Abstract
Rationale
Cigarette demand is a behavioral economic measure of the relative value of cigarettes. Decreasing the value of cigarette reinforcement may help with quitting smoking.
Objectives
This study aimed to evaluate the effects of initial use of varenicline (VAR) versus nicotine replacement therapy (NRT) on demand for cigarettes on quit day among smokers with substance use disorders (SUD) and to determine whether reduced demand was associated with subsequent abstinence from smoking at 1 and 3 months.
Methods
Participants (N = 110) were randomized to double-blind, double-placebo conditions: VAR with placebo NRT or NRT with placebo capsules. The cigarette purchase task (CPT) was used to assess demand for cigarettes at baseline and on quit day, following a 1-week medication dose run-up/placebo capsule lead-in and first day use of the patch.
Results
Demand for cigarettes decreased from baseline to quit day without significant differences between medications. Reductions in CPT intensity (number of cigarettes that would be smoked if they were free) and CPT breakpoint (lowest price at which no cigarettes would be purchased) predicted greater likelihood of abstaining on quit day. Reduced intensity predicted length of abstinence at 1 and 3 months while reduced breakpoint predicted only 1 month length of abstinence.
Conclusions
Initial therapeutic doses of VAR and NRT resulted in similar reductions in cigarette reinforcement. Larger initial reductions in demand on quit day were associated with early success with abstaining from cigarettes. Behavioral economic approaches may be useful for identifying individuals who benefit less from pharmacotherapy and may need additional treatment resources.
Trial registration
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Smokers with substance use disorders (SUD) have quit rates that are half that of non-substance users (Richter et al. 2002). In general population studies, the probability of sustained abstinence from smoking using varenicline (VAR) is greater than for any other pharmacotherapy except combination nicotine replacement therapy (NRT) (Anthenelli et al. 2016; Cahill et al. 2014); however, the research on the effectiveness of smoking cessation pharmacotherapies among individuals with SUDs is limited. A recent review of 17 randomized controlled trials of smoking cessation interventions for patients with SUD reported some support for the effectiveness of NRT in this population but not for VAR (Thurgood et al. 2015). This review was limited in that it only included one VAR study in which VAR, NRT, and placebo were compared among methadone-maintained opiate-dependent smokers; smoking cessation rates were low (5.4%) and did not differ significantly between groups (Stein et al. 2013). Given the difficulty of maintaining long-term cessation among individuals with SUD, identifying mechanisms underlying treatment efficacy is important for developing effective cessation treatments for this high-risk group (Prochaska et al. 2004).
Given that addiction pathology is associated with dysregulation of reward circuitry in the brain (Koob and Le Moal 2001), understanding mechanisms by which pharmacotherapy may impact abstinence, particularly if medications are able to reduce nicotine reinforcement, has the potential to inform the development of more effective interventions and ultimately improve smoking cessation outcomes. A growing body of work has incorporated behavioral economic laboratory paradigms in the study of mediating mechanisms of pharmacological treatments for SUD. VAR is an α4β2 nicotinic acetylcholine receptor partial agonist with selective activation of these receptors proposed to be involved in the reinforcing effects of nicotine (Tapper et al. 2004). To date, two studies have examined the effects of VAR on smoking reinforcement using the cigarette purchase task (CPT; Jacobs and Bickel 1999; MacKillop et al. 2008). In the first, following a week of medication induction, but before abstaining from smoking, participants randomized to either VAR or placebo reported reduced intensity of demand (the parameter reflecting purchases when cigarettes are free), without significant differences between conditions (McClure et al. 2013); at higher prices, however, participants in the VAR condition reported a steeper decline in the number of cigarettes purchased resulting in increased demand elasticity (i.e., sensitivity to price) relative to placebo. The second study collected data from smokers in their natural environment using handheld devices for 4 weeks prior to a target quit date (week 1: premedication baseline; week 2: dose run-up; weeks 3–4: full dose) (Schlienz et al. 2014). Two indices of demand (elasticity and intensity) were reduced, suggesting decreases in smoking reinforcement over the 3 weeks, whereas a third breakpoint (the price at which purchases fall to zero) was unaffected. Findings did not support VAR causing the reduced reinforcement from cigarettes as changes over time tended to be comparable or superior in the placebo condition.
