Abstract
Rationale
Hypofunction of striatal dopamine neurotransmission, or hypodopaminergia, is a consequence of excessive ethanol use and is hypothesized to be a critical component of alcoholism, driving alcohol intake in an attempt to restore dopamine levels; however, the neurochemical mechanisms involved in these dopaminergic deficiencies are not fully understood.
Objective
Here we examined the specific dopaminergic adaptations that produce hypodopaminergia and contribute to alcohol use disorders using direct, sub-second measurements of dopamine signaling in nonhuman primates following chronic ethanol self-administration.
Methods
Female rhesus macaques completed 1 year of daily (22 h/day) ethanol self-administration. Subsequently, fast-scan cyclic voltammetry was used in nucleus accumbens core brain slices to determine alterations in dopamine terminal function, including release and uptake kinetics, and sensitivity to quinpirole (D2/D3 dopamine receptor agonist) and U50,488 (kappa opioid receptor agonist) induced inhibition of dopamine release.
Results
Ethanol drinking greatly increased uptake rates, which were positively correlated with lifetime ethanol intake. Furthermore, the sensitivity of dopamine D2/D3 autoreceptors and kappa opioid receptors, which both act as negative regulators of presynaptic dopamine release, was moderately and robustly enhanced in ethanol drinkers.
Conclusions
Greater uptake rates and sensitivity to D2-type autoreceptor and kappa opioid receptor agonists could converge to drive a hypodopaminergic state, characterized by reduced basal dopamine and an inability to mount appropriate dopaminergic responses to salient stimuli. Together, we outline the specific alterations to dopamine signaling that may drive ethanol-induced hypofunction of the dopamine system and suggest that the dopamine and dynorphin/kappa opioid receptor systems may be efficacious pharmacotherapeutic targets in the treatment of alcohol use disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eighteen million people in the United States currently meet the criteria for an alcohol use disorder, and alcohol abuse results in over 100,000 deaths per year (McGinnis and Foege 1999; SAMHSA, 2012). In the search for treatment interventions, animal models are critical to investigating the neurochemical basis of alcohol use disorders. Due to their neuroanatomical, genetic, and behavioral similarities with humans, nonhuman primate (NHP) models of ethanol self-administration provide high translational validity (Grant and Bennett 2003; Kroenke et al. 2014). In this regard, the inclusion of female subjects is integral to understanding risks for alcohol use disorders, as previous work has shown divergent rates of alcohol use and addiction between sexes, with greater rates of alcohol dependence occurring in males (Grant 1996; Haberstick et al. 2014) as well as differential long-term effects of ethanol in animal studies (Devaud et al. 2003; Wiren et al. 2006). While investigations of NHP models of ethanol self-administration have provided a wealth of valuable information on behavior and endocrine outcomes, far fewer studies have examined ethanol-induced changes on a cellular level and even fewer in female monkeys. Thus, the inclusion of female NHPs is an important step in elucidating factors underlying alcoholism and, if there is a sex difference in the neural mechanisms underlying excessive drinking, for selecting pharmacotherapeutic agents to specifically treat female alcoholics.
Reduced dopaminergic responsiveness in the striatum is a hallmark of alcohol use in human and animal models, and it is hypothesized that this hypodopaminergic state leads to negative affective states, reduced salience of nondrug stimuli, and excessive drinking (Volkow et al. 2007; Koob 2013; Melis et al. 2005); however, the exact mechanisms resulting in reduced dopamine function, especially in female subjects, have not been elucidated. When administered acutely to rodents, ethanol augments dopamine in striatal regions by increasing the firing rate of dopaminergic cells projecting from the ventral tegmental area and substantia nigra (Brodie et al. 1990). However, after repeated administration, ethanol can induce long-term changes in dopamine neuron projection regions, such as the NAc, including reductions in basal dopamine levels as measured by microdialysis (Rossetti et al. 1992, 1999) and increased dynorphin peptide levels during abstinence from ethanol as measured by radioimmunoassays (Lindholm et al. 2000). Both kappa opioid receptors (KOR) and D2-type dopamine autoreceptors are located on the dopamine neurons/terminals and act to negatively regulate dopamine cell firing, release, and synthesis (Werling et al. 1988; Walker et al. 1987; Manzanares et al. 1991; Ford 2014); thus, dysregulation of these receptors could explain, at least in part, the reduced dopamine responsiveness in the striatum during withdrawal.
