Abstract
Rationale
Several model organisms have been employed to study the impacts of stress on biological systems. Different models of unpredictable chronic stress (UCS) have been established in rodents; however, these protocols are expensive, long-lasting, and require a large physical structure. Our group has recently reported an UCS protocol in zebrafish with several advantages compared to rodent models. We observed that UCS induced behavioral, biochemical, and molecular changes similar to those observed in depressed patients, supporting the translational relevance of the protocol.
Objectives
Considering that a pharmacological assessment is lacking in this zebrafish model, our aim was to evaluate the effects of anxiolytic (bromazepam) and antidepressant drugs (fluoxetine and nortriptyline) on behavioral (novel tank test), biochemical (whole-body cortisol), and molecular parameters (cox-2, tnf-α, il-6, and il-10 gene expression) in zebrafish subjected to UCS.
Results
We replicated previous data showing that UCS induces behavioral and neuroendocrine alterations in zebrafish, and we show for the first time that anxiolytic and antidepressant drugs are able to prevent such effects. Furthermore, we extended the molecular characterization of the model, revealing that UCS increases expression of the pro-inflammatory markers cox-2 and il-6, which was also prevented by the drugs tested.
Conclusions
This study reinforces the use of zebrafish as a model organism to study the behavioral and physiological effects of stress. The UCS protocol may also serve as a screening tool for evaluating new drugs that can be used to treat psychiatric disorders with stress-related etiologies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the seminal work of Hans Selye (1936), the impact of stress on biological systems has been studied in different model organisms. Several other researchers have subsequently established the pillars of the stress response by understanding the relationship between neuroendocrine axes and behavioral phenotypes (Sapolsky 1982; Sapolsky et al. 1984; McEwen et al. 1988; McEwen 2007). More recently, the links between stress and inflammation began to be uncovered, with accumulating evidence on the role of pro-inflammatory cytokines in mood and anxiety disorders (Miller et al. 2009; Haroon et al. 2012; Hou and Baldwin 2012).
The deleterious effects of stress are especially relevant in the context of modern life, as dysfunctions in stress response pathways are increasingly involved in the etiology of peripheral and central nervous system disorders that contribute to a significant share of the global burden of disease (Global Burden of Disease Study 2013 Collaborators 2015). When submitted to a stressful situation, the body promotes a series of neuroendocrine changes seeking to adequately respond to the demand. However, when the stress exceeds the adaptive capacity of the body, it can predispose the individual to diseases. In humans, this is particularly related to the etiology of affective and anxiety disorders (Crowley and Girdler 2014; McEwen et al. 2015). It is thus necessary to further develop experimental models to better understand the neurobiology of stress and to evaluate potential therapies aimed at preventing and/or treating stress-related disorders.
In this attempt, different models of unpredictable chronic stress (UCS) have been established in rodents (Willner et al. 1992; D’Aquila et al. 1994; Mineur et al. 2006; Yalcin et al. 2008). However, such protocols are expensive, long lasting (up to 4–6 weeks), and require a large physical structure to be implemented (Willner 1997, 2005). We have recently established an UCS protocol in zebrafish with several advantages compared to rodent models (Piato et al. 2011). We observed behavioral and physiological alterations with translational relevance for neuropsychiatric disorders, including anxiety-like behavior, cognitive impairment, increased cortisol and corticotrophin-releasing factor (CRF) levels, and decreased glucocorticoid receptor (GR) expression (Piato et al. 2011). These alterations were evident in zebrafish after only 7 or 14 days of UCS, contrasting with the 4 to 6 weeks of stress required in rodent protocols, which is an interesting and advantageous interspecies difference. Studies using similar UCS protocols in zebrafish were published after our initial report, extending the knowledge on the effects of chronic stress in this model organism (Chakravarty et al. 2013; Manuel et al. 2014; Zimmermann et al. 2015; Pavlidis et al. 2015). Although these data collectively support the validity of the UCS model in zebrafish, a pharmacological assessment is still lacking.
Our purpose was to evaluate the effects of psychotropic drugs on behavioral, biochemical, and molecular parameters in zebrafish subjected to UCS. Since stress leads to behavioral alterations relevant to both anxiety and depression, we chose to evaluate the effects of bromazepam (a benzodiazepine anxiolytic), fluoxetine (a selective serotonin reuptake inhibitor with both antidepressant and anxiolytic properties), and nortriptyline (a tricyclic antidepressant). Specifically, we evaluated the effects of treatment, stress, and their combination on the novel tank test, cortisol levels, and inflammation-related markers (cox-2, tnf-α, il-6 and il-10 gene expression). IL-6 and TNF-α are among the pro-inflammatory cytokines most frequently reported to be increased in studies with depressed patients—both are acute-phase response proteins (Dantzer 2006; Dantzer and Kelley 2007; Dowlati et al. 2010; Haroon et al. 2012). But since it is the balance between anti- and pro-inflammatory cytokines that determines the extent of the inflammatory response, we also chose to measure IL-10 as an important representative of anti-inflammatory cytokines. COX-2 was selected because of its involvement in a different inflammatory pathway (mediated by prostaglandin production), which was also shown to be altered in rodent (Li et al. 2015; Wang et al. 2015) and human studies (Gałecki et al. 2014).