While the two studies differed in notable ways (e.g., duration of time VAR was taken, naturalistic versus laboratory design), decreases in two demand indices were found over time in both, without evidence of VAR having a greater effect on demand intensity when compared to placebo and lack of consensus on whether changes in elasticity could be attributable to VAR. A limitation of these studies is that neither examined the associations between cigarette demand and abstinence. Additionally, individuals with a history of past year SUD were either excluded or not recruited in large enough numbers to examine in these trials; this reflects the larger trend of smokers who drink heavily or have SUDs being excluded from or underrepresented in studies of varenicline and other pharmacotherapies (Fucito et al. 2011; Leeman et al. 2007). However, smokers with SUD have more difficulty quitting smoking than smokers in general (Richter et al. 2002) so need to be studied. We have previously reported that, among smokers with SUD, lower cigarette demand at baseline (intensity and O max) was associated with greater abstinence during treatment, although not at follow-up points (MacKillop et al. 2016). Therefore, evaluating the effects of established pharmacotherapies on demand for cigarettes on quit day can determine if one medication reduces demand more, and if reductions in demand are associated with greater likelihood of success in abstaining. This is the first study assessing the effects of VAR vs. NRT on relative reinforcing efficacy among smokers with SUD. It is a secondary analysis from a clinical trial that reported significant effects of VAR versus NRT on 3-month smoking abstinence (Rohsenow et al. in press).
Method
Participants
Participants were smokers with SUD recruited from the community as part of a clinical trial (the parent study) that compared the effects of VAR to nicotine patch on smoking, substance use, and depressive symptoms (Rohsenow et al. in press). Inclusion criteria were (a) smoking 10+ cigarettes per day for the past 6 months, (b) SUD diagnosis, (c) current participation in SUD treatment, and (d) being 18–75 years old. Exclusionary criteria included (a) current smoking cessation treatment, (b) medical contraindications or use of medications that could be affected by smoking cessation (e.g., antipsychotics, warfarin, insulin), (c) recent use of substances (i.e., self-report of having used alcohol or drugs within the 48 h before recruitment or positive breath alcohol at screening), (d) evidence of hallucinations or delusions, (e) recent (i.e., past 5 years) suicide plan or attempt, (f) not willing to quit smoking, and (g) inability to understand informed consent. Participants needed to endorse being “willing to try to quit” smoking; however, there was no eligibility requirement regarding motivation or intention to quit smoking or for participants to be seeking treatment.
In order to validly assess medication effects on the CPT following the dose run-up period, participants who took <50% of assigned capsules in either condition during the dose run-up/lead-in week were excluded from analyses (n = 24), leaving a sample of 113. Three additional participants did not complete the CPT at either baseline or quit day and were excluded. Thus, the final sample (N = 110) included 59 participants randomized to VAR + placebo NRT and 51 participants randomized to NRT + placebo capsules. No participants were excluded for low effort responding on the CPT.
Procedures
All procedures were approved by the university Institutional Review Board as part of a clinical medication trial (clinicaltrials.gov identifier: NCT00756275). Using a double-blind double-placebo design, participants were randomized to one of two medication conditions for 13 weeks with quit day following a 7-day dose run-up/capsule lead-in period. Figure 1 illustrates the study design and procedures. Participants provided written informed consent to participate. They completed screening questionnaires and interviews, a physical exam, and laboratory tests to assess full study eligibility criteria. Eligible participants completed baseline questionnaires prior to urn randomization (stratified by gender, nicotine dependence, and depression diagnosis) to medication condition. Participants in both conditions participated in 10 weekly manualized “brief advice” sessions. This included one 20-min session at the start of the dose run-up week occurring between the baseline and quit day administrations of the CPT. Participants attended a 30-min counseling session on quit day, then eight weekly 5–10 min sessions. Participants also attended in-person assessment sessions on quit day and at 1- and 3-month post-quit by blinded assessors. Total compensation for completing all assessments was $185.
Study design schematic of procedures by week. Note: Brief weekly counseling sessions scheduled in weeks 1–10. Double asterisks indicate cigarette purchase task (CPT) administered. Single asterisk indicates assessments of smoking abstinence outcome at 1 and 3 months. QD quit day (begin use of patch), VAR varenicline, NRT nicotine replacement therapy, PBO placebo
Medication conditions
Participants in both conditions received two capsules (VAR or matching placebo) to take daily and were instructed to take one in the morning and one in the evening (except the first 3 days when they took one in the morning only). In both conditions, participants were instructed to apply the patch (NRT or matching placebo) upon waking daily and to wear it for 24 h or until going to sleep (if sleep interference was reported) beginning on quit day.
