Abstract
Rationale
In depressive disorders, one of the mechanisms proposed for antidepressant drugs is the enhancement of synaptic plasticity in the hippocampus and cerebral cortex. Previously, we showed that the muscarinic acetylcholine receptor (mAChR) agonist oxotremorine (Oxo) increases neuronal plasticity in hippocampal neurons via FGFR1 transactivation.
Objectives
Here, we aimed to explore (a) whether Oxo exerts anxiolytic effect in the rat model of anxiety-depression-like behavior induced by chronic restraint stress (CRS), and (b) if the anxiolytic effect of Oxo is associated with the modulation of neurotrophic factors, brain-derived neurotrophic factor (BDNF) and fibroblast growth factor-2 (FGF2), and phosphorylated Erk1/2 (p-Erk1/2) levels in the dorsal or ventral hippocampus and in the medial prefrontal cortex.
Methods
The rats were randomly divided into four groups: control unstressed, CRS group, CRS group treated with 0.2 mg/kg Oxo, and unstressed group treated with Oxo. After 21 days of CRS, the groups were treated for 10 days with Oxo or saline. The anxiolytic role of Oxo was tested by using the following: forced swimming test, novelty suppressed feeding test, elevated plus maze test, and light/dark box test. The hippocampi and prefrontal cortex were used to evaluate BDNF and FGF2 protein levels and p-Erk1/2 levels.
Results
Oxo treatment significantly attenuated anxiety induced by CRS. Moreover, Oxo treatment counteracted the CRS-induced reduction of BDNF and FGF2 levels in the ventral hippocampus and medial prefrontal cerebral cortex
Conclusions
The present study showed that Oxo treatment ameliorates the stress-induced anxiety-like behavior and rescues FGF2 and BDNF levels in two brain regions involved in CRS-induced anxiety, ventral hippocampal formation, and medial prefrontal cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The muscarinic acetylcholine receptors (mAChRs), a family of metabotropic G protein-coupled receptors, include five subtypes (M1–5) with discrete distribution throughout the brain and whose activation regulates synaptic plasticity, neurogenesis, neuroprotection (Di Liberto et al. 2014; Veena et al. 2011), and many aspects of cognitive functions and behaviors (Drever et al. 2011; Luchicchi et al. 2014). Moreover, mAChR activation can modulate the production or/and the release of neurotrophic factors in neurons, such as brain-derived neurotrophic factor (BDNF) and fibroblast growth factor-2 (FGF2), which in turn could mediate synaptic plasticity, axon outgrowth, and neurogenesis (Barathi et al. 2009; Lindholm et al. 1994; Mudo et al. 1996; Navakkode and Korte 2012). Several of these effects involve mainly M1 receptor in adult hippocampus (Dennis et al. 2016; Mitsushima et al. 2013).
In recent years, chronic exposure to stressful life events has become an established risk factor for the development of depression and anxiety (Caspi et al. 2003; Colman and Ataullahjan 2010; Hill et al. 2012). The brain regions mainly involved in the alterations found in anxiety and depressive disorders are the hippocampus and the frontal cerebral cortex, which are usually accompanied by atrophy, closely related to the loss of neurons and their connections (Sheline et al. 2003). Therefore, one of the mechanisms proposed as antidepressant drug effects is the improvement of synaptic connections and plasticity in the hippocampus and cerebral cortex. In this context, there is evidence that the therapeutic action of antidepressant drugs is mediated by growth factors, such as BDNF and FGF2, which can exert trophic effects on neurons and reverse the neuronal damage and atrophy in the affected brain regions (Warner-Schmidt and Duman 2007).
Accordingly, the neurotrophic factor BDNF, which is highly expressed in the hippocampus (Mudo et al. 1996), was found to exert anxiolytic and antidepressant-like effects and to increase resistance to stress (Berton et al. 2006; Duman and Monteggia 2006; Govindarajan et al. 2006; Martinowich et al. 2007; Sairanen et al. 2005; Shirayama et al. 2002). Direct infusion of BDNF leads to recovery of behavioral deficits in depressed rats (Sairanen et al. 2005; Siuciak et al. 1997) and chronic administration of antidepressants increases BDNF levels in cortical and hippocampal areas (Nibuya et al. 1995; Russo-Neustadt et al. 2001). Conversely, long-term stress reduces hippocampal BDNF, which, by contributing to the atrophy and even the death of pyramidal neurons in the hippocampus, may play a role in stress-related psychiatric illnesses (Bennett and Lagopoulos 2014; Monteggia et al. 2007; Shi et al. 2010; Smith et al. 1995). In human, a post-mortem study of depressed patients reported a reduction in BDNF expression (Karege et al. 2005) and polymorphisms of BDNF gene were associated with increased susceptibility to depression (Neves-Pereira et al. 2002; Sen et al. 2003).
Similarly to BDNF, the neurotrophic factor FGF2, which is expressed in the hippocampus (Belluardo et al. 1998), has also been included among trophic factors that mediate therapeutic actions of antidepressants (Mallei et al. 2002). Several evidences have demonstrated the relevance of FGF2 to anxiety (Perez et al. 2009; Turner et al. 2006). FGF2 has been found decreased in post-mortem brains of depressed individuals (Evans et al. 2004; Gaughran et al. 2006) and in social defeat, an animal model of depression (Turner et al. 2008a). Conversely, treatment with FGF2 exerts antidepressant effects (Elsayed et al. 2012; Turner et al. 2008b). Recently, we reported that treatment with FGF2 and 8-OH-DPAT, a 5HT1A receptor agonist, produces antidepressant actions in the forced swimming test (Borroto-Escuela et al. 2012). Finally, it has been shown that chronic treatment with antidepressants increases FGF2 expression (Bachis et al. 2008; Mallei et al. 2002). Thus, FGF2 is considered as an endogenous anxiolytic and antidepressant factor, and therefore, it is implicated in anxiety disorders (Eren-Kocak et al. 2011; Perez et al. 2009).
All the above listed data provided the basis for the hypothesis that anxiety and depression may be associated to a deficit in neurotrophic factors, and that antidepressants may neutralize this deficit (Duman and Monteggia 2006). Therefore, in the present study, we aimed to explore (a) whether mAChR activation by oxotremorine M (Oxo), a nonselective mAChR agonist, may exert anxiolytic effects in the rat model of anxiety-depression-like behavior induced by chronic restraint stress (CRS), and (b) if the anxiolytic effect of Oxo is associated with the modulation of neurotrophic factors, BDNF and FGF2, in the hippocampus and medial prefrontal cortex.
In the first aim, the anxiolytic role of Oxo in CRS rat was tested using four paradigms: forced swimming test (FST), novelty suppressed feeding test (NSFT), elevated plus maze (EPM) test, and light/dark box (LDB) test.
In the second aim, we decided to evaluate the protein levels of BDNF and FGF2, including related cell signaling by assessment of phosphorylated Erk1/2 (p-Erk1/2) levels, in the hippocampus and medial prefrontal cortex, two brain areas involved in anxiety-related disorders. In addition, since it has been largely accepted that the dorsal hippocampus has a preferential role in spatial learning and memory, whereas the ventral hippocampus may have a preferential role in anxiety-related behaviors (O’Leary and Cryan 2014; Strange et al. 2014), we analyzed the levels of BDNF, FGF2, and p-Erk1/2 separately in dorsal and ventral hippocampus.
Material and methods
Animals
Adult female Wistar rats (4 months old) were used. Rats were housed in polypropylene cages in a temperature (23 ± 2 °C), humidity (50–55%), and light-controlled (12-h light/dark cycle) environment, with access to food and water ad libitum. The experiments were carried out in accordance with the National Institute of Health Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academics Press, WA, USA), with the rules and principles of the European Communities Council Directive 2010/63/EU revising Directive 86/609/EEC, in accordance with the national D.L. March 4, 2014, no. 26, and were approved by the local Bioethical Committee. All efforts were made to minimize both the suffering and the number of animals used.