Material and methods
Animals
A total of 450 wild-type short-fin strain adult zebrafish (Danio rerio, 6 months old, 50:50 male/female ratio) were obtained from the heterogeneous breeding stock of Universidade Federal do Rio Grande do Sul. The fish were kept in 40-L aquariums (2.5 fish per liter), filled with non-chlorinated filtered water, with a light/dark cycle of 14/10 h (lights on at 07:00 a.m.), for at least 2 weeks before experiments. Tank water was partially changed once per week and maintained under appropriate conditions (temperature 26 ± 1 °C; pH 7.0 ± 0.3; dissolved oxygen at 7.0 ± 0.4 mg/L; total ammonia at <0.01 mg/L; total hardness at 5.8 mg/L; and alkalinity at 22 mg/L CaCO3). The fish were fed twice a day with a commercial flake fish food (Alcon BASIC®, Alcon, Brazil). All protocols were approved by the Ethics Committee of Universidade Federal do Rio Grande do Sul (#27614/2014).
Drugs
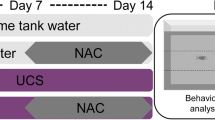
Bromazepam (BMZ, 0.5 mg/L) was acquired from Roche (Rio de Janeiro, Brazil), fluoxetine (FLU, 0.01 mg/L) from Sigma Pharma (São Paulo, Brazil), and nortriptyline (NOR, 0.01 mg/L) from Novartis (São Paulo, Brazil). Drug concentrations were determined based on pilot experiments that tested different concentrations reported in acute or subacute studies available in the literature. We initially tested 1.0, 0.25, and 0.05 mg/L for fluoxetine, and 2.0, 0.1, and 0.05 mg/L for nortriptyline; due to observations of toxic effects (deaths, motor retardation, or other gross behavioral alterations) during the 7 days of drug exposure, different concentrations had to be tested in different sets of animals until the safe concentrations used in this study were reached (0.01 mg/L for fluoxetine and nortriptyline). For bromazepam, we used 0.5 mg/L, which is the same concentration reported by Schaefer et al. (2015). To evaluate whether the chosen drugs were able to prevent the effects induced by UCS, drugs were delivered through water immersion concomitantly to the 7 days of stressor presentation. Fish were daily transferred at 08:00 a.m. to tanks containing fresh water (control groups) or fresh drug solutions (treated groups). Tanks were not connected to a recirculation system.
UCS protocol
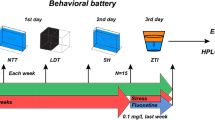
The experimental design is shown in Fig. 1. The animals were divided into control (non-stressed) and UCS groups. Within each group, the animals were divided into control (without treatment), bromazepam (0.5 mg/L), fluoxetine (0.01 mg/L), or nortriptyline (0.01 mg/L). The UCS protocol followed our previous study with slight adaptations (Piato et al. 2011; Zimmermann et al. 2015). Stressors were presented randomly twice a day during 7 days (day 0 to day 7) to avoid habituation. The stressors used were (i) heating tank water up to 33 °C (30 min); (ii) social isolation (45 min); (iii) cooling tank water to 23 °C (30 min); (iv) crowding of 10 animals in a 250-mL beaker (50 min); (v) low water level on housing tanks until dorsal body wall was exposed (2 min); (vi) tank change, three consecutive times with 30-min interval; and (vii) chasing with a net (8 min). All stressors were applied between 08:00 a.m. and 06:00 p.m. The non-stressed group was left undisturbed throughout the experiments. Separate sets of fish were used for the behavioral, cortisol, and molecular analyses. Samples were collected from animals that were not behaviorally tested in order for gene expression and cortisol assessments to reflect the status of the animals 24 h after the last stressor and to avoid possible interference resulting from the acute exposure to the novel tank test apparatus.
Experimental design. Fish were submitted to a 7-day UCS protocol or remained undisturbed. Throughout the UCS protocol, they were exposed to bromazepam (BMZ), fluoxetine (FLU), nortriptyline (NOR), or normal tank water (control). Twenty-four hours after the last stressor, different sets of fish were submitted to behavioral test or euthanized for the biochemical and molecular analysis
Novel tank test
On day 8, all animals were individually submitted to the novel tank test (Levin et al. 2007; Egan et al. 2009) between 08:00 and 11:00 a.m. Briefly, animals were placed for 6 min in 24 × 8 × 20-cm (length × width × height) tanks with 15 cm of water level. The water in the test apparatus was changed for each animal. The tanks were virtually divided into three equal horizontal sections (bottom, middle, and upper zones). Behavioral tests were video recorded and analyzed with the ANY-Maze tracking software (Stoelting Co., USA). The following parameters were quantified: total distance traveled, time spent in the upper zone, and number of transitions to the upper zone. Total distance moved was used as an indicative of overall locomotor activity. Time spent in the upper zone and transitions to the upper zone are distinct measures of similar and generally related phenomena, and correspond in rodents to the time spent and number of entries in the center of the open-field arena. A decrease in any of these parameters is thus mainly used as a proxy for anxiety behavior. Researchers blinded to experimental groups conducted all behavioral tests and analysis.