Varenicline and placebo patches
Those in the VAR condition underwent a standard 1-week VAR dose run-up (0.5 mg per day for 3 days, followed by 0.5 mg twice per day for 4 days), followed by 12 weeks of VAR (1 mg twice per day). Starting on quit day, after the dose run-up week, all received placebo patches (Rejuvenation Labs, Inc., Midvale, UT) that matched active transdermal NRT patches.
Nicotine replacement therapy and placebo capsules
Those in the NRT condition underwent a placebo capsule lead-in on the same schedule as the VAR group. Starting on quit day, after the 7-day lead-in, they received 12 weeks of NicoDerm CQ® transdermal patches (4 weeks each of 21, 14, and 7 mg/day) with placebo capsules (one capsule twice per day).
Measures
Demographics
Age, sex, race, education, and income were assessed at baseline.
Structured clinical interview for DSM-IV diagnosis (SCID)-patient version (First et al. 1997)
This interview was completed to establish a SUD diagnosis and to assess history of Major Depressive Disorder for the purpose of stratified randomization.
Fagerström test of nicotine dependence (FTND) (Heatherton et al. 1991)
This was used to assess nicotine dependence at baseline. This 6-item measure is scored 0–10 with higher scores indicating greater dependence.
Participant medication adherence
In both conditions, adherence was assessed weekly using participant self-report, Medication Event Monitoring System caps (MEMSCaps™; Aardex) for capsules, and count of returned used patches.
Cigarette purchase task (CPT) (Jacobs and Bickel 1999; MacKillop et al. 2008)
Hypothetical demand for cigarettes in a 24-h period was assessed at baseline and quit day at 41 escalating prices (0.00, 0.02, 0.04, 0.06, 0.08, 0.1, 0.12, 0.14, 0.16, 0.18, 0.2, 0.22, 0.24, 0.26, 0.28, 0.3, 0.32, 0.34, 0.36, 0.38, 0.4, 0.42, 0.44, 0.46, 0.48, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, and 35 $/cigarette). At baseline, participants were asked, “If you were smoking today according to your typical habits, how many cigarettes would you smoke at the following prices?” On quit day, they were asked “If you were smoking today at this level of the medication, how many cigarettes would you smoke at the following prices?” The remainders of the instructions for the CPT were identical during both administrations: “The available cigarettes are your favorite brand. Assume that you have the same income/savings that you have now, and NO ACCESS to any cigarettes or nicotine products other than those offered at these prices. In addition, assume that you would consume cigarettes that you request on that day, that is, you cannot save or stockpile cigarettes for a later date.”
Three behavioral economic indices of demand were calculated using an observed values approach: (1) breakpoint—the first price to suppress cigarette consumption to zero, (2) intensity—the number of cigarettes consumed at zero cost, and (3) O max—the maximum amount of money allocated to cigarettes. P max (i.e., the price at which expenditure is maximized) was not generated given typical collinearity (rs > .80) with breakpoint (MacKillop et al. 2008; MacKillop and Tidey 2011; Murphy et al. 2011). The fourth (4), elasticity of demand—overall proportionate price sensitivity, was generated using non-linear regression and defined as the α parameter in the exponential demand equation, log10 Q = log10 Q 0 + k(e -αQ0C–1) (Hursh and Silberberg 2008). In this equation, Q = consumption at a given price; Q 0 = maximum consumption (consumption at $.00); k = 3, a constant that denotes the range of consumption values in log10 across individuals; C = cost/price; and α = the derived elasticity parameter reflecting the rate of decline in consumption across price.
Smoking abstinence
Point prevalence abstinence from smoking was assessed on quit day and at 1- and 3-month post-quit dichotomously coded (abstinent/smoked). On quit day, abstinence was based on self-reported abstinence that day and biochemically verified with alveolar carbon monoxide (CO) levels using a Bedfont Micro Smokerlyzer®. An abstinence criterion of ≤6 ppm was used because participants had been abstinent for less than a day. At 1- and 3-month post-quit, abstinence was based on self-report of no smoking during the past week and biochemically verified by CO ≤ 4 ppm. At 3-month post-quit, use of NRT had been discontinued and salivary cotinine ≤15 ng/ml was used as an additional source of biochemical verification.
Longest continuous abstinence from smoking was assessed using a calendar-facilitated interview. Smoking on each day was self-reported by the participant from quit day to 1 month (1-month assessment) and from 1 to 3 months (3-month assessment) with the greatest consecutive number of days of abstinence calculated within each period.