Experimental design
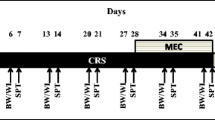
This study, according to the first aim, was designed to explore the anxiolytic efficacy of Oxo (O100, Sigma-Aldrich Chemical Co., St. Louis, MO, USA) treatment against CRS-induced anxiety-depression-like behavior in rats. The Oxo dose (0.2 mg/kg) used in the present study was chosen based mainly on literature data (Srikumar et al. 2006; Veena et al. 2011) and on preliminary experiments in which the anxiolytic efficacy of 0.2 mg/kg b.w. was assessed by FST and EPM tests. The rats were randomly divided into four groups as follows: (i) control unstressed group treated with saline (Ctrl); (ii) chronic restraint-stressed group treated with saline (CRS); (iii) restraint stressed plus 0.2 mg/kg Oxo-treated group (CRS + Oxo); (iv) unstressed group treated with Oxo. Control- and Oxo-treated groups of rats remained undisturbed in their home cages. The CRS procedure was carried out once daily for 6 h, from 10:00 a.m. to 16:00 p.m., and for 21 consecutive days. In brief, rats were placed in a transparent plastic restrainer (350-ml cubage water bottle, 20 × 7 cm), which has several 3 mm holes for breathing, under 200 lx light conditions at 22 °C. The animals have ample air but were unable to move within the restrainer. After 21 days of CRS the experimental groups were treated for 10 days with Oxo or saline according to the above mentioned groups. Oxo was dissolved in 0.9% physiological saline solution before use, and a dose of 0.2 mg/kg was given intraperitoneally (i.p.) in a volume of 0.5 ml, as well as saline treatment. After 10 days, four different behavioral tests for anxiety-like behavior were randomly performed as follows: NSFT, FST, EPM, and LDB. The entire experimental schedule is shown in Fig. 1a. After the behavioral testing, rats were sacrificed by decapitation to collect blood and to quickly remove the brain. Hippocampus or sampled medial prefrontal cortex was dissected under stereomicroscopy with refrigerate support. Dissected tissues were quickly frozen in isopentane, cooled in liquid nitrogen, and stored at −80 °C for later use. The dissection of hippocampus was performed as described below. Cerebellum and part of rostral frontal lobes were removed using the scalpel blade; therefore, the midline of the brain was incised in order to separate the hemispheres. From each hemisphere, using the surgical scissors, the brainstem/midbrain/thalamus was removed away, revealing the medial surface of the hippocampus. Using the sharp edge of the spatula, the fornix and white fiber bundle located at the anterior/dorsal portion of the hippocampus were detached, and subsequently, the hippocampus was overturned with spatula and separated from the cortex. The sampled brain regions were used to analyze, according to the second aim of this study, the BDNF and FGF2 protein levels together with p-Erk1/2 levels.
Schedule of treatment experimental procedures and illustration of ventral and dorsal hippocampal dissection. a Experimental schedule of chronic restraint stress (CRS), treatment with oxotremorine (Oxo), and sequential behavioral tests performed: novelty suppressed feeding test (NSF), forced swimming test (FST), elevated plus maze test (EPM), and light/dark box test (LDB). b Illustration showing the right part of coronal brain section at antero-posterior plane AP −4.80 mm relative to Bregma in the rat brain (Paxinos and Watson 2014). The upper gray area indicates approximately the sampled area of the dorsal hippocampus. The lower gray area indicates approximately the sampled area of the ventral hippocampus. The remaining middle part of the hippocampus was discarded. The dotted line tracks the empirical boundary line between dorsal and ventral hippocampus that should be referred to dorso-ventral plane (Paxinos and Watson 2014) at horizontal plane −4.50 according to previous report (Christensen et al. 2010)
Behavioral tests
Behavioral tests were performed between 9:00 and 11:00 a.m. Before CRS experiments, each rat was habituated for 5 days and twice per day to be handled for 3 min, during which the experimenter interacts with the animal by stroking its fur and cupping the rat in hand. The rats were also habituated to enter inside the bottle used for CRS and to be transported in their home cage on a cart into behavioral test room. The control- and Oxo-treated groups were handled per 3 min twice per day during the experimental procedure. Animals were tested in NSFT, LDB, FST, and EPM to examine anxiety-related behaviors.
Novelty suppressed feeding test
Approximately 24 h after the removal of the food, the rats were transferred to the testing room, placed in a clean holding cage, and allowed to habituate for 30 min. Food deprived rats were placed in an open field (60 cm × 40 cm) illuminated (60 W), with its floor covered with wooden bedding and with a small amount of food in the center of the box on a white circular filter paper (10 cm in diameter). Each rat was placed in the corner of the testing arena and the latency to the first feeding episode was recorded. The NSF test was performed during a 15-min period, and food intake was measured at the end of this period by weighting the piece of chow after the test.
Forced swimming test
The FST was done by placing a rat in a transparent Plastic cylinder (30 cm diameter × 50 cm height) filled up to a depth of 35 cm with water at 25 °C. At this depth, rats could not touch the bottom of the cylinder with their tails or hind limbs. Rats were introduced to pre-swimming for 15 min on the day before experimentation. The rats were subjected to 5 min of forced swim, and behavior was continuously recorded with an overhead video camera for later manual scoring. Immobility time and escape behaviors, as well as number of climbing and swimming, were determined. The rats were judged to be immobile when they remained in the water without struggling or making only those movements necessary to keep the head above water. Climbing behavior was defined as upward-directed movements of the forepaws alone the side of the swim chamber, while swimming behavior was considered as movements throughout the swim chamber including crossing into another quadrant. Immobility behavior was calculated as the length of time in which the animal did not show escape responses (e.g., total time of the test minus time spent in climbing and swimming behaviors). After the test, the rat was removed from the tank, dried with a towel, and placed back in its home cage. The water in the swim tank was changed between rats.
Elevated plus maze test
The EPM test was conducted in an apparatus consisting of two open arms (50 × 10 cm each), two closed arms (50 × 10 × 40 cm each) and a central platform (10 × 10 cm), arranged in a way such that the two arms of each type were opposite to each other. The maze was made from dark gray PVC and elevated 70 cm above the floor. Exploration of the open arms was encouraged by testing under indirect dim light (100 lx). Each animal was placed on the central platform facing one of the open arms, and during a 5-min test period, the following parameters were recorded: (a) number of open arm entries, (b) number of closed arm entries, (c) time spent in open arms, (d) time spent in closed arms, and (e) ratio time in open arms and total time in arms. Entry by an animal into an arm was defined as the condition in which the animal has placed his four paws in that arm. The behavior in the maze was recorded using a computerized video tracking system (Any-Maze software), with a video camera mounted on the ceiling above the center of the maze. Anxiety reduction, indicated by open arm exploration in the EPM, was defined as an increase in the numbers of entries into the open arms relative to total entries into either open or closed arm and as an increase in the proportion of time spent in the open arms relative to total spending time in either open or closed arm. The apparatus was cleaned after each trial.
Light/dark box test
The LDB apparatus consisted of a wooden box (60 cm length × 30 cm height × 30 cm width) divided into two equal-sized compartments by a barrier that contained a doorway (10 cm height × 10 cm width). One of the compartments was painted black and was covered with a lid, and the other compartment was painted white and illuminated with a 45-W light bulb positioned 40 cm above the box. The rats were placed in the middle of the lit compartment, facing away from the dark chamber and allowed to freely explore the apparatus for 5 min. The number of transitions and time spent in the light compartments were measured. The apparatus was cleaned after each trial.