Cortisol measurement
The extraction and quantification of whole-body cortisol was based on previously described methodology (Piato et al. 2011) using a commercially available enzyme-linked immunosorbent assay kit (EIAgen™ CORTISOL test, BioChem ImmunoSystems). Briefly, 24 h after the last stressor, fish were gently captured and immediately frozen in liquid nitrogen, followed by storage at −20 °C until cortisol extraction. In order to prevent a possible stress response induced by manipulation, the time elapsed between capture and killing was less than 10 s. Each zebrafish was weighed, and a pool of two fish was minced and placed into a disposable stomacher bag with 2 mL of phosphate buffered saline (PBS, pH 7.4) for 6 min. Ethyl ether was added and samples were centrifuged for 10 min at 3000 rpm, then immediately frozen in liquid nitrogen. The unfrozen portion (ethyl ether containing cortisol) was decanted and the ethyl ether was transferred to a new tube and completely evaporated under a gentle stream of nitrogen for 2 h, yielding a lipid extract containing the cortisol. The extract was stored at −20 °C until the ELISA was conducted on the samples suspended with 1 mL of PBS buffer.
Gene expression
The gene expression of cox-2 (cyclooxygenase 2), tnf-α (tumor necrosis factor alpha), il-6 (interleukin 6), and il-10 (interleukin 10) was determined by a quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR) assay. 24 h after the last stressor, fish were cryoanesthetized, euthanized by decapitation, and brains were dissected out. Three independent assays for each group were performed, and a pool of five whole zebrafish brains was used for each independent experiment. The total RNA was isolated with Trizol® reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. RNA concentration and purity were measured by NanoDrop Spectrophotometer (Thermo Fisher Scientific) and then treated with Deoxyribonuclease I, Amplification Grade (Invitrogen) to prevent trace amounts of genomic DNA contamination in accordance with the manufacturer’s instructions. The cDNA was synthesized with ImProm-II™ Reverse Transcription System (Promega) from 1 μg total RNA. Quantitative PCR was performed using SYBR® Green I (Invitrogen) to detect double-strand cDNA synthesis. Reactions were done in a volume of 25 μL using 12.5 μL of diluted cDNA (cDNAs were replaced by ultrapure water in the negative controls), containing a final concentration of 0.2× SYBR® Green I (Invitrogen), 100 μM dNTP, 1× PCR Buffer, 3 mM MgCl2 0.25 U Platinum® Taq DNA Polymerase (Invitrogen), 0.5 M of betaine (for il-10), 2 % of the reaction of DMSO (for il-6, tnf-α), and 200 nM of each reverse and forward primers (Table 1). The PCR cycling conditions were an initial polymerase activation step for 5 min at 95 °C, 40 cycles of 15 s at 95 °C for denaturation, 35 s at 60 °C for annealing, and 15 s at 72 °C for elongation. At the end of cycling protocol, a melting-curve analysis was included and fluorescence measured from 60 to 99 °C and showed in all cases one single peak. Each sample was analyzed in four technical replicates, and mean Cq values were used for further analysis. ef1α and β-actin were used as reference genes for normalization. Relative mRNA expression levels were determined with 7500 Real-Time Systems Software v.2.0.6 (Applied Biosystems). The efficiency per sample was calculated using LinRegPCR 2012.3 Software (http://LinRegPCR.nl) and the stability of the reference gene, and the optimal number of reference genes according to the pairwise variation (V) was analyzed by GeNorm 3.5 Software (http://medgen.ugent.be/genorm/). Relative mRNA expression levels were determined using the 2-ΔΔCq method (Bustin et al. 2013).
Statistical analysis
The normal distribution of the data was confirmed by Kolmogorov-Smirnov and Levene tests. Results were analyzed by two-way ANOVA (stress and treatment as independent factors) followed by Newman-Keuls post hoc test. Differences were considered significant at p < 0.05. The data are expressed as mean + standard error of mean (SEM). SPSS 20.0 for Windows was used to run the analyses.