Data analysis approach
Baseline and quit day responses on CPT items were initially examined for outlying values (Z > 3.29) and these outliers were Winsorized to one unit above the next non-outlying value to minimize excessive leverage on individual items (Tabachnick and Fidell 2007). Responses on the CPT at baseline and again on quit day were assessed for invariant responding. While no participant demonstrated an invariant response pattern at baseline, 30 participants did so on quit day including 29 participants who reported no demand (i.e., reported they would smoke zero cigarettes at every cigarette price including $0). As these responses were believed to reflect change in demand for cigarettes rather than low effort responding, these participants were not excluded from analyses (Smith et al. 2016). Given the large proportion of individuals reporting no cigarette purchases on quit day (i.e., invariant responders), elasticity of demand could only be validly calculated at baseline. Thus, changes in elasticity from baseline to quit day could not be assessed. Outlying values for the four calculated demand indices were also Winsorized and log10 transformed to improve normality. A trivial non-zero value (0.1) was used to permit modeling of zero consumption values.
Group comparisons on demographic and smoking measures at baseline were conducted using independent sample t tests for continuous variables and chi-square tests for categorical variables. Independent sample t tests were conducted to verify group equivalence at baseline on demand indices. Correlation analyses were conducted to examine relationships between variables and to identify any collinearity between CPT indices.
To examine changes in demand from baseline to quit day and whether assigned pharmacotherapy resulted in differential changes between groups mixed ANOVA was used. Cohen’s f is reported to show effect sizes with medium effect sizes beginning at f = 0.25 and large effect sizes beginning at f = 0.40 (Cohen 1988). Logistic regression was used to determine the extent to which baseline demand or reduction in demand from baseline to quit day predicted biochemically verified point prevalence abstinence from smoking on quit day, 1-month post-quit, or 3-month post-quit, covarying the effects of medication condition. Regression analyses were repeated using the longest period of continuous abstinence at 1 and 3 months due to limited power to detect dichotomous abstinence outcomes.
Results
Preliminary analyses
Baseline characteristics of participants are reported in Table 1; there were no significant differences at baseline with regard to SUD diagnoses, smoking severity, or demographic characteristics. Similarly, baseline demand indices as measured by the CPT did not differ significantly by medication condition, ts(108) = 0.65–0.41, ps > 0.52. Mean capsule adherence during dose run-up was 90%, from quit day to 1-month follow-up was 57%, and from 1- to 3-month follow-up was 35%, with no significant differences by medication condition during any time interval, ts(108) = −0.95–0.91, ps > 0.35. Capsule and patch adherence at 1 and 3-month follow-ups was highly collinear, rs = 0.84–0.87, ps < .01.
Associations between demand indices at baseline and at quit day are shown in Table 2. Baseline smoking and level of nicotine dependence were associated with initial CPT responses but unassociated with responses on quit day or the degree of change from baseline to quit day. Breakpoint was not correlated with intensity at baseline but was positively correlated with intensity on quit day. Elasticity was significantly negatively associated with the other demand indices at baseline. Associations between elasticity and other demand indices were not assessed on quit day due to the large number of invariant responses, as noted above. O max was highly positively correlated with the other demand indices at both time points (rs = .47–.90). Therefore, O max was not used for analyses of hypotheses in order to reduce potential error inflation due to multi-collinearity. Relationships observed in other studies suggest O max may often be confounded with the one or more CPT demand indices (MacKillop et al. 2008; MacKillop and Tidey 2011; Murphy et al. 2011). As a result, evaluation of primary study aims was conducted using intensity of demand and breakpoint.
Primary analyses
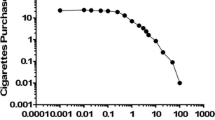
Figure 2 shows the demand curves generated from CPT data at baseline and quit day. There was a significant effect of time on breakpoint F(1, 108) = 53.57, p < .01, f = 0.70, but no effect of medication condition F(1, 108) = 0.00, p = .97, f = 0.00, nor an interaction between medication and time F(1, 108) = 0.75, p = .39, f = 0.10. Similarly, there was a significant effect of time on intensity F(1, 108) = 60.95, p < .01, f = 0.75, but no effect of medication condition F(1, 108) = 0.02, p = .88, f = 0.00, nor an interaction between medication and time F(1, 108) = 0.06, p = .80, f = 0.00. Figure 3 depicts intensity and breakpoint by condition and time point.