Cortisol levels
After the behavioral tests, the rats of all experimental groups were decapitated under anesthesia between 11:00 and 12:00 a.m. and blood was collected in tubes coated with EDTA. Blood samples were centrifuged at 4000×g at 4 °C for 10 min, and the supernatant was stored at −80 °C. The plasma levels of cortisol were measured using automated electrochemiluminescence immunoassay (Roche Diagnostics Elecsys assays and modulator analytics E 170), and values were expressed in nanograms per milliliter. The minimum level of detection for assays of cortisol was 0.15 ng/ml.
Western blotting
In order to explore the hypothesis of potential different molecular changes between the dorsal and ventral region of the hippocampus, both left and right hippocampi were dissected from the brain under stereomicroscopy, keeping distinct the rostro-septal pole (dorsal part) and the caudal-temporal pole (ventral part) in order to dissect each hippocampus in a dorsal part (2.5 mm size from the rostral pole) and in a ventral part (2.5 mm size from the caudal-pole), as well as in a middle part (about 3 mm) that was discarded to obtain a safe separation between dorsal and ventral region (Fig. 1b). The prefrontal cortex was sampled from the right hemisphere approximately at the following cortex levels: antero-posterior planes from Bregma (from AP +4.70 to AP +2.00 mm) and mediolateral planes (from L 0 to L 2.5 mm).
Dissected tissues, dorsal or ventral hippocampus of both side and medial prefrontal cortex, were processed as reported in Frinchi et al. (2010). Shortly, tissues were homogenized at 4 °C in cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton, SDS 0.1%), supplemented with protease inhibitor cocktail (Sigma-Aldrich P8340) and phosphatase inhibitor cocktail (Sigma-Aldrich P5726). Samples were sonicated (30 pulsations/min), quantified by the Lowry method (Lowry et al. 1951), and stored at −80 °C. Western blotting was performed as previously described (Di Liberto et al. 2011). Protein samples (50 μg per lane) and molecular weight marker (161–0375 BIO-RAD) were run on polyacrylamide gel and electrophoretically transferred onto nitrocellulose membrane (RPN303E, Hybond-C-extra, GE Healthcare Europe GmbH). The membranes were incubated for 1 h in blocking buffer (1× TBS, 0.1% Tween-20, 5% w/v nonfat dry milk) and thereafter incubated with gentle shaking overnight at −4 °C with specific antibody in blocking buffer. For detection of BDNF, FGF2, and phosphorylated Erk1/2 (p-Erk1/2), the following antibodies were used: rabbit anti-BDNF (N-20) (SC-546, Santa Cruz Biotechnology), rabbit anti-FGF2 (147) (SC-79, Santa Cruz Biotechnology), and rabbit anti-p-Erk1/2 (Thr202/Tyr204) (9102, Cell Signaling). The day after, membranes were washed three times for 10 min with TBS/T and incubated for 1 h at room temperature with goat anti-rabbit IgG (sc-2004, Santa Cruz Biotechnology) horseradish peroxidase-conjugated diluted 1:10,000. After three washings with TBS-T, immune complexes were visualized with chemiluminescence reagent (RPN2236, GE Healthcare Europe GmbH) according to the manufacturer’s instructions. The Hyperfilm (ECL-films 28906837, GE Healthcare Europe GmbH) was developed using Kodak developer and fixer (catalog no. 1900984 and 1902485, Kodak GBX, Eastman Kodak). Each film was exposed for at least three different times to evaluate saturation problems. For the normalization of quantitative evaluation of bands, each membrane was stripped at 37 °C for 30 min in buffer containing glycine 200 mM, SDS 3 mM, 1% of Tween-20, pH 2.2. After two washings with TBST, the membranes were re-probed with rabbit anti-Erk1/2 antibody (9102, Cell Signaling) and anti-β-actin antibody (sc-47778, Santa Cruz Biotechnology). The densitometric evaluation of bands was performed by measuring the optical density (O.D.) after background subtraction using the ImageJ software (Rasband WS, ImageJ, US National Institutes of Health, Bethesda, Maryland, USA; http://rsb.info.nih.gov/ij/; 1997–2016).
Statistical analysis
Data are the results of the average of 4–9 animals for experimental groups. For the analysis of body, hippocampal and adrenal gland weights, cortisol levels, and behavioral tests, all experimental rats of each group were used. For BDNF, FGF2, and p-Erk1/2 analysis of the hippocampus and medial prefrontal cortex, the following number of rats for group were used: Ctrl (n = 5), Oxo (n = 4), CRS + Oxo (n = 5), and CRS (n = 5) rats, randomly sampled by following the sequential number order assigned to each rat at the beginning of the experiments.
Statistical analyses were performed using one-way analysis of variance (ANOVA). Two-way repeated measures of ANOVA were performed to compare the modification of body weight using the experimental groups (Ctrl, CRS, CRS + Oxo, Oxo) and experimental time (0, 7, 14, 21, 31 days) as main effects, and the assumption of sphericity was assessed using Mauchly’s test. Significant effects were further evaluated by Fisher’s protected last significant difference (PLSD test). Values are shown as mean ± SE. Differences in p value less than 0.05 were considered statistically significant.
Results
Weight of body, hippocampus, and adrenal glands
Because weight changes have been observed in rodent models of CRS, we measured weekly (0, 7,14, 21, 31 days) the body weight of rat during the whole experimental time. Mauchly’s sphericity test revealed that the two main effects, i.e., experimental groups and time, as well as the interaction effect (experimental groups × time) met the assumption of sphericity (p > 0.05). Thus, there was no need of any correction of F ratios. Two-way repeated measurement ANOVA showed a significant experimental groups × experimental time interaction [F (12,104) = 48.6, p < 0.0001] and significant experimental groups and experimental time main effects [F (3,104) = 8.1, p < 0.001; F (4,104) = 7.8, p < 0.0001] in the body weight change (Fig. 2a). As expected, the body weight of Ctrl group (n = 8) increased throughout the experimental period, becoming significant at 21 (post-hoc p < 0.05) and 31 (post-hoc p < 0.01) days as compared to starting body weight. The CRS groups (n = 9) showed a progressive reduction becoming significant at 14, 21, and 31 (post-hoc p < 0.01, p < 0.001, p = 0.0001) vs starting body weight. The reduction of body weight observed at 14 and 21 days (post-hoc p < 0.01, p < 0.001) in the CRS + Oxo group (n = 9) was recovered to starting body weight at 31 days (Fig. 2a). However, the recovery at 31 days of body weight in the CRS + Oxo group was still significantly lower compared to Ctrl group (post-hoc p < 0.001). Similarly, to Ctrl group, the body weight in the Oxo-treated rats (n = 4) showed a progressive increase before the Oxo treatment, becoming significant at 21 days (post-hoc p > 0.05), and thereafter undergoes a reduction with achievement of the starting body weight (Fig. 2a).
Changes in body weight, hippocampi, and adrenal glands weight and quantitative analysis of serum cortisol levels in Ctrl-, CRS-, CRS + Oxo-, and Oxo-treated rats. Evaluation of body weight (a), expressed for each group as percentage of variation of body weight registered at the beginning of the experimental procedure and thereafter every week (0, 7, 14, 21, 31 days). Evaluation of hippocampi weight (b), adrenal glands weight (c), and serum cortisol levels (d). Data represent mean ± S.E.M. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs Ctrl group; #p < 0.05, ##p < 0.01, ###p < 0.001, ####p < 0.0001 vs Oxo group. Concerning evaluation of body weight (a), there were also some significant points in the various experimental days among animals stressed and unstressed. (i) At the day 7: CRS vs Ctrl p < 0.05 and CRS + Oxo vs Ctrl p < 0.05. (ii) At the day 14: CRS vs Ctrl p < 0.0001, CRS + Oxo vs Ctrl p < 0.001, CRS vs Oxo p < 0.01, CRS + Oxo vs Oxo p < 0.01. (iii) At the day 21: CRS vs Ctrl p < 0.0001, CRS + Oxo vs Ctrl p < 0.0001, CRS vs Oxo p < 0.001, CRS + Oxo vs Oxo p < 0.001. (iv) At the day 31: CRS vs Ctrl p < 0.0001, CRS + Oxo vs Ctrl p < 0.001, CRS vs Oxo p < 0.0001, Oxo vs Ctrl p < 0.05
Concerning the hippocampi weight, one-way ANOVA showed significant modification [F (3,24) = 4.1, p < 0.02], with intergroup difference in the CRS group (n = 8) when compared to Oxo-treated group (n = 4; p < 0.05) and CRS + Oxo group (n = 8; p < 0.02), but not significant when compared to Ctrl (n = 8) group (Fig. 2b). In the CRS group treated with Oxo, a significant recovery of the hippocampi weight when compared to CRS group was observed (Fig. 2b). As shown in Fig. 2c, the adrenal glands weight did not show significant changes in all the experimental groups [F (3,23) = 1.2, p = 0.32].
