Abstract
Rationale
Development of new drugs for treatment of Alzheimer’s disease (AD) requires valid paradigms for testing their efficacy and sensitive tests validated in translational research.
Objectives
We present validation of a place-navigation task, a Hidden Goal Task (HGT) based on the Morris water maze (MWM), in comparable animal and human protocols.
Methods
We used scopolamine to model cognitive dysfunction similar to that seen in AD and donepezil, a symptomatic medication for AD, to assess its potential reversible effect on this scopolamine-induced cognitive dysfunction. We tested the effects of scopolamine and the combination of scopolamine and donepezil on place navigation and compared their effects in human and rat versions of the HGT. Place navigation testing consisted of 4 sessions of HGT performed at baseline, 2, 4, and 8 h after dosing in humans or 1, 2.5, and 5 h in rats.
Results
Scopolamine worsened performance in both animals and humans. In the animal experiment, co-administration of donepezil alleviated the negative effect of scopolamine. In the human experiment, subjects co-administered with scopolamine and donepezil performed similarly to subjects on placebo and scopolamine, indicating a partial ameliorative effect of donepezil.
Conclusions
In the task based on the MWM, scopolamine impaired place navigation, while co-administration of donepezil alleviated this effect in comparable animal and human protocols. Using scopolamine and donepezil to challenge place navigation testing can be studied concurrently in animals and humans and may be a valid and reliable model for translational research, as well as for preclinical and clinical phases of drug trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD) is characterized by gradual worsening of cognitive functions, which reflect structural brain changes due to the AD pathology. Various standardized neuropsychological tests are used to detect cognitive impairment in the dementia and pre-dementia stages of AD (Weintraub et al. 2009; Albert et al. 2011). Besides these standardized neuropsychological tests, experimental tests derived from animal behavioral tasks have been increasingly used in an effort to reliably detect subtle cognitive deficits in the very early stages of AD (Rentz et al. 2013). Among these, tests focused on place navigation and spatial memory play a very important role, tapping the functions of the most early affected brain areas in AD—the hippocampus, retrosplenial cortex, posterior parietal cortex including the precuneus, and the prefrontal cortex (Kesner and Hopkins 2006; Vlček and Laczó 2014). These place navigation and spatial memory tests may be useful for the early diagnosis of AD and monitoring the effect of AD therapy (Hort et al. 2014), but also for the reliable measurement of cognitive changes in clinical trials with new promising compounds. In addition, place navigation and spatial memory tests can be administered to both animals and humans. Direct comparison of constructs in humans and animals using similar pharmacological and testing procedures may provide a sound basis for comparing the effect of neurocognitive agents on cognitive functions than post hoc analysis of studies using different methodologies.
Scopolamine, a centrally active anticholinergic drug with a high specificity for muscarinic receptors (Frey et al. 1992), has been commonly employed in both animals (Bartolini et al. 1992; Herrera-Morales et al. 2007; von Linstow Roloff et al. 2007) and humans (Snyder et al. 2005; Antonova et al. 2011) to model cognitive dysfunctions similar to that seen in AD (Whitehouse et al. 1982; Whitehouse and Au 1986), and for this reason, it is commonly used in behavioral studies (Herrera-Morales et al. 2007; Snyder et al. 2014). In humans, scopolamine consistently induces deficits in memory, learning (Molchan et al. 1992; Robbins et al. 1997; Koller et al. 2003; Sherman et al. 2003; Ellis et al. 2006; Thomas et al. 2008; Voss et al. 2010), and attention (Molchan et al. 1992; Koller et al. 2003; Ellis et al. 2006). However, results of studies with scopolamine are ambiguous for working memory and executive functions (Thomas et al. 2008; Voss et al. 2010). The inconsistent results may be explained by the heterogeneity of tests used, as well as the fact that they were performed with healthy volunteers (Snyder et al. 2005; Ellis et al. 2006). A recent experiment applied a cognitive stress test using a low dose of scopolamine in older adults with known risk factors for AD (Snyder et al. 2014). In this study, a low dose caused a detectable cognitive decline in executive function and working memory in the at-risk subjects and might have thus revealed latent cognitive deficits in these participants.
In animals, numerous behavioral studies with scopolamine have employed place navigation tasks, with convincing results about the impairment of hippocampus-based learning and memory (Cassel and Kelche 1989; Baxter and Gallagher 1996; Vales and Stuchlik 2005; Entlerova et al. 2013). Scopolamine is thought to disrupt the functions of the hippocampus, an essential structure for various types of memory (Stuchlik 2014). Although place navigation impairment is well established early in the course of AD (Hort et al. 2007), just one experiment with a place navigation task has been conducted in humans (Antonova et al. 2011). In that study (Antonova et al. 2011), scopolamine had an attenuating effect on hippocampal activity as shown by reduced activity on functional magnetic resonance imaging during navigation in a virtual reality arena task. However, there was no effect of scopolamine on spatial navigation performance.
Donepezil, an active acetylcholinesterase inhibitor, is used as a symptomatic medication for AD that increases the level of acetylcholine in the brain cholinergic system (Scali et al. 2002) and has been shown to improve cognitive functions in both animal models (Guo et al. 2015) and human subjects (Greenberg et al. 2000; Winblad et al. 2001; Bianchetti et al. 2006). Moreover, donepezil has the potential to reverse scopolamine-induced cognitive deficits (Snyder et al. 2005). In one study, the drug improved place navigation in AD patients (Hort et al. 2014).
To our knowledge, a direct comparison of the effect of scopolamine and donepezil on place navigation concurrently in human subjects and an animal model has not yet been performed. The aim of our study, therefore, was to evaluate the influence of scopolamine on hippocampal-based place navigation and the modulatory effect of donepezil on such performance in human subjects and an animal model. In our main analyses, we address the hypothesis that administration of scopolamine will impair spatial navigation performance and that donepezil co-administered with scopolamine should alleviate this deficit. These changes should be observable in both animals and humans. We employed the Morris water maze (MWM) task, which is a classical task of precise place navigation and memory, widely used in animal models of cognitive disorders (Morris 1981; Morris 1984; Czéh et al. 2001; Stuchlik et al. 2007; Svoboda et al. 2015). In order to be able to compare the results of human and animal experiments, we created a version which parallels the Hidden Goal Task (HGT; a human analog of the MWM) designed for the Blue Velvet Arena, a real-space circular environment for spatial navigation testing (Kalová et al. 2005).
Materials and methods
Animal experiment
Subjects
Thirty-nine naive adult male rats of the Wistar strain (3–4 months old, weighing 250–350 g) were obtained from the breeding colony of the Institute of Physiology ASCR. Animals were housed in pairs and triplets in 30 × 30 × 40-cm transparent plastic cages in an air-conditioned animal room with a constant temperature (22 °C) and 12:12 light/dark cycle (lights on at 7:00). Water and food was available ad libitum. All animal procedures were approved by the Local Animal Care Committee of the Institute of Physiology (No. 139/2013) and complied with the Animal Protection Act of the Czech Republic and EU directive 2010/63/EC.