On quit day, 9/59 (15%) participants in the VAR condition and 11/51 (22%) participants in the NRT condition had biochemically verified abstinence from smoking. At 1-month post-quit, 6/59 (10%) participants in the VAR condition and 9/51 (18%) participants in the NRT condition had biochemically verified abstinence from smoking. At 3-month follow-up, 9/59 (15%) participants in the VAR condition and 2/51 (4%) participants in the NRT condition had biochemically verified abstinence from smoking. In the subset of participants in this study, medication conditions did not differ in abstinence on quit day (OR = 0.66, p = .39), 1-month post-quit (OR = .53, p = .26), or 3-month post-quit (OR = 4.41, p = .07). Similarly, there were no significant differences as a function of medication condition on the longest number of days of continuous abstinence reported at 1-month (VAR: M = 5.36, SD = 8.58; NRT: M = 7.27, SD = 9.91; t = −1.09, p = .28) or 3-month (VAR: M = 9.61, SD = 19.25; NRT: M = 10.18, SD = 17.62; t = −.16, p = .87) follow-ups. Longest continuous abstinence was significantly positively correlated with CO-confirmed point prevalence abstinence at both time points (rs = .70, ps < .01). Mediational models (i.e., medication affecting abstinence via changes in cigarette reinforcement) were considered but rejected given lack of association between medication and abstinence.
Breakpoint measured at baseline did not predict abstinence on quit day (OR = 1.35, p = .50), 1-month post-quit (OR = 0.47, p = .18), or 3-month post-quit (OR = 0.49, p = .29). Similarly, intensity of demand at baseline did not predict abstinence on quit day (OR = 0.52, p = .62), 1-month post-quit (OR = 0.48, p = .62), or 3-month post-quit (ORs = 0.16, p = .29).
Results predicting abstinence from initial reductions in demand from baseline to quit day are shown in Table 3. Having larger initial decreases in intensity of demand and in breakpoint were associated with increased likelihood of abstinence on quit day. Reduced demand intensity, but not breakpoint, predicted greater likelihood of point prevalence abstinence at 1-month post-quit. The decreases in intensity of demand and in breakpoint observed on quit day were not associated with increased likelihood of point prevalence abstinence at 3-month post-quit. Results predicting longest period of continuous abstinence are shown in Table 3. Both reduced demand intensity and breakpoint predicted longest number of days of continuous abstinence at 1-month post-quit. Reduced demand intensity, but not breakpoint, predicted the longest number of days of continuous abstinence at 3-month post-quit.
Discussion
The current study used a behavioral economic framework to test the effects of pharmacotherapy (VAR vs. NRT) on demand for cigarettes in smokers with SUD. Demand for cigarettes was significantly reduced on quit day compared to baseline for both CPT indices studied: intensity of demand and breakpoint. The reductions were comparable on quit day in both medication conditions. These findings suggest either that both medications reduced demand equivalently or that demand is reduced by quit day as a function of time rather than medication. The latter explanation is likely given that past research has suggested that some of the changes in demand observed during a dose run-up regimen are comparable whether taking VAR or placebo and that indices of smoking reinforcement may diminish as smokers approached their quit date more generally (McClure et al. 2013; Schlienz et al. 2014).
If changes in demand were not due to medication effects, possibly some cognitive or motivational changes (e.g., shift in decision balance (Prochaska et al. 1994)) resulted in the reductions observed on quit day. Another possible explanation is that demand was reduced by the provision of cessation resources including medication and brief advice to individuals who were willing to try to quit smoking but not seeking treatment. Research has suggested that more than three quarters of smokers in outpatient treatment for alcohol said they would be willing to consider stopping smoking during or after alcohol treatment (Ellingstad et al. 1999). Thus, engaging a population of smokers with SUD in treatment and providing medication in conjunction with brief counseling may be an important step in changing the perceived value of smoking among a population characterized by higher levels of nicotine dependence. Understanding what is driving changes in demand remains an important future direction that may shed light on how to facilitate reducing the reinforcing value of cigarettes and possibly improve smoking cessation outcomes. Nonetheless, as participants in both conditions were taking the recommended therapeutic dose of medication (i.e., 2 mg split dose of VAR; 21 mg transdermal dose of NRT) on quit day when CPT was reassessed, it is also possible that the change in demand was secondary to the effects of pharmacotherapy, with NRT and VAR not differing significantly in their effects.
