 2012). Despite this, few studies have examined the effects of synthetic cathinones on neurochemistry. As noted previously, when methylone is administered to adolescent rats in a warm ambient environment, it induces an acute hyperthermic response and persistent depletions of serotonin and deficits in reference memory. These decrements were also selective, as there were no changes in dopamine levels or spatial memory (Lopez-Arnau et al. 2014). An interesting recent study reported the effects of exposure to MDPV, methylone, or mephedrone, using the same dosing regimen that we used in the present study (30–40 mg/kg, QID, q2h, IP), in female C57BL/6 mice, housed at five per cage. There was no significant change in striatal dopamine levels, dopamine transporter density, tyrosine hydroxylase immunoreactivity, or glial fibrillary acidic protein immunoreactivity 2 days after treatment (Anneken et al. 2015). We report that α-PPP persistently depletes dopamine, DOPAC, serotonin, and norepinephrine levels in the striatum, as well as norepinephrine levels in the prefrontal cortex, in male mice 5 days after treatment, using an 80-mg/kg unit dose of α-PPP. Our findings suggest that the striatum may be a brain region that is particularly sensitive to the untoward effects of α-PPP, and possibly other synthetic cathinones. Moreover, at least for dopamine, these changes do not appear to be related to changes in neurotransmitter production or metabolism, as we did not detect any changes in dopamine turnover, suggesting that they are related to poisoning of the dopamine system rather than temporary changes in dopamine metabolism. Notwithstanding these findings, much work remains to be completed to elucidate the role of sex, age, species, strain, dose, drug class, structural modification, and pharmacological mechanism in the putative neurotoxicity effects of cathinones, including the second-generation pyrrolidines.
The effects of α-PPP on serotonin levels are somewhat surprising, as it has reported selectivity for the dopamine and norepinephrine transporters over the serotonin transporter (Eshleman et al. 2017). However, we used a relatively high-dose regimen to ensure near maximal levels of toxicity, and α-PPP likely loses some selectivity at such doses. Given the paucity of literature on the neurotoxic effects of synthetic cathinones, it is difficult to overly generalize or compare our findings to the literature. Nonetheless, our findings add to a new literature demonstrating the propensity of synthetic cathinones to induce persistent changes in monoamine neurochemistry. The role of serotonin in spontaneous alternations is even more poorly understood than the role of catecholamines. For example, previous studies have shown that depletion of serotonin through provision of a tryptophan-deficient diet to rats results in impaired spontaneous alternations (González-Burgos et al. 1995). Electrolytic lesioning of the raphe in rats resulted in response perseveration in a T-maze spontaneous alternation task, whereas administration of the serotonin neurotoxin 5,7-dihydroxytryptamine had no effect on spontaneous alternations, despite the fact that both treatments resulted in comparable decreases in forebrain serotonin levels (Asin and Fibiger 1984). In rhesus macaques, provision of a tryptophan-deficient diet resulted in significant reductions in cerebrospinal fluid biomarkers of serotonin tone, yet no significant change in recognition memory and significant improvement in spatial working memory (Taffe et al. 2003). The role of serotonin systems in working and recognition memory is an area ripe for additional research.
As with neurochemistry, there has been little study of the persistent effects of synthetic cathinone exposure on learning, memory, and behavior. A recent study examined the effects of binge-like self-administration of MDPV using five 96-h self-administration sessions in rats. Three weeks after the last session, the subjects showed both neurodegeneration and deficits in NOR performance (Sewalia et al. 2018). We report that, 5 days after exposure to α-PPP, mice exhibited decreased exploratory behavior as well as significantly impaired Y-maze performance. Although spontaneous alternation behavior has been closely linked to brain cholinergic systems (Lalonde 2002), previous studies have documented a role for catecholamines, as dopamine depletions in the striatum or septum result in impaired spontaneous alternations (Taghzouti et al. 1985). Likewise, norepinephrine levels in the hippocampus are elevated during spontaneous alternations (Men et al. 1999) and depletion of norepinephrine from forebrain projections results in impaired spontaneous alternation (Pisa and Fibiger 1983). Relating specific neurochemical depletions to specific behavior impairments presents rich research opportunities.
In the present study, we report that exposure to the second-generation pyrrolidine synthetic cathinone α-PPP has persistent effects on monoamine neurochemistry, spontaneous alternations in the Y-maze, and exploratory behavior in the NOR. These changes are consistent with drug-induced neurotoxicity. Moreover, the deleterious effects of α-PPP were apparent even in the absence of acute drug-induced hyperthermia, and despite the fact that it is a reuptake inhibitor rather than a substrate-based releaser. Our findings provide new evidence regarding the dangers associated with synthetic cathinones. Moreover, they suggest that neurotoxicity associated with synthetic cathinones may be distinct from that induced by amphetamines, as it does not appear to depend on hyperthermia or penetration of the synaptic terminal by the compound of interest. In future studies, we intend to extend these results by investigating neuroinflammation as a novel mechanism through which synthetic cathinones may induce their untoward effects as well as determining the role of ambient temperature and pharmacological mechanism in their neurotoxic effects.