We assessed the relationship between ethanol drinking and dopaminergic function by examining the effects of 1 year of daily voluntary ethanol drinking on dopamine release, uptake, and presynaptic regulators of dopamine signaling in female rhesus macaques. Using fast-scan cyclic voltammetry (FSCV) in brain slices containing the NAc core, we examined the effects of KOR agonist U50,488 and the dopamine D2/D3 receptor agonist quinpirole on dopamine neurotransmission in ethanol self-administering female macaques and age-matched controls. These data provide novel insights into dopaminergic changes induced by chronic voluntary ethanol consumption in female macaques that may mediate the aberrant drinking behaviors observed in human ethanol abusers.
Methods and materials
Animals
Young adult female rhesus macaques (Macaca mulatta) between 5.5 and 6.0 years of age and weighing 5.12–6.08 kg were housed in quadrant cages (0.8 × 0.8 × 0.9 m) with constant temperature (20–22 °C), humidity (65 %), and an 11-h light cycle (lights on at 08:00 a.m.). A total of eight animals were used in this study, which included five ethanol drinkers and three controls. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Oregon National Primate Research Center IACUC.
Ethanol self-administration
Each cage had a panel on one wall that dispensed food and fluids, as previously described (Vivian et al. 2001; Grant et al. 2008). Briefly, the panels had two spouts, each below a set of three stimulus lights (white, red, and green) that indicated an active session and food or fluid availability, respectively. A centrally located recessed dowel activated the fluid spouts and an infrared finger-poke detector activated the pellet dispenser. Each spout was connected via Nalgene tubing to a 1-l fluid reservoir set on a digital scale. Dowel pulls, finger pokes, and fluid consumption were recorded every 500 ms via a computerized system using custom hardware and programing using National Instruments interface and Labview Software (Austin, TX). Schedule-induced polydipsia, as previously described (Vivian et al. 2001), was used to induce ethanol self-administration in daily 16-h sessions. Briefly, a 1-g banana food pellet was dispensed every 300 s (fixed time 300 s) until a water volume equivalent to 1.5 g/kg of 4 % (w/v) ethanol was consumed. Following water induction, 4 % ethanol replaced water. In 30-day increments, each animal consumed increasing doses of 4 % ethanol; 0.5, 1.0, then 1.5 g/kg/day. Following consumption of the programmed volume, water was immediately available and any remaining pellets were available on a fixed-ratio 1 (FR-1) schedule after 2 h. Following completion of ethanol induction, there were daily 22-h sessions during which water and ethanol were concurrently available.
Control monkeys were selected from the same breeding colony and matched on age. Controls were housed in the same housing rooms as the experimental subjects and underwent the same training for awake blood draws, medical check-ups, and MRI imaging. However, control monkeys did not receive access to ethanol. Instead, at baseline, controls were yoked to future ethanol monkeys based on weight and received a quantity of 10 % maltose-dextrin solution matched to the previous day’s intake of their yoked ethanol monkey. In addition, controls were induced to drink water at the same time as the ethanol monkeys but were only given water during the induction volume equivalent to 0.5, 1.0, or 1.5 g/kg of 4 % (w/v) ethanol. During the open access condition, control monkeys had water available from both spouts, but all other conditions were matched to ethanol drinkers.
Necropsy and tissue collection
Following ethanol self-administration, each animal was sedated with 10 mg/kg ketamine, intubated, and maintained with isoflurane and pentobarbital. A craniotomy was performed followed by perfusion with chilled artificial cerebral spinal fluid. Once the perfusion was complete (within 4 min), the brain was immediately removed, placed into a brain block, and dissected into 4-mm coronal sections, as described previously (Daunais et al. 2010). A section containing the nucleus accumbens was removed and placed into oxygenated artificial CSF. The approximate area dissected is shown in Fig. 1c.