Greater reductions in demand intensity and breakpoint were associated with greater likelihood of abstaining from smoking on quit day. Conversely, individuals for whom demand was unwavering despite pharmacotherapy were less likely to be abstinent on quit day. For that reason, individuals who do not show reductions in demand by quit day are at risk and may need stronger treatments to succeed. This may be particularly relevant among individuals with SUD given disappointingly low quit rates despite reportedly high levels of motivation and first-line therapy being provided free of charge and on-site in the context of SUD treatment (Stein et al. 2013). In addition, because individuals with SUD are likely to have experienced changes in the reinforcing effects of nicotine and other substances from their concurrent exposure (Littleton et al. 2007), understanding differences between the individuals who report reductions in smoking reinforcement while preparing to quit and those who do not is an important next step.
To date, evidence does not support an effect of VAR on either intensity of demand or breakpoint using the CPT. Demand intensity is a measure of the number of cigarettes an individual would purchase over the course of a day if they were “free.” In two previous studies of VAR compared to placebo in general populations of smokers (McClure et al. 2013; Schlienz et al. 2014), as well as in the current study of smokers with SUD, initial intensity was approximately 21–26 cigarettes/day and decreased by 20–50% when assessed on or immediately before quit day, without differences between conditions. Only one previous study has assessed changes in breakpoint secondary to medication use using the CPT; this study reported no effects of either time or medication on breakpoint (Schlienz et al. 2014). The authors note that rapidly increasing price intervals (i.e., $5, $6, $11, $35/cigarette) may have contributed to non-significant findings regarding breakpoint. In the current study, the use of a greater number of prices within this range (i.e., $5, $10, $15, $20, $25, $30, $35/cigarette) likely provided greater resolution and contributed to the significant effect of time on breakpoint. Nonetheless, the current study replicates the finding that there were no effects of medication on breakpoint, and extends findings to a sample of smokers with SUD. Therefore, there is considerable evidence to suggest that intensity of demand is reduced as one prepares to quit smoking and initial evidence that breakpoint may also be reduced, without evidence suggesting reductions are attributable to VAR.
Change in intensity but not breakpoint predicted 1-month point prevalence abstinence. While the reductions in both intensities of demand and breakpoint observed on quit day were associated with initial length of abstinence from smoking, changes in intensity predicted length of smoking abstinence that persisted at 3 months, unlike changes in breakpoint. Previous factor analytic research has suggested that different demand indices may aggregate to form two separate dimensions of reinforcement (MacKillop et al. 2009). Intensity is a component of the “amplitude” dimension reflecting how much of something is wanted, depicted on the y-axis of the demand curve. In contrast, breakpoint is a component of the “persistence” dimension of the demand curve reflecting how far an individual will go in terms of defending cigarette purchasing against rising costs before the finally ceasing purchasing cigarettes completely, depicted on the x-axis (MacKillop et al. 2009). The fact that change in intensity predicted abstinence for a longer time than did breakpoint may indicate that changes on the x-axis (persistence) of the demand curve when quitting smoking are less clinically relevant than changes on the y-axis (amplitude). Within the context of smoking cessation, it seems logical that greater likelihood of future abstinence would be associated with a larger decrease in intensity (i.e., “I won’t smoke as much or as many cigarettes as I used to (even if they are free)”) than breakpoint (i.e., “I won’t go as far as I used to in order to smoke; I won’t persist with smoking at high prices”).
It should be noted that the shrinking number of abstainers 20 (18%) on quit day, 15 (14%) at 1-month post-quit, and 11 (10%) at 3-month post-quit likely reduced the power to detect effects on abstinence when dichotomously coded. Rates of medication adherence decreased considerably over the course of the study which likely contributed to low rates of abstinence as has been show previously among smokers with SUD treated with NRT (Stein et al. 2006) and with VAR (Stein et al. 2013). While medication condition was not associated with greater likelihood of abstinence, differences in abstinence detected between the VAR (15%) and NRT (4%) conditions at 3-month follow-up just missed the threshold for statistical significance in this secondary analysis of a subset; VAR was associated with significantly greater likelihood of abstinence at 3 months in the larger parent trial (Rohsenow et al. in press). Therefore, the combined effects of low levels of medication adherence contributing to reduced rates of abstinence with increased outcome variance likely attributable to VAR effects over time makes the decreased associations between demand and abstinence outcomes not surprising.
