Abstract
Ketamine is emerging as a new hope against depression, but ketamine-associated psychotomimetic effects limit its clinical use. An adjunct therapy along with ketamine to alleviate its adverse effects and even potentiate the antidepressant effects might be an alternative strategy. Betaine, a methyl derivative of glycine and a dietary supplement, has been shown to have antidepressant-like effects and to act like a partial agonist at the glycine site of N-methyl-D-aspartate receptors (NMDARs). Accordingly, betaine might have potential to be an adjunct to ketamine treatment for depression. The antidepressant-like effects of ketamine and betaine were evaluated by forced swimming test and novelty suppressed feeding test in mice. Both betaine and ketamine produced antidepressant-like effects. Furthermore, we determined the effects of betaine on ketamine-induced antidepressant-like and psychotomimetic behaviors, motor incoordination, hyperlocomotor activity, and anesthesia. The antidepressant-like responses to betaine combined with ketamine were stronger than their individual effects. In contrast, ketamine-induced impairments in prepulse inhibition, novel object recognition test, social interaction, and rotarod test were remarkably attenuated, whereas ketamine-induced hyperlocomotion and loss of righting reflex were not affected by betaine. These findings revealed that betaine could enhance the antidepressant-like effects, yet block the psychotomimetic effects of ketamine, suggesting that betaine can be considered as an add-on therapy to ketamine for treatment-resistant depression and suitable for the treatment of depressive symptoms in patients with schizophrenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ketamine is clinically used for analgesia, sedation, and anesthetic induction. Recent clinical studies have shown that low-dose ketamine produces a rapid-acting and sustained antidepressant effect in major depressive disorder (Murrough et al. 2013; Wan et al. 2014), in bipolar depression (Ionescu et al. 2015; Nugent et al. 2014; Rybakowski et al. 2013), and in depression with suicidal ideation (Aligeti et al. 2014; Thakurta et al. 2012; Zigman and Blier 2013).
Despite ketamine can induce a rapid onset of antidepressant effect, the adverse mental status associated with ketamine use including psychosis, dissociative, hallucinogenic, and amnesic effects (Krystal et al. 1994; Perry et al. 2007) leads to discontinuation. Accordingly, research attempts have been focusing on developing new compounds with more specific rapid-acting antidepressant treatments but free of ketamine’s adverse effects (Browne and Lucki 2013; Burgdorf et al. 2013). Alternatively, an adjunct treatment which can promote the therapeutic efficacy and concomitantly avoid the adverse effects of ketamine has also been considered (Chiu et al. 2015; Ibrahim et al. 2012).
The mechanisms underlying the antidepressant- and psychosis-inducing effects of ketamine have been suggested to be associated with blockade of NMDARs. Several drugs that effectively either block or antagonize NMDAR activity, such as the competitive NMDAR antagonists CGP 37849 and CGP 40116 (Papp and Moryl 1994), the noncompetitive, nonsubunit selective NMDAR antagonist MK-801 (Autry et al. 2011; Lima-Ojeda et al. 2013; Maeng et al. 2008), and the NR2B selective antagonist RO-25-6981 (Lima-Ojeda et al. 2013; Maeng et al. 2008), have repeatedly and consistently been shown to have antidepressant-like properties in rodent models. NMDAR blockade is also the main contributor of the psychotomimetic effects of ketamine, as most NMDAR antagonist compounds have the same properties (Nakao et al. 2003; Neymotin et al. 2011; Thomson et al. 1985; Vollenweider and Geyer 2001). On the other hand, enhancement of NMDAR function, via activation of glycine binding site or modulation of metabotropic glutamate receptors, represents a promising approach to reverse psychotomimetic effects of ketamine (Chan et al. 2008; Krystal et al. 2005; Roberts et al. 2010; Yang et al. 2010) or other NMDAR antagonists (Kanahara et al. 2008; Kawaura et al. 2015; Lipina et al. 2005; Santini et al. 2014). Therefore, NMDAR glycine binding site partial agonists, being developed with the goal of achieving the antidepressant efficacy and rapid onset seen with ketamine, but without their limiting side effects, might also have the capability of being agonists to ameliorate the manifestations of NMDAR hypofunction state, such as ketamine-induced psychotic effects and addiction.
Betaine, a methyl glycine derivative, is an important unit in one-carbon metabolism and a commonly used nutrient supplement. In addition to being a methyl donor, betaine has been shown to attenuate glutamate-induced neurotoxicity in primary cultured brain cells (Park et al. 1994). Based on the structural similarity to glycine, we have examined if betaine can affect the NMDAR function and demonstrated that betaine acts like a NMDAR glycine binding site partial agonist (Lee and Chen 2014). Furthermore, betaine exhibits antidepressant-like effects in rats (Kim et al. 2013). Accordingly, it is hypothesized that betaine can promote the antidepressant-like but antagonize the psychotomimetic effect of ketamine.
The present study used mouse models to evaluate the effects of betaine on antidepressant-like and psychosis-like behaviors of ketamine to test the hypothesis. At first, the forced swimming test (FST), novelty suppressed feeding test (NSF), and emergence test were used to compare the depression-like and anxiolytic properties of betaine with ketamine. Thereafter, the combined effects of betaine and ketamine on the forced swimming test were examined. Moreover, we determined whether betaine can abolish or attenuate the ketamine-induced psychotomimetic effects including prepulse inhibition (PPI) deficits in acoustic startle reflex, cognitive dysfunction in the novel object recognition test, and social withdrawal. Finally, the motor incoordination, hyperlocomotor activity, and sedative effects of ketamine were monitored by the rotarod test, open field, and loss of righting reflex, respectively.