Study design and drug treatment
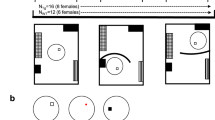
The study was conducted using a randomized, 3-way crossover within-subject design with repeated measures (Fig. 1a). Animals were assigned to treatment groups prior to the experiment: a saline group, a scopolamine (0.8 mg/kg) group, and a scopolamine (0.8 mg/kg) + donepezil (1 mg/kg) group. Each animal was assigned to each treatment group (i.e., went through all the treatment conditions) but in a different order. The sequence of the three treatment conditions was assigned randomly for each animal. Each treatment administered during the spatial navigation test was separated from the next one with a 7-day wash-out period to eliminate effects of the previous treatment. One day prior to the spatial navigation test, all animals were subjected to visible platform training to familiarize them with the basic requirements for the MWM. On each day of testing, animals completed the HGT in 4 spatial navigation sessions—baseline, after which the treatment was given, and then again at 1, 2.5, and 5 h after treatment.
Design of the experiment and apparatus. a The subjects completed the spatial navigation test (Hidden Goal Task) in 4 sessions: baseline, after which treatment was applied, and then again 2/1, 4/2.5, and 8/5 h after treatment in the animal/human experiments, respectively. Each session consisted of 8 spatial navigation trials in which the subjects were trained to locate the position of the hidden goal in relation to the distal orientation cues in the room; and 1/2 probe trials in rats/humans, when the hidden goal was removed and distal orientation cues were used for navigation. b The Morris Water Maze apparatus consisted of a circular pool filled with opaque water in a room providing extra-maze cues. A small, submerged escape platform was placed in one of the center of arbitrarily defined quadrants of the pool. c The Blue Velvet Arena is a real-space human analog of the Morris water maze. A fully enclosed circular arena is surrounded by a dark blue velvet curtain, where subjects were trained to locate the position of the hidden platform relative to the distal orientation cues
Scopolamine hydrobromide was dissolved in sterile saline (0.9 % NaCl) at a concentration of 0.8 mg/ml. Donepezil was dissolved in distilled water at a concentration of 1 mg/ml. Both drugs were purchased from Sigma-Aldrich, Czech Republic, and their solutions were prepared fresh on the day of testing. The saline group received sterile saline. All solutions were injected intraperitoneally at a volume of 1 ml/kg of body weight. All animals thus received the same volume of liquid per body weight.
Behavioral apparatus and design of the test
The MWM consisted of a blue-painted metallic circular pool (180 cm in diameter, 50 cm high) filled with water (20 °C, 40 cm deep) (Fig. 1b). The water was rendered opaque by adding small amount of a non-toxic white paint (Primalex, PPG Deco, Czech Republic). A small, transparent Plexiglas escape platform (10 cm in diameter) was placed in one of the centers of arbitrarily defined quadrants in the pool (NE, NW, SE, SW). The platform was either elevated above the water surface and marked with a 2-cm-high blue plastic cap (the visible goal condition) or submerged 1.5 cm below the water surface (the hidden-goal condition). The maze was located in a room providing an abundance of extra-maze cues. Rats were tracked by a computer-based tracking system (Tracker, Biosignal Group, USA) via an overhead TV camera. The tracking system recorded the rat’s position every 40 ms. Position time series were stored for off-line analysis (TrackAnalysis, Biosignal Group, USA).
In the visible platform training, rats underwent 4 swim trials to search for the marked visible platform. Rats were released facing the wall from the “south” point of the pool, and the platform position varied between trials.
In the spatial navigation session (hidden-goal condition), animals were trained to locate the position of the hidden platform in 8 consecutive swims (spatial navigation trials). The trial stopped when the rat found the hidden escape platform and climbed upon it. If the rat failed to find the escape platform in 60 s, it was gently guided to the platform by the experimenter. The rat was allowed to stay on the platform for 15 s, and then, it was placed in a warmed waiting cage. Rats were released facing the wall from the pseudo-randomly chosen 8 compass directions. To ensure a rat was learning the spatial location of the platform and not the path itself, the sequence of release points was unique for each spatial navigation test. The probe trial was administered approximately 1.5 h after the first spatial navigation trial and 10 min after the last trial. In the probe trial, the platform was removed, and the rat, always released from south, was allowed to swim freely for 60 s to display a preference for quadrants. The probe trial was used as a retention test after completion of the spatial navigation trial in order to provide an additional way how to detect effects of drugs on memory because the acquisition training with the platform may not always show the effects of drugs on memory (D’Hooge and De Deyn 2001). The platform position remained unchanged in all 4 spatial navigation sessions and was then changed between testing days.
Human experiment
Subjects
Twenty-three healthy young volunteers (20–28 years old) were recruited for the study through advertisements at the Charles University in Prague. All participants were medical or psychology students and received credits as compensation for their participation in the study. All subjects were in good health as determined by a medical history, physical examination, and vital signs, took no other medication, and all of them had a body weight of greater than 50 kg. The study design was approved by the Motol University Hospital Ethics Committee, and all subjects gave written informed consent to participate. The research was carried out according to The Code of Ethics of the World Medical Association (Declaration of Helsinki), informed consent was obtained, and the author’s institutional review board had approved the study.
The demographic characteristics of study participants are described in Table 1.
Study design and drug treatment
This study employed a randomized, double-blind, placebo-controlled, 3-way crossover within-subject design using scopolamine (0.6 mg), scopolamine (0.6 mg) + donepezil (5 mg), and placebo. Thus, each participant went through all the treatment conditions (i.e., was assigned to each treatment group) but in a different order. The sequence of the three treatment conditions was assigned randomly for each participant using a Latin Square design. Medical history including a dichotomic (yes/no) question about subjectively perceived spatial navigation difficulties, physical examinations, and vital sign measurements was performed at a screening assessment that occurred 1 week prior to administration of the first dose of study medication. At the screening visit, subjects also performed the spatial navigation test (see below) to familiarize themselves with the test requirements. Each treatment administered during the spatial navigation test was separated from the next one with a 7- to 14-day wash-out period. Trial medication was administered between 9:00 and 11:30 on the day of testing. In each test, subjects completed 4 spatial navigation sessions: at baseline before medication and then again at 2, 4, and 8 h after dosing (Fig. 1a).
