Abstract
Introduction
The use of second-generation antipsychotics (SGA) has been associated with metabolic changes. However, there are differences in the metabolic profile between SGAs. We have previously observed that ziprasidone had a more benign early metabolic profile compared to aripiprazole and quetiapine. However, a long-term follow-up is preferred to detect clinically relevant impairment in metabolic parameters. We aimed to compare the effect of aripiprazole, ziprasidone, and quetiapine on metabolic measures in first-episode non-affective psychosis patients after 1 year of treatment.
Material and methods
One hundred and sixty-five drug-naïve patients, suffering from a first episode of non-affective psychosis, were randomly assigned to receive quetiapine, ziprasidone, or aripiprazole. Weight and glycemic/lipid parameters were recorded at baseline and after 1 year of treatment.
Results
After 1 year of antipsychotic treatment, we found significant increments in weight, BMI, total cholesterol, LDL-cholesterol, triglycerides, and the triglyceride/HDL index in the sample as a whole. These changes produced a significant rise in the percentage of patients with obesity, hypercholesterolemia, and hypertriglyceridemia. However, when comparing the differential effect of each antipsychotic medication, we found no significant differences in any of the metabolic parameters between antipsychotics groups after 1 year of treatment.
Conclusion
We concluded that the antipsychotics studied present similar metabolic profiles. However, the primary exposure to SGAs during the first year of psychosis was associated with significant increases in weight and metabolic parameters, leading to increments in obesity, hypertriglyceridemia, and hypercholesterolemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia is a severe brain disorder with an excess morbidity and mortality, leading to a reduced life expectancy (Hjorthøj et al. 2017; De Hert et al. 2011). A significant portion of this excess mortality is due to cardiometabolic disorders (Foley and Morley 2011).
Recent articles showed that cardiometabolic risk factor and abnormalities are present early in the illness (Pillinger et al. 2017; Zhai et al. 2017; Correll et al. 2014), regardless of antipsychotic exposure (Pillinger et al. 2017; Jensen et al. 2017), suggesting that cardiometabolic alterations may be in part explained by the underlying illness.
However, it is well established that antipsychotic medication produce, by itself, cardiovascular and metabolic side effects of relevance (Stahl et al. 2009). The metabolic disturbances described related to antipsychotic exposure include weight gain and obesity, dyslipidemia, insulin resistance, or new onset diabetes mellitus (type 2) (Kahn et al. 2008; Correll et al. 2009; De Hert et al. 2011). These effects have been widely described after a short term of antipsychotic treatment (Malla et al. 2016; Parabiaghi et al. 2016; Pérez-Iglesias et al. 2014a; Perez-Iglesias et al. 2007). Nevertheless, these disturbances keep progressing and their clinical implications are even more evident after mid- and long-term antipsychotic treatments (Pérez-Iglesias et al. 2014b; Mackin et al. 2012).
Previous studies have reported differences in the metabolic profile between second-generation antipsychotics (SGAs), showing differentiated potential for metabolic adverse effects (Maayan and Correll 2011; Crespo-Facorro et al. 2016). In this line, ziprasidone and aripiprazole have been proposed to have a “neutral” metabolic effect when compared to placebo in previous studies (Daniel et al. 1999; Kane et al. 2002; McEvoy et al. 2007; Pappadopulos et al. 2012). Moreover, several studies described ziprasidone or aripiprazole producing less metabolic alterations than other SGAs such as olanzapine or risperidone (Breier et al. 2005; Kinon et al. 2006; Komossa et al. 2009; Grootens et al. 2011). And recent studies have shown that switching to aripiprazole (Spurling et al. 2007; Takeuchi et al. 2010; Stroup et al. 2011) or ziprasidone (Stroup et al. 2006; Alptekin et al. 2009) from other SGAs led to improvements in the metabolic parameters in psychosis. However, there are to our knowledge no previous studies comparing the longitudinal metabolic effect of these SGAs with a low or neutral effect on metabolism (i.e., aripiprazole, ziprasidone, and quetiapine), on samples of drug-naïve patients, thus avoiding the confounding effect of chronicity and long-term exposure to medication.