These experiments support that betaine can be considered as an add-on treatment to reduce the discontinuation rates for patients who have a dramatic response to ketamine therapy.
Materials and methods
Animals and drugs
Male ICR mice (8 weeks, 30–45 g) were supplied from the BioLASCO Charles River Technology (Taiwan) and housed 4–5 per cage in a 12-h light/dark cycle with ad libitum access to water and food. All the experiments were in accordance with the Republic of China animal protection law (Chapter III: Scientific Application of Animals) and approved by the Review Committee of Tzu Chi University and the institutional animal care and use committees of National Health Research Institutes.
Ketamine and betaine monohydrate (Sigma Chemical Co., St. Louis, MO, USA) were dissolved in saline and intraperitoneally injected in volumes of 10 ml/kg. Low doses of ketamine (3, 10, and 15 mg/kg, i.p.) were chosen for the forced swimming test based on previous studies (Garcia et al. 2008). Ketamine (10 mg/kg, i.p.) was used in novelty suppressed feeding test (Li et al. 2010). Ketamine (30 mg/kg, i.p.) was applied in the open field test, rotarod performance, novel object recognition memory, social interaction, and prepulse inhibition (Chan et al. 2008). The anesthetic dose of ketamine (100 mg/kg i.p.) was used for loss of righting reflex.
Forced swimming test
In the pilot study, the dose-dependent effects of ketamine and betaine on FST were explored by a time-sampling method. Mice were placed in a Plexiglas cylinder (33.5 cm height, 20 cm diameter) filled with 25 ± 1 °C water to a height of 18–20 cm for 15 min (pretest session) followed by two subsequent tests 1 week apart. Twenty-four hours later (day 1 test session), mice were treated with various doses of betaine or ketamine 30 min prior to the test. The mice were placed in the water again for 6 min, the first 2 min has elapsed, and the observation was made every 5 s to score the presence of immobility, which was defined as a lack of motion of the whole body, when mice remained floating motionless in the water, making only those movements necessary to keep the head above water. The sustained effects were examined 8 days later (day 8 retest session). The mice were retested again without any pretreatment. After each test, animals were dried by towels and under a lamp for 30 min.
In order to determine the interaction between ketamine and betaine, the duration of immobility, struggling, and swimming was measured in another set of experiments. Struggling behavior consisted of upward directed movements of the forepaws along the side of the swim chamber. Swimming behavior was defined as movement (usually horizontal) throughout the swim chamber. Immobility was assigned when no additional activity was observed other than that required to keep the head above the water. The test sessions were videotaped and each session was stored as a video file. The mice were tested 1 and 7 days after the 15-min pretest session. Ketamine and/or betaine was administered 30 min prior to day 1 test session only.
Novelty suppressed feeding test
The NSF test was measured in 24-hr food-deprived mice. Betaine (10, 20, and 30 mg/kg), ketamine (10 mg/kg), or saline was administered 1 h prior to the test. At the time of testing, a single food pellet placed in the middle of a novel environment (a test box 40 × 40 × 40 cm). The latency to start feeding was recorded.
Emergence test
The emergence test was examined in a test box (35 × 35 × 30 cm) contained an aluminum cylinder (10 cm deep × 6.5 cm diameter) located lengthwise along one wall, with the open end 10 cm from the corner. Betaine (0, 30, and 100 mg/kg) or ketamine (10 mg/kg) was administered 30 min prior to the test. Mice were placed into the cylinder and tested for 10 min and scored three behavioral parameters: the latency to leave the cylinder, the number of entries into the cylinder, and the total time spent inside the cylinder.
Prepulse inhibition test
SR-LAB (San Diego Instruments, San Diego, CA, USA) acoustic startle chambers were used. SR-LAB software controlled the delivery of all stimuli to the animals and recorded the response. The animals were initially moved from the home cage, weighed, and then placed into the restrainers in the startle chambers for 30-min habituation.
Betaine (0, 30, and 100 mg/kg) was administered 30 min prior to the ketamine (30 mg/kg) or saline injection. After administration of ketamine or saline, the experiment started with a 5-min adaptation period during which the animals were exposed to 67-dB background white noise, and this background noise was continued throughout the session. Then, the following adaptation period startle session began with five initial startle stimuli (120 dB bursts of white noise, 40 ms duration). After the first five initial stimuli, mice received five different trial types: pulse alone trials (120 dB bursts of white noise, 40 ms duration); three prepulse and pulse trials in which 76, 81, or 86 dB white noise bursts (9, 14, and 19 dB above background) of 20 ms duration preceded 120 dB pulse by 100 ms prepulse onset to pulse onset; and no-stimuli trials during which only background noise was applied. Each of these trial types was presented five times in randomized order. The intertrial interval was 7–23 s, and the test lasted 15 min in total. Prepulse inhibition was calculated as the percent inhibition of the startle amplitude evoked by the pulse alone: % PPI = (magnitude on pulse alone trial − magnitude on prepulse + pulse trial / magnitude on pulse alone trial) × 100.