Scopolamine hydrobromide was dissolved in purified water at a concentration of 3.0 mg/ml, which is equivalent to 0.1 mg in 1 drop. The participants received 0.6 mg of scopolamine (i.e., 6 drops) or the same volume of liquid placebo (purified water PhEur) instilled on a teaspoon of sugar after baseline testing. The participants were instructed to let the sugar dissolve sublingually. The 5-mg dose of donepezil (or placebo—corn starch PhEur) was given orally immediately after dissolution of the sugar. The scopolamine, donepezil, and placebo used in this study were supplied by the Motol University Hospital Pharmacy and dispensed by an unblinded third party (research pharmacist) at the study site. Scopolamine was given sublingually, and donepezil was given orally as non-invasive forms of drug administration according to the requirements of the ethics committee.
Behavioral apparatus and design of the test
The spatial navigation test was performed in the Laboratory of Spatial Cognition, a joint workplace of the Department of Neurology, 2nd Faculty of Medicine, Charles University, Prague, Czech Republic and the Institute of Physiology, Academy of Sciences of the Czech Republic, Prague, Czech Republic. We used the real-space version of the HGT (Kalová et al. 2005), a human analog of the MWM test that was performed in a real-space navigation apparatus named the Blue Velvet Arena, a fully enclosed circular arena 2.8 m in diameter and 2.9 m high surrounded by a dark blue velvet curtain with 8 large digital numerical displays used as distal orientation cues (Fig. 1c). The two subtests of the HGT used in this study mimic the conditions in the original MWM task, where animals are trained to locate the position of the hidden platform: (a) in relation to the distal orientation cues in the room while being released from the pool periphery in 8 trials (spatial navigation trial) and (b) in the probe trial when the hidden platform is removed and distal orientation cues are used for navigation (probe trial).
In both, the spatial navigation trial and probe trial, the participants were asked to locate an invisible goal using two distal orientation cues at the perimeter of the arena (Fig. 2a). The spatial navigation session consisted of 8 spatial navigation trials performed in direct sequence and the probe including 2 consecutive trials (Fig. 1a). The positions of the goal (i.e., 12 cm circle of laser light) on the arena floor were constant across all trials relative to the positions of both orientation cues (Fig. 2b). To begin the task, participants were asked to enter the arena, and they were given a long standing pole with an infrared light-emitting diode. The position of the goal on the arena floor was shown to them. Before each trial, they were asked to stand at the side of the arena, go directly as quickly as possible from their start position to the goal, and to place the pole directly on the presumed goal position. In the spatial navigation session, the goal was briefly shown after each trial in order to facilitate learning. The probe trial was then administered 30 min after the spatial navigation trial was completed, and no feedback through showing the hidden goal was provided. Performance was recorded automatically by the computer as the distance error between the standing pole’s final position and the actual goal location (in centimeters).
Scheme of orientation cues in the human experiment. a Two distal orientation cues were at the perimeter of the arena in the Hidden Goal Task. b The position of the goal on the arena floor was constant across all trials relative to the positions of both distal orientation cues in the Hidden Goal Task
Measured parameters and statistical analyses in the animal and human experiments
In the animal experiment, we analyzed the distance (m) traveled to reach the platform in the spatial navigation trials, the time (s) spent in the quadrant where the platform had been previously positioned (target quadrant), and number of rats which failed to find the platform in 60 s.
In the human experiment, the distance between the participant’s final position and the correct goal location (distance error) measured in centimeters was used as the measure of navigational accuracy (dependent variable), whereas group status was the independent variable.
The first set of analyses included an assessment of between-treatment differences in spatial navigation trials (averaged across each session) and probe trials in four sessions. We used two general linear models (GLMs) with repeated measures (RM) with one between-subject factor (group: scopolamine versus scopolamine-donepezil versus placebo in the human experiment or saline in the animal experiment) and one within-subject factor. In the first GLM analysis, we used spatial navigation trials of sessions 1–4 (in animal experiment: baseline, 1, 2.5, and 5 h after dosing; in human experiment: baseline, 2, 4, and 8 h after dosing) as a within-subject factor. In the second GLM analysis, we used probe trials of sessions 1–4 (in animal experiment: baseline, 1, 2.5, and 5 h after dosing; in human experiment: baseline, 2, 4, and 8 h after dosing) as a within-subject factor. Post hoc pairwise comparisons with Sidak’s test were used to compare individual groups.
The second set of analyses included an assessment of the differences between 8 consecutive spatial navigation trials within each of the 4 sessions (effect of trial). We used 4 GLMs with RM with one between-subject factor (group: scopolamine versus scopolamine-donepezil versus placebo or saline) and one within-subject factor (trial: trials 1–8) for each session separately. Again, post hoc pairwise comparisons were used to compare individual groups.
All quantitative data from animal probe trials and from the human experiment were found to be adequate for parametric analysis. Data from the distance traveled in spatial navigation trials in the animal experiment had skewed (not normal) distribution; therefore, we transformed them with the natural logarithm. In the human experiment, the participants with subjectively perceived spatial navigation difficulties (n = 2) did not differ in spatial navigation performance at the screening visit from those who did not report spatial navigation difficulties. Therefore, all participants were included in the analyses. Statistical significance was set at two-tailed (alpha) of 0.05. Effect sizes are reported using partial eta2 for GLMs with RM (Tabachnick and Fidell 2007). Partial eta2 of 0.2 corresponds to Cohen’s d of 1.0. All analyses were conducted by using IBM SPSS for Windows version 20.0.
Results
Results of the animal experiment
For the rats’ performance in spatial navigation trials of sessions 1–4 (Fig. 3a), we found a significant main effect for group performance (F(2, 104) = 12.50, p < .001, partial eta2 = 0.19), which was driven by a significantly higher distance traveled by the scopolamine group compared to the scopolamine-donepezil group and the saline group (p’s ≤ .001, Cohen’s d’s > 0.32). There were no differences between the saline group and the scopolamine-donepezil group (p = 0.900). We found a significant main effect for session (F(3, 312) = 83.30, p < 0.001, partial eta2 = 0.44), which showed a decrease of distance traveled in sessions 2, 3, and 4 (p’s ≤ .009, Cohen’s d’s > 0.28). There were no differences between performances in session 3 and session 4 (p = .190). However, there was a significant group-by-session interaction (F(6, 312) = 6.20, p < 0.001, partial eta2 = 0.11), with improvement of the saline group and the scopolamine-donepezil group compared to the scopolamine group in sessions 2 and 4 (p’s ≤ .005, Cohen’s d’s > 1.18). The saline group improved its performance throughout sessions 2 to 4 (p’s ≤ .014, Cohen’s d’s > 0.45), while the scopolamine group and the scopolamine-donepezil group improved their performance only in sessions 3 and 4 (p’s < .001, Cohen’s d’s > 0.43).