In this sense, we have previously conducted a study of SGAs with a low or neutral effect on metabolic parameters in a sample of drug-naïve patients suffering from a first episode of non-affective psychosis (Pérez-Iglesias et al. 2014a). In that study, we observed that ziprasidone had a more benign metabolic profile compared to aripiprazole and quetiapine, at short term (12 weeks). However, a long-term follow-up would be more appropriate to detect clinically relevant impairments in metabolic parameters. We hypothesized that, in the first place, the metabolic alterations and weight gain observed at short term in the sample as a whole in our previous study (Pérez-Iglesias et al. 2014a) will be even greater after 1 year of antipsychotic treatment. Secondly, we hypothesized that, according to the results obtained in our previous study (Pérez-Iglesias et al. 2014a), and previous lines of evidence described above, ziprasidone will maintain a more benign metabolic profile compared to quetiapine and aripiprazole.
Therefore, the present study was aimed to evaluate and compare the longitudinal effect of aripiprazole, ziprasidone, and quetiapine on metabolic parameters and weight in medication-naïve first-episode non-affective psychosis patients after 1 year of treatment.
Material and methods
This study was conducted in the outpatient and inpatient psychiatric units of the University Hospital Marques de Valdecilla, located in the province of Cantabria, in the north of Spain. The hospital is a reference center of a catchment area population of 555,000 people and provides the only psychiatric acute inpatient unit and 24-h emergency care service for the whole province. To guarantee the inclusion of all first episodes of psychosis, regular meetings with all mental health care services of Cantabria were maintained. The study protocol was approved by the ethics committee of our hospital.
Subjects
The sample for the present study was recruited from October 2005 to January 2011 and formed part of a larger prospective longitudinal study on first episode non-affective psychosis (Pelayo-Terán et al. 2008; Crespo-Facorro et al. 2013). These patients were referred to the program when presenting a first episode of non-affective psychosis and were admitted if they fulfilled the following criteria: (1) aged 15–60 years; (2) met the DSM-IV criteria (according to the Structured Clinical Interview for DSM-IV, SCID-I) 6 months after inclusion for a principal diagnosis of schizophrenia, schizophreniform disorder, schizoaffective disorder, brief reactive psychosis, or psychosis nonotherwise specified; (3) habitually living in the catchment area; (4) no prior treatment with antipsychotic medication; and (5) current psychotic symptoms of at least of moderate severity, as assessed by one of the five items of the Scale for the Assessment of Positive Symptoms (SAPS). Patients were not admitted to the program if they presented any of the following: (1) mental retardation, (2) neurological disorder, or (3) drug dependence (DSM-IV criteria). All subjects provided written informed consent prior to their inclusion in the study, which was approved by the local ethics committee (University Hospital Marques de Valdecilla Ethics Committee).
Study design
Patients were randomly assigned to receive quetiapine, ziprasidone, or aripiprazole. A computer-generated randomization list was drawn up by a statistician. Treatment dose ranges were 100–600 mg/day for quetiapine, 40–160 mg/day for ziprasidone, and 5–30 mg/day for aripiprazole. Doses could be adjusted as clinically indicated within the prescribed range in an attempt to target the lowest effective dose. Certain concomitant medications (lormetazepam and clonazepam) were permitted for the management of agitation, general behavior disturbances, and/or insomnia. Only if clinically significant extrapyramidal signs occurred was anticholinergic medication (biperiden at a dose of up to 8 mg/day) allowed. Antidepressants (sertraline) and mood stabilizers (lithium) were permitted if clinically needed.
Patients were clinically evaluated by a senior psychiatrist, at least, at baseline and at the following time points after the treatment was initiated: 6 weeks, 12 weeks, 6 months, and 1 year.
Those patients who did not respond after 6 weeks of treatment (< 30% total improvement) measured by SAPS (Andreasen 1984) and SANS (Andreasen 1983) scales or who had significant side effects, measured by UKU scale (Lingjaerde et al. 1987), were changed to a different antipsychotic. The study’s main outcome measures were changes in fasting glucose, total cholesterol, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides, and insulin plasma levels after 1 year of antipsychotic treatment.
Laboratory analysis
All determinations were performed in our hospital, including both biochemical and endocrinology analyses. All measurements were obtained at first visit and at 1 year after an overnight fast. Fasting state, as well as treatment compliance, was reported by patients and their family members. Glucose, total cholesterol, HDL-cholesterol, and triglycerides were measured by automated methods on a TechniconDax (Technicon Instruments Corp, Tarrytown, NY, USA), using the reagents supplied by Boehringer-Mannheim (Mannheim, Germany). LDL-cholesterol was determined by the Friedewald et al. calculation (Friedewald et al. 1972): LDL = total cholesterol − (HDL + [triglycerides/5]). Insulin levels were measured by an immunoradiometric assay (IRMA) (Immunotech, Beckman Coulter Company, Prague, Czech Republic) with an average interassay coefficient of variation (CV) of 3.3% and intraassay CV of 2.8%. The sensitivity of the method was 0.5 μU/ml. Values for normal weight subjects are 2–17 μU/ml. This assay does not show any cross-reactivity with human proinsulin and C-peptide. Homeostasis model assessment (HOMA) was used to assess insulin resistance (IR). The HOMA index was calculated by means of a previously described formula (Matthews et al. 1985): HOMA = [fasting insulin (μU/ml) × fasting glucose (mmol/l)]/22.5. In addition, as proposed by McLaughlin et al. (2005), we calculated the triglyceride/HDL-cholesterol (TG/HDL-cholesterol) ratio as a predictor of insulin resistance using the cutpoint of 3.5 described by them as the optimal one.