Novel object recognition test
The experimental apparatus consisted of a Plexiglas open field box (35 × 35 × 30 cm) located in a sound-attenuated room and illuminated with a 20-W light bulb. The novel object recognition procedure consisted of habituation, training, and retention sessions. Habituation was conducted in two consecutive daily sessions, during which each mouse was allowed to individually explore the box in the absence of objects for 20 min. The animal is then removed from the arena and placed in its home cage. During the training session, each animal was placed in the box, and after 5 min, two identical sample objects (A + A) were simultaneously introduced in two corners. Each animal was allowed to explore the objects for 5 min. An animal was considered to explore the object when its head was facing the object at a distance of approximately 1 cm or less between the head and object or when it was touching or sniffing the object. The time spent exploring each object was recorded using stopwatches by an experimenter blind to the treatment condition. After the training session, the mice were immediately returned to their home cages. The next day, the mice are allowed to explore the open field with one identical sample object and a novel object to assess the recognition memory (A + B). The animals were allowed to explore the box freely for 5 min, and the time spent exploring each object was recorded as described above. The objects and chambers were cleaned with 70 % ethanol after each use. The preference index in the retention session, defined as the ratio of the amount of time spent exploring the novel object and total time spent exploring both objects, was used to evaluate memory retention. In the training session, the preference index was defined as the ratio of the time spent exploring the object that replaced the original object in the retention session and the total exploration time. Betaine (0, 30, and 100 mg/kg) was administered 30 min prior to ketamine (30 mg/kg) or saline (vehicle) 20 min prior to the training session.
Social interaction test
This protocol was modified from the original social interaction test (Lin et al. 2010; Qiao et al. 2001). The social interaction between pairs of mice was examined in an open field box (35 × 35 × 30 cm) under normal room lighting. The paired mice were randomly assigned from different home cages with the same drug treatment. Betaine (0, 100, and 300 mg/kg) was administered 30 min prior to ketamine (30 mg/kg). Five minutes later, each pair of unfamiliar mice was placed in the apparatus for 10 min and the total time that a pair spent in social interaction was recorded by an observer who was blind to the drug treatments.
Rotarod test
Motor coordination was assessed by an automated rotarod device (Singa; Technology Co., Ltd, Taiwan) for a maximum of six mice. A computer recorded the latency to fall in seconds. During the training period, the mice were first trained on the rotarod at a constant speed of 20 rotations per minute (rpm) until all of the mice were able to spend at least 3 min on the rod. Betaine (0, 30, and 100 mg/kg) was administered 30 min prior to the ketamine (30 mg/kg) or saline injection. Subsequently, the mice were tested at 20 rpm 10 min after ketamine injection at 5 min intervals for 30 min.
Loss of righting reflex
Betaine (0, 300, and 600 mg/kg) was administered 30 min prior to the anesthetic doses of ketamine (100 mg/kg). Then, the mice were placed in a clean cage until the righting reflex was lost. They were then placed in the supine position until recovery and the onset and duration of the loss of righting reflex was recorded. Recovery of the righting reflex was defined as the ability to perform three successive rightings.
Open field test
The dose-dependent effect of betaine and the effect of betaine prior to ketamine on locomotor activity were assessed. The animals were habituated in the activity cages (Columbus Auto-Track System, Version 3.0 A, Columbus Institute, Columbus, OH, USA) for 2 h. Betaine (30 and/or 100 mg/kg) or saline was given 30 min prior to ketamine (30 mg/kg). The distance (cm) traveled was recorded for a total of 180 min.
Statistical analyses
All of the data are expressed as mean ± SEM. The data from the forced swimming test, rotarod test, the percentage of PPI, and novel object recognition test were analyzed by mixed-design ANOVAs with test session, time, prepulse intensity, and session as the within subject factor, respectively. Differences among experimental groups in the social interaction test, the duration of loss of righting reflex, and the total distance in locomotor activity test were analyzed by one-way ANOVA. Multiple comparisons were performed using the Fisher’s LSD test. p < 0.05 was considered statistically significant.
Results
Dose-dependent effects of ketamine and betaine on FST scored by the time-sampling method
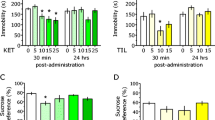
The acute and sustained effects of various doses of ketamine (3, 10, 15 mg/kg) and betaine (10, 20, 30 mg/kg) on FST were assessed on days 1 and 8, respectively (Fig. 1a, b). A mixed-design ANOVA on the count of immobility demonstrated significant main effects of ketamine (F 3, 28 = 13.295, p < 0.001) and betaine (F 3, 25 = 11.362, p < 0.001). There was no significant effect of test session or interaction. Post hoc comparisons showed that ketamine (3, 10, and 15 mg/kg) and betaine (20 and 30 mg/kg) significantly decreased the count of immobility.
Dose-dependent effects of ketamine and betaine on FST scored by time-sampling method. The acute and sustained effects of various doses of ketamine (3, 10, 15 mg/kg) and betaine (10, 20, 30 mg/kg) on FST were assessed on days 1 (a) and 8 (b), respectively. All values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the respective control
Dose-dependent effects of betaine on novelty suppressed feeding test
The effects of ketamine (10 mg/kg) and betaine (10, 20, 30 mg/kg) on NSF were examined (Fig. 2a). One-way ANOVA revealed a significant treatment effect (F 4, 35 = 5.3, p < 0.01). Post hoc comparisons demonstrated that betaine (30 mg/kg) and ketamine (10 mg/kg) significantly reduced the latency to feed in the NSF compared with saline-treated mice.
Effects of betaine and ketamine on the novelty suppressed feeding test and emergence test. The animals were food restricted for 24 h. Betaine (0, 10, 20, and 30 mg/kg, i.p.) or ketamine (10 mg/kg) was administered 1 h prior to the test. The latency to feed was measured in NSF (a). The latency to leave the cylinder (b), the number of entries into the cylinder (c), and the total time spent inside the cylinder (d) were measured in the emergency test. All values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 compared with the saline group
Dose-dependent effects of betaine on the emergence test
The effects of ketamine (10 mg/kg) and betaine (30 and 100 mg/kg) on the emergence test were examined (Fig. 2b–d). One-way ANOVA revealed that there was a significant difference in the total time spent inside the cylinder (F 3, 30 = 4.079, p < 0.05), but not in the latency to leave the cylinder (F 3, 30 = 0.262, p = 0.852) and the number of entries into the cylinder (F 3, 30 = 1.592, p = 0.212). Post hoc tests indicated that only ketamine significantly reduced the total time spent inside the cylinder.