Drinking data and anatomical location of voltammetry experiments. a Lifetime sum of ethanol intake for each animal. b There was a strong positive correlation between BEC and ethanol intake at the time of sample collection (n = 61 per animal; individual animals are separated by color). The dotted line at 80 mg/dl marks the legal limit for intoxication in the United States. c Photograph of brain section containing the caudate (cd), putamen (pu), and nucleus accumbens (NAc) core (dotted line). All voltammetric recordings were performed in the NAc core
In vitro voltammetry
Necropsy began approximately 3.5–6.5 h after discontinuation of ethanol access. As previously described (Siciliano et al. 2014), a vibrating tissue slicer fitted with a ceramic blade was used to prepare coronal brain sections containing the NAc (250 μm thick). Once sliced, the tissue was incubated in oxygenated artificial cerebrospinal fluid (containing (in mM) 126 NaCl, 20 HEPES acid, 25 NaHCO3, 11 glucose, 2.5 KCl, 4.4 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 0.4 l-ascorbic acid, pH adjusted to 7.4). Dopamine release and uptake were recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4 V vs Ag/AgCl, 400 V/s, scanning every 100 ms) to a carbon fiber microelectrode (100–200 μM length, 7 μM diameter). Dopamine release was evoked by one pulse stimulation (350 μA, 4 ms, monophasic) applied to the tissue every 5 min through a bipolar stimulating electrode, which was placed in close proximity to the recording electrode on the surface of the tissue in the core of the NAc. Stimulations occurred until the peak height of evoked dopamine release was stable (three collections within 10 % variability). After pre-drug measures were taken, U50,488 (0.3 μM, 1 μM) or quinpirole (30 nM) were bath applied to separate slices.
Data analysis
Demon Voltammetry and Analysis software was used for all analysis of FSCV data (Yorgason et al. 2011). To convert current to dopamine concentration, electrodes were calibrated by recording responses to a known concentration of dopamine (3 μM) using a flow injection system. To evaluate dopamine kinetics, FSCV data were modeled using Michaelis-Menten kinetics, which allows for the determination of dopamine release and the maximal rate of dopamine uptake (V max), as previously described (Ferris et al. 2013). K m was held constant at 160 nM, and V max was manipulated to fit the curve.
Statistics
Comparisons of dopamine release and uptake as well as D2/D3 receptor sensitivity were made using Student’s t test. The effects of KOR activation between groups were subject to a repeated measures two-way analysis of variance (ANOVA) with concentration as the within-subjects factor and treatment group as the between-subjects factor. Correlational (Pearson’s) and linear regression analyses were used to assess the associations between multiple measures of presynaptic terminal function and lifetime ethanol intake.
Results
Drinking behaviors correlated with measured blood ethanol concentration
After an induction period, animals were allowed access to ethanol for 22 h/day for approximately 1 year. Blood ethanol concentrations (BECs) were measured every 5–7 days, 7 h after session onset, as previously described (Vivian et al. 2001). Lifetime sums of ethanol intake ranged from 533.9 to 1670.9 g/kg (Fig. 1a; mean = 959.2 ± 208.0). We found that BECs were highly positively correlated with ethanol intake at the time of sample collection (Fig. 1b; r = 0.88, p = 0.018); however, because the ethanol self-administration is voluntary and the time of sampling is 7 h after the onset of the 22-h session, BECs collected every fifth day over the course of 1 year show within-subject variability (Fig. 1b).
Ethanol self-administration increased dopamine uptake in the nucleus accumbens
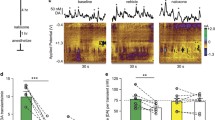
Animals were allowed access to ethanol until the morning of necropsy, and the effect of voluntary ethanol self-administration on dopamine terminals was examined using FSCV in brain slices containing the NAc core (Fig. 1c). Compared to age- and sex-matched controls, electrically evoked dopamine release was unchanged by chronic ethanol drinking (Fig. 2a, b), while dopamine uptake was increased (Fig. 2c; t 6 = 2.22, p = 0.034). Additionally, there were strong positive correlations between dopamine release and lifetime ethanol intake (Fig. 2d; r = 0.96, p = 0.005, β = 0.0003 ± 0.0001) and between dopamine uptake rate and lifetime ethanol consumption (Fig. 2e; r = 0.90, p = 0.02, β = 0.001 ± 0.0003). Further, we found similar positive correlations between each animal’s daily average intakes during either the first (dopamine release r = 0.91, p = 0.02; dopamine uptake r = 0.89, p = 0.02) or second (dopamine release r = 0.96, p = 0.004; dopamine uptake r = 0.88, p = 0.03) 6-month ethanol access period (data not shown).