Statistical analysis
We investigated the relationship between antipsychotic treatment and changes in metabolic parameters. First, an analysis of variance (ANOVA) for continuous variables and a chi-square test for categorical variables were used for comparison between groups. An analysis of covariance was performed to compare the differences in metabolic side effects between treatment groups. Change in each parameter between baseline and endpoint was used as the dependent variable, the type of medication (treatment group) was used as the independent variable, and body mass index (BMI) at baseline, parameter level at baseline, sex, and age were used as covariates. When the analysis revealed significance between group effects, additional post hoc analysis (Sidak test) was conducted. This analysis was performed according to the intention to treat (per protocol analysis was also performed).
A paired t test was used to determine if there were statistically significant changes in glycemic and lipid parameters after 1 year of antipsychotic treatment. When exploring differences in longitudinal changes between antipsychotic groups, we compared mean differences resulting from 1-year data minus baseline data.
We additionally calculated the percentage of subjects with pathologic values in glucose and lipid parameters (according to the reference values of our laboratory) at baseline and at 1 year. To evaluate significant changes in these percentages, we used the McNemar test for repeated measures.
Finally, we studied the association between weight gain and metabolic changes. First, we performed a partial correlation analysis controlling sex, age, and BMI at baseline. Second, we compared the changes in glycemic and lipid parameters in three different groups: patients with less than 7% increase in their BMI at baseline, patients with an increase between 7 and 20% of the BMI at baseline, and patients with an increase of more than 20% of the BMI at baseline. The three groups were compared using a covariance analysis.
We conducted post hoc analysis to explore a possible differentiated effect of antipsychotic treatment regarding gender. For this purpose, we repeated the ANCOVA model described above in the female and male subsamples.
The Statistical Package for Social Science (SPSS), version 12.0, was used for statistical analyses. All statistical tests were two tailed and significance was determined at the 0.05 level. We calculated the sample size (≥ 131) for the study population (n = 198), using a confidence level of 95% and a confidence interval of 5%.
Results
Enrollment and characteristics of study population
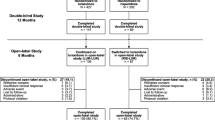
A total of 222 patients were enrolled between October 2005 and January 2011. Of these, 24 were excluded for not meeting the inclusion criteria or refusing to participate in the study. A total of 198 patients were included and randomized to receive (Fig. 1) quetiapine (n = 62; 31.3%), ziprasidone (n = 58; 29.3%), and aripiprazole (n = 78; 39.4%). We had a 16.7% dropout rate at 1 year (33 patients were lost to follow-up or refused to be evaluated). Finally, 165 patients were included in the study to assess the long-term changes of the main metabolic values. Nine subjects with outlier values at baseline, for at least one of the glycemic/lipid parameters (value higher than three standard deviations), were excluded of the analysis to avoid data distortion; eight of these patients were on aripiprazole and one on ziprasidone. Clinical and sociodemographic characteristics of the sample are shown in Table 1. The three treatment groups were comparable on a wide range of baseline data, including age, ethnicity, alcohol, tobacco smoking, and other drug consumption. However, there was a significant difference in sex between treatment groups, with the quetiapine group being formed by more men than the other groups.
At the end of follow-up, 40.6% (n = 67) of the 165 patients evaluated continued with the same antipsychotic treatment assigned at baseline (17.8% of the quetiapine group, 43.1% of the ziprasidone group, and 61.7% of the aripiprazole group). Eight-nine patients evaluated after 1 year of treatment did not complete the protocol. The data regarding what treatments were prescribed to those patients discontinuing the initial assigned (randomized) antipsychotic is available as Supplementary material (S1). The reasons for treatment switch were inefficacy (22.4%), side effects (14.7%), and nonadherence (10.2%). A complete analysis of treatment discontinuation at 1 year in this sample of patients has been described elsewhere (Crespo-Facorro et al. 2014). The mean doses of antipsychotic drugs at 1 year were 252.6 mg (SD = 203.8) of quetiapine, 60.0 mg (SD = 23.9) of ziprasidone, and 9.9 mg (SD = 6.3) of aripiprazole.
