Abstract
Rationale
3,4 Methylenedioxymethamphetamine (MDMA) preferentially stimulates the release of serotonin (5-HT) that subsequently produces behavioral responses by activation of post-synaptic receptor mechanisms. The 5-HT1A and 5-HT1B receptors are both well localized to regulate dopamine (DA) release, and have been implicated in modulating the reinforcing effects of many drugs of abuse, but a role in acquisition of self-administration has not been determined.
Objectives
This study was designed to determine the effect of pharmacological manipulation of 5-HT1A and 5-HT1B receptor mechanisms on the acquisition of MDMA self-administration.
Methods
The 5-HT1B/1A receptor agonist, RU 24969 (0.0 or 3.0 mg/kg, bid), was administered for 3 days in order to down-regulate both 5-HT1A and 5-HT1B receptors. Following the pretreatment phase, latency to acquisition of MDMA self-administration was measured.
Results
Repeated administration of RU 24969 significantly decreased the latency to acquisition and increased the proportion of animals that acquired MDMA self-administration. Dose-effect curves for the 5-HT1A-mediated hyperactivity produced by the 5-HT1A agonist, 8-OH-DPAT, and the 5-HT1B-mediated adipsic response produced by RU 24969 were shifted rightward, suggesting a desensitization of 5-HT1A and 5-HT1B receptor mechanisms.
Conclusions
These data suggest that the initial reinforcing effects of MDMA are modulated by 5-HT1A and/or 5-HT1B receptor mechanisms. The potential impact of these changes on the DAergic response relevant to self-administration and a possible role in conditioned reinforcement pertaining to acquisition of self-administration are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
3,4 Methylenedioxymethamphetamine (MDMA) is the primary psychoactive component of the internationally popular street drug, ecstasy. Ecstasy is widely used in developed and developing countries (United Nations Office on Drugs and Crime 2015), and a subset of regular ecstasy users met criteria for dependence (Cottler et al. 2001; Degenhardt et al. 2004; Topp et al. 1997).
MDMA is self-administered by laboratory animals (Ball et al. 2007; Fantegrossi et al. 2002; Lile et al. 2005; Reveron et al. 2010; Schenk et al. 2007; Wang and Woolverton 2007), but when compared to self-administration of other psychostimulants, latency to acquisition of MDMA self-administration is relatively long. For example, cocaine and amphetamine self-administration are usually acquired within a few test sessions (Carroll and Lac 1997), whereas MDMA self-administration requires around 15 daily test sessions (Schenk et al. 2012). Additionally, only about 50 % of rats met a criterion for MDMA self-administration (Schenk et al. 2012), whereas virtually all rats acquire cocaine or amphetamine self-administration (Carroll and Lac 1997). This behavioral profile might reflect a unique pharmacology of MDMA.
Most drugs that are self-administered preferentially increase synaptic dopamine (DA) levels in the nucleus accumbens (NAc) (Di Chiara and Imperato 1988; Kalivas et al. 1993; Koob 1992). In contrast, MDMA preferentially stimulates serotonin (5-HT) release and produces smaller increases in synaptic DA (Green et al. 2003). This pharmacological effect might be expected to limit MDMA self-administration. For example, stimulation of 5-HT release inhibited (Rothman et al. 2005) while neurotoxic 5,7-dihydroxytryptamine (DHT) lesions enhanced (Bradbury et al. 2014; Loh and Roberts 1990) self-administration. Self-administration of amphetamine-type drugs was inversely related to affinity for the 5-HT transporter (Ritz and Kuhar 1989) or potency to stimulate 5-HT release (Wee et al. 2005). With specific reference to MDMA, the (+) isomer that selectively releases DA was more readily self-administered than the (−) isomer that selectively releases 5-HT (Wang and Woolverton 2007).
A recent study (Bradbury et al. 2014) further tested the idea that MDMA-produced 5-HT release might be inhibitory to MDMA self-administration. The MDMA-produced increase in synaptic 5-HT was measured by in vivo microdialysis before MDMA self-administration began. As has been observed in many studies, about 50 % of the rats acquired MDMA self-administration. Of interest, MDMA-stimulated 5-HT release was lower for the rats that ultimately met the acquisition criteria. In order to determine whether MDMA-produced 5-HT release inhibited the acquisition of MDMA self-administration, the effect of a neurotoxic, 5,7-DHT, lesion on MDMA self-administration was measured. The lesion greatly facilitated the acquisition of MDMA self-administration, as evidenced by a leftward and upward shift in the acquisition curve. These findings strengthen the idea that variability in the acquisition of MDMA self-administration is due to variability in sensitivity to MDMA-produced 5-HT release. A question remains as to the mechanism for the inhibitory effect of 5-HT on MDMA self-administration. One possibility is that high levels of synaptic 5-HT produced by MDMA during initial self-administration sessions led to neuroadaptive changes in post-synaptic 5-HT receptor mechanisms that modulate DA responses.
There is ample evidence that activation of some 5-HT receptors modulates DA release in the NAc. Of particular relevance to this study are the 5-HT1A and 5-HT1B receptor subtypes. The preferential 5-HT1A agonist, 8-hydroxy-2-(n-dipropylamino)tetralin (8-OH-DPAT), inhibited DA cell activity (Arborelius et al. 1993) and decreased extracellular DA levels in the NAc, as measured by in vivo microdialysis (Ichikawa and Meltzer 2000). Systemic administration of 8-OH-DPAT also attenuated the amphetamine-produced increase in extracellular DA in the NAc (Ichikawa et al. 1995). These findings suggest that 5-HT1A receptor activation is inhibitory to mesolimbic DA neurotransmission. A down-regulation of these receptors would, therefore, be expected to facilitate the acquisition of MDMA self-administration (Müller et al. 2007).
In contrast, activation of 5-HT1B receptors increased DA activity (Alex and Pehek 2007). For example, systemic administration of the 5-HT1B/1A agonist, RU 24969, increased extracellular DA in the NAc (Boulenguez et al. 1996). Local infusion of 5-HT in the NAc increased extracellular DA levels, and this effect was attenuated by the 5-HT1B/1D antagonist, GR 127935 (Hållbus et al. 1997). Activation of 5-HT1B receptors in the ventral tegmental area (VTA) also increased NAc DA (O’Dell and Parsons 2004; Yan and Yan 2001a, 2001b; Yan et al. 2004), and RU 24969 and the more selective 5-HT1B agonist CP 93129 both potentiated the increase in synaptic DA produced by cocaine (