Results
Table 2 summarizes the main statistical data. Figure 2 shows the effects of BMZ (0.5 mg/L), FLU (0.01 mg/L), and NOR (0.01 mg/L) on behavioral parameters in zebrafish submitted to UCS. The UCS protocol did not alter the total distance moved (Fig. 2a), while it decreased time spent (Fig. 2b) and number of entries in the upper zone of the tank (Fig. 2c). Treatment with BMZ, FLU, or NOR did not induce general motor alterations as indicated by total distance traveled (Fig. 2a). BMZ increased time spent and entries in the upper zone, but only in stressed fish, and the antidepressants FLU and NOR prevented the effects of UCS in these parameters (Fig. 2b, c). Treatment with FLU and NOR in non-stressed animals led to a decrease in the number of transitions to the upper zone of the tank (Fig. 2c).
Effects of bromazepam (BMZ), fluoxetine (FLU), and nortriptyline (NOR) on behavioral parameters (novel tank test) in zebrafish submitted to unpredictable chronic stress (S+) or not (S−). Data are expressed as mean + standard error of mean (SEM). n = 13–18. Two-way ANOVA followed by Newman-Keuls post hoc test
Figure 3 shows that UCS increased cortisol levels; BMZ, FLU, and NOR prevented this effect and were devoid of effects in non-stressed animals. Figure 4 shows the results of gene expression analyses. The UCS protocol increased cox-2 and il-6 levels, which was prevented by all the drugs tested (Fig. 4a, c). Treatment with NOR increased tnf-α and il-6 in non-stressed animals (Fig. 4b, c) and increased il-10 in both stressed and non-stressed animals (Fig. 4d).
Effects of bromazepam (BMZ), fluoxetine (FLU), and nortriptyline (NOR) on brain gene expression in zebrafish submitted to unpredictable chronic stress (S+) or not (S−). a cyclooxygenase-2 (cox-2), b tumor necrosis factor alpha (tnf-α), c interleukin 6 (il-6), and d interleukin 10 (il-10). Data are expressed as mean + standard error of mean (SEM) of three independent experiments (n = 3). Two-way ANOVA followed by Newman-Keuls post hoc test
Discussion
In this study, we confirmed previous data showing that unpredictable chronic stress induces behavioral and neuroendocrine alterations in zebrafish, and we show for the first time that our model predicted the anti-stress activity of both anxiolytic and antidepressant drugs. The behavioral analysis was refined using an automated tracking system, since in our previous report we evaluated behavior through manual observation. Furthermore, we extended the molecular characterization of the model, revealing UCS-induced gene expression changes in the pro-inflammatory markers cox-2 and il-6, which were also prevented by the drugs tested.
Interestingly, while FLU and NOR exposure in stressed animals restored swimming in the upper zone of the tank to control levels, BMZ further increased this parameter in stressed animals, thus decreasing basal anxiety levels only when in conjunction with the stress protocol. Although FLU and NOR per se decreased the number of transitions to the upper zone, time spent in this zone was not significantly altered by these drugs; cortisol levels, which are highly correlated with anxiety-like behavior in zebrafish models (Egan et al. 2009; Herculano and Maximino 2014), were also not altered by FLU and NOR per se. Together, these data indicate that the behavioral effects of chronic treatment with psychotropic drugs may differ depending on the stress condition. Another factor that has been shown to determine whether anxiogenic or anxiolytic effects are induced by antidepressants in zebrafish and rodents is treatment duration (Borsini et al. 2002; Herculano and Maximino 2014; Gray and Hughes 2015). This is in agreement with the fact that monoaminergic pathways, especially serotonergic, are implicated in anxiety circuits (Tovote et al. 2015), and the effects of reuptake inhibitors involve changes in synaptic plasticity that develop gradually over long-term use (Santarelli et al. 2003; Djavadian 2004; Martinowich and Lu 2007). However, regarding drug effects only in stressed animals, we observe that all the compounds we tested had the same preventive effects, i.e., all three drugs were effective in counteracting stress-induced anxiety behavior.
The effects of chronic stress on the hypothalamus–pituitary–interrenal (HPI) axis have already been studied in zebrafish (Piato et al. 2011; Manuel et al. 2014; Pavlidis et al. 2015). Now, we show that BMZ, FLU, and NOR completely prevented the 3-fold increase in cortisol levels induced by the UCS protocol. This is in agreement with previous work that explored the effects of benzodiazepines and antidepressants on this neuroendocrine parameter in rodents (Song et al. 2006; Banasr et al. 2007; Zhao et al. 2012) and humans (Pomara et al. 2005; Piwowarska et al. 2012).
Substantial evidence supports a role for the immune system in the pathogenesis of depression, and the relationship between stress and neuroinflammation has been established in both clinical and preclinical studies (Dowlati et al. 2010; Maes et al. 2011; Leonard and Maes 2012). This led to the cytokine hypothesis of depression, which postulates that the activation of the peripheral immune system, with release of pro-inflammatory cytokines, is involved in the neuroendocrine and neurochemical changes associated with the disease (Schiepers et al. 2005; Maes 2011). Indeed, immune activation caused by injection of cytokines, lipopolysaccharide (LPS), or interferon-α (IFN-α) induces mood and cognitive impairments in humans and in animal models (Reichenberg et al. 2001; Dantzer 2006). Furthermore, the effects of cytokines on microglia activity may affect the maintenance of neuronal and synaptic health (Hanisch and Kettenmann 2007).