Metabolic changes and different antipsychotic treatments
The three groups of treatment were comparable in all the main sociodemographic and clinical variables (Crespo-Facorro et al. 2013). However, patients in the ziprasidone group were more frequently prescribed antidepressant medication at 1 year, compared with those patients in the other antipsychotic groups (31 vs 18 and 11.3% for quetiapine and aripiprazole, respectively; χ 2 = 6.937, p = 0.031).
When we explored the possible differential effect of treatments on metabolic side effects following an intention-to-treat analysis, we found no significant differences between treatment groups (Table 2). Baseline and 1-year mean values for anthropometric and metabolic parameters of each treatment group and the results from per protocol analysis are available as supplementary material (see Supplementary materials 2 and 3).
Metabolic changes after 1 year of antipsychotic therapy
As shown in Table 3, mean fasting glucose and insulin levels did not change after 1 year of antipsychotic treatment. In the same line, the HOMA index of insulin resistance remained similar to baseline values. However, the other insulin resistance index, the triglyceride/HDL, significantly increased. Regarding lipid measures, total cholesterol, LDL-cholesterol, and triglyceride levels showed a statistically significant increase after 1 year of treatment. No variations in HDL-cholesterol levels were observed.
On comparing the percentage of patients with pathological levels before and 1 year after the antipsychotic treatment (Table 4), the more noticeable changes detected were the higher percentage of patients with obesity (BMI ≥ 30 kg/m2) (5.1 vs 15.3%; p < 0.001) and the higher percentage of patients with hypercholesterolemia (cholesterol > 200 mg/dl) (23.2 vs 39.6%; p < 0.001) and hypertriglyceridemia (triglycerides > 150 mg/dl) (5.8 vs 14.2%; p = 0.021), after 1 year of treatment. The increase in the percentage of patients with pathological values of LDL-cholesterol (> 130 mg/dl) (21.6 vs 31.6%; p = 0.029) and HOMA index in the men’s subgroup (> 3.5) (1.8 vs 17.8%; p = 0.021) was also statistically significant.
Metabolic changes and weight gain
Weight gain was positively correlated with insulin increase (r = 0.25, p = 0.014) and with the insulin resistance indexes, HOMA index (r = 0.30, p = 0.003) and triglycerides/HDL index (r = 0.23, p = 0.007). There was also a positive correlation between weight gain and triglyceride increase (r = 0.20, p = 0.018) and with total cholesterol increase (r = 0.23, p = 0.004). A significant negative correlation was observed between the weight and glucose changes (r = − 0.20, p = 0.012).
A relationship between weight increase and changes in metabolic parameters was also detected when we compared three groups of patients according to the increase in their BMI (Table 5). Significant differences were observed for glucose, insulin, HOMA index, cholesterol, and LDL-cholesterol increase, between the groups of patients with differential BMI gain after 1 year of treatment.
Post hoc analyses exploring sex-treatment interaction on metabolic and weight changes
Secondary analysis in the female subsample showed a sex-treatment interaction in weight (F = 3.997; p = 0.023) and BMI (F = 3.860; p = 0.026) changes after 1 year of treatment. Post hoc analyses (Sidak test) revealed that those female patients treated with aripiprazole presented a higher weight gain than those treated with quetiapine (p = 0.067) and ziprasidone (0.055) and a significantly higher increase in BMI than those on ziprasidone (p = 0.038). The statistical analyses carried out on the male subsample showed no significant sex-treatment interaction in any of the anthropometric or metabolic parameters. The complete data is available as supplementary material (Supplementary material 4).
Discussion
The results of this study provide further evidence of the effect of long-term treatment with aripiprazole, ziprasidone, and quetiapine on metabolism in patients suffering from a first episode of non-affective psychosis.
Contrary to our hypothesis and to previous results at short term where ziprasidone showed a better metabolic profile (Pérez-Iglesias et al. 2014a), we failed to detect statistically significant differences between treatments in any of the metabolic parameters evaluated and body weight or BMI. The three antipsychotic drugs (i.e., aripiprazole, ziprasidone, and quetiapine) produced, after 1 year of treatment, similar increments in weight, BMI, and glucose, cholesterol, and triglyceride levels, suggesting that none of them can be considered metabolically “neutral.” However, they showed a less detrimental effect on metabolism than other antipsychotics previously studied. For instance, the mean weight gain with ziprasidone after 1 year of treatment was 5.3 kg; half of the mean weight increase observed with other antipsychotic drugs such as olanzapine, risperidone, or haloperidol (Perez-Iglesias et al. 2008).