Effects of scopolamine and donepezil on HGT in rats. a Distance traveled to the target platform in spatial navigation trials according to treatment condition and time of testing after treatment (session 1 at baseline, session 2 at 1 h, session 3 at 2.5 h, and session 4 at 5 h). b Time spent in a target quadrant of the Morris water maze in probe trials according to treatment condition and time of testing after treatment (session 1 at baseline, session 2 at 1 h, session 3 at 2.5 h, and session 4 at 5 h). c Example of rats’ trajectories in probe trials of session 1 at baseline, session 2 at 1 h, session 3 at 2.5 h, and session 4 at 5 h according to treatment condition. The area surrounded by a dotted line shows the quadrant of the maze in which the platform was positioned in spatial navigation sessions. d Distance traveled to the target platform in spatial navigation trials according to treatment condition and time of testing after treatment (session 1 at baseline, session 2 at 1 h, session 3 at 2.5 h, and session 4 at 5 h) shown in detail for each session
In probe trials of sessions 1–4 (Fig. 3b), we found a significant main effect for group performance (F(2, 99) = 4.03, p = 0.021, partial eta2 = 0.08), which was driven by significant impairment of the scopolamine group compared to the saline group (p = 0.017, Cohen’s d = 0.69). There were no differences between the saline group and the scopolamine-donepezil group (p = .630) nor between the scopolamine group and the scopolamine-donepezil group (p = 0.153). Examples of the rats’ trajectories are shown in Fig. 3c. We also found a significant main effect for session (F(3, 297) = 2.90, p = 0.034, partial eta2 = 0.03), revealing an improvement between sessions 3 and 4 (p = .019, Cohen’s d = 0.26). There was no significant group-by-session interaction, suggesting no differences in the time spent in the target quadrant across 4 consecutive sessions among the groups (F(6, 297) = 0.94, p = 0.470).
Next, in the analysis of spatial navigation trials for each of the 4 sessions separately to determine the effect of 8 consecutive spatial navigation trials in each session (Fig. 3d), we found a significant main effect for group performance in session 2 (F(2, 94) = 17.80, p < 0.001, partial eta2 = 0.30), session 3 (F(2, 94) = 6.50, p = .002, partial eta2 = 0.13), and in session 4 (F(2, 94) = 15.80, p < 0.001, partial eta2 = 0.32). In all these sessions, the scopolamine group exhibited poorer performance than both the saline group and the scopolamine-donepezil group (p’s ≤ .034, Cohen’s d’s > 0.70). The main effect for trial was significant in session 1, indicating significant learning across the 8 consecutive spatial navigation trials in the sample overall (F(7, 623) = 19.70, p < 0.001, partial eta2 = 0.20). The subsequent analysis revealed that there was a significant decrease of traveled distance in trials 2–8 (p’s ≤ .001, Cohen’s d’s > 0.72). We also found a significant main effect for trial in session 2 (F(7, 623) = 8.36, p < 0.001, partial eta2 = 0.09) and in session 4 (F(7, 623) = 3.50, p = .001, partial eta2 = 0.05). In session 2, there was a significant decrease of traveled distance in trials 4–8 (p’s ≤ 0.014, Cohen’s d’s > 0.20), while in session 4, traveled distance decreased only in trial 7 (p < .001, Cohen’s d’s > 0.66). There was no significant main effect for trial in session 3, indicating no significant change across 8 consecutive trials in the sample overall (F(7, 623) = 0.91, p = 0.500). We found a significant group-by-trial interaction in session 4, suggesting a decrease in traveled distance across 8 consecutive trials in the saline and the scopolamine-donepezil groups compared to the scopolamine group (F(14, 623) = 2.60, p = 0.002, partial eta2 = 0.07).
If the rat failed to find the platform in 60 s, the trial was stopped. Analyzing the effect of treatment on the number of rats, which failed to find the hidden platform, we found a significant main effect for group performance (F(2, 104) = 8.12, p < .001, partial eta2 = 0.12). In average, 1.93 rats per session in the scopolamine group failed to find the platform compared to 0.15 rats per session in the saline group and 0.86 rats per session in the scopolamine-donepezil group (p’s < .019, Cohen’s d’s > 0.54). We did not find any difference between the saline group and the scopolamine-donepezil group (p = 0.109).
Results of the human experiment
For the humans’ performance in spatial navigation trials of sessions 1–4 (Fig. 4a), we found a significant main effect for group performance (F(2, 60) = 3.40, p = .042, partial eta2 = 0.13), which was driven by a significantly higher distance error for the scopolamine group compared to the placebo group (p = .023, Cohen’s d = 0.63). There were no differences between the scopolamine-donepezil group and the placebo group or between the scopolamine-donepezil group and the scopolamine group. The main effect for session was not significant, indicating no significant change across 4 consecutive sessions in the sample overall (F(3, 58) = 0.20, p = 0.794). Further, there was no significant group-by-session interaction, suggesting no differences in the change of distance errors across 4 consecutive sessions among the groups (F(6, 118) = 1.83, p = 0.140).
Effects of scopolamine and donepezil on HGT in humans. a Effects of treatment condition on distance error made by subjects in spatial navigation trials according to time of testing after treatment (session 1 at baseline, session 2 at 2 h, session 3 at 4 h, and session 4 at 8 h). b Distance error in probe trials according to treatment condition and time of testing after treatment (session 1 at baseline, session 2 at 2 h, session 3 at 4 h, and session 4 at 8 h). c Distance error made by subjects in spatial navigation trials according to treatment condition and time of testing after treatment (session 1 at baseline, session 2 at 2 h, session 3 at 4 h, and session 4 at 8 h) shown in detail for each session
In probe trials of sessions 1–4 (Fig. 4b), we found a significant main effect for group performance (F(2, 60) = 3.37, p = 0.044, partial eta2 = 0.14), which was driven by a significantly higher distance error for the scopolamine group compared to the placebo group (p = .023, Cohen’s d = 0.62). The differences were most pronounced in session 3, where the placebo group consistently outperformed the scopolamine group (p = 0.040, Cohen’s d = 0.58). There were no differences between the scopolamine-donepezil group and the placebo group or between the scopolamine-donepezil group and the scopolamine group. The main effect for session was not significant, indicating no significant change across 4 consecutive sessions in the sample overall (F(3, 58) = 0.44, p = .656). Further, there was no significant group-by-session interaction, suggesting no differences in the change of distance errors across 4 consecutive sessions among the groups (F(6, 118) = 0.66, p = .633).