Contrary to prediction, pretreatment levels of demand did not predict subsequent abstinence at any time point. Much of the research examining the predictive validity of the CPT on smoking abstinence more generally has relied on baseline assessment of cigarette demand for prediction and results have been inconsistent and specific to population and intervention (MacKillop et al. 2016; Secades-Villa et al. 2016), possibly because demand was not reassessed after treatment was initiated. Many changes can result in treatment that would affect outcomes. A novel aspect of this study is that it examined whether changes in demand during the initial week of treatment predicted abstinence. Findings suggest that the degree to which demand is reduced initially, rather than absolute levels of demand at baseline, may play an important role in early success with abstaining from smoking. This is consistent with other research of smokers with SUD that found that increased elasticity during the initial week of treatment, but not baseline demand, predicted smoking cessation (Madden and Kalman 2010).
Strengths and limitations
The strengths of this study included its use of an at-risk group of smokers, its prospective double-blind double-placebo design for medication assignment, its use of biochemically verified measures of abstinence from smoking, and its repeated administration of the CPT at baseline and quit day. This approach served to overcome previously reported limitations related to assessing demand only at baseline (Secades-Villa et al. 2016). Measuring demand for cigarettes at more than one time point permits greater understanding of the ways demand changes during the process of quitting smoking and the ways that these changes may impact subsequent smoking outcomes. Nonetheless, the use of a population of smokers with SUD, a group known to have particular difficulty quitting (Prochaska et al. 2004; Richter et al. 2002), combined with participants not being treatment-seeking, and decreased medication adherence over time, resulted in low rates of abstinence in this study and reduced prediction power. Similarly, participants were smokers with SUD from one urban setting and less racially diverse than those from public substance treatment facilities nationally (National Institute on Drug Abuse 2011). Finally, as this study included two active medication conditions plus smoking cessation counseling, it is impossible to determine whether the reductions in demand reported by participants across conditions were a function of taking medication more generally or whether these effects would have been present in the absence of either medication and were due to counseling, the provision of cessation resources, or some other aspect of the quitting process. As both conditions received manualized brief advice, it is impossible to know the effects of the medication in the absence of counseling and counseling may have contributed to the reduction in demand observed.
Conclusions
Behavioral economic methodology has been used extensively as a way to better understand why certain populations are more vulnerable to the reinforcing efficacy of drugs of abuse and possible mechanistic effects of pharmacotherapy (Hursh and Silberberg 2008). The data from the present study indicated that there was a reduction in intensity of demand and breakpoint from baseline to quit day among smokers with SUD. This reduction was comparable across medications and did not suggest beneficial effects of VAR relative to NRT in reducing the value of cigarettes. While pretreatment demand did not predict subsequent abstinence, changes in demand, particularly intensity of demand, secondary to initial medication use, predicted short-term abstinence. Thus, changes in behavioral economic indices were predictive of early smoking abstinence. Future studies might consider investigating demand throughout treatment to better understand how cessation affects demand and whether those changes are associated with long-term quitting.
References
Anthenelli RM, Benowitz NL, West R, et al. (2016) Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. The Lancet
Cahill K, Stevens S, Lancaster T (2014) Pharmacological treatments for smoking cessation. JAMA : the journal of the American Medical Association 311(2):193
Cohen J (1988) Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates.