In addition, our results are also in disagreement with previous studies which provide data on the differential metabolic profile of antipsychotic drugs. For instance, ziprasidone has been recently described to have a neutral effect on fasting plasma lipids and glucose levels and to produce a minimal effect on weight at short term (≤ 12 weeks) (Pappadopulos et al. 2012). Besides, it has been associated with decreasing levels of triglycerides and cholesterol after 8 weeks of treatment (Grootens et al. 2011). Moreover, switching from quetiapine to ziprasidone showed a significant decrease in weight as well as improved metabolic profile (Karayal et al. 2011). Similarly, this beneficial “switching effect” on metabolic parameters and weight has been described with ziprasidone at the long term (1 year) in a recent open-label prospective study (Chue et al. 2014); therefore, ziprasidone has been repeatedly proposed as the antipsychotic drug with the most benign metabolic profile (Kahn et al. 2008; Lieberman et al. 2005; Stroup et al. 2006). Regarding aripiprazole, previous studies have shown that this drug has a better metabolic profile than other SGAs. Kerwin et al. (2007) reported that short-term treatment with aripiprazole produced a decrease in weight from baseline values, while those patients treated with other SGAs presented increments in weight. Furthermore, switching from other SGA to aripiprazole has shown to produce an improvement in metabolic measures and weight reduction (Stroup et al. 2011). In contrast, our results showed a mean weight gain of 8.5 kg after 1 year of treatment with aripiprazole, taking into account that 66.7% of the patients in this group were treated over the year only with aripiprazole at low doses (mean dose 9.9 mg). Finally, quetiapine has been reported to induce higher LDL-cholesterol levels (Rummel-Kluge et al. 2010) and higher triglycerides to HDL-cholesterol ratio (Correll et al. 2014), and greater weight gain (Lieberman et al. 2005; Komossa et al. 2009) than other antipsychotics.
There are several possible reasons that may explain the lack of significant differences on the metabolic profile of the three treatment groups observed. For some of the metabolic parameters, we did not have a sufficient sample size to detect significant differences. Another relevant issue is the concomitant medication prescribed to the patients. In this sense, the patients in the ziprasidone group were significantly more frequently treated with antidepressants, most of which are known to produce weight gain as a frequent adverse event (Carvalho et al. 2016). Other nonpsychiatric drugs with a direct effect on metabolism (e.g., lipid-lowering drugs) may had been prescribed elsewhere (e.g., GP), thus altering the study results. It is more difficult to control these possible confounding factors in long-term naturalistic studies.
Interestingly, we have previously reported differences in the metabolic profile of antipsychotic medications at short term (3 months) that disappeared when evaluated after 1 year of treatment. Thus, Perez-Iglesias et al. (2007) compared the effect of haloperidol, olanzapine, and risperidone after 3 months of treatment and observed a higher weight gain and higher increase in triglyceride levels for the olanzapine group compared with haloperidol. However, this differential effect on metabolic and weight parameters disappeared after 1 year of treatment (Perez-Iglesias et al. 2009). These results suggest that other clinical variables and interventions may moderate the differences in metabolic profile of antipsychotics at the long term. Subjects showing early increments in weight and alterations in metabolic parameters in the early stages of the treatment may be provided with lifestyle counseling (e.g., exercise, diet) and even with pharmacological assistance, since these interventions have proved to be effective (Chacón et al. 2011; Bonfioli et al. 2012).
Post hoc analyses revealed a sex-treatment interaction on weight gain. Those women treated with aripiprazole presented a significantly higher increase in BMI than those treated with ziprasidone after 1 year of treatment. No significant differences were observed between treatment groups in the male subsample. Although gender differences in the metabolic effect of antipsychotics have been previously described (Li et al. 2016; Lee et al. 2011), there is scarce data comparing sex-tolerability interaction of antipsychotics due to limited number of direct head-to-head data from clinical trials.