References
Achat-Mendes C, Ali SF, Itzhak Y (2005) Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology 30:1128–1137
Anneken JH, Angoa-Perez M, Kuhn DM (2015) 3,4-Methylenedioxypyrovalerone prevents while methylone enhances methamphetamine-induced damage to dopamine nerve endings: beta-ketoamphetamine modulation of neurotoxicity by the dopamine transporter. J Neurochem 133:211–222
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13:93–110
Asin KE, Fibiger HC (1984) Spontaneous and delayed spatial alternation following damage to specific neuronal elements within the nucleus medianus raphe. Behav Brain Res 13:241–250
Banks ML, Czoty PW, Sprague JE, Nader MA (2008) Influence of thyroid hormones on 3,4-methylenedioxymethamphetamine-induced thermogenesis and reinforcing strength in monkeys. Behav Pharmacol 19:167–170
Baumann MH, Ayestas MA Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–1203
Broening HW, Bowyer JF, Slikker W Jr (1995) Age-dependent sensitivity of rats to the long-term effects of the serotonergic neurotoxicant (+/−)-3,4-methylenedioxymethamphetamine (MDMA) correlates with the magnitude of the MDMA-induced thermal response. J Pharmacol Exp Ther 275:325–333
Eshleman AJ, Wolfrum KM, Reed JF, Kim SO, Swanson T, Johnson RA, Janowsky A (2017) Structure-activity relationships of substituted Cathinones, with transporter binding, uptake, and release. J Pharmacol Exp Ther 360:33–47
Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R 2nd, Traynor JR, Woods JH (2008) A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience 151:533–543
Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR (2007) New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol 47:681–698
Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT (2018) Relative reinforcing effects of second-generation synthetic cathinones: acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology 134:28–35
Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE (2016) Stereoselective effects of abused "Bath salt" constituent 3,4-Methylenedioxypyrovalerone in mice: drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther 356:615–623
Gatch MB, Dolan SB, Forster MJ (2017) Locomotor activity and discriminative stimulus effects of a novel series of synthetic cathinone analogs in mice and rats. Psychopharmacology 234:1237–1245
González-Burgos I, Olvera-Cortés E, Del Angel-Meza AR, Feria-Velasco A (1995) Serotonin involvement in the spontaneous alternation ability: a behavioral study in tryptophan-restricted rats. Neurosci Lett 190:143–145
Gordon CJ, Watkinson WP, O'Callaghan JP, Miller DB (1991) Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav 38:339–344
Hirata H, Ladenheim B, Rothman RB, Epstein C, Cadet JL (1995) Methamphetamine-induced serotonin neurotoxicity is mediated by superoxide radicals. Brain Res 677:345–347
John ME, Thomas-Rozea C, Hahn D (2014) Bath salts abuse leading to new onset psychosis and potential for violence. Clin Schizophr Relat Psychoses 1–14
Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS (2000) Striatal serotonin is depleted in brain of a human MDMA (ecstasy) user. Neurology 55:294–296
Lalonde R (2002) The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev 26:91–104
Laviola G, Macri S, Morley-Fletcher S, Adriani W (2003) Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev 27:19–31
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T (2013) Object recognition test in mice. Nat Protoc 8:2531–2537
Lopez-Arnau R, Martinez-Clemente J, Pubill D, Escubedo E, Camarasa J (2014) Serotonergic impairment and memory deficits in adolescent rats after binge exposure of methylone. J Psychopharmacol 28:1053–1063
Malberg JE, Seiden LS (1998) Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J Neurosci 18:5086–5094
Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH (2014) Pharmacology of novel synthetic stimulants structurally related to the "bath salts" constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87:206–213
McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA (1998) Positron emission tomographic evidence of toxic effect of MDMA ("ecstasy") on brain serotonin neurons in human beings. Lancet 352:1433–1437
Men D, McCarty R, Gold PE (1999) Enhanced release of norepinephrine in rat Hippocampus during spontaneous alternation tests. Neurobiol Learn Mem 71:289–300
Miller DB, O'Callaghan JP (1995) The role of temperature, stress, and other factors in the neurotoxicity of the substituted amphetamines 3,4-methylenedioxymethamphetamine and fenfluramine. Mol Neurobiol 11:177–192
Murnane KS, Perrine SA, Finton BJ, Galloway MP, Howell LL, Fantegrossi WE (2012) Effects of exposure to amphetamine derivatives on passive avoidance performance and the central levels of monoamines and their metabolites in mice: correlations between behavior and neurochemistry. Psychopharmacology 220:495–508