Effects of ketamine and betaine on the duration of immobility, struggling, and swimming in FST
This experiment included a control group and various doses of ketamine (3, 10, 15 mg/kg), betaine (10, 20, 30 mg/kg), and betaine (10, 20, 30 mg/kg) pretreatment prior to ketamine (fixed dose at 10 mg/kg). The duration of immobility, struggling, and swimming is shown in Fig. 3. A mixed-design ANOVA revealed that there was a significant main effect of treatment on the duration of immobility (F 9, 75 = 5.42, p < 0.001). There was no significant effect of test session or interaction. All pairwise multiple comparisons indicated that ketamine (10 and 15 mg/kg), betaine (20 and 30 mg/kg), and betaine (10, 20, and 30 mg/kg) pretreatment prior to ketamine (10 mg/kg) significantly decreased the duration of immobility. Furthermore, the mice with betaine (30 mg/kg) pretreatment prior to ketamine (10 mg/kg) had significantly shorter duration of immobility compared with the mice that received ketamine (10 mg/kg) alone.
Effects of ketamine and betaine on the duration of immobility, struggling, and swimming in FST. This experiment included groups with various doses of ketamine (3, 10, 15 mg/kg), betaine (10, 20, 30 mg/kg), and betaine (10, 20, 30 mg/kg) pretreatment prior to ketamine (fixed dose at 10 mg/kg). Tests were conducted on days 1 and 7 and the duration of immobility (a), struggling (b), and swimming (c) were recorded. All values are expressed as mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 vs. saline/saline, # p < 0.05 vs. saline/ketamine
During day 1 test session, ketamine (3, 10, and 15 mg/kg), betaine (20 and 30 mg/kg), and betaine (10, 20, and 30 mg/kg) prior to ketamine (10 mg/kg) significantly reduced the duration of immobility compared with the vehicle control group. Further, the mice with betaine (30 mg/kg) pretreatment prior to ketamine (10 mg/kg) had significantly shorter duration of immobility compared with the mice that received ketamine (10 mg/kg) alone. During day 7 retest session, the duration of immobility in the groups of ketamine (15 mg/kg), betaine (20 and 30 mg/kg), and betaine (10, 20 and 30 mg/kg) pretreatment prior to ketamine (10 mg/kg) was significantly decreased compared with the vehicle control group (Fig. 3a).
For the duration of struggling, a mixed-design ANOVA revealed significant main effects of treatment (F 9, 75 = 2.586, p < 0.05) and test session (F 1, 75 = 24.517, p < 0.001). All pairwise multiple comparisons indicated that ketamine (15 mg/kg), betaine (20 and 30 mg/kg), and betaine (20 mg/kg) prior to ketamine (10 mg/kg) significantly increased the duration of struggling. During day 1 test session, ketamine (15 mg/kg) and betaine (20 mg/kg) prior to ketamine (10 mg/kg) significantly increased the duration of struggling compared with the control group. During day 7 retest session, ketamine (15 mg/kg) and betaine (30 mg/kg) significantly increased the duration of struggling compared with the control group (Fig. 3b).
A mixed-design ANOVA revealed that there were significant effects of treatment (F 9, 75 = 3.096, p < 0.01) and test session (F 1, 75 = 4.918, p < 0.05) on the duration of swimming. All pairwise multiple comparisons demonstrated that the ketamine (10 and 15 mg/kg), betaine (20 and 30 mg/kg), and betaine (10, 20, and 30 mg/kg) pretreatment prior to ketamine (10 mg/kg) significantly increased the duration of swimming. During day 1 test session, betaine (20 and 30 mg/kg), ketamine (10 and 15 mg/kg), and betaine (10, 20, and 30 mg/kg) pretreatment prior to ketamine (10 mg/kg) significantly increased the duration of swimming compared with the control group. Further, betaine (30 mg/kg) pretreatment prior to ketamine (10 mg/kg) showed longer duration of swimming compared with ketamine (10 mg/kg). During day 7 retest session, ketamine (15 mg/kg) and betaine (10, 20 and 30 mg/kg) prior to ketamine (10 mg/kg) significantly increased the duration of swimming compared with the control group (Fig. 3c).
Effects of betaine and ketamine on motor coordination in the rotarod test
In the experiment for assessing the effect of betaine and ketamine on rotarod performance, a mixed-design ANOVA revealed significant main effects of treatment (F 4, 205 = 107.477, p < 0.001) and time (F 4, 205 = 23.938, p < 0.001) on rotarod performance and a significant treatment × time interaction (F 16, 205 = 8.48, p < 0.001). Post hoc multiple comparisons indicated that ketamine significantly decreased the latency to stay on the rotarod, and betaine (30 and 100 mg/kg) significantly reduced the ketamine-induced motor incoordination (Fig. 4a).
Effects of betaine on ketamine-induced motor incoordination in the rotarod test and prepulse inhibition deficits in the acoustic startle reflex. Mice were pretreated with various doses of betaine (0, 30, and 100 mg/kg, i.p.). The latency to fall in the rotarod was recorded 10, 15, 20, 25, and 30 min after administration of ketamine (30 mg/kg, i.p.) (a). Startle amplitude and PPI were measured 15 min after ketamine (30 mg/kg, i.p.) administration (b). All values are expressed as mean ± SEM. *p < 0.05, ***p < 0.001 vs. saline/saline, # p < 0.05, ### p < 0.001 vs. saline/ketamine
Effects of betaine on ketamine-induced prepulse inhibition deficits
As for PPI, a mixed-design ANOVA revealed main effects of treatment (F 4, 90 = 5.338, p = 0.001) and prepulse intensity (F 2, 90 = 27.215, p < 0.001) and a significant treatment × prepulse intensity interaction (F 8, 90 = 2.292, p < 0.05). Ketamine alone significantly reduced the PPI. Multiple comparisons revealed that pretreatment of betaine (100 mg/kg) significantly attenuated the ketamine-induced disruption of PPI (Fig. 4b).