Here, we show that UCS increased gene expression of the pro-inflammatory markers IL-6 and COX-2, without altering TNF-α or IL-10. The lack of activation of IL-10 expression suggests that increase expression of pro-inflammatory cytokines is unopposed, so the net effect is of increased immune activation in stressed animals. While COX-2 is an enzyme induced by cytokines (Kubera et al. 2011), it also influences cytokine expression, since the main product of COX-2, prostaglandin E2 (PGE2), stimulates the production of IL-6 (Hinson et al. 1996; Müller et al. 2009). PGE2 concentration is also increased in depressed patients and decreases following antidepressant treatment (Calabrese et al. 1986). Anti-inflammatory drugs that inhibit COX-2 activity are being investigated as a possible treatment for depression, but the results obtained so far are mixed (Eyre et al. 2015).
We also observed that all the drugs tested in this study prevented the UCS-induced increase in brain inflammatory markers, which is in agreement with studies using in vivo LPS or IFN-α rat models (Castanon et al. 2004; Myint et al. 2007; O’Sullivan et al. 2009). Antidepressants have also been shown to modulate cytokine levels in rodent models of chronic stress and in humans (Kubera et al. 2011; Hannestad et al. 2011). While all drugs were equally effective in preventing the inflammatory effects of stress, NOR also had induced robust effects by itself. This tricyclic antidepressant increased transcript levels of all the three cytokines in non-stressed animals, and il-10 also in stressed ones. Similar effects have already been reported for imipramine, an antidepressant chemically related to NOR (Kubera et al. 2004). Considering that different psychotropic drugs have been shown to influence NF-κB signaling at different levels of the pathway (Troib and Azab 2015), it is possible that the non-specific increase of cytokine expression induced by NOR is due to modulation of this transcription factor that regulates cytokine production. Antidepressants may influence immune responses through other pathways as well, such as macrophage activation (Nazimek et al. 2016b; Nazimek et al. 2016a). Although the overall results are mixed and the exact mechanisms remain to be elucidated (Bartholomä et al. 2002; Troib and Azab 2015), clinical observations have led researchers to speculate that the therapeutic activity of antidepressant drugs may be associated with their effects on cytokine production (Kenis and Maes 2002; Hannestad et al. 2011; Leonard 2014).
We have previously demonstrated that UCS increases both cortisol and CRF expression levels, while it decreases GR expression (Piato et al. 2011). A body of work provides evidence that stress-induced GR reduction may be mediated by inflammatory cytokines such as IL-6 (Pace et al. 2007). Although we did not measure GR in this study, we can infer that the UCS-induced decrease in GR expression may be related to the observed increase in il-6.
Following our first report on the effects of this UCS protocol in zebrafish (Piato et al. 2011), several other groups have investigated different outcomes induced by this model. Stressed zebrafish showed anxiety-like behavior, impaired neurogenesis, and mitochondrial toxicity (Chakravarty et al. 2013). Moreover, chronic stress protocols increased pro-opiomelanocortin, mineralocorticoid receptors, prolactin, brain-derived neurotrophic factor, hypocretin/orexin, and c-fos expression (Manuel et al. 2014; Pavlidis et al. 2015). This work further extends the molecular characterization of the UCS model to include increased brain expression of cox-2 and il-6, and is the first report on the effects of psychotropic drugs counteracting chronic stress in zebrafish, in agreement with rodent models (Muscat et al. 1992; Willner 2005; Zhao et al. 2012). A full pharmacological validation is yet to be achieved; however, this is an important first step to better characterize the predictive validity of the model.
There are several possible variations in the experimental design to investigate drug effects in zebrafish submitted to UCS. Since the 7-day protocol induces the same behavioral and endocrine changes observed after 14 days of UCS (Piato et al. 2011) and is significantly less time- and space-consuming, we chose the shorter protocol as the starting path to look at drug effects in this model. We then chose to apply the drugs from the beginning of UCS because antidepressant drugs require chronic administration before people can experience its positive effects. Further studies with decreased treatment duration may be carried out to determine the minimum length of treatment required for each drug to revert the effects of stress.
A limitation of our study is that we cannot, at this point, determine whether the 7-day UCS protocol in zebrafish more closely models anxiety or depression, since the behavioral endpoints employed here are sensitive to the administration of both anxiolytic and antidepressant drugs. Further studies and the use of other behavioral measures are necessary to better dissect this issue, though most patients suffering from mood disorders present with both depression and anxiety symptoms (Belzer and Schneier 2004; Kessler et al. 2005; Beesdo et al. 2010). Important next steps also include evaluating different treatment schedules in zebrafish submitted to a more prolonged period of UCS. The behavioral tests should also be extended to include long-term memory testing, for example, in order to elucidate whether these drugs can also prevent the cognitive deficits induced by UCS. Phenotypes more specific to depressive disorders, such as anhedonia, should also be addressed through tasks that involve reward processing. Although in this study we assessed the gene expression of inflammatory markers, investigating whether the findings would be similar for the protein content is also important and a goal for future studies.