Dopamine release and uptake predicted ethanol intake. a Representative concentration versus time traces and pseudo-color plots from a control (left) and ethanol self-administration (right) animal indicating the presence of dopamine, as indicated by its oxidation at +0.6 V and reduction at −0.2 V. b Group data indicating evoked dopamine release was not changed by ethanol self-administration. c Following ethanol self-administration, dopamine uptake rates were increased as compared to controls. d There was a positive correlation between dopamine release and lifetime ethanol intake. e Correlation analysis showing a positive relationship between dopamine uptake rate and lifetime ethanol intake. *p < 0.05 vs control
Ethanol self-administration increased D2/D3 autoreceptor sensitivity
D2/D3 autoreceptors located on presynaptic dopamine terminals in the NAc regulate dopamine release and play an important role in addictive behaviors. We therefore sought to examine the responsiveness to the selective D2/D3 receptor agonist quinpirole to determine ethanol-induced changes in autoreceptor regulation of dopamine release following ethanol self-administration. Following bath application of 30 nM quinpirole, there was a greater decrease in dopamine release in ethanol drinkers as compared to controls (Fig. 3a; t 6 = 2.14, p = 0.038), indicating an increased sensitivity of presynaptic autoreceptors. Additionally, correlation analysis revealed no relationship between D2/D3 sensitivity and lifetime ethanol intake (Fig. 3b). While there was a trend towards a correlation between autoreceptor sensitivity and average daily ethanol intake during the first 6-month ethanol access period (r = −0.76, p = 0.07), there was no relationship during the second 6-month access period (data not shown).
Ethanol self-administration produced supersensitive D2/D3 autoreceptors. a Following ethanol self-administration, the ability of quinpirole to reduce dopamine release was increased as compared to controls demonstrating that augmented autoreceptor sensitivity is a consequence of ethanol use. b D2/D3 receptor sensitivity was not correlated with lifetime ethanol intake. *p < 0.05 vs control
Ethanol self-administration augments the ability of KORs to inhibit dopamine release
KORs have been shown to play a critical role in stress-induced adaptations as well as ethanol drinking in rodent models of ethanol abuse (Sirohi et al. 2012). Here we determined whether KOR regulation of dopamine neurotransmission was altered by ethanol use by examining the ability of the selective KOR agonist U50,488 to decrease dopamine release. Following bath application of 0.3 and 1 μM U50,488, a two-way repeated measures ANOVA revealed a main effect of concentration (Fig. 4a; F 1, 6 = 109.0, p = 0.0001) and a main effect of ethanol history (F 1, 6 = 6.45, p = 0.04) demonstrating a functional increase in the sensitivity of KORs. Correlation analysis showed no relationship between U50,488-induced decrease in dopamine release at 300 nM (data not shown) or 1 μM and ethanol intake (Fig. 4b). Further, there was no relationship between the effects of U50,488 on dopamine release and average daily ethanol intakes for the first or second 6-month access period (data not shown). While previous investigations have highlighted ethanol-induced alterations in dynorphin/KOR system function by showing changes in the behavioral effects of KOR agonists following ethanol exposure (Walker and Koob 2008; Nealey et al. 2011), the current findings demonstrate that chronic ethanol intake results in increases in KOR sensitivity at the NAc core dopamine terminals whereby KORs are likely more sensitive to dynorphin following chronic voluntary consumption of ethanol in female monkeys.
Kappa opioid receptors (KOR) were supersensitive following ethanol self-administration. a Following bath application of the selective KOR agonist U50,488, we observed a greater reduction in dopamine release in animals with a history of ethanol use, indicating that supersensitivity of KORs is a consequence of ethanol use. b Correlation analysis showing no relationship between KOR sensitivity and lifetime ethanol intake. *p < 0.05 main effect of group
To ensure that the observed effects in ethanol self-administering animals were due to pharmacological effects of ethanol rather than gross differences in caloric intake, we compared fluid intakes as well as weight gain over the ethanol access period. We found there was a trend towards increased fluid intake in the ethanol self-administration animals (Fig. 5a; p = 0.08). Importantly, while both groups gained weight over the access period, there was no difference in weight gain between groups (Fig. 5b).