Finally, our results demonstrate that the exposure to SGAs, during the first year of treatment, is associated with significant increments in weight, BMI, alterations in metabolic parameters, such as insulin resistance index (triglyceride/HDL), total cholesterol, LDL-cholesterol, and triglyceride levels. These results are in agreement with previous studies supporting the understanding that antipsychotic medication produce, by itself, cardiovascular and metabolic side effects of relevance (Malla et al. 2016; Parabiaghi et al. 2016; Pérez-Iglesias et al. 2014a; Perez-Iglesias et al. 2009). However, recent articles have shown that cardiometabolic risk factor and abnormalities are present early in the illness in psychosis (Pillinger et al. 2017; Zhai et al. 2017; Correll et al. 2014), even regardless of antipsychotic exposure (Pillinger et al. 2017; Jensen et al. 2017), and suggesting that the cardiometabolic alterations may be in part explained by the underlying illness itself.
The clinical relevance of the metabolic and anthropometric variations observed in group means is revealed by a significant increment in the proportion of patients with obesity (5.1 to 15.3%) and with hypertriglyceridemia (5.8 to 14.2%), hypercholesterolemia (23.2 to 39.6%), and pathological values of LDL-cholesterol (21.6 to 31.6%) after 1 year of treatment. It is worth to mention that, as described before (Perez-Iglesias et al. 2009), those patients who experienced a higher weight increase also showed a significantly greater increase in glucose and insulin plasma levels, insulin resistance index (HOMA), and total cholesterol and LDL-cholesterol levels. Therefore, the changes in metabolic parameters after the first year of antipsychotic treatment appear to be related to the magnitude of weight gain rather than a direct toxic effect of the antipsychotic treatment.
The study has several strengths. First, its design is a practical RCT using a sample of drug-naïve patients recently diagnosed of a first episode of non-affective psychosis, thus avoiding the confounding effect of chronicity and long-term exposure to previous medications. Second, the long-term follow-up period (1 year) facilitates to detect the long-range impact of antipsychotics on metabolic parameters. And finally, the low proportion of dropouts for a long-term follow-up study makes us to consider the results highly generalizable.
However, the study counts with several relevant limitations, including the open-label design. Another possible limitation may be the differences in treatment discontinuation between treatment groups (quetiapine > ziprasidone > aripiprazole), producing unbalanced groups that may have influenced the statistical analyses. Following international recommendations, our hospital has incorporated more rigorous normal levels for metabolic parameters. This may have influenced the variations in pathologic proportion of patients and may be difficult comparing with previous studies with less stringent parameter limits. Finally, early adverse effects of treatment on metabolic parameters observed individually at the clinic are subject to be modulated by other clinical interventions in the long run (e.g., diet, prescribed exercise, medication), therefore moderating differences in metabolic profile between treatment groups.
Conclusions
The primary exposure to SGAs during the first year of non-affective psychosis was associated with significant increments in weight and usual metabolic parameters leading to a significant increment in the proportion of obesity, hypertriglyceridemia, and hypercholesterolemia in our sample. When we compared the metabolic profile of the three antipsychotics studied (i.e., quetiapine, ziprasidone, and aripiprazole), we observed no significant differences in any of the main metabolic and weight parameters studied between treatments, concluding that they present similar metabolic profiles.
References
Alptekin K, Hafez J, Brook S et al (2009) Efficacy and tolerability of switching to ziprasidone from olanzapine, risperidone or haloperidol: an international, multicenter study. Int Clin Psychopharmacol 24:229–238
Andreasen N (1983) Scale for the assessment of negative symptoms (SANS). University of Iowa, Iowa City
Andreasen N (1984) Scale for the assessment of positive symptoms (SAPS). University of Iowa, Iowa City
Bonfioli E, Berti L, Goss C et al (2012) Health promotion lifestyle interventions for weight management in psychosis: a systematic review and meta-analysis of randomized controlled trials. BMC Psychiatry 12:78
Breier A, Berg PH, Thakore JH et al (2005) Olanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophrenia. Am J Psychiatr 162:1879–1887
Carvalho AF, Sharma MS, Brunoni AR et al (2016) The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother. Psychosomatics 85(5):270–288
Chacón F, Mora F, Gervás-Ríos A et al (2011) Efficacy of lifestyle interventions in physical health management of patients with severe mental illness. Ann Gen Psychiatry 10:22