Murphy CM, Dulaney AR, Beuhler MC, Kacinko S (2013) "Bath salts" and "plant food" products: the experience of one regional US poison center. J Med Toxicol 9:42–48
Nic Dhonnchadha BA, Bourin M, Hascoet M (2003) Anxiolytic-like effects of 5-HT2 ligands on three mouse models of anxiety. Behav Brain Res 140:203–214
O'Callaghan JP, Miller DB (1994) Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther 270:741–751
Penders TM, Gestring RE, Vilensky DA (2012) Excited delirium following use of synthetic cathinones (bath salts). Gen Hosp Psychiatry 34:647–650
Pisa M, Fibiger HC (1983) Evidence against a role of the rat's dorsal noradrenergic bundle in selective attention and place memory. Brain Res 272:319–329
Prosser JM, Nelson LS (2012) The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol 8:33–42
Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den Brink W (2001) Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet 358:1864–1869
Renoir T, Paizanis E, Yacoubi ME, Saurini F, Hanoun N, Melfort M, Lesch KP, Hamon M, Lanfumey L (2008) Differential long-term effects of MDMA on the serotoninergic system and hippocampal cell proliferation in 5-HTT knock-out vs. wild-type mice. Int J Neuropsychopharmacol 11:1149–1162
Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA (2012) Psychoactive "bath salts" intoxication with methylenedioxypyrovalerone. Am J Med 125:854–858
Sanders-Bush E, Bushing JA, Sulser F (1975) Long-term effects of p-chloroamphetamine and related drugs on central serotonergic mechanisms. J Pharmacol Exp Ther 192:33–41
Sewalia K, Watterson LR, Hryciw A, Belloc A, Ortiz JB, Olive MF (2018) Neurocognitive dysfunction following repeated binge-like self-administration of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 134:36–45
Shekar A, Aguilar JI, Galli G, Cozzi NV, Brandt SD, Ruoho AE, Baumann MH, Matthies HJG, Galli A (2017) Atypical dopamine efflux caused by 3,4-methylenedioxypyrovalerone (MDPV) via the human dopamine transporter. J Chem Neuroanat 83-84:69–74
Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME (2013) Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 168:458–470
Steranka L, Bessent R, Sanders-Bush E (1977) Reversible and irreversible effects of p-chloroamphetamine on brain serotonin in mice. Commun Psychopharmacol 1:447–454
Steranka LR, Sanders-Bush E (1980) Long-term effects of continuous exposure to amphetamine on brain dopamine concentration and synaptosomal uptake in mice. Eur J Pharmacol 65:439–443
Stoica MV, Felthous AR (2013) Acute psychosis induced by bath salts: a case report with clinical and forensic implications. J Forensic Sci 58:530–533
Stone DM, Hanson GR, Gibb JW (1987) Differences in the central serotonergic effects of methylenedioxymethamphetamine (MDMA) in mice and rats. Neuropharmacology 26:1657–1661
Taffe MA, Huitron-Resendiz S, Schroeder R, Parsons LH, Henriksen SJ, Gold LH (2003) MDMA exposure alters cognitive and electrophysiological sensitivity to rapid tryptophan depletion in rhesus monkeys. Pharmacol Biochem Behav 76:141–152
Taghzouti K, Louilot A, Herman JP, Le Moal M, Simon H (1985) Alternation behavior, spatial discrimination, and reversal disturbances following 6-hydroxydopamine lesions in the nucleus accumbens of the rat. Behav Neural Biol 44:354–363
Timar J, Gyarmati S, Szabo A, Furst S (2003) Behavioural changes in rats treated with a neurotoxic dose regimen of dextrorotatory amphetamine derivatives. Behav Pharmacol 14:199–206
Von Huben SN, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA (2007) Impact of ambient temperature on hyperthermia induced by (+/−)3,4-methylenedioxymethamphetamine in rhesus macaques. Neuropsychopharmacology 32:673–681
Walker DL, Gold PE (1994) Intrahippocampal administration of both the D- and the L-isomers of AP5 disrupt spontaneous alternation behavior and evoked potentials. Behav Neural Biol 62:151–162
Wang X, Baumann MH, Xu H, Rothman RB (2004) 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse 53:240–248
Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ (1996) Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med 2:699–703
Wood DM, Hunter L, Measham F, Dargan PI (2012) Limited use of novel psychoactive substances in South London nightclubs. Qjm 105:959–964
Funding
These studies were supported by the National Institutes of Health [DA040907 (CEC and KSM) and NS100512 (KSM)] and by funding from the Mercer University College of Pharmacy. These studies represent partial fulfillment of AR’s PhD dissertation research project at Mercer University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
This article belongs to a Special Issue on Bath Salts
Rights and permissions
About this article
Cite this article
Ray, A., Chitre, N.M., Daphney, C.M. et al. Effects of the second-generation "bath salt" cathinone alpha-pyrrolidinopropiophenone (α-PPP) on behavior and monoamine neurochemistry in male mice. Psychopharmacology 236, 1107–1117 (2019). https://doi.org/10.1007/s00213-018-5044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-018-5044-z