Discussion
NHPs are uniquely translational preclinical models for alcohol use disorders (Kroenke et al. 2014; Grant and Bennett 2003); however, there has been a paucity of investigations into the neurochemical consequences of ethanol use at a cellular level in NHPs. Of these investigations, fewer have examined the effects of ethanol use in females, which appear to have distinct neurochemical and behavioral responses to drugs of abuse from males (Grant 1996; Haberstick et al. 2014; Devaud et al. 2003; Wiren et al. 2006). Here we demonstrate that in female rhesus macaques, long-term ethanol consumption produces robust changes in dopamine terminal function in the NAc core. Specifically, ethanol increased function of three important inhibitory presynaptic regulators of dopamine release: D2/D3 autoreceptors, KORs, and the dopamine transporter. While changes in dopamine uptake rate tightly tracked individual differences in ethanol consumption, autoreceptor and KOR sensitivity were not related to the amount of ethanol consumed, suggesting that these effects may be evoked after a certain threshold of ethanol intake and do not become more severe with greater amounts of intake. These changes likely converge in vivo to reduce synaptic dopamine levels by attenuating presynaptic release and increasing clearance from the synapse.
The prevalence of alcoholism as well as the effects of alcohol are sexually dimorphic, making it critical that the neurobiological basis of alcoholism are studied in both sexes. For example, epidemiological studies have shown that alcohol dependence is more common in males (Grant 1996; Haberstick et al. 2014), although women are more susceptible to the impairing effects of alcohol (Wilsnack et al. 1984) and develop alcohol-induced peripheral organ damage at a faster rate (Ammendola et al. 2000; Loft et al. 1987). Further, alcohol-induced morphological changes appear more quickly and result from less alcohol in females as compared to males (Mann et al. 1992; Schweinsburg et al. 2003). In preclinical studies, the effects of ethanol have also been shown to vary between sexes. In female rats, ethanol induces greater increases in dopamine levels, and female rats consume more ethanol than males (Blanchard et al. 1993). Further, female rats are less sensitive to the anxiogenic effects of ethanol withdrawal (Jung et al. 1999). Given differences in the acute actions of ethanol as well as the differential rate of alcoholism between sexes, it is likely that the ethanol-induced adaptations that drive excessive ethanol intake are at least partly divergent between sexes; for this reason, it is critical that both sexes are studied.
Although ethanol self-administration did not alter stimulated dopamine release compared to controls, we found a positive correlation between dopamine release and lifetime ethanol intake. One possible explanation for this finding is that pre-existing differences in dopamine release may be a factor in determining an animal’s preferred intake level. Differences in preferred intake have strong implications for the development of problematic drinking, as the amount of ethanol consumed in humans is closely tied to the development of alcohol dependence (Grant and Harford 1990). A possible explanation for the observed relationship between dopamine release and ethanol intake is that higher dopamine release during phasic signaling could facilitate the acquisition and strength of conditioned associations (Steinberg et al. 2013) and thus lead to an increased propensity to consume alcohol in the presence of alcohol-associated cues. While this study utilized the strengths of ex vivo voltammetry to probe the sensitivity of presynaptic regulators of dopamine release in isolation, future studies may further explore changes in cue-evoked, phasic dopamine signaling following chronic ethanol self-administration using in vivo voltammetry. Additionally, this interpretation relies on the lack of effect between the controls and drinkers; however, it should be noted that the low sample size may be a contributing factor. Previous studies in these same animals have suggested a similar relationship whereby density of corticotropin-releasing hormone expressing terminals in the hypothalamic paraventricular nucleus may also be a pre-existing factor in determining animal’s alcohol intake (Jimenez et al. 2015), and future studies will aim to explore these putative biomarkers.