Since small hydrophilic molecules are readily taken up through the fish gills or skin, we took advantage of this fact and used water immersion for drug delivery. However, a drawback of this method is the possibility that the drug being administered may influence gill physiology and thus its own absorption. This is another limitation of our study, although we did not observe any visual or behavioral signs of irritation to the fish gills in the treated groups, and no such effects have been reported in the literature for the drugs we tested.
Zebrafish is an adequate model organism to study the effects of stress on behavioral and physiological parameters, and further studies are warranted. Moreover, the UCS protocol may serve as a screening tool for evaluating new drugs that can be used to combat stress. Future studies are necessary to further develop the UCS protocol in zebrafish as a model to study the neurobiological mechanisms involved in several psychiatric disorders with stress-related etiologies.
References
Banasr M, Valentine GW, Li X-Y, et al. (2007) Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry 62:496–504. doi:10.1016/j.biopsych.2007.02.006
Bartholomä P, Erlandsson N, Kaufmann K, et al. (2002) Neuronal cell death induced by antidepressants: lack of correlation with Egr-1, NF-kappa B and extracellular signal-regulated protein kinase activation. Biochem Pharmacol 63:1507–1516
Beesdo K, Pine DS, Lieb R, Wittchen H-U (2010) Incidence and risk patterns of anxiety and depressive disorders and categorization of generalized anxiety disorder. Arch Gen Psychiatry 67:47–57. doi:10.1001/archgenpsychiatry.2009.177
Belzer K, Schneier FR (2004) Comorbidity of anxiety and depressive disorders: issues in conceptualization, assessment, and treatment. J Psychiatr Pract 10:296–306
Borsini F, Podhorna J, Marazziti D (2002) Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology 163:121–141. doi:10.1007/s00213-002-1155-6
Bustin SA, Benes V, Garson J, et al. (2013) The need for transparency and good practices in the qPCR literature. Nat Methods 10:1063–1067. doi:10.1038/nmeth.2697
Calabrese JR, Skwerer RG, Barna B, et al. (1986) Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res 17:41–47
Castanon N, Médina C, Mormède C, Dantzer R (2004) Chronic administration of tianeptine balances lipopolysaccharide-induced expression of cytokines in the spleen and hypothalamus of rats. Psychoneuroendocrinology 29:778–790. doi:10.1016/S0306-4530(03)00142-2
Chakravarty S, Reddy BR, Sudhakar SR, et al. (2013) Chronic unpredictable stress (CUS)-induced anxiety and related mood disorders in a zebrafish model: altered brain proteome profile implicates mitochondrial dysfunction. PLoS One 8:e63302. doi:10.1371/journal.pone.0063302
Crowley SK, Girdler SS (2014) Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: what is the current state of knowledge in humans? Psychopharmacology 231:3619–3634. doi:10.1007/s00213-014-3572-8
D’Aquila PS, Brain P, Willner P (1994) Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav 56:861–867
Dantzer R (2006) Cytokine, sickness behavior, and depression. Neurol Clin 24:441–460. doi:10.1016/j.ncl.2006.03.003
Dantzer R, Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21:153–160. doi:10.1016/j.bbi.2006.09.006
Djavadian RL (2004) Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol Exp (Warsz) 64:189–200
Dowlati Y, Herrmann N, Swardfager W, et al. (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. doi:10.1016/j.biopsych.2009.09.033
Egan RJ, Bergner CL, Hart PC, et al. (2009) Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res 205:38–44. doi:10.1016/j.bbr.2009.06.022
Eyre HA, Air T, Proctor S, et al. (2015) A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuro-Psychopharmacol Biol Psychiatry 57:11–16. doi:10.1016/j.pnpbp.2014.10.003
Faikoh EN, Hong Y-H, S-Y H (2014) Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio Vulnificus and Streptococcus agalactiae. Fish Shellfish Immunol 38:15–24. doi:10.1016/j.fsi.2014.02.024
Gałecki P, Talarowska M, Bobińska K, Szemraj J (2014) COX-2 gene expression is correlated with cognitive function in recurrent depressive disorder. Psychiatry Res 215:488–490. doi:10.1016/j.psychres.2013.12.017
Global Burden of Disease Study 2013 Collaborators (2015) Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. doi:10.1016/S0140-6736(15)60692-4
Gray VC, Hughes RN (2015) Drug-, dose- and sex-dependent effects of chronic fluoxetine, reboxetine and venlafaxine on open-field behavior and spatial memory in rats. Behav Brain Res 281:43–54. doi:10.1016/j.bbr.2014.12.023