Cortisol levels
To test the efficiency of the stress paradigm, the levels of cortisol were assessed at the end of experimental protocol and the result is showed in Fig. 2d. One-way ANOVA showed a not significant change (F (3,24) = 1.8, p = 0.18) in cortisol level among experimental groups, even though post-hoc PLSD test showed significant increase (p < 0.05) in CRS-Oxo-treated group as compared to Ctrl group.
Effect of Oxo treatment in NSF test
The NSF test revealed a significant increase in the latency of first feeding [F (3,24) = 4.1, p < 0.0001], with intergroup significant difference between CRS group (n = 8) and Ctrl group (n = 8) (Fig. 3a), indicating increased anxiety. Oxo treatment in the CRS group (n = 8) produced a recovery of the latency period of first feeding, that becomes similar to control group (Fig. 3a). The latency of first feeding did not show significant changes in the Oxo group (n = 4) as compared to Ctrl group (Fig. 3a). The analysis of food consumed (Fig. 3b) within 15 min revealed a significant reduction of food intake [F (3,24) = 6.3, p < 0.002] with intergroup difference among CRS group and other groups (post-hoc vs Ctrl p < 0.05; vs CRS + Oxo p < 0.01; vs Oxo p < 0.05). In the CRS group, the decreased food intake was reversed by Oxo treatment (Fig. 3b), becoming even significantly increased when compared to Ctrl group (post-hoc: p < 0.05). Oxo group did not show significant change of food consumption as compared to Ctrl group.
Behavioral evaluation by novelty-suppressed feeding test and by light/dark box test. a, b Evaluation of CRS-induced anxiety-like behavior and the anxiolytic role of Oxo by novelty-suppressed feeding test: a latency period of first feeding or b amount of consumed food within 15 min. c, d Evaluation of CRS-induced anxiety-like behavior and the anxiolytic role of Oxo by light/dark box test: c time spent in the light compartments or d number of entries within 5 min. Data represent mean ± S.E.M.; *p < 0.05, **p < 0.01, ***p < 0.001
Effect of Oxo treatment in LDB
In the test that analyzes the time spent in light/dark box, the one-way ANOVA test revealed significant differences among the experimental groups [F (3,24) = 3.8, p < 0.01] (Fig. 3c, d). The CRS group (n = 8) spent significantly more time in the dark compartment and showed significant fewer light/dark transitions as compared to the Ctrl group (n = 8; post-hoc p < 0.05), and this anxiety-related behavior of CRS group was reversed in CRS + Oxo (n = 8)-treated group (Fig. 3c). Oxo treatment alone (n = 4) did not affect behavior as compared to Ctrl group. The number of entries was significantly different [F (3,24) = 7.9, p < 0.001] among the groups: it was significantly reduced in CRS group (vs Ctrl, post-hoc p < 0.01), completely restored in CRS treated with Oxo (vs CRS, post-hoc p < 0.001), whereas Oxo treatment alone did not affect this parameter (Fig. 3d).
Effect of Oxo treatment in FST
In this test, in addition to examining total immobility time, we also assessed the latency of first episode of immobility and the climbing number. One-way ANOVA revealed difference in immobility time [F (3,27) = 11.3, p < 0.0001], latency of first episode of immobility [F (3,27) = 6.8, p < 0.01], and climbing number [F (3,27) = 6.3, p < 0.01] among the experimental groups. Rats of CRS group (n = 9), as compared to Ctrl group (n = 9), showed a significant increase of immobility time (post-hoc p < 0.01; Fig. 4a), which was in a good correlation (Fig. 4b, c) with the reduced latency of first immobility (post-hoc p < 0.01) and the decreased climbing number (post-hoc p < 0.01). CRS + Oxo group (n = 9) showed a strong reduction of immobility time, becoming even significant when compared to Ctrl sgroup (n = 9; post-hoc, p < 0.05; Fig. 4a). This result was in good correlation with the increase of both latency of first immobility and climbing number, as compared to CRS (Fig. 4b, c). Oxo (n = 4) treatment alone did not significantly affect any of the examined parameters (Fig. 4a, c).
Behavioral evaluation by forced swimming test and by elevated plus maze test. a–c Evaluation of CRS-induced anxiety-like behavior and the anxiolytic role of Oxo by forced swimming test: a total immobility time, b latency to first episode of immobility, and c number of climbings within 5 min. d–f Evaluation of CRS-induced anxiety-like behavior and the anxiolytic role of Oxo by elevated plus maze test: d time spent in open arms, e ratio time in open arms and total time in arms, and f number of open arm entries within 5 min. Data represent mean ± S.E.M.; *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Effect of Oxo treatment in EPM test
In the EPM test, one-way ANOVA showed significant differences in time spent in open arms [F (3,27) = 3.1, p < 0.05] among experimental groups, with intergroup significant difference in the CRS group as compared to the Ctrl group (post-hoc p < 0.05) and these effects were reversed by Oxo treatment (Fig. 4d). One-way ANOVA showed that there were no significant changes in the entries in open arms [F (3,27) = 1.8, p = 0.12] among groups (Fig. 4f), although post-hoc test showed significant reduction in CRS compared to Ctrl group (p < 0.05). Oxo treatment alone did not affect significantly any of the examined parameters (Fig. 4d, f). Results, when expressed as ratio of time spent in open arms/total time spent in arms (Fig. 4e), confirmed for all experimental groups the course of time spent in open arms [F (3,27) = 3.3, p < 0.05].
Oxo restored the CRS-induced deficit of BDNF and FGF2 protein levels in the ventral hippocampus and medial prefrontal cortex
Once established the anxiolytic effects of Oxo treatment, we next analyzed the protein levels of BDNF and FGF2 by Western blot analysis, both in the dorsal or ventral hippocampus and in the medial prefrontal cortex. Levels of BDNF and FGF2 proteins in the ventral hippocampus (Fig. 5a, b) were found significantly changed [F (3,15) = 3.4, p < 0.05, F (3,15) = 11.8, p < 0.001 respectively], with a significant decrease in the CRS group (n = 5) as compared to Ctrl group (n = 5) (post-hoc: BDNF, p < 0.05; FGF2, p < 0.001). In CRS + Oxo group (n = 5), the treatment with Oxo was able to significantly recover to control levels (Fig. 5a, b) both neurotrophic factors as compared to CRS group (post-hoc: BDNF, p < 0.05; FGF2, p < 0.01). However, the treatment with Oxo alone did not affect the basal levels of both neurotrophic factors (Fig. 5a, b). In contrast, the same analysis in the dorsal hippocampus revealed that CRS (Fig. 5d, e) did not affect the basal levels of both neurotrophic factors [FGF2 F (3,15) = 0.24, p = 0.87; BDNF F (3,15) = 1.5, p = 0.26]. Similarly, in the dorsal hippocampus, Oxo treatment in CRS group or in unstressed group (Oxo, n = 4) did not change the neurotrophic factors levels as compared to control group (Fig. 5d, e).