Chue P, Mandel FS, Therrien F (2014) The effect of ziprasidone on metabolic syndrome risk factors in subjects with schizophrenia: a 1 year, open-label, prospective study. Curr Med Res Opin 30(6):997–1005
Correll CU, Manu P, Olshanskiy V et al (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302:1765–1773
Correll CU, Robinson DG, Schooler NR et al (2014) Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 71(12):1350–1363
Crespo-Facorro B, Ortiz-Garcia d l FV, Mata I et al (2013) Aripiprazole, ziprasidone and quetiapine in the treatment of first-episode non-affective psychosis: a 12-week randomized, flexible-dose, open-label trial. Schizophr Res 147:375–382
Crespo-Facorro B, de la Foz VO, Mata I et al (2014) Treatment of first-episode non-affective psychosis: a randomized comparison of aripiprazole, quetiapine and ziprasidone over 1 year. Psychopharmacology 231(2):357–366
Crespo-Facorro B, Pelayo-Teran JM, Mayoral-van Son J (2016) Current data on and clinical insights into the treatment of first episode nonaffective psychosis: a comprehensive review. Neurol Ther 5(2):105–130. https://doi.org/10.1007/s40120-016-0050-8
Daniel DG, Zimbroff DL, Potkin SG et al (1999) Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology 20:491–505
De Hert M, Detraux J, vanWinkel R et al (2011) Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 8:114–126
Foley DL and Morley KI (2011) Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry 68:609–616
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Grootens KP, van Veelen NM, Peuskens J et al (2011) Ziprasidone vs olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial. Schizophr Bull 37(2):352–361
Hjorthøj C, Stürup AE, McGrath JJ et al (2017) Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 4(4):295–301
Jensen KG, Correll CU, Rudå D et al (2017) Pretreatment cardiometabolic status in youth with early-onset psychosis: baseline results from the TEA trial. J Clin Psychiatry. https://doi.org/10.4088/JCP.15m10479
Kahn RS, Fleischhacker WW, Boter H et al (2008) Effectiveness of antipsychotic drugsin first-episode schizophrenia and schizophreniform disorder: an openrandomised clinical trial. Lancet 371(9618):1085–1097
Kane JM, Carson WH, Saha AR et al (2002) Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 63:763–771
Karayal ON, Glue P, Bachinsky M et al (2011) Switching from quetiapine to ziprasidone: a sixteen-week, open-label, multicenter study evaluating the effectiveness and safety of ziprasidone in outpatient subjects with schizophrenia or schizoaffective disorder. J Psychiatr Pract 17(2):100–109
Kerwin R, Millet B, Herman E et al (2007) A multicentre, randomized, naturalistic, open-label study between aripiprazole and standard of care in the management of community-treated schizophrenic patients Schizophrenia Trial of Aripiprazole: (STAR) study. Eur Psychiatry 22:433–443
Kinon BJ, Lipkovich I, Edwards SB et al (2006) A 24-week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. J Clin Psychopharmacol 26:157–162
Komossa K, Rummel-Kluge C, Hunger H et al (2009) Ziprasidone versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 4:CD006627
Lee SY, Park MH, Patkar AA et al (2011) A retrospective comparison of BMI changes and the potential risk factors among schizophrenic inpatients treated with aripiprazole, olanzapine, quetiapine or risperidone. Prog Neuropsychopharmacol Biol Psychiatry 35(2):490–496
Li Q, Chen D, Liu T et al (2016) Sex differences in body mass index and obesity in Chinese patients with chronic schizophrenia. J Clin Psychopharmacol 36(6):643–648
Lieberman JA, Stroup TS, McEvoy JP et al (2005) Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223
Lingjaerde O, Ahlfors UG, Bech P et al (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100
Maayan L, Correll CU (2011) Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 21(6):517–535
Mackin P, Waton T, Watkinson HM et al (2012) A four-year naturalistic prospective study of cardiometabolic disease in antipsychotic-treated patients. Eur Psychiatry 27(1):50–5
Malla A, Mustafa S, Rho A et al (2016) Therapeutic effectiveness and tolerability of aripiprazole as initial choice of treatment in first episode psychosis in an early intervention service: a one-year outcome study. Schizophr Res 174(1–3):120–125. https://doi.org/10.1016/j.schres.2016.04.036
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and betacell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
McEvoy JP, Daniel DG, Carson WH Jr et al (2007) A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res 41:895–905
McLaughlin T, Reaven G, Abbasi F et al (2005) Is there a simple way to identify insulinresistant individuals at increased risk of cardiovascular disease? Am J Cardiol 96(3):399–404
Pappadopulos E, Newcomer JW, Kolluri S (2012) Changes in weight, plasma lipids, and glucose in adults treated with ziprasidone: a comprehensive analysis of Pfizer-initiated clinical trials. J Clin Psychiatry 73:e742–e748