Effects of betaine on ketamine-induced recognition memory deficits in the novel object recognition test
A mixed-design ANOVA revealed significant main effects of treatment (F 4, 29 = 7.114, p < 0.001) and session (F 1, 29 = 72.776, p < 0.001) and a significant treatment × session interaction (F 4, 29 = 3.684, p < 0.05). There was no significant difference in the recognition index between treatment groups in the training session. Post hoc tests revealed that ketamine significantly reduced the recognition index and betaine (30 and 100 mg/kg) significantly reversed the recognition impairing effects of ketamine in the retention session (Fig. 5a).
Effects of betaine on ketamine-induced deficits in the novel object recognition test and social interaction test. Mice were pretreated with saline or betaine (30 and 100 mg/kg, i.p.) 30 min prior to ketamine (30 mg/kg). After 5 min, the training session in the novel object recognition test started. The retention session was conducted 24 h later. The amount of time spent exploring the novel object and total exploring time were measured (a). For the social interaction test, two mice with the same treatment but from different cages were introduced into the testing arena. The total time that a pair spent in social interaction was recorded (b). All values are expressed as mean ± SEM. ***p < 0.001 vs. saline/saline, # p < 0.05, ### p < 0.001 vs. saline/ketamine
Effects of betaine on ketamine-induced social withdrawal
One-way ANOVA indicated a significant effect of treatment (total duration: F 4, 39 = 6.608, p < 0.001). Post hoc tests indicated that betaine (30 and 100 mg/kg, i.p.) significantly attenuated the reduction in social interaction duration induced by ketamine (Fig. 5b).
Effects of betaine on ketamine-induced loss of righting reflex
Ketamine (100 mg/kg, i.p.) produced loss of righting reflex (LORR). One-way ANOVA revealed that pretreatment with betaine (300 and 600 mg/kg) did not affect the onset (F 2, 18 = 0.76, p = 0.482) and duration (F 2, 18 = 0.191, p = 0.828) of ketamine-induced loss of righting reflex (Fig. 6).
Effects of betaine on locomotor activity and locomotor hyperactivity induced by ketamine
One-way ANOVA revealed that betaine (30 and 100 mg/kg) did not affect the travel distances (F 2, 17 = 0.862, p = 0.44) (Fig. 7a) and the time in the center (F 2, 17 = 0.149, p = 0.863) after betaine administration (Fig. 7b). The effect of betaine on ketamine-induced locomotor hyperactivity was examined by administration of ketamine (30 mg/kg) 30 min after betaine (0, 30, and 100 mg/kg, i.p.) injection (Fig. 7c). One-way ANOVA demonstrated that there was a significant effect of treatment (F 3, 32 = 5.157, p < 0.01) on the total travel distances after ketamine administration (Fig. 7d). Post hoc tests indicated that ketamine increased the total travel distances, while the ketamine-induced locomotor hyperactivity was not affected by betaine (30 and 100 mg/kg) treatment.
Effects of betaine on locomotor activity in the open field test and locomotor hyperactivity induced by ketamine. Spontaneous locomotor activity was recorded for 2 h, then betaine (0, 30, and 100 mg/kg, i.p.) was administered, and the distance moved (a) and the time in the center (b) were recorded for 60 min. The effect of betaine on ketamine-induced locomotor hyperactivity was examined by administration of ketamine (30 mg/kg) 30 min after betaine (0, 30, and 100 mg/kg, i.p.) injection (c). Total distances after ketamine administration were measured for 30 min (d). All values are expressed as the mean ± SEM. *p < 0.05, **p < 0.01, compared with saline/saline
Discussion
The present study revealed that betaine dose-dependently produced not only rapid but also sustained antidepressant-like effects in the FST. In addition, betaine reduced the latency to feed in the NSF, supporting its antidepressant-like effect. Unlike ketamine, betaine did not show anxiolytic effect in the emergence test. It is of note that betaine had an additive effect when combined with a low dose of ketamine in the FST. On the contrary, betaine remarkably attenuated the ketamine-evoked disruption of PPI, novel object recognition impairment, and motor incoordination in the rotarod test, but did not influence ketamine-induced locomotor hyperactivity and anesthesia. It appears that betaine could enhance the antidepressant-like yet disrupt the psychotomimetic effects of ketamine.
It is not surprising that betaine could produce rapid and sustained antidepressant effects because betaine has been found to act like an NMDAR glycine binding site partial agonist. In fact, the NMDAR glycine site partial agonists D-cycloserine, 1-aminocyclopropanecarboxylic acid (ACPC) (Papp and Moryl 1996), and GLYX-13 (Burgdorf et al. 2013; Moskal et al. 2014) as well as NMDAR glycine site antagonist 7-chlorokynurenic acid (Zhu et al. 2013) have shown potential antidepressant-like effects. Among them, GLYX-13 has been proved to have sustained effects. On the other hand, D-serine (Malkesman et al. 2012) and glycine transporter-I inhibitor sarcosine (Chen et al. 2015; Huang et al. 2013), which are assumed to potentiate NMDAR function through glycine site, can also improve depression-like behaviors in rodent models and in human depression (Huang et al. 2013). Modulation of NMDAR glycine site might be majorly contributed to the rapid and sustained antidepressant-like effect of betaine, although betaine elevated 5-HT levels and has been suggested to be like a traditional antidepressant (Kim et al. 2013).