Next, in the analysis of spatial navigation trials for each of the 4 sessions separately to determine the effect of 8 consecutive spatial navigation trials in each session (Fig. 4c), we found a significant main effect for group performance in session 3 (F(2, 60) = 4.08, p = 0.024, partial eta2 = 0.15), where the scopolamine group consistently exhibited higher distance error than the placebo group (p = 0.017, Cohen’s d = 0.76), in contrast to the scopolamine-donepezil group. There was no significant main effect for group in the other sessions (F(2, 60) ≤ 2.57, p’s ≥ .088). The main effect for trial was significant in session 1, indicating a significant learning effect across 8 consecutive spatial navigation trials in the sample overall (F(7, 54) = 3.59, p = 0.006, partial eta2 = 0.07). The subsequent analysis revealed that there was a significant decrease of distance errors in trials 3–8 (F(1, 60) = 13.71, p = 0.001, partial eta2 = 0.23, quadratic effect). There was no significant main effect for trial in other sessions, indicating no significant change across 8 consecutive trials in the sample overall (F(7, 54) ≤ 0.72, p’s ≥ .544). There was no significant group-by-trial interaction in any session, suggesting no differences in the change of distance errors across 8 consecutive trials among the groups (F(14, 110) ≤ 1.53, p’s ≥ 0.140).
Discussion
Our results here show that a centrally active antagonist of muscarinic acetylcholine receptors, scopolamine, significantly impairs place navigation in both the rat and human versions of the MWM (HGT). In rats, we found a clear ameliorative effect of donepezil on spatial navigation trials in all sessions. In probe trials, the performance of the group co-administered scopolamine and donepezil was statistically indistinguishable from the performance of the placebo group. In humans, in spatial navigation trials and probe trials, the performance of the group co-administered scopolamine and donepezil was also statistically indistinguishable from the performance of the placebo group. This may indicate a potential modulatory effect of donepezil on place navigation performance influenced by scopolamine. However, in probe trials in rats and in spatial navigation trials and probe trials in humans, the performance of the groups co-administered scopolamine and donepezil did not significantly differ from the scopolamine groups; so, a clear ameliorative effect of donepezil was not demonstrated.
The effect of scopolamine on place navigation in rats and humans
Our aim was to design a translational testing protocol using the MWM that could be validated by scopolamine and donepezil treatment in both rats and humans. Acetylcholine is known to facilitate place learning. Its action is mediated by facilitation of synaptic plasticity in the hippocampus (Mitsushima et al. 2013). Next, cholinergic projections from the medial septum to the hippocampus maintain theta rhythm, which is crucial for learning and memory (Jezek et al. 2011). The systemic administration of anticholinergic drugs such as scopolamine is used frequently as a fast screening model for cognitive dysfunction resembling dementia in patients with AD, despite the fact that the peripheral application of scopolamine yields many non-mnemonic adverse effects (von Linstow Roloff et al. 2007). However, the responsivity of these models to pro-cognitive drugs such as acetylcholinesterase inhibitors (Ogura et al. 2000) or even nootropics (Christoffersen et al. 1998; Marisco et al. 2013) suggests some predictive validity of this simple model. The fact that central muscarinic blockade can impair learning is well established (Koller et al. 2003; Ellis et al. 2006; Thomas et al. 2008), although caution must be taken to separate amnestic effects from procedural, motivational, and other general functions.
In our experiment, we used place navigation tasks adopted for rat and human versions of the MWM (HGT). The advantage of using place navigation tasks in the MWM is that these tasks can be administered to both rats and humans and thus provide a direct comparison between rat and human cognitive performance. Place navigation tasks sensitive for the detection of subtle hippocampal dysfunction (Nedelska et al. 2012) are useful for the early diagnosis of cognitive disturbances occurring very early in the course of AD (Hort et al. 2007) and are useful for monitoring the effect of treatment with acetylcholinesterase inhibitors (Hort et al. 2014).
In rats, we found an inhibitory effect of scopolamine on place navigation. The saline group outperformed the scopolamine group consistently after drug administration in both spatial navigation trials and probe trials. The rats treated with scopolamine exhibited poor navigation performance particularly 1 h after drug administration. Separate analysis of individual sessions showed a disruptive effect of scopolamine in all sessions after drug administration, though we found a decrease in the distance traveled to the hidden goal in scopolamine groups 2.5 and 5 h after treatment, indicating some learning capability. This observed learning effect indicates that scopolamine in lower doses did not impair the spatial navigation completely, and that the scopolamine-treated rats were still able to learn the hidden goal position.
In the human experiment, we found a deteriorating effect of scopolamine on place navigation in all spatial navigation sessions, but the largest effect was observed during the session 3–4 h after dosing. The effect was not as clear 2 and 8 h after dosing, as documented by the separate analyses of individual sessions. These results are congruent with findings showing the maximum effect of scopolamine on cognitive performance 3 or 5 h after dosing (Fredrickson et al. 2008; Lim et al. 2015). However, a previous study on young healthy volunteers did not demonstrate any effect of scopolamine on place navigation performance and showed only reduced activity of the hippocampus, which is a crucial structure for place navigation (Antonova et al. 2011). They used a lower dose of scopolamine (0.4 mg), and the tests were performed only 1.5 h after scopolamine treatment. In addition, navigation tests in that study were performed in virtual space, where it may have been easier for subjects to use alternative non-allocentric strategies (Iaria et al. 2003). On the other hand, a low dose of scopolamine (0.2 mg) administered subcutaneously may be sufficient to reveal spatial learning deficits in older adults with preclinical AD as demonstrated by the recent study using a 2D hidden maze task (Lim et al. 2015).
The effect of donepezil on scopolamine-induced deficits in rats and humans
Co-administration of donepezil and scopolamine in the rat experiment improved place navigation in spatial navigation trials, but not in probe trials. In spatial navigation trials, rats treated with donepezil and scopolamine outperformed the scopolamine-treated rats and did not differ from the saline-treated rats, showing alleviating effects of donepezil on scopolamine-induced impairment. However, in probe trials, we only found significant differences between the saline and the scopolamine groups, but no improvement in the donepezil-treated group. Our results agree with Buccafusco et al. (2008), who reported only a partial alleviating effect of donepezil on scopolamine-induced impairment in a delayed stimulus discrimination task and in the MWM. Similar to our experiment, they did not find any effect of donepezil in probe trials. Takahata et al. (2005) and Chen et al. (2002) also found an alleviating effect of donepezil on scopolamine-induced impairment in the MWM, but did not test the rats’ performance in probe trials. On the contrary, Lindner et al. (2006) failed to find any mitigating effects of donepezil on spatial navigation trials and probe trials in the MWM using various doses of scopolamine and donepezil. Still, they reported that donepezil inhibits the effects of scopolamine on other tasks involving mainly psychomotor functions and classical and attention conditioning. Similar to these findings, we observed some effects of donepezil on scopolamine-induced hyper locomotion (data not shown). However, an effect of donepezil on swimming speed cannot explain the decrease in distance traveled to the hidden goal in the scopolamine-donepezil group.