Ellingstad TP, Sobell LC, Sobell MB, Cleland PA, Agrawal S (1999) Alcohol abusers who want to quit smoking: implications for clinical treatment. Drug Alcohol Depend 54(3):259–265
First MB, Spitzer RL, Williams JBW, Gibbon M (1997) Structured clinical interview of DSM-IV disorders (SCID). American Psychiatric Association, Washington, DC
Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS (2011) A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology 215(4):655–663
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict 86:1119–1127
Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115(1):186–198. doi:10.1037/0033-295x.115.1.186
Jacobs EA, Bickel WK (1999) Modeling drug consumption in the clinic using simulation procedures: demand for heroin and cigarettes in opioid-dependent outpatients. Exp Clin Psychopharmacol 7(4):412–426. doi:10.1037/1064-1297.7.4.412
Koob GF, Le Moal M (2001) Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24(2):97–129
Leeman RF, Huffman CJ, O'Malley SS (2007) Alcohol history and smoking cessation in nicotine replacement therapy, bupropion sustained release and varenicline trials: a review. Alcohol Alcohol 42(3):196–206
Littleton J, Barron S, Prendergast M, Nixon SJ (2007) Smoking kills (alcoholics)! Shouldn’t we do something about it? Alcohol Alcohol 42(3):167–173
MacKillop J, Tidey JW (2011) Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: an initial study. Psychopharmacology 216(1):91–99
MacKillop J, Murphy JG, Ray LA et al (2008) Further validation of a cigarette purchase task for assessing the relative reinforcing efficacy of nicotine in college smokers. Exp Clin Psychopharmacol 16(1):57–65. doi:10.1037/1064-1297.16.1.57
MacKillop J, Murphy JG, Tidey JW, Kahler CW, Ray LA, Bickel WK (2009) Latent structure of facets of alcohol reinforcement from a behavioral economic demand curve. Psychopharmacology 203(1):33–40. doi:10.1007/s00213-008-1367-5
MacKillop J, Murphy CM, Martin RA et al (2016) Predictive validity of a cigarette purchase task in a randomized controlled trial of contingent vouchers for smoking in individuals with substance use disorders. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 18(5):531–537. doi:10.1093/ntr/ntv233
Madden GJ, Kalman D (2010) Effects of bupropion on simulated demand for cigarettes and the subjective effects of smoking. Nicotine Tob Res 12(4):416–422
McClure EA, Vandrey RG, Johnson MW, Stitzer ML (2013) Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine Tob Res 15(1):139–148. doi:10.1093/ntr/nts101
Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM (2011) Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend 113(2):207–214
National Institute on Drug Abuse (2011) Treatment statistics. Revised March 2011 edn. National Institute on Drug Abuse
Prochaska JO, Velicer WF, Rossi JS et al (1994) Stages of change and decisional balance for 12 problem behaviors. Health Psychol 13(1):39–46. doi:10.1037/0278-6133.13.1.39
Prochaska JJ, Delucchi K, Hall SM (2004) A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. American Psychological Assn
Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS (2002) A population-based study of cigarette smoking among illicit drug users in the United States. Addiction 97(7):861–869. doi:10.1046/j.1360-0443.2002.00162.x
Rohsenow DJ, Tidey JW, Martin RA, et al. (in press) Varenicline versus nicotine patch with brief advice for smokers with substance use disorders with or without depression: effects on smoking, substance use and depressive symptoms. Addiction
Schlienz NJ, Hawk LW Jr, Tiffany ST, O'Connor RJ, Mahoney MC (2014) The impact of pre-cessation varenicline on behavioral economic indices of smoking reinforcement. Addict Behav 39(10):1484–1490. doi:10.1016/j.addbeh.2014.05.008
Secades-Villa R, Pericot-Valverde I, Weidberg S (2016) Relative reinforcing efficacy of cigarettes as a predictor of smoking abstinence among treatment-seeking smokers. Psychopharmacology. doi:10.1007/s00213-016-4350-6
Smith TT, Cassidy RN, Tidey JW, et al. (2016) Impact of smoking reduced nicotine content cigarettes on sensitivity to cigarette price: further results from a multi-site clinical trial. Addiction
Stein MD, Anderson BJ, Niaura R (2006) Nicotine replacement therapy: patterns of use after a quit attempt among methadone-maintained smokers. J Gen Intern Med 21(7):753–757
Stein M, Caviness C, Kurth M, Audet D, Olson J, Anderson B (2013) Varenicline for smoking cessation among methadone-maintained smokers: a randomized clinical trial. Drug Alcohol Depend 133(2):486–493
Tabachnick BG, Fidell LS (2007) Using multivariate statistics, 5th edn. Allyn & Bacon/Pearson Education, Boston
Tapper AR, McKinney SL, Nashmi R et al (2004) Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science 306(5698):1029–1032
Thurgood SL, McNeill A, Clark-Carter D, Brose LS (2015) A systematic review of smoking cessation interventions for adults in substance abuse treatment or recovery. Nicotine & Tobacco Research:ntv127
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Participants provided written informed consent to participate.
Financial support
This research was supported by a grant from the National Institute on Drug Abuse (R01DA024652 and T32DA016184), the Peter Boris Chair in Addictions Research, and a Senior Research Career Scientist Award from the Department of Veterans Affairs.
Rights and permissions
About this article
Cite this article
Murphy, C.M., MacKillop, J., Martin, R.A. et al. Effects of varenicline versus transdermal nicotine replacement therapy on cigarette demand on quit day in individuals with substance use disorders. Psychopharmacology 234, 2443–2452 (2017). https://doi.org/10.1007/s00213-017-4635-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4635-4