A recent clinical study has demonstrated that S-adenosyl-methionine (SAMe) plus betaine is more effective than SAMe alone when administered as an add-on therapy to subjects, affected by mild to moderate depression, who were low responders to conventional antidepressants (Di Pierro et al. 2015). Although a betaine-alone treatment group was not included in this clinical report and the exact mechanisms underlying the antidepressant effects of betaine remain to be further investigated, apparently betaine has beneficial effects for patients with treatment-resistant depression.
Betaine pretreatment increased the antidepressant-like effects of ketamine at a dose below the ceiling effect. The possible mechanisms underlying the antidepressant effects of ketamine have been reviewed and suggested that in addition to acting on NMDAR, ketamine at low doses increases glutamate neurotransmission by both increased glutamate release and increased AMPA receptor expression and insertion into the synaptic site. This causes secondary increased BDNF release and hence activation of ERK signaling which then stimulates the protein kinase mammalian target of rapamycin (mTOR) and thus via a complex signaling path leads to increased synaptic protein expression (GluR1) and increased insertion and density of synapses, leading to increased structural connectivity between neurons, particularly in the prefrontal cortex (Dwyer and Duman 2013). Betaine and ketamine might have the convergent influence on downstream synaptic plasticity cascades in their sustained antidepressant effects.
The adverse mental status associated with ketamine use including psychosis, dissociative, hallucinogenic, and amnesic effects (Krystal et al. 1994; Perry et al. 2007) may lead to discontinuation of ketamine therapy in the treatment of depression. The effects of betaine on ketamine model of psychosis were determined. As shown in previous studies (Chan et al. 2008, 2012), ketamine at a higher dose (30 mg/kg) produced psychotomimetic behaviors. Betaine abolished or significantly attenuated these behavioral abnormalities in a dose-dependent manner. The psychotomimetic effects of ketamine are mainly attributed to the NMDAR blockade, as it is a prominent feature of most NMDAR antagonist compounds (Nakao et al. 2003; Neymotin et al. 2011; Thomson et al. 1985; Vollenweider and Geyer 2001). Betaine might counteract the psychotomimetic effects of ketamine through modulating the NMDAR glycine binding site since the beneficial effect of betaine in the NORT was significantly attenuated by 7-chlorokynurenic acid (7-CTKA), an antagonist for the NMDAR glycine binding site (Supplement 1). The effective doses of betaine to reverse the psychotomimetic behaviors induced by ketamine ranged from 30 to 100 mg/kg, which were significantly lower than other NMDAR modulators, such as glycine (1.6 g/kg), D-serine (0.6, 1.8, and 2.7 g/kg) (Katsuki et al. 2007; Lipina et al. 2005; Zhou et al. 2013), and sarcosine (100 and 300 mg/kg) (Chen et al. 2010; Yang et al. 2010). It is possible but still needs to be verified whether the antipsychotic effects of betaine are more potent than glycine, D-serine, and sarcosine clinically.
Ketamine anesthesia has been attributed to the NMDAR blockade. In fact, other molecular actions, in particular, the HCN1 channels, have been indicated as a critical molecular substrate for hypnotic actions of ketamine (Chen et al. 2009). Betaine did not affect the ketamine-induced righting reflex, which might implicate that betaine is devoid of interaction with HCN1 channels. The diverse interactions between ketamine and betaine in the behavioral manifestations indicated the distinct neural circuits participating in the antidepressant, psychotomimetic, and anesthetic effects of ketamine. It is of interest to differentiate the particular brain regions for the psychotomimetic or antidepressant-like effects of ketamine. Comparison of ketamine, betaine, and their combined effects on c-Fos expression might be able to provide more information to address this issue.
Betaine is the methyl donor exclusively in the betaine homocysteine methyltransferase pathway and causes both homocysteine reduction and increased blood SAMe levels (Obeid 2013). Thus, betaine is approved for the treatment of homocystinuria. Growing evidence shows that betaine has the potential to treat neurological disorders. Betaine has been reported to prevent seizures in rodents (Freed 1984; 1985), to improve symptoms of Rett syndrome (Percy and Lane 2005), and to delay the onset of neurologic impairment due to vitamin B12 deficiency (van der Westhuyzen and Metz 1984) clinically. Furthermore, betaine attenuates memory deficits induced by homocysteine (Chai et al. 2013) and LPS (Miwa et al. 2011). It appears that betaine plays a critical role in the regulation of brain functions.
A recent clinical study has shown that the combined action of SAMe and betaine is more effective than the administration of SAMe alone in patients with mild to moderate depression (Di Pierro et al. 2015). The present animal study revealed that betaine has acute and sustained antidepressant-like effects. Taken together, betaine might be beneficial in the treatment of different types of depression and involve more than one mechanism. Moreover, treatment with betaine prior to ketamine produced additive antidepressant-like effects and avoided the psychotomimetic effects of ketamine, suggesting that betaine is an ideal candidate for use as an add-on therapy with ketamine in patients with treatment-resistant depression and bipolar disorder. Finally, betaine might be especially suitable for the treatment of depression in patients with schizophrenia as it exhibits antidepressant-like and antipsychotic-like properties.