While ethanol self-administration did not alter stimulated dopamine release compared to controls, it did have a large effect on multiple presynaptic regulators of dopamine neurotransmission. First, dopamine uptake rates, a measure of dopamine transporter function, were increased after chronic daily ethanol consumption, consistent with previous findings in rodents following ethanol vapor exposure (Budygin et al. 2007). Dopamine transporters are a major regulator of presynaptic dopamine terminal function and controls extracellular dopamine levels, as increased uptake decreases extracellular dopamine levels (Jones et al. 1999; Ferris et al. 2014). Consistent with these results, several studies have found decreased dopamine dialysate levels during withdrawal from chronic ethanol exposure in rodents (Rossetti et al. 1992, 1999) and decreased dopaminergic responsiveness in human alcoholics as measured by PET imaging (Volkow et al. 2007). Second, the sensitivity of inhibitory D2-type autoreceptors was augmented following ethanol self-administration, which may also help explain ethanol-induced reductions in basal dopamine levels observed in rodents and humans. Third, in further support of the notion that excessive ethanol use results in decreased dopamine levels, we report that in female NHPs, as in males (Siciliano et al. 2015a), the ability of KORs to attenuate dopamine release was significantly increased following ethanol self-administration. KORs are located on the presynaptic terminals of dopaminergic afferents from the VTA and act both to reduce the excitability of the dopamine terminal and to decrease dopamine synthesis, which converge to reduce the magnitude of presynaptic dopamine release events as well as release probability (Walker et al. 1987; Werling et al. 1988; Manzanares et al. 1991). In addition to the increase in KOR function reported here, dynorphin, the endogenous ligand for the KOR, has been shown to be increased during ethanol exposure and in withdrawal (Lindholm et al. 2000; Sirohi et al. 2012); thus, KOR supersensitivity paired with an increase in agonist peptide is likely to contribute significantly to decreased dopamine neurotransmission. Previous studies show that ethanol drinking is modulated by KORs in NHPs and ethanol-dependent rodents and that KOR and dynorphin genes are upregulated in human alcoholics, further supporting our hypothesis that increased KOR sensitivity and subsequent hypodopaminergia drive continued ethanol use (Walker and Koob 2008; Nealey et al. 2011).
Together, increased uptake, KOR sensitivity, and autoregulation of dopamine release, with no compensatory alterations in presynaptic dopamine release events, likely converge to produce a hypodopaminergic state, a phenomenon which is a signature of excessive ethanol drinking, as well as escalated intake of other drugs of abuse (Koob 2013; Melis et al. 2005). It is important to note that while we do not see decreases in dopamine release, this is likely due to the lack of tonic activity in acute slice preparations (Phillips et al. 2002). Without tonic activity, there is no agonist activity at the autoreceptors or KORs; however, in vivo endogenous activation of these receptors is likely to lead to greatly decreased dopamine release. Thus, we hypothesize that ethanol-induced changes in dopamine transporters, KORs, and presynaptic autoreceptors and the resultant hypodopaminergia, at least in part, drive aberrant drinking behaviors. Future studies should also aim to determine the lasting impact of ethanol consumption on these measures. Although it is possible that these findings reflect acute withdrawal, we hypothesize that these alterations will last long into the withdrawal period, based on the lasting hypodopaminergia seen in detoxified alcoholics.
Similar to the findings here, we have shown previously in male monkeys that ethanol self-administration produces an increased ability of KORs to regulate dopamine release (Siciliano et al. 2015a). Comparison of the current study with findings from male monkeys revealed two major differences in the neurochemical adaptations induced by ethanol self-administration that may be due to sex. First, in male monkeys, ethanol increased dopamine release in the core of the accumbens (Siciliano et al. 2015a), as compared to no change in the current study. Second, there was no change in the overall sensitivity of D2/D3 autoreceptors in males (Siciliano et al. 2015b), as compared to an increase in sensitivity in females. However, both previous studies in males were recordings taken from cynomolgus macaques after 6 months of free ethanol access as opposed to rhesus macaques with 12 months of free access used here. Thus, these differences may also be due to exposure length or strain; future studies will aim to systematically examine the hypothesis that these differences are due to sex. A second issue that should be addressed in future studies is the differences in dopamine release changes induced by ethanol between these findings and rodent models, which have shown decreased dopamine release following chronic intermittent ethanol exposure via vapor inhalation (Karkhanis et al. 2015). These differences may be due to exposure length or route of administration; however, in all three studies (Siciliano et al. 2015a; Karkhanis et al. 2015; current study), dopamine uptake rate was increased by ethanol exposure, suggesting that alterations to the dopamine transporter may be a critical neurochemical adaptation induced by ethanol.