Evaluation in the ventral and dorsal hippocampi of BDNF and FGF2 protein levels or p-Erk1/2 levels in CRS-induced anxiety-like behavior and in the Oxo anxiolytic treatment. CRS induced reduction of BDNF and FGF2 protein levels or p-Erk1/2 levels in the ventral hippocampus (a–c) but not in the dorsal hippocampus (d–f). In the ventral hippocampus, Oxo treatment in CRS restored FGF2 and BDNF protein levels, but not p-Erk1/2 levels (a–c). Oxo treatment alone did not change the basal levels of FGF2, BDNF, and p-Erk1/2. Data represent mean ± S.E.M.; *p < 0.05, **p < 0.01, ***p < 0.001
The same analysis in the medial prefrontal cortex revealed that either BDNF and FGF2 levels had significant differences [F (3,15) = 8.9, p < 0.002; F (3,15) = 4.8, p < 0.02, respectively] among the experimental groups, with a decrease in CRS group (n = 5; post-hoc vs Ctrl: BDNF, p < 0.01; FGF2, p < 0.05) and, again, treatment with Oxo in CRS + Oxo group (n = 5) reversed CRS-induced changes in BDNF and FGF2, revealing for BDNF a significant increase over the control (n = 5; post-hoc p < 0.001; Fig. 6a, b). As for the hippocampus, the treatment with Oxo alone (n = 4) did not affect the basal levels of both neurotrophic factors (Fig. 6a, b)
Evaluation in the medial prefrontal cortex of BDNF and FGF2 protein levels or p-Erk1/2 levels in the CRS-induced anxiety-like behavior and in the Oxo anxiolytic treatment. a–c CRS induced reduction of BDNF and FGF2 protein levels or p-Erk1/2 levels as compared to control. a–c Oxo treatment in CRS restored FGF2 and BDNF protein levels, but not p-Erk1/2 levels. Oxo treatment alone did not change the basal levels of FGF2, BDNF, and p-Erk1/2. Data represent mean ± S.E.M.; *p < 0.05, ***p < 0.001, ****p < 0.0001
p-Erk1/2 levels in the ventral hippocampus and the medial prefrontal cortex were decreased in CRS group and reversed by Oxo treatment
Next, we examined the potential signaling involved in the BDNF and FGF2-mediated anxiolytic action of Oxo treatment. Among the main signaling activated by BDNF and FGF2 binding to their receptors, we examined the Erk1/2 phosphorylation. This analysis showed that the levels of p-Erk1/2 were significantly changed both in the ventral hippocampus [F (3,15) = 3.8, p < 0.03] and in the medial prefrontal cortex [F (3,15) = 15.3, p < 0.0001]. The post-hoc analysis revealed that the CRS group (n = 5) shows a significant reduction of p-Erk1/2 in the ventral hippocampus (p < 0.01) and medial prefrontal cortex (p < 0.001) compared to Ctrl group (n = 5). Oxo treatment in CRS + Oxo group (n = 5) failed to recover the decreased p-Erk1/2 levels in the ventral hippocampus (Fig. 5c). Similarly, the decreased p-Erk1/2 levels in the medial prefrontal cortex of CRS group were not recovered by Oxo treatment in CRS + Oxo group (Fig. 6c). In Oxo group, there was no significant change of p-Erk1/2 levels as compared to control group in both the brain regions examined (Figs. 5c and 6c). The analyses of Erk1/2 phosphorylation in the dorsal hippocampus revealed that all experimental treatments were ineffective [F (3,15) = 1.1, p = 0.38] (Fig. 5f).
Discussion
By several studies, CRS has been suggested as an animal model of anxiety/depression-like behavior (Veena et al. 2011), and here, the degree of anxiety-induced by CRS was measured using four different paradigms, which confirmed that the CRS protocol used was able to induce anxiety. Thus, the increased latency to feed in NSF test, the increased immobility in FST, and the decreased time spent both in light in LDB test or in open arms in EPM test clearly indicated that CRS rats were anxious. However, the increased immobility in FST may also indicate that CRS rats were also depressed (Matthews and Robbins 2003; Ruedi-Bettschen et al. 2005; Strekalova et al. 2004; Willner et al. 1987).
In the present work, although we used female rats, we did not measure estrous cycle and its impact on the outcome of behavioral tests, since the handling to determine estrous status may have an impact on rat performance in the behavior paradigms (Hoffman et al. 2010), and both a large number of rats and chronic stress and treatment were made difficult to take account of estrous cycle. However, in specific studies, there are conflicting reports on the impact of estrous cycle on the outcome of several behavioral tests and, based on previous literature, there is conclusion that the estrous cycle effect on FST behavior is small and perhaps negligible (Eskelund et al. 2016; Kokras et al. 2015).
In addition to different tests used to establish the anxiety-like behavior induced by CRS, further data supporting anxiety-depressive-like behavior profile in CRS group are provided indirectly by body weight reduction and hippocampi weight reduction (Bremner et al. 2000; MacQueen et al. 2003; Orlovsky et al. 2014; Sheline et al. 1996; Veena et al. 2011). In the present study, according to previous report (Veena et al. 2011), Oxo treatment in CRS and unstressed rats significantly increased hippocampal weight when compared to CRS group, showing that Oxo may not only restore the hippocampal volumes but also increase it beyond the normal control.
To further test the efficiency of our stress paradigm, we assessed cortisol levels as evaluation of hypothalamic-pituitary-adrenal axis activation. Although corticosterone is considered the main glucocorticoid involved in regulation of stress responses in rodents, we have chosen to detect cortisol as stress indicator in consideration of kits and procedure availability in the laboratory. On the other hand, previous studies have showed that the dynamics of serum cortisol is closely correlated with that of corticosterone under several tested conditions, such as CRS (Gong et al. 2015). In the present work, cortisol levels in CRS group, although showing a trend of increase, were not significantly changed as compared to control rats, probably because the interruption of CRS treatment between 21 and 34 days has partially recovered them to control levels.
mAChR subtypes mediating anxiolytic effects of Oxo
By using the CRS model of anxiety-/depression-like behavior, we have tested anxiolytic effect of Oxo treatment and we could show that Oxo significantly ameliorates stress induced anxiety-like behavior, which is a relevant finding since anxiety is frequently co-morbid in patients with major depression (Zimmerman et al. 2002). The anxiolytic effect of Oxo was in agreement with previous data demonstrating that Oxo counteracts the CRS induced anxiolytic-like behavior as tested by FST and sucrose consumption test (Veena et al. 2011). Although here we did not examine the receptor subtype involved in the Oxo anxiolytic effect, several reports may give suggestions on antidepressant role of M1 receptor, which has been found throughout the brain with the highest concentrations in cerebral cortex and hippocampus (Hohmann et al. 1995; Levey et al. 1995). Previous works have shown that disturbances in the cholinergic activity may be associated to neurobiology of depression (Janowsky et al. 1972), and muscarinic receptors have been found downregulated in the prefrontal cortex of depressed patients (Gibbons et al. 2009), or upregulated in the hippocampus following mood stabilizers like lithium treatment (Marinho et al. 1998). Recent studies have identified an activity-dependent long-term depression in deep layers of the rat medial prefrontal cortex mediated by the M1 receptor, which may be relevant for neurological disorders (Caruana et al. 2011). In an another study, local microinjection in the nucleus accumbens of arecoline, an M2 nonspecific agonist, decreased swimming time in the Porsolt swim test, while pirenzepine, an M1 receptor agonist, increased the swim time (Chau et al. 2001). However, specific studies are needed to define the mAChR subtypes and the related mechanism(s) involved in these effects.