In the human experiment, co-administration of donepezil normalized performance in spatial navigation trials and probe trials when compared to the placebo group. This may indicate that donepezil may mitigate the negative effects of scopolamine on place navigation. However, a clear ameliorating effect of donepezil was not found as there were no significant differences between the scopolamine and scopolamine-donepezil groups. In a recent study by Snyder et al. (2005), administration of donepezil alleviated a spatial navigation impairment associated with scopolamine injection in a computer-screen maze. However, that study used a lower dose of scopolamine (0.3 mg) and also pretreated the subject with donepezil 3 h before scopolamine treatment. Further, the alleviating effect of donepezil emerged after 7 h of scopolamine administration; such an effect could not have been demonstrated in our study, because the effect of scopolamine on place navigation was no longer present 8 h after administration of the medication. In addition, the dose of scopolamine used in our study (0.6 mg) can induce higher sedation and lower psychomotor speed (Robbins et al. 1997). These adverse effects, which are not reversible by donepezil, may have further negatively influenced place navigation. Finally, our previous study found improvements in place navigation among subjects with mild dementia due to AD after daily administration of donepezil over 3 months (Hort et al. 2014). This may indicate that long-term donepezil treatment may be more beneficial for cognitive functioning compared to administration of a single dose.
Differences between animal and human experimental designs
In spite of the fact that our experiment was designed to be as similar as possible for rats and humans, there were still some minor differences that should be mentioned. In the human experiment, we used a 0.6-mg dose of scopolamine, which showed a robust impact on cognitive functioning, but also higher side effects of sedation, lower psychomotor speed, and attention (Robbins et al. 1997; Fredrickson et al. 2008). Similarly, the subjects on scopolamine in our current study reported more adverse effects than subjects on placebo (Table 1). Thus, we cannot fully exclude that the adverse side effects of scopolamine may have influenced spatial navigation performance to a certain extent in our subjects. For the animal experiment, we calculated the ideal dose of scopolamine in rats according to Reagan-Shaw et al. (2008) and Shin et al. (2010) to be 0.05 mg/kg, but we failed to detect any effect of this dose on place navigation (unpublished results). Based on our previous findings (Vales and Stuchlik 2005; Entlerova et al. 2013), we carefully increased the dose of scopolamine to 0.8 mg/kg, as this low dose should have lower impacts on psychomotor functions but still impair cognitive functioning. The same situation was encountered in the administration of donepezil, where for human subjects, we used a well-tolerated initial 5-mg dose of donepezil, and for rats, we used a 1-mg dose of donepezil. Again to convert the dose for humans to suitable dose for rats, we used the guidelines of Reagan-Shaw et al. (2008) and Shin et al. (2010). Another difference between the animal and the human design was the method of drug administration. Rats were injected both scopolamine and donepezil intraperitoneally while human participants received scopolamine sublingually and donepezil orally. In general, the sublingual administration of scopolamine and the oral administration of donepezil may result in slower pharmacokinetics compared to intraperitoneal administration. However, this was taken into account when designing the experiment and thus may not significantly influence the results of our study. In rats and human subjects, the design of baseline testing followed by 3 consecutive sessions was the same, although the timing of the sessions was slightly different. In the human experiment, we adopted a well-established design—2, 4, and 8 h after dosing (Fredrickson et al. 2008; Snyder et al. 2014). In the rat experiment, we used a timing of 1, 2.5, and 5 h after dosing, adjusting the timing of the sessions according to the different half-life of scopolamine in humans and rats (Golding et al. 1991; Gupta 2012), which is approximately 1.5 times shorter in rats upon intraperitoneal administration. Next, the accumulative effect of learning may be a possible limitation. However, due to different platform positions between testing days, we observed no motor strategy to solve the task. Rats searched the platform position by a random search in trial 1 of the session 1 (data not reported).
Finally, the sample in the human experiment was highly educated and inhomogeneous in demographic characteristics (i.e., consisted of males and females and right- and left-handed participants). Although the 3-way cross-over design should be resistant to this inhomogeneity since each participant went through all 3 treatment conditions (Snyder et al. 2005), we cannot fully exclude that high education and inhomogeneity of the sample may mitigate the differences between the treatment conditions. This may be caused by the potentially unequal cognitive susceptibility to the effect of scopolamine and donepezil among the participants and should be a focus of future studies.
Concluding remarks
In conclusion, this study demonstrated that the model using scopolamine and donepezil to challenge place navigation testing can be studied concurrently in laboratory animals and in human volunteers. The animal and human versions of the HGT in the MWM and in the real maze for humans, respectively, may thus be useful and reliable tools for measuring the effect of other pro-cognitive or anti-cognitive drugs on spatial navigation. This approach may help reduce inconsistent results in preclinical and clinical phases of drug trials, which may be partially caused by using different measures of cognitive outcome. However, place navigation testing in the real space requires special equipment that is not generally available. Therefore, easy-to-use and inexpensive 2D computerized spatial navigation tests, which provide similar results to real-space setting (Laczó et al. 2012; Laczó et al. 2014) and a 3D virtual reality route learning task (Tippett et al. 2009), may be a reliable alternative to the real mazes for humans.