These studies provide insights into the molecular mechanisms underlying drinking behaviors in a NHP model of ethanol abuse with unparalleled translational value. These data demonstrate a strong role for dopamine terminal changes in mediating the neurochemical consequences of ethanol abuse in female rhesus macaques. All of the changes shown here are likely to converge to significantly reduce dopaminergic responsiveness during withdrawal and, combined with decreased postsynaptic dopamine receptor expression (Volkow et al. 2002; Volkow et al. 1996), may result in greatly restricted dopaminergic modulation of accumbal outputs. Interventions that target these systems to restore presynaptic terminal function may reduce drinking and serve as pharmacotherapies in the treatment of alcoholism.
References
Ammendola A, Gemini D, Iannaccone S, Argenzio F, Ciccone G, Ammendola E, Serio L, Ugolini G, Bravaccio F (2000) Gender and peripheral neuropathy in chronic alcoholism: a clinical-electroneurographic study. Alcohol Alcohol 35(4):368–371
Blanchard BA, Steindorf S, Wang S, Glick SD (1993) Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res 17(5):968–973
Brodie MS, Shefner SA, Dunwiddie TV (1990) Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res 508(1):65–69
Budygin EA, Oleson EB, Mathews TA, Läck AK, Diaz MR, McCool BA, Jones SR (2007) Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacol (Berl) 193(4):495–501
Daunais JB, Kraft RA, Davenport AT, Burnett EJ, Maxey VM, Szeliga KT, Rau AR, Flory GS, Hemby SE, Kroenke CD, Grant KA, Friedman DP (2010) MRI-guided dissection of the nonhuman primate brain: a case study. Methods 50(3):199–204
Devaud LL, Alele P, Ritu C (2003) Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol 15(1):41–59
Ferris MJ, Calipari ES, Yorgason JT, Jones SR (2013) Examining the complex regulation and drug-induced plasticity of dopamine release and uptake using voltammetry in brain slices. ACS Chem Neurosci 4(5):693–703
Ferris MJ, España RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, Jones SR, 26 (2014) Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci U S A 111:E2751–E2759
Ford CP (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 282C:13–22
Grant BF (1996) Prevalence and correlates of drug use and DSM-IV drug dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse 8(2):195–210
Grant KA, Bennett AJ (2003) Advances in nonhuman primate alcohol abuse and alcoholism research. Pharmacol Ther 100(3):235–255
Grant BF, Harford TC (1990) The relationship between ethanol intake and DSM-III-R alcohol dependence. J Stud Alcohol 51(5):448–456
Grant KA et al (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res 32(10):1824–1838
Haberstick BC, Young SE, Zeiger JS, Lessem JM, Hewitt JK, Hopfer CJ (2014) Prevalence and correlates of alcohol and cannabis use disorders in the United States: results from the national longitudinal study of adolescent health. Drug Alcohol Depend 136:158–161
Jimenez VA, Helms CM, Cornea A, Meshul CK, Grant KA (2015) An ultrastructural analysis of the effects of ethanol self-administration on the hypothalamic paraventricular nucleus in rhesus macaques. Front Cell Neurosci 9:260
Jones SR, Gainetdinov RR, Caron MG (1999) Application of microdialysis and voltammetry to assess dopamine functions in genetically altered mice: correlation with locomotor activity. Psychopharmacology 147(1):30–32
Jung ME, Wallis CJ, Gatch MB, Lal H (1999) Sex differences in the pentylenetetrazol-like stimulus induced by ethanol withdrawal. J Pharmacol Exp Ther 291(2):576–582
Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK, Jones SR (2015) Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend 150:24–30
Koob GF (2013) Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci 13:3–30
Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA (2014) Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology 39(4):823–830
Lindholm S, Ploj K, Franck J, Nylander I (2000) Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol 22(3):165–171
Loft S, Olesen KL, Døssing M (1987) Increased susceptibility to liver disease in relation to alcohol consumption in women. Scand J Gastroenterol 22(10):1251–1256
Mann K, Batra A, Günthner A, Schroth G (1992) Do women develop alcoholic brain damage more readily than men? Alcohol Clin Exp Res 16(6):1052–1056