References
Aligeti S, Quinones M, Salazar R (2014) Rapid resolution of suicidal behavior and depression with single low-dose ketamine intravenous push even after 6 months of follow-up. J Clin Psychopharmacol 34:533–535
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95
Browne CA, Lucki I (2013) Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol 4:161
Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR (2013) GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 38:729–742
Chai GS, Jiang X, Ni ZF, Ma ZW, Xie AJ, Cheng XS, Wang Q, Wang JZ, Liu GP (2013) Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J Neurochem 124:388–396
Chan MH, Chiu PH, Sou JH, Chen HH (2008) Attenuation of ketamine-evoked behavioral responses by mGluR5 positive modulators in mice. Psychopharmacology (Berl) 198:141–148
Chan MH, Chiu PH, Lin CY, Chen HH (2012) Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr Res 136:96–103
Chen X, Shu S, Bayliss DA (2009) HCN1 channel subunits are a molecular substrate for hypnotic actions of ketamine. J Neurosci 29:600–609
Chen HH, Stoker A, Markou A (2010) The glutamatergic compounds sarcosine and N-acetylcysteine ameliorate prepulse inhibition deficits in metabotropic glutamate 5 receptor knockout mice. Psychopharmacology (Berl) 209:343–350
Chen KT, Tsai MH, Wu CH, Jou MJ, Wei IH, Huang CC (2015) AMPA receptor-mTOR activation is required for the antidepressant-like effects of sarcosine during the forced swim test in rats: insertion of AMPA receptor may play a role. Front Behav Neurosci 9:162
Chiu CT, Scheuing L, Liu G, Liao HM, Linares GR, Lin D, Chuang DM (2015) The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int J Neuropsychopharmacol 18. doi: 10.1093/ijnp/pyu102
Di Pierro F, Orsi R, Settembre R (2015) Role of betaine in improving the antidepressant effect of S-adenosyl-methionine in patients with mild-to-moderate depression. J Multidiscip Healthc 8:39–45
Dwyer JM, Duman RS (2013) Activation of mammalian target of rapamycin and synaptogenesis: role in the actions of rapid-acting antidepressants. Biol Psychiatry 73:1189–1198
Freed WJ (1984) N, N-dimethylglycine, betaine, and seizures. Arch Neurol 41:1129–1130
Freed WJ (1985) Prevention of strychnine-induced seizures and death by the N-methylated glycine derivatives betaine, dimethylglycine and sarcosine. Pharmacol Biochem Behav 22:641–643
Garcia LS, Comim CM, Valvassori SS, Reus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J (2008) Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry 32:140–144
Huang CC, Wei IH, Huang CL, Chen KT, Tsai MH, Tsai P, Tun R, Huang KH, Chang YC, Lane HY, Tsai GE (2013) Inhibition of glycine transporter-I as a novel mechanism for the treatment of depression. Biol Psychiatry 74:734–741
Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA Jr (2012) Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology 37:1526–1533
Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CA Jr (2015) A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord 17:438–443
Kanahara N, Shimizu E, Ohgake S, Fujita Y, Kohno M, Hashimoto T, Matsuzawa D, Shirayama Y, Hashimoto K, Iyo M (2008) Glycine and D-serine, but not D-cycloserine, attenuate prepulse inhibition deficits induced by NMDA receptor antagonist MK-801. Psychopharmacology (Berl) 198:363–374
Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A (2007) Contribution of endogenous glycine and d-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci 81:740–749
Kawaura K, Koike H, Kinoshita K, Kambe D, Kaku A, Karasawa J, Chaki S, Hikichi H (2015) Effects of a glycine transporter-1 inhibitor and D-serine on MK-801-induced immobility in the forced swimming test in rats. Behav Brain Res 278:186–192
Kim SJ, Lee L, Kim JH, Lee TH, Shim I (2013) Antidepressant-like effects of lycii radicis cortex and betaine in the forced swimming test in rats. Biomol Ther (Seoul) 21:79–83
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214
Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A (2005) Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 179:303–309
Lee M-Y, Chen H-H (2014) Modulatory role for sarcosine, N, N-dimethylglycine and betaine in NMDA receptor activation. Basic Clin Pharmacol Toxicol 115:203 (Abstract)
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
Lima-Ojeda JM, Vogt MA, Pfeiffer N, Dormann C, Kohr G, Sprengel R, Gass P, Inta D (2013) Pharmacological blockade of GluN2B-containing NMDA receptors induces antidepressant-like effects lacking psychotomimetic action and neurotoxicity in the perinatal and adult rodent brain. Prog Neuropsychopharmacol Biol Psychiatry 45:28–33
Lin BF, Ou MC, Chung SS, Pang CY, Chen HH (2010) Adolescent toluene exposure produces enduring social and cognitive deficits in mice: an animal model of solvent-induced psychosis. World J Biol Psychiatry 11:792–802
Lipina T, Labrie V, Weiner I, Roder J (2005) Modulators of the glycine site on NMDA receptors, D-serine and ALX 5407, display similar beneficial effects to clozapine in mouse models of schizophrenia. Psychopharmacology (Berl) 179:54–67
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK (2008) Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63:349–352
Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB, Cui Z, Young WS, Nakazawa K, Zarate CA, Manji HK, Chen G (2012) Acute D-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol 15:1135–1148
Miwa M, Tsuboi M, Noguchi Y, Enokishima A, Nabeshima T, Hiramatsu M (2011) Effects of betaine on lipopolysaccharide-induced memory impairment in mice and the involvement of GABA transporter 2. J Neuroinflammation 8:153
Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, Leander JD (2014) GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert Opin Investig Drugs 23:243–254
Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV (2013) Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 74:250–256