Parabiaghi A, Tettamanti M, D’Avanzo B et al (2016) Metabolic syndrome and drug discontinuation in schizophrenia: a randomized trial comparing aripiprazole olanzapine and haloperidol. Acta Psychiatr Scand 133(1):63–75
Pelayo-Terán JM, Pérez-Iglesias R, Ramírez-Bonilla M et al (2008) Epidemiological factors associated with treated incidence of first-episode non-affective psychosis in Cantabria: insights from the Clinical Programme on Early Phases of Psychosis. Early Interv Psychiatry 2(3):178–187
Perez-Iglesias R, Crespo-Facorro B, Amado JA et al (2007) A 12-week randomized clinical trial to evaluate metabolic changes in drug-naive, first-episode psychosis patients treated with haloperidol, olanzapine, or risperidone. J Clin Psychiatry 68(11):1733–1740
Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O et al (2008) Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naïve population. Schizophr Res 99(1–3):13–22
Perez-Iglesias R, Mata I, Pelayo-Teran JM et al (2009) Glucose and lipid disturbances after 1 year of antipsychotic treatment in a drug-naïve population. Schizophr Res 107(2–3):115–121
Pérez-Iglesias R, Ortiz-Garcia de la Foz V, Martínez García O et al (2014a) Comparison of metabolic effects of aripiprazole, quetiapine and ziprasidone after 12 weeks of treatment in first treated episode of psychosis. Schizophr Res 159(1):90–94
Pérez-Iglesias R, Martínez-García O, Pardo-Garcia G et al (2014b) Course of weight gain and metabolic abnormalities in first treated episode of psychosis: the first year is a critical period for development of cardiovascular risk factors. Int J Neuropsychopharmacol 17(1):41–51
Pillinger T, Beck K, Gobjila C et al (2017) Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry 74(3):261–269
Rummel-Kluge C, Komossa K, Schwarz S et al (2010) Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 123:225–233
Spurling RD, Lamberti JS, Olsen D et al (2007) Changes in metabolic parameters with switching to aripiprazole from another second-generation antipsychotic: a retrospective chart review. J Clin Psychiatry 68:406–409
Stahl SM, Mignon L, Meyer JM (2009) Which comes first: atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiatr Scand 119(3):171–9
Stroup TS, Lieberman JA, McEvoy JP et al (2006) Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatr 163:611–622
Stroup TS, McEvoy JP, Ring KD et al (2011) A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry 168:947–956
Takeuchi H, Uchida H, Suzuki T et al (2010) Changes in metabolic parameters following a switch to aripiprazole in Japanese patients with schizophrenia: one-year follow-up study. Psychiatry. Clin Neurosci 64:104–106
Zhai D, Lang Y, Feng Y et al (2017) Early onset of cardiometabolic risk factor profiles in drug naïve adolescents and young adults with first-episode schizophrenia. Schizophr Res pii: S0920-9964(17):30126–30123
Acknowledgements
This study was conducted as part of a clinical trial “Comparative Study of Aripiprazole, Quetiapine and Ziprasidone in the Treatment of First Episode Non-affective Psychosis (AZQ2005).” ClinicalTrials.gov Identifier: NCT02305823.
The authors wish to thank all “Programa Asistencial de las Fases Iniciales de Psicosis” (PAFIP) research team and all patients and family members who participated in the study.
Funding
The present study was carried out at the Hospital Marqués de Valdecilla, University of Cantabria, Santander, Spain, under the following grant support: Instituto de Salud Carlos III PI020499, PI050427, PI060507; Plan Nacional de Drogas Research Grant 2005-Orden sco/3246/2004; SENY Fundació Research Grant CI 2005–0308007; and Fundación Marqués de Valdecilla API07/011. Unrestricted educational and research grants from AstraZeneca, Pfizer, Bristol-Myers Squibb, and Johnson & Johnson provided support for PAFIP activities. No pharmaceutical industry or institutional sponsors participated in the study concept and design, data collection, analysis and interpretation of the results, and drafting the manuscript.
Author information
Authors and Affiliations
Contributions
BC-F designed the study and wrote the protocol. PSP evaluated the patients and collected the study variables. VO-G built and maintained the database and helped with the statistical analyses. RP-I and JV-B managed the literature searches. JV-B undertook the statistical analysis and wrote the first draft of the manuscript. ADM, RP-I, and BC-F contributed to the interpretation of the data and revised the manuscript critically. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Vázquez-Bourgon, J., Pérez-Iglesias, R., Ortiz-García de la Foz, V. et al. Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: a pragmatic clinical trial in drug-naïve patients with a first-episode of non-affective psychosis. Psychopharmacology 235, 245–255 (2018). https://doi.org/10.1007/s00213-017-4763-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4763-x