Hanisch U-K, Kettenmann H (2007) Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci 10:1387–1394. doi:10.1038/nn1997
Hannestad J, DellaGioia N, Bloch M (2011) The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 36:2452–2459. doi:10.1038/npp.2011.132
Haroon E, Raison CL, Miller AH (2012) Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 37:137–162. doi:10.1038/npp.2011.205
Herculano AM, Maximino C (2014) Serotonergic modulation of zebrafish behavior: towards a paradox. Prog Neuro-Psychopharmacol Biol Psychiatry 55:50–66. doi:10.1016/j.pnpbp.2014.03.008
Hinson RM, Williams JA, Shacter E (1996) Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2. Proc Natl Acad Sci U S A 93:4885–4890
Hou R, Baldwin DS (2012) A neuroimmunological perspective on anxiety disorders. Hum Psychopharmacol 27:6–14. doi:10.1002/hup.1259
Kenis G, Maes M (2002) Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP 5:401–412. doi:10.1017/S1461145702003164
Kessler RC, Chiu WT, Demler O, et al. (2005) Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627. doi:10.1001/archpsyc.62.6.617
Kubera M, Kenis G, Bosmans E, et al. (2004) Stimulatory effect of antidepressants on the production of IL-6. Int Immunopharmacol 4:185–192. doi:10.1016/j.intimp.2003.11.006
Kubera M, Obuchowicz E, Goehler L, et al. (2011) In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35:744–759. doi:10.1016/j.pnpbp.2010.08.026
Leite CE, Maboni Lde O, Cruz FF, Rosemberg DB, Zimmermann FF, Pereira TC, Bogo MR, Bonan CD, Campos MM, Morrone FB, Battastini AM (2013) Involvement of purinergic system in inflammation and toxicity induced by copper in zebrafish larvae. Toxicol Appl Pharmacol 272(3):681–689. doi:10.1016/j.taap.2013.08.001
Leonard BE (2014) Impact of inflammation on neurotransmitter changes in major depression: an insight into the action of antidepressants. Prog Neuro-Psychopharmacol Biol Psychiatry 48:261–267. doi:10.1016/j.pnpbp.2013.10.018
Leonard B, Maes M (2012) Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 36:764–785. doi:10.1016/j.neubiorev.2011.12.005
Levin ED, Bencan Z, Cerutti DT (2007) Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90:54–58. doi:10.1016/j.physbeh.2006.08.026
Li R, Zhao D, Qu R, et al. (2015) The effects of apigenin on lipopolysaccharide-induced depressive-like behavior in mice. Neurosci Lett 594:17–22. doi:10.1016/j.neulet.2015.03.040
Maes M (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuro-Psychopharmacol Biol Psychiatry 35:664–675. doi:10.1016/j.pnpbp.2010.06.014
Maes M, Kubera M, Obuchowiczwa E, et al. (2011) Depression’s multiple comorbidities explained by (neuro) inflammatory and oxidative & nitrosative stress pathways. Neuro Endocrinol Lett 32:7–24
Manuel R, Gorissen M, Zethof J, et al. (2014) Unpredictable chronic stress decreases inhibitory avoidance learning in Tuebingen long-fin zebrafish: stronger effects in the resting phase than in the active phase. J Exp Biol 217:3919–3928. doi:10.1242/jeb.109736
Martinowich K, Lu B (2007) Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 33:73–83. doi:10.1038/sj.npp.1301571
McEwen BS (2007) Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904. doi:10.1152/physrev.00041.2006
McEwen BS, Brinton RE, Sapolsky RM (1988) Glucocorticoid receptors and behavior: implications for the stress response. Adv Exp Med Biol 245:35–45
McEwen BS, Bowles NP, Gray JD, et al. (2015) Mechanisms of stress in the brain. Nat Neurosci 18:1353–1363. doi:10.1038/nn.4086
Miller AH, Maletic V, Raison CL (2009) Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry 65:732–741. doi:10.1016/j.biopsych.2008.11.029
Mineur YS, Belzung C, Crusio WE (2006) Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res 175:43–50. doi:10.1016/j.bbr.2006.07.029
Müller N, Myint A-M, Schwarz MJ (2009) The impact of neuroimmune dysregulation on neuroprotection and neurotoxicity in psychiatric disorders—relation to drug treatment. Dialogues Clin Neurosci 11:319–332
Muscat R, Papp M, Willner P (1992) Reversal of stress-induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacology 109:433–438
Myint AM, O’Mahony S, Kubera M, et al. (2007) Role of paroxetine in interferon-alpha-induced immune and behavioural changes in male Wistar rats. J Psychopharmacol Oxf Engl 21:843–850. doi:10.1177/0269881107077165
Nazimek K, Kozlowski M, Bryniarski P, et al. (2016a) Repeatedly administered antidepressant drugs modulate humoral and cellular immune response in mice through action on macrophages. Exp Biol Med Maywood NJ 241:1540–1550. doi:10.1177/1535370216643769
Nazimek K, Strobel S, Bryniarski P, et al. (2016b) The role of macrophages in anti-inflammatory activity of antidepressant drugs. Immunobiology. doi:10.1016/j.imbio.2016.07.001