Role of BDNF and FGF2 on anxiolytic action of Oxo
BDNF and FGF2 implications in depression and anxiety have been extensively documented and discussed in the “Introduction” section (Duman and Monteggia 2006; Eren-Kocak et al. 2011; Govindarajan et al. 2006; Martinowich et al. 2007; Perez et al. 2009).
In the present study, we found that CRS may induce anxiety-like behavior, and this effect is associated to the significant reduction of both BDNF and FGF2 protein levels in the ventral hippocampus and medial prefrontal cortex. Our data are in line with published data showing reduced levels of BDNF in the hippocampal formation in different models of stress including CRS (Bennett and Lagopoulos 2014; Monteggia et al. 2007; Murakami et al. 2005; Russo-Neustadt et al. 2001; Schaaf et al. 2000; Shi et al. 2010; Smith et al. 1995; Xu et al. 2002; Yamaura et al. 2015; Yulug et al. 2009) and of FGF2 in the hippocampus and frontal cortex of depressed patients and in animal model of depression (Evans et al. 2004; Gaughran et al. 2006; Turner et al. 2008a; Turner et al. 2008b). However, it has been also reported that CRS did not change FGF2 and BDNF in the rat hippocampus (Chiba et al. 2012; Kuroda and McEwen 1998). Overall, there is a general approval that a deficiency in growth factors increases vulnerability to depression (Duman and Monteggia 2006). Accordingly, silencing of endogenous hippocampal FGF2 increased anxiety-like behavior in rats (Eren-Kocak et al. 2011).
In the present study, the finding that Oxo administration may restore the levels of either BDNF or FGF2 suggests that anxiolytic effects of Oxo treatment could be correlated to restored neurotrophic factors levels. As a support to this correlation, several studies have suggested that hippocampal regulation of mood may derive directly from neurotrophic factors. Accordingly, antidepressant treatments increase the expression of BDNF and FGF2 in the hippocampus (Mallei et al. 2002; Maragnoli et al. 2004; Nibuya et al. 1995), and hippocampal BDNF and its TrkB receptor are enhanced in patients with depression following treatment with antidepressant drugs (Dwivedi et al. 2003; Saarelainen et al. 2003). Therefore, there is a general agreement that induction of these factors is relevant to the effectiveness of antidepressants (Neto et al. 2011; Warner-Schmidt and Duman 2007), as also suggested by chronic administration of FGF2 inducing anxiolytic and antidepressant effects in rodents (Turner et al. 2008b)
Like in the hippocampus, in the medial prefrontal cortex, the most documented effects of chronic stress are dendritic atrophy and reduction of dendritic spines (Brown et al. 2005; Cook and Wellman 2004; Liu and Aghajanian 2008; Shansky et al. 2009), which are associated to a parallel impairment of behavior (Liston et al. 2006). The prefrontal cortex displays functional impairments in patients suffering mood disorders (Drevets et al. 1997; Johnstone et al. 2007), and chronic stress induces alterations in the prefrontal cortex related to behavioral dysfunctions (Holmes and Wellman 2009; Liston et al. 2006). Patients with depressive disorders are usually accompanied with atrophy of medial prefrontal cortex (McEwen 2005), in which BDNF and FGF2 are highly expressed (Belluardo et al. 1998; Mudo et al. 1996).
Overall, accordingly to the above listed data, Oxo treatment may exert anxiolytic effects that could be associated to BDNF and FGF2 modulation in the hippocampus and medial prefrontal cortex. However, in addition to BDNF and FGF2, multiple neurotrophic factors may likely contribute to the beneficial actions of anxiolytic treatment (Martinowich et al. 2007; Warner-Schmidt and Duman 2007); therefore, a correlation between the neurotrophic factors and the morphological changes in the hippocampus following Oxo treatment needs more detailed studies.
In order to investigate the relationship between BDNF and FGF2 changes in rats subject to CRS and cell signaling involved in the anxiolytic action of Oxo treatment, we measured p-Erk1/2, one of the three main signaling pathways stimulated by tyrosine kinase receptors (Santos et al. 2010). Our results have shown that CRS induces a significant decrease in Erk1/2 phosphorylation content both in the ventral hippocampus and in the prefrontal cortex and that this change was not significantly reversed by Oxo treatment. However, modulation of this neurotrophic signaling pathway, which has been shown to be important for cell survival, neurogenesis, and synaptic plasticity in the brain, may play a role in mediating anxiolytic effects of Oxo treatment (First et al. 2011).
BDNF and FGF2 are differentially regulated in dorsal and ventral hippocampus
A relevant result in this study was the finding that CRS-induced anxiety differentially modifies the levels of BDNF and FGF2 in the ventral hippocampus as compared to the dorsal part. Several reports support the conclusion that the ventral hippocampus is preferential linked to anxiety-related behaviors (Bannerman et al. 2004; Degroot and Treit 2004; Eadie et al. 2009; Engin and Treit 2007), while the dorsal hippocampus appears to be subservient to spatial learning and memory (Engin and Treit 2007; Fanselow and Dong 2010; Maurer et al. 2005; O’Leary and Cryan 2014; Small et al. 2011; Strange et al. 2014). In addition, direct ventral hippocampal-medial prefrontal cortex input is required for anxiety-related neural activity and behavior (Padilla-Coreano et al. 2016). Overall, the changes of neurotrophic factors found in the ventral hippocampus following CRS correlate with these segregated functions along longitudinal dorso-ventral axis of the hippocampus. By contrast, in the dorsal hippocampus, we could not observe modification of BDNF and FGF2 protein levels, suggesting that CRS per se may differentially affect the hippocampus along with the longitudinal axis.
Conclusion
In conclusion, the present study showed that Oxo treatment ameliorates the stress induced anxiety-like behavior and rescues FGF2 and BDNF levels in two brain regions involved in CRS-induced anxiety, the ventral hippocampal formation and medial prefrontal cortex.