Nakao S, Nagata A, Masuzawa M, Miyamoto E, Yamada M, Nishizawa N, Shingu K (2003) NMDA receptor antagonist neurotoxicity and psychotomimetic activity. Masui 52:594–602
Neymotin SA, Lazarewicz MT, Sherif M, Contreras D, Finkel LH, Lytton WW (2011) Ketamine disrupts theta modulation of gamma in a computer model of hippocampus. J Neurosci 31:11733–11743
Nugent AC, Diazgranados N, Carlson PJ, Ibrahim L, Luckenbaugh DA, Brutsche N, Herscovitch P, Drevets WC, Zarate CA Jr (2014) Neural correlates of rapid antidepressant response to ketamine in bipolar disorder. Bipolar Disord 16:119–128
Obeid R (2013) The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 5:3481–3495
Papp M, Moryl E (1994) Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. Eur J Pharmacol 263:1–7
Papp M, Moryl E (1996) Antidepressant-like effects of 1-aminocyclopropanecarboxylic acid and D-cycloserine in an animal model of depression. Eur J Pharmacol 316:145–151
Park MJ, SRK HH, JHJ YCK (1994) Betaine attenuates glutamate-induced neurotoxicity in primary cultured brain cells. Arch Pharm Res 17:343–347
Percy AK, Lane JB (2005) Rett syndrome: model of neurodevelopmental disorders. J Child Neurol 20:718–721
Perry EB Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, O’Donnell E, Krystal JH, D’Souza DC, Yale Ketamine Study G (2007) Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology (Berl) 192:253–260
Qiao H, Noda Y, Kamei H, Nagai T, Furukawa H, Miura H, Kayukawa Y, Ohta T, Nabeshima T (2001) Clozapine, but not haloperidol, reverses social behavior deficit in mice during withdrawal from chronic phencyclidine treatment. Neuroreport 12:11–15
Roberts BM, Shaffer CL, Seymour PA, Schmidt CJ, Williams GV, Castner SA (2010) Glycine transporter inhibition reverses ketamine-induced working memory deficits. Neuroreport 21:390–394
Rybakowski JK, Permoda-Osip A, Skibinska M, Adamski R, Bartkowska-Sniatkowska A (2013) Single ketamine infusion in bipolar depression resistant to antidepressants: are neurotrophins involved? Hum Psychopharmacol 28:87–90
Santini AC, Pierantoni GM, Gerlini R, Iorio R, Olabinjo Y, Giovane A, Di Domenico M, Sogos C (2014) Glix 13, a new drug acting on glutamatergic pathways in children and animal models of autism spectrum disorders. BioMed research international 2014:234295
Thakurta RG, Das R, Bhattacharya AK, Saha D, Sen S, Singh OP, Bisui B (2012) Rapid response with ketamine on suicidal cognition in resistant depression. Indian J Psychol Med 34:170–175
Thomson AM, West DC, Lodge D (1985) An N-methylaspartate receptor-mediated synapse in rat cerebral cortex: a site of action of ketamine? Nature 313:479–481
van der Westhuyzen J, Metz J (1984) Betaine delays the onset of neurological impairment in nitrous oxide-induced vitamin B-12 deficiency in fruit bats. J Nutr 114:1106–1111
Vollenweider FX, Geyer MA (2001) A systems model of altered consciousness: integrating natural and drug-induced psychoses. Brain Res Bull 56:495–507
Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW (2014) Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry
Yang SY, Hong CJ, Huang YH, Tsai SJ (2010) The effects of glycine transporter I inhibitor, N-methylglycine (sarcosine), on ketamine-induced alterations in sensorimotor gating and regional brain c-Fos expression in rats. Neurosci Lett 469:127–130
Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ (2013) Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry
Zhu WL, Wang SJ, Liu MM, Shi HS, Zhang RX, Liu JF, Ding ZB, Lu L (2013) Glycine site N-methyl-D-aspartate receptor antagonist 7-CTKA produces rapid antidepressant-like effects in male rats. J Psychiatry Neurosci 38:306–316
Zigman D, Blier P (2013) Urgent ketamine infusion rapidly eliminated suicidal ideation for a patient with major depressive disorder: a case report. J Clin Psychopharmacol 33:270–272
Acknowledgments
This work was supported by the National Health Research Institutes (NP-103-PP-02) and the Ministry of Science and Technology (MOST-104-2314-B-400-007-MY22).
Authors’ contributions
MHC, YCC, and HHC were responsible for the study concept and design. JCL and MYL contributed to the acquisition and analysis of the animal data and interpretation of the findings. JCL drafted the manuscript. YCC and HHC provided critical revision of the manuscript for important intellectual content. MHC contributed to reviewing and editing of the manuscript. All of the authors critically reviewed the content and approved the final version for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
NMDAR glycine binding site antagonist 7-chlorokynurenic acid (7-CTKA) abolished the reversing effect of betaine on ketamine-induced cognitive deficits in the novel object recognition test. 7-CTKA (0, 0.3 and 1 mg/kg, i.p.) was given 30 min prior to betaine (30 mg/kg, i.p.) or saline, followed by administration of ketamine (30 mg/kg) or saline 30 min later. The training session was conducted 5 min after ketamine administration. The retention session was performed 24 h later. All values are expressed as the mean ± SEM. A mixed-design ANOVA revealed significant main effects of treatment (F5, 43 = 4.361, p < 0.01) and session (F1, 43 = 47.944, p < 0.001) and a significant treatment x session interaction (F5, 43 = 8.677, p < 0.001). 7-CTKA (1 mg/kg, i.p.) affected the novel object preferences and disrupted the reversing effect of betaine on ketamine-induced impairment in the retention session. ***p < 0.001 vs. Saline/Saline/Saline, ## p < 0.01, ### p < 0.001 vs. Saline/Saline /Ketamine, $$$ p < 0.001 vs. Saline /Betaine/Ketamine. (PPTX 110 kb)
Rights and permissions
About this article
Cite this article
Lin, JC., Lee, MY., Chan, MH. et al. Betaine enhances antidepressant-like, but blocks psychotomimetic effects of ketamine in mice. Psychopharmacology 233, 3223–3235 (2016). https://doi.org/10.1007/s00213-016-4359-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4359-x