Manzanares J, Lookingland KJ, Moore KE (1991) Kappa opioid receptor-mediated regulation of dopaminergic neurons in the rat brain. J Pharmacol Exp Ther 256(2):500–505
McGinnis JM, Foege WH (1999) Mortality and morbidity attributable to use of addictive substances in the United States. Proc Assoc Am Physicians 111(2):109–118
Melis M, Spiga S, Diana M (2005) The dopamine hypothesis of drug addiction: hypodopaminergic state. Int Rev Neurobiol 63:101–154
Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM (2011) κ-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology 61(1–2):35–42
Phillips PE, Hancock PJ, Stamford JA (2002) Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse 44(1):15–22
Rossetti ZL, Melis F, Carboni S, Diana M, Gessa GL (1992) Alcohol withdrawal in rats is associated with a marked fall in extraneuronal dopamine. Alcohol Clin Exp Res 16(3):529–532
Rossetti ZL, Isola D, De Vry J, Fadda F (1999) Effects of nimodipine on extracellular dopamine levels in the rat nucleus accumbens in ethanol withdrawal. Neuropharmacology 38(9):1361–1369
Schweinsburg BC, Alhassoon OM, Taylor MJ, Gonzalez R, Videen JS, Brown GG, Patterson TL, Grant I (2003) Effects of alcoholism and gender on brain metabolism. Am J Psychiatry 160(6):1180–1183
Siciliano CA, Calipari ES, Ferris MJ, Jones SR (2014) Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci 34(16):5575–5582
Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA, Jones SR (2015a) Voluntary ethanol intake predicts κ-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J Neurosci 35(15):5959–5968
Siciliano CA, Calipari ES, Yorgason JT, Mateo Y, Helms CM, Lovinger DM, Grant KA, Jones SR (2015b) Chronic ethanol self-administration in macaques shifts dopamine feedback inhibition to predominantly D2 receptors in nucleus accumbens core. Drug Alcohol Depend 158:159–163
Sirohi S, Bakalkin G, Walker BM (2012) Alcohol-induced plasticity in the dynorphin/kappa-opioid receptor system. Front Mol Neurosci 5:95
Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH (2013) A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci 16(7):966–973
Substance Abuse and Mental Health Services Administration (2012) National Survey on Drug Use and Health (NSDUH): http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/DetTabs/NSDUH-DetTabsSect2peTabs43to84-2012.htm#Tab2.71B.
Vivian JA et al (2001) Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res 25:1087–1097
Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K (1996) Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 20(9):1594–1598
Volkow ND, Fowler JS, Wang GJ (2002) Role of dopamine in drug reinforcement and addiction in humans: results from imaging studies. Behav Pharmacol 13(5–6):355–366
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C (2007) Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 27(46):12700–12706
Walker BM, Koob GF (2008) Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology 33(3):643–652
Walker JM, Thompson LA, Frascella J, Friederich MW (1987) Opposite effects of mu and kappa opiates on the firing-rate of dopamine cells in the substantia nigra of the rat. Eur J Pharmacol 134(1):53–59
Werling LL, Frattali A, Portoghese PS, Takemori AE, Cox BM (1988) Kappa receptor regulation of dopamine release from striatum and cortex of rats and guinea pigs. J Pharmacol Exp Ther 246(1):282–286
Wilsnack RW, Wilsnack SC, Klassen AD (1984) Women’s drinking and drinking problems: patterns from a 1981 national survey. Am J Public Health 74(11):1231–1238
Wiren KM, Hashimoto JG, Alele PE, Devaud LL, Price KL, Middaugh LD, Grant KA, Finn DA (2006) Impact of sex: determination of alcohol neuroadaptation and reinforcement. Alcohol Clin Exp Res 30(2):233–242
Yorgason JT, España RA, Jones SR (2011) Demon Voltammetry and Analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202(2):158–164
Acknowledgments
This work was funded by NIH grants U01 AA014091, P01 AA021099 (SRJ), F31 DA031533 (ESC), F31 DA037710, T32 AA007565 (CAS), F31 AA020439 (JTY), P51 OD011092, R24 AA019431, P60 AA10760 (KAG), Division of Intramural Clinical and Biomedical Research NIAAA (DML), and Integrative Neuroscience Initiative on Alcoholism AA 13510 (KAG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Siciliano, C.A., Calipari, E.S., Yorgason, J.T. et al. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology 233, 1435–1443 (2016). https://doi.org/10.1007/s00213-016-4239-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4239-4