References
Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I (2008) Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology 55:1114–1120. doi:10.1016/j.neuropharm.2008.07.014
Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J (2004) Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev 28:273–283. doi:10.1016/j.neubiorev.2004.03.004
Barathi VA, Weon SR, Beuerman RW (2009) Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol Vis 15:1277–1293
Belluardo N, Blum M, Mudo G, Andbjer B, Fuxe K (1998) Acute intermittent nicotine treatment produces regional increases of basic fibroblast growth factor messenger RNA and protein in the tel- and diencephalon of the rat. Neuroscience 83:723–740
Bennett MR, Lagopoulos J (2014) Stress and trauma: BDNF control of dendritic-spine formation and regression. Prog Neurobiol 112:80–99. doi:10.1016/j.pneurobio.2013.10.005
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ (2006) Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311:864–868. doi:10.1126/science.1120972
Borroto-Escuela DO, Romero-Fernandez W, Mudo G, Perez-Alea M, Ciruela F, Tarakanov AO, Narvaez M, Di LV, Agnati LF, Belluardo N, Fuxe K (2012) Fibroblast growth factor receptor 1-5-hydroxytryptamine 1A heteroreceptor complexes and their enhancement of hippocampal plasticity. Biol Psychiatry 71:84–91. doi:10.1016/j.biopsych.2011.09.012
Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS (2000) Hippocampal volume reduction in major depression. Am J Psychiatry 157:115–118. doi:10.1176/ajp.157.1.115
Brown SM, Henning S, Wellman CL (2005) Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex 15:1714–1722. doi:10.1093/cercor/bhi048
Caruana DA, Warburton EC, Bashir ZI (2011) Induction of activity-dependent LTD requires muscarinic receptor activation in medial prefrontal cortex. J Neurosci 31:18464–18478. doi:10.1523/JNEUROSCI.4719-11.2011
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389. doi:10.1126/science.1083968
Chau DT, Rada P, Kosloff RA, Taylor JL, Hoebel BG (2001) Nucleus accumbens muscarinic receptors in the control of behavioral depression: antidepressant-like effects of local M1 antagonist in the Porsolt swim test. Neuroscience 104:791–798
Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H (2012) Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 39:112–119. doi:10.1016/j.pnpbp.2012.05.018
Christensen T, Bisgaard CF, Nielsen HB, Wiborg O (2010) Transcriptome differentiation along the dorso-ventral axis in laser-captured microdissected rat hippocampal granular cell layer. Neuroscience 170:731–741. doi:10.1016/j.neuroscience.2010.07.016
Colman I, Ataullahjan A (2010) Life course perspectives on the epidemiology of depression 3. Can J Psychiatry 55:622–632
Cook SC, Wellman CL (2004) Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60:236–248. doi:10.1002/neu.20025
Degroot A, Treit D (2004) Anxiety is functionally segregated within the septo-hippocampal system. Brain Res 1001:60–71. doi:10.1016/j.brainres.2003.10.065
Dennis SH, Pasqui F, Colvin EM, Sanger H, Mogg AJ, Felder CC, Broad LM, Fitzjohn SM, Isaac JT, Mellor JR (2016) Activation of muscarinic M1 acetylcholine receptors induces long-term potentiation in the hippocampus. Cereb Cortex 26:414–426. doi:10.1093/cercor/bhv227
Di Liberto V, Mudo G, Belluardo N (2011) mGluR2/3 agonist LY379268, by enhancing the production of GDNF, induces a time-related phosphorylation of RET receptor and intracellular signaling Erk1/2 in mouse striatum. Neuropharmacology 61:638–645. doi:10.1016/j.neuropharm.2011.05.006
Di Liberto V, Mudo G, Fuxe K, Belluardo N (2014) Interactions between cholinergic and fibroblast growth factor receptors in brain trophism and plasticity. Curr Protein Pept Sci 15:691–702
Drever BD, Riedel G, Platt B (2011) The cholinergic system and hippocampal plasticity. Behav Brain Res. 221:505–514. doi:10.1016/j.bbr.2010.11.037
Drevets WC, Price JL, Simpson JR Jr, Todd RD, Reich T, Vannier M, Raichle ME (1997) Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824–827. doi:10.1038/386824a0
Duman RS, Monteggia LM (2006) A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59:1116–1127. doi:10.1016/j.biopsych.2006.02.013
Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN (2003) Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 60:804–815. doi:10.1001/archpsyc.60.8.804
Eadie BD, Zhang WN, Boehme F, Gil-Mohapel J, Kainer L, Simpson JM, Christie BR (2009) Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol Dis 36:361–373. doi:10.1016/j.nbd.2009.08.001
Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS (2012) Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol Psychiatry 72:258–265. doi:10.1016/j.biopsych.2012.03.003
Engin E, Treit D (2007) The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol 18:365–374. doi:10.1097/FBP.0b013e3282de7929
Eren-Kocak E, Turner CA, Watson SJ, Akil H (2011) Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry 69:534–540. doi:10.1016/j.biopsych.2010.11.020
Eskelund A, Budac DP, Sanchez C, Elfving B, Wegener G (2016) Female flinders sensitive line rats show estrous cycle-independent depression-like behavior and altered tryptophan metabolism. Neuroscience 329:337–348. doi:10.1016/j.neuroscience.2016.05.024
Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, Lopez JF, Thompson RC, Meng F, Stead JD, Walsh DM, Myers RM, Bunney WE, Watson SJ, Jones EG, Akil H (2004) Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci USA 101:15506–15511. doi:10.1073/pnas.0406788101
Fanselow MS, Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19. doi:10.1016/j.neuron.2009.11.031
First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A (2011) The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J Mol Neurosci 45:246–255. doi:10.1007/s12031-011-9515-5
Frinchi M, Di LV, Olivieri M, Fuxe K, Belluardo N, Mudo G (2010) FGF-2/FGFR1 neurotrophic system expression level and its basal activation do not account for the age-dependent decline of precursor cell proliferation in the subventricular zone of rat brain. Brain Res 1358:39–45. doi:10.1016/j.brainres.2010.08.083
Gaughran F, Payne J, Sedgwick PM, Cotter D, Berry M (2006) Hippocampal FGF-2 and FGFR1 mRNA expression in major depression, schizophrenia and bipolar disorder. Brain Res Bull 70:221–227. doi:10.1016/j.brainresbull.2006.04.008
Gibbons AS, Scarr E, McLean C, Sundram S, Dean B (2009) Decreased muscarinic receptor binding in the frontal cortex of bipolar disorder and major depressive disorder subjects. J Affect Disord 116:184–191. doi:10.1016/j.jad.2008.11.015
Gong S, Miao YL, Jiao GZ, Sun MJ, Li H, Lin J, Luo MJ, Tan JH (2015) Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 10:e0117503. doi:10.1371/journal.pone.0117503
Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S (2006) Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA 103:13208–13213. doi:10.1073/pnas.0605180103
Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J (2012) Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev 36:2085–2117. doi:10.1016/j.neubiorev.2012.07.001
Hoffman AN, Armstrong CE, Hanna JJ, Conrad CD (2010) Chronic stress, cyclic 17beta-estradiol, and daily handling influences on fear conditioning in the female rat. Neurobiol Learn Mem 94:422–433. doi:10.1016/j.nlm.2010.08.010
Hohmann CF, Potter ED, Levey AI (1995) Development of muscarinic receptor subtypes in the forebrain of the mouse. J Comp Neurol 358:88–101. doi:10.1002/cne.903580106
Holmes A, Wellman CL (2009) Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev. 33:773–783. doi:10.1016/j.neubiorev.2008.11.005
Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ (1972) A cholinergic-adrenergic hypothesis of mania and depression. Lancet 2:632–635
Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ (2007) Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 27:8877–8884. doi:10.1523/JNEUROSCI.2063-07.2007
Karege F, Vaudan G, Schwald M, Perroud N, La HR (2005) Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 136:29–37. doi:10.1016/j.molbrainres.2004.12.020
Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C (2015) Forced swim test: what about females? Neuropharmacology 99:408–421. doi:10.1016/j.neuropharm.2015.03.016
Kuroda Y, McEwen BS (1998) Effect of chronic restraint stress and tianeptine on growth factors, growth-associated protein-43 and microtubule-associated protein 2 mRNA expression in the rat hippocampus. Brain Res Mol Brain Res 59:35–39
Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995) Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15:4077–4092
Lindholm D, da Penha BM, Cooper J, Thoenen H, Castren E (1994) Brain-derived neurotrophic factor and neurotrophin-4 increase neurotrophin-3 expression in the rat hippocampus. Int J Dev Neurosci 12:745–751
Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS (2006) Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 26:7870–7874. doi:10.1523/JNEUROSCI.1184-06.2006
Liu RJ, Aghajanian GK (2008) Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA 105:359–364. doi:10.1073/pnas.0706679105
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luchicchi A, Bloem B, Viana JN, Mansvelder HD, Role LW (2014) Illuminating the role of cholinergic signaling in circuits of attention and emotionally salient behaviors. Front Synaptic Neurosci 6:24. doi:10.3389/fnsyn.2014.00024
MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT (2003) Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA 100:1387–1392. doi:10.1073/pnas.0337481100
Mallei A, Shi B, Mocchetti I (2002) Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol 61:1017–1024
Maragnoli ME, Fumagalli F, Gennarelli M, Racagni G, Riva MA (2004) Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biol Psychiatry 55:1095–1102. doi:10.1016/j.biopsych.2004.02.003
Marinho MM, de Sousa FC, de Bruin VM, Vale MR, Viana GS (1998) Effects of lithium, alone or associated with pilocarpine, on muscarinic and dopaminergic receptors and on phosphoinositide metabolism in rat hippocampus and striatum. Neurochem Int 33:299–306
Martinowich K, Manji H, Lu B (2007) New insights into BDNF function in depression and anxiety. Nat Neurosci 10:1089–1093. doi:10.1038/nn1971
Matthews K, Robbins TW (2003) Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev 27:45–55
Maurer AP, Vanrhoads SR, Sutherland GR, Lipa P, McNaughton BL (2005) Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus 15:841–852. doi:10.1002/hipo.20114
McEwen BS (2005) Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism 54:20–23. doi:10.1016/j.metabol.2005.01.008
Mitsushima D, Sano A, Takahashi T (2013) A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun 4:2760. doi:10.1038/ncomms3760
Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ (2007) Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry 61:187–197. doi:10.1016/j.biopsych.2006.03.021
Mudo G, Jiang XH, Timmusk T, Bindoni M, Belluardo N (1996) Change in neurotrophins and their receptor mRNAs in the rat forebrain after status epilepticus induced by pilocarpine. Epilepsia 37:198–207
Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E (2005) Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res 53:129–139. doi:10.1016/j.neures.2005.06.008
Navakkode S, Korte M (2012) Cooperation between cholinergic and glutamatergic receptors are essential to induce BDNF-dependent long-lasting memory storage. Hippocampus 22:335–346. doi:10.1002/hipo.20902
Neto FL, Borges G, Torres-Sanchez S, Mico JA, Berrocoso E (2011) Neurotrophins role in depression neurobiology: a review of basic and clinical evidence. Curr Neuropharmacol 9:530–552. doi:10.2174/157015911798376262
Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL (2002) The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet 71:651–655. doi:10.1086/342288
Nibuya M, Morinobu S, Duman RS (1995) Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 15:7539–7547
O’Leary OF, Cryan JF (2014) A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends Pharmacol Sci 35:675–687. doi:10.1016/j.tips.2014.09.011
Orlovsky MA, Dosenko VE, Spiga F, Skibo GG, Lightman SL (2014) Hippocampus remodeling by chronic stress accompanied by GR, proteasome and caspase-3 overexpression. Brain Res 1593:83–94. doi:10.1016/j.brainres.2014.09.059
Padilla-Coreano N, Bolkan SS, Pierce GM, Blackman DR, Hardin WD, Garcia-Garcia AL, Spellman TJ, Gordon JA (2016) Direct ventral hippocampal-prefrontal input is required for anxiety-related neural activity and behavior. Neuron. doi:10.1016/j.neuron.2016.01.011
Paxinos G, Watson C (2014) The Rat Brain in Stereotaxic Coordinates, 7th edn. San Diego, CA, Academic Press
Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H (2009) A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci 29:6379–6387. doi:10.1523/JNEUROSCI.4829-08.2009
Ruedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR (2005) Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res 156:297–310. doi:10.1016/j.bbr.2004.06.001
Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP (2001) Physical activity-antidepressant treatment combination: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res 120:87–95
Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E (2003) Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 23:349–357
Sairanen M, Lucas G, Ernfors P, Castren M, Castren E (2005) Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci 25:1089–1094. doi:10.1523/JNEUROSCI.3741-04.2005
Santos AR, Comprido D, Duarte CB (2010) Regulation of local translation at the synapse by BDNF. Prog Neurobiol 92:505–516. doi:10.1016/j.pneurobio.2010.08.004
Schaaf MJ, De Kloet ER, Vreugdenhil E (2000) Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress 3:201–208
Sen S, Nesse RM, Stoltenberg SF, Li S, Gleiberman L, Chakravarti A, Weder AB, Burmeister M (2003) A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology 28:397–401. doi:10.1038/sj.npp.1300053
Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH (2009) Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex 19:2479–2484. doi:10.1093/cercor/bhp003
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93:3908–3913
Sheline YI, Gado MH, Kraemer HC (2003) Untreated depression and hippocampal volume loss. Am J Psychiatry 160:1516–1518. doi:10.1176/appi.ajp.160.8.1516
Shi SS, Shao SH, Yuan BP, Pan F, Li ZL (2010) Acute stress and chronic stress change brain-derived neurotrophic factor (BDNF) and tyrosine kinase-coupled receptor (TrkB) expression in both young and aged rat hippocampus. Yonsei Med J 51:661–671. doi:10.3349/ymj.2010.51.5.661
Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS (2002) Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 22:3251–3261
Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM (1997) Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav 56:131–137. doi:10.1016/S0091-3057(96)00169-4
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601. doi:10.1038/nrn3085
Smith MA, Makino S, Kvetnansky R, Post RM (1995) Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 15:1768–1777
Srikumar BN, Raju TR, Shankaranarayana Rao BS (2006) The involvement of cholinergic and noradrenergic systems in behavioral recovery following oxotremorine treatment to chronically stressed rats. Neuroscience 143:679–688. doi:10.1016/j.neuroscience.2006.08.041
Strange BA, Witter MP, Lein ES, Moser EI (2014) Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15:655–669. doi:10.1038/nrn3785
Strekalova T, Spanagel R, Bartsch D, Henn FA, Gass P (2004) Stress-induced anhedonia in mice is associated with deficits in forced swimming and exploration. Neuropsychopharmacology 29:2007–2017. doi:10.1038/sj.npp.1300532
Turner CA, Akil H, Watson SJ, Evans SJ (2006) The fibroblast growth factor system and mood disorders. Biol Psychiatry 59:1128–1135. doi:10.1016/j.biopsych.2006.02.026
Turner CA, Calvo N, Frost DO, Akil H, Watson SJ (2008a) The fibroblast growth factor system is downregulated following social defeat. Neurosci Lett 430:147–150. doi:10.1016/j.neulet.2007.10.041
Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H (2008b) Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res 1224:63–68. doi:10.1016/j.brainres.2008.05.088
Veena J, Srikumar BN, Mahati K, Raju TR, Shankaranarayana Rao BS (2011) Oxotremorine treatment restores hippocampal neurogenesis and ameliorates depression-like behaviour in chronically stressed rats. Psychopharmacology 217:239–253. doi:10.1007/s00213-011-2279-3
Warner-Schmidt JL, Duman RS (2007) VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA 104:4647–4652. doi:10.1073/pnas.0610282104
Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93:358–364
Xu H, Qing H, Lu W, Keegan D, Richardson JS, Chlan-Fourney J, Li XM (2002) Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett 321:65–68
Yamaura K, Tanaka R, Bi Y, Fukata H, Oishi N, Sato H, Mori C, Ueno K (2015) Protective effect of young green barley leaf (Hordeum vulgare L.) on restraint stress-induced decrease in hippocampal brain-derived neurotrophic factor in mice. Pharmacogn Mag 11:S86–S92. doi:10.4103/0973-1296.157702
Yulug B, Ozan E, Gonul AS, Kilic E (2009) Brain-derived neurotrophic factor, stress and depression: a mini review. Brain Res Bull 78:267–269. doi:10.1016/j.brainresbull.2008.12.002
Zimmerman M, Mattia JI, Posternak MA (2002) Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? Am J Psychiatry 159:469–473. doi:10.1176/appi.ajp.159.3.469
Acknowledgements
This work was supported by grants from “Fondi Finanziamento della Ricerca (FFR),” University of Palermo.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were carried out in accordance with the National Institute of Health Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academics Press, WA, USA), with the rules and principles of the European Communities Council Directive 2010/63/EU revising Directive 86/609/EEC, in accordance with the national D.L. March 4, 2014, no. 26, and were approved by the local Bioethical Committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Natale Belluardo and Giuseppa Mudò are co-senior authors.
Rights and permissions
About this article
Cite this article
Di Liberto, V., Frinchi, M., Verdi, V. et al. Anxiolytic effects of muscarinic acetylcholine receptors agonist oxotremorine in chronically stressed rats and related changes in BDNF and FGF2 levels in the hippocampus and prefrontal cortex. Psychopharmacology 234, 559–573 (2017). https://doi.org/10.1007/s00213-016-4498-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4498-0