O’Sullivan JB, Ryan KM, Curtin NM, et al. (2009) Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP 12:687–699. doi:10.1017/S146114570800967X
Pace TWW, Hu F, Miller AH (2007) Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 21:9–19. doi:10.1016/j.bbi.2006.08.009
Pavlidis M, Theodoridi A, Tsalafouta A (2015) Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio. Prog Neuro-Psychopharmacol Biol Psychiatry 60:121–131. doi:10.1016/j.pnpbp.2015.02.014
Piato ÂL, Capiotti KM, Tamborski AR, et al. (2011) Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog Neuro-Psychopharmacol Biol Psychiatry 35:561–567. doi:10.1016/j.pnpbp.2010.12.018
Piwowarska J, Chimiak A, Matsumoto H, et al. (2012) Serum cortisol concentration in patients with major depression after treatment with fluoxetine. Psychiatry Res 198:407–411. doi:10.1016/j.psychres.2012.01.029
Pomara N, Willoughby LM, Sidtis JJ, et al. (2005) Cortisol response to diazepam: its relationship to age, dose, duration of treatment, and presence of generalized anxiety disorder. Psychopharmacology 178:1–8. doi:10.1007/s00213-004-1974-8
Reichenberg A, Yirmiya R, Schuld A, et al. (2001) Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58:445–452
Santarelli L, Saxe M, Gross C, et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301:805–809. doi:10.1126/science.1083328
Sapolsky RM (1982) The endocrine stress-response and social status in the wild baboon. Horm Behav 16:279–292
Sapolsky RM, Krey LC, McEwen BS (1984) Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology 114:287–292. doi:10.1210/endo-114-1-287
Schaefer IC, Siebel AM, Piato AL, et al. (2015) The side-by-side exploratory test: a simple automated protocol for the evaluation of adult zebrafish behavior simultaneously with social interaction. Behav Pharmacol 26:691–696. doi:10.1097/FBP.0000000000000145
Schiepers OJG, Wichers MC, Maes M (2005) Cytokines and major depression. Prog Neuro-Psychopharmacol Biol Psychiatry 29:201–217. doi:10.1016/j.pnpbp.2004.11.003
Selye H (1936) A syndrome produced by diverse nocuous agents. Nature 138:32
Song C, Horrobin DF, Leonard BE (2006) The comparison of changes in behavior, neurochemistry, endocrine, and immune functions after different routes, doses and durations of administrations of IL-1beta in rats. Pharmacopsychiatry 39:88–99. doi:10.1055/s-2006-941557
Tang R, Dodd A, Lai D, et al. (2007) Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim Biophys Sin 39:384–390
Tovote P, Fadok JP, Lüthi A (2015) Neuronal circuits for fear and anxiety. Nat Rev Neurosci 16:317–331. doi:10.1038/nrn3945
Troib A, Azab AN (2015) Effects of psychotropic drugs on nuclear factor kappa B. Eur Rev Med Pharmacol Sci 19:1198–1208
Varela M, Dios S, Novoa B, Figueras A (2012) Characterisation, expression and ontogeny of interleukin-6 and its receptors in zebrafish (Danio rerio. Dev Comp Immunol 37:97–106. doi:10.1016/j.dci.2011.11.004
Wang J-M, Yang L-H, Zhang Y-Y, et al. (2015) BDNF and COX-2 participate in anti-depressive mechanisms of catalpol in rats undergoing chronic unpredictable mild stress. Physiol Behav 151:360–368. doi:10.1016/j.physbeh.2015.08.008
Willner P (1997) Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology 134:319–329
Willner P (2005) Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52:90–110. doi:10.1159/000087097
Willner P, Muscat R, Papp M (1992) Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16:525–534
Yalcin I, Belzung C, Surget A (2008) Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res 193:140–143. doi:10.1016/j.bbr.2008.04.021
Zhao Y, Wang Z, Dai J, et al. (2012) Beneficial effects of benzodiazepine diazepam on chronic stress-induced impairment of hippocampal structural plasticity and depression-like behavior in mice. Behav Brain Res 228:339–350. doi:10.1016/j.bbr.2011.12.013
Zimmermann FF, Altenhofen S, Kist LW, et al. (2015) Unpredictable chronic stress alters adenosine metabolism in zebrafish brain. Mol Neurobiol. doi:10.1007/s12035-015-9270-7
Acknowledgments
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico-Brazil (CNPq, Proc. 472715/2012-7). L.W.K. is the recipient of fellowship CAPES/PNPD Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Matheus Marcon and Ana P. Herrmann contributed equally for this article.
Rights and permissions
About this article
Cite this article
Marcon, M., Herrmann, A.P., Mocelin, R. et al. Prevention of unpredictable chronic stress-related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology 233, 3815–3824 (2016). https://doi.org/10.1007/s00213-016-4408-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4408-5