Abbreviations
- AD:
-
Alzheimer’s disease
- MWM:
-
Morris water maze
- HGT:
-
Hidden Goal Task
- GLM:
-
General linear model
- RM:
-
Repeated measures
References
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging and Alzheimer’s association workgroup. Alzheimers Dement 7:270–279. doi:10.1016/j.jalz.2011.03.008
Antonova E, Parslow D, Brammer M et al (2011) Scopolamine disrupts hippocampal activity during allocentric spatial memory in humans: an fMRI study using a virtual reality analogue of the Morris water maze. J Psychopharmacol 25:1256–1265. doi:10.1177/0269881110379285
Bartolini L, Risaliti R, Pepeu G (1992) Effect of scopolamine and nootropic drugs on rewarded alternation in a T-maze. Pharmacol Biochem Behav 43:1161–1164
Baxter MG, Gallagher M (1996) Intact spatial learning in both young and aged rats following selective removal of hippocampal cholinergic input. Behav Neurosci 110:460–467
Bianchetti A, Ranieri P, Margiotta A, Trabucchi M (2006) Pharmacological treatment of Alzheimer’s disease. Aging Clin Exp Res 18:158–162
Buccafusco JJ, Terry AV, Webster SJ et al (2008) The scopolamine-reversal paradigm in rats and monkeys: the importance of computer-assisted operant-conditioning memory tasks for screening drug candidates. Psychopharmacology 199:481–494. doi:10.1007/s00213-007-0887-8
Cassel JC, Kelche C (1989) Scopolamine treatment and fimbria-fornix lesions: mimetic effects on radial maze performance. Physiol Behav 46:347–353
Czéh B, Stuchlik A, Wesierska M et al (2001) Effect of neonatal dentate gyrus lesion on allothetic and idiothetic navigation in rats. Neurobiol Learn Mem 75:190–213. doi:10.1006/nlme.2000.3975
D’Hooge R, De Deyn PP (2001) Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev 36:60–90
Ellis JR, Ellis KA, Bartholomeusz CF et al (2006) Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol 9:175–189. doi:10.1017/S1461145705005407
Entlerova M, Lobellova V, Hatalova H et al (2013) Comparison of long-Evans and Wistar rats in sensitivity to central cholinergic blockade with scopolamine in two spatial tasks: an active place avoidance and the Morris water maze. Physiol Behav 120:11–18. doi:10.1016/j.physbeh.2013.06.024
Fredrickson A, Snyder PJ, Cromer J et al (2008) The use of effect sizes to characterize the nature of cognitive change in psychopharmacological studies: an example with scopolamine. Hum Psychopharmacol 23:425–436. doi:10.1002/hup.942
Frey KA, Koeppe RA, Mulholland GK et al (1992) In vivo muscarinic cholinergic receptor imaging in human brain with [11C]scopolamine and positron emission tomography. J Cereb Blood Flow Metab 12:147–154. doi:10.1038/jcbfm.1992.18
Golding JF, Gosden E, Gerrell J (1991) Scopolamine blood levels following buccal versus ingested tablets. Aviat Space Environ Med 62:521–526
Greenberg SM, Tennis MK, Brown LB et al (2000) Donepezil therapy in clinical practice: a randomized crossover study. Arch Neurol 57:94–99
Guo HB, Cheng YF, Wu JG et al (2015) Donepezil improves learning and memory deficits in APP/PS1 mice by inhibition of microglial activation. Neuroscience 290:530–542. doi:10.1016/j.neuroscience.2015.01.058
Gupta R (2012) Veterinary toxicology: basic and clinical principles, chapter 85. Academic Press, United States
Herrera-Morales W, Mar I, Serrano B, Bermúdez-Rattoni F (2007) Activation of hippocampal postsynaptic muscarinic receptors is involved in long-term spatial memory formation. Eur J Neurosci 25:1581–1588. doi:10.1111/j.1460-9568.2007.05391.x
Hort J, Andel R, Mokrisova I et al (2014) Effect of donepezil in Alzheimer disease can be measured by a computerized human analog of the Morris water maze. Neurodegener Dis 13:192–196. doi:10.1159/000355517
Hort J, Laczó J, Vyhnálek M et al (2007) Spatial navigation deficit in amnestic mild cognitive impairment. Proc Natl Acad Sci U S A 104:4042–4047. doi:10.1073/pnas.0611314104
Chen Z, Xu A-J, Li R, Wei E-Q (2002) Reversal of scopolamine-induced spatial memory deficits in rats by TAK-147. Acta Pharmacol Sin 23:355–360
Christoffersen GR, von Linstow Roloff E, Nielsen KS (1998) Effects of piracetam on the performance of rats in a delayed match-to-position task. Prog Neuro-Psychopharmacol Biol Psychiatry 22:211–228
Iaria G, Petrides M, Dagher A et al (2003) Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci 23:5945–5952
Jezek K, Henriksen EJ, Treves A et al (2011) Theta-paced flickering between place-cell maps in the hippocampus. Nature 478:246–249. doi:10.1038/nature10439
Kalová E, Vlcek K, Jarolímová E, Bures J (2005) Allothetic orientation and sequential ordering of places is impaired in early stages of Alzheimer’s disease: corresponding results in real space tests and computer tests. Behav Brain Res 159:175–186. doi:10.1016/j.bbr.2004.10.016
Kesner RP, Hopkins RO (2006) Mnemonic functions of the hippocampus: a comparison between animals and humans. Biol Psychol 73:3–18. doi:10.1016/j.biopsycho.2006.01.004
Koller G, Satzger W, Adam M et al (2003) Effects of scopolamine on matching to sample paradigm and related tests in human subjects. Neuropsychobiology 48:87–94
Laczó J, Andel R, Vyhnalek M et al (2012) From Morris water maze to computer tests in the prediction of Alzheimer’s disease. Neurodegener Dis 10:153–157. doi:10.1159/000333121
Laczó J, Andel R, Vyhnalek M et al (2014) APOE and spatial navigation in amnestic MCI: results from a computer-based test. Neuropsychology 28:676–684. doi:10.1037/neu0000072
Lim YY, Maruff P, Schindler R et al (2015) Disruption of cholinergic neurotransmission exacerbates Aβ-related cognitive impairment in preclinical Alzheimer’s disease. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2015.07.009
Lindner MD, Hogan JB, Hodges DB et al (2006) Donepezil primarily attenuates scopolamine-induced deficits in psychomotor function, with moderate effects on simple conditioning and attention, and small effects on working memory and spatial mapping. Psychopharmacology 188:629–640. doi:10.1007/s00213-006-0556-3
Marisco PC, Carvalho FB, Rosa MM et al (2013) Piracetam prevents scopolamine-induced memory impairment and decrease of NTPDase, 5′-nucleotidase and adenosine deaminase activities. Neurochem Res 38:1704–1714. doi:10.1007/s11064-013-1072-6
Mitsushima D, Sano A, Takahashi T (2013) A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun. doi:10.1038/ncomms3760
Molchan SE, Mellow AM, Hill JL et al (1992) The effects of thyrotropin-releasing hormone and scopolamine in Alzheimer’s disease and normal volunteers. J Psychopharmacol 6:489–500. doi:10.1177/026988119200600404
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11:47–60
Morris RG (1981) Spatial localization does not require the presence of local cues. Learn Motiv 12:239–260
Nedelska Z, Andel R, Laczó J et al (2012) Spatial navigation impairment is proportional to right hippocampal volume. Proc Natl Acad Sci U S A 109:2590–2594. doi:10.1073/pnas.1121588109
Ogura H, Kosasa T, Kuriya Y, Yamanishi Y (2000) Donepezil, a centrally acting acetylcholinesterase inhibitor, alleviates learning deficits in hypocholinergic models in rats. Methods Find Exp Clin Pharmacol 22:89–95
Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22:659–661. doi:10.1096/fj.07-9574LSF
Rentz DM, Parra Rodriguez MA, Amariglio R et al (2013) Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: a selective review. Alzheimers Res Ther 5:58. doi:10.1186/alzrt222
Robbins TW, Semple J, Kumar R et al (1997) Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology 134:95–106
Scali C, Casamenti F, Bellucci A et al (2002) Effect of subchronic administration of metrifonate, rivastigmine and donepezil on brain acetylcholine in aged F344 rats. J Neural Transm 109:1067–1080. doi:10.1007/s007020200090
Sherman SJ, Atri A, Hasselmo ME et al (2003) Scopolamine impairs human recognition memory: data and modeling. Behav Neurosci 117:526–539
Shin J, Seol I, Son C (2010) Interpretation of animal dose and human equivalent dose for drug development. J Korean Orient Med 31:351–357
Snyder PJ, Bednar MM, Cromer JR, Maruff P (2005) Reversal of scopolamine-induced deficits with a single dose of donepezil, an acetylcholinesterase inhibitor. Alzheimers Dement 1:126–135. doi:10.1016/j.jalz.2005.09.004
Snyder PJ, Lim YY, Schindler R et al (2014) Microdosing of scopolamine as a “cognitive stress test”: rationale and test of a very low dose in an at-risk cohort of older adults. Alzheimers Dement 10:262–267. doi:10.1016/j.jalz.2014.01.009
Stuchlik A (2014) Dynamic learning and memory, synaptic plasticity and neurogenesis: an update. Front Behav Neurosci 8:106. doi:10.3389/fnbeh.2014.00106
Stuchlik A, Rehakova L, Telensky P, Vales K (2007) Morris water maze learning in long-Evans rats is differentially affected by blockade of D1-like and D2-like dopamine receptors. Neurosci Lett 422:169–174. doi:10.1016/j.neulet.2007.06.012
Svoboda J, Telensky P, Blahna K et al (2015) The role of rat posterior parietal cortex in coordinating spatial representations during place avoidance in dissociated reference frames on a continuously rotating arena (carousel). Behav Brain Res 292:1–9. doi:10.1016/j.bbr.2015.05.008
Tabachnick B, Fidell L (2007) Using multivariate statistics, 6th edn. Pearson/Allyn & Bacon, Boston
Takahata K, Minami A, Kusumoto H et al (2005) Effects of selegiline alone or with donepezil on memory impairment in rats. Eur J Pharmacol 518:140–144. doi:10.1016/j.ejphar.2005.06.024
Thomas E, Snyder PJ, Pietrzak RH et al (2008) Specific impairments in visuospatial working and short-term memory following low-dose scopolamine challenge in healthy older adults. Neuropsychologia 46:2476–2484. doi:10.1016/j.neuropsychologia.2008.04.010
Tippett WJ, Lee J-H, Mraz R et al (2009) Convergent validity and sex differences in healthy elderly adults for performance on 3D virtual reality navigation learning and 2D hidden maze tasks. Cyberpsychol Behav 12:169–174. doi:10.1089/cpb.2008.0218
Vales K, Stuchlik A (2005) Central muscarinic blockade interferes with retrieval and reacquisition of active allothetic place avoidance despite spatial pretraining. Behav Brain Res 161:238–244. doi:10.1016/j.bbr.2005.02.012
Vlček K, Laczó J (2014) Neural correlates of spatial navigation changes in mild cognitive impairment and Alzheimer’s disease. Front Behav Neurosci 8:89. doi:10.3389/fnbeh.2014.00089
von Linstow Roloff E, Harbaran D, Micheau J et al (2007) Dissociation of cholinergic function in spatial and procedural learning in rats. Neuroscience 146:875–889. doi:10.1016/j.neuroscience.2007.02.038
Voss B, Thienel R, Reske M et al (2010) Cognitive performance and cholinergic transmission: influence of muscarinic and nicotinic receptor blockade. Eur Arch Psychiatry Clin Neurosci 260(Suppl):S106–S110. doi:10.1007/s00406-010-0160-8
Weintraub S, Salmon D, Mercaldo N et al (2009) The Alzheimer’s disease centers’ uniform data set ({UDS):} the neuropsychological test battery. Alzheimer Dis Assoc Disord 23:91–101. doi:10.1097/WAD.0b013e318191c7dd
Whitehouse PJ, Au KS (1986) Cholinergic receptors in aging and Alzheimer’s disease. Prog Neuro-Psychopharmacol Biol Psychiatry 10:665–676
Whitehouse PJ, Price DL, Struble RG et al (1982) Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239
Winblad B, Engedal K, Soininen H et al (2001) A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 57:489–495
Acknowledgments
Scientists from the Institute of Physiology CAS were supported by GAČR Center of Excellence P304/12/G069. Tereza Nekovarova and Ales Stuchlik were supported also by MSMT project LH14053 KONTAKT ll.
Scientists from Motol University Hospital were supported by the Grant Agency of Charles University in Prague, grant nos. 624012, 546113, 1108214, and 135215. Ministry of Health, Czech Republic—conceptual development of research organization, University Hospital Motol, Prague, Czech Republic 00064203 provided additional support of scientists from Motol University Hospital.
Scientists from the National Institute of Mental Health were also supported by the project “National Institute of Mental Health (NIMH-CZ),” grant number ED2.1.00/03.0078, and the European Regional Development Fund through the project “Sustainability for the National Institute of Mental Health,” under grant number LO1611, with additional financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the NPU I program.
Other support included the European Regional Development Fund—Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123) and project ICRC-ERA-HumanBridge (No. 316345), and project no. LQ1605 from the National Program of Sustainability II (MEYS CR).
Institutional support was provided by Laboratory Research Grant No. 2/2012 (699002), Excellence Grant and research project RVO: 67985823. We would like to thank Mr. F. Safar, Dr. D. Pechackova, Dr. O. Lerch, Ms. B. Zemlickova, Mr. R. Martinko, Mr. A. Chadima, and Dr. E. Hyncicova for help with data collection and Dr. M. Petrzelova for drug preparation.
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. Dr. Harrison has received consultancy from Abbvie, Astra-Zeneca, Avraham, Boehringer Ingelheim, Bracket (Clinical), CRF Health, EnVivo Pharma, ePharmaSolutions, Eisai, Eli Lilly, Heptares, Janssen AI, Kyowa Hakko Kirin, Lundbeck, MedAvante, Merck, MyCognition, Novartis, Nutricia, Orion Pharma, Pharmanet/i3, Pfizer, Prana Biotech, ProStrakan, Reviva, Servier, Shire, TCG and TransTech Pharma & Velacor. Dr. Windisch is owner and CEO at NeuroScios GmbH. Dr. Hort has consulted for Pfizer, Janssen, Merck, Axon, Sotio, Novartis, Elan, Zentiva and Ipsen. Other authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jan Laczó, Hana Markova, and Veronika Lobellova contributed equally to this study.
Rights and permissions
About this article
Cite this article
Laczó, J., Markova, H., Lobellova, V. et al. Scopolamine disrupts place navigation in rats and humans: a translational validation of the Hidden Goal Task in the Morris water maze and a real maze for humans. Psychopharmacology 234, 535–547 (2017). https://doi.org/10.1007/s00213-016-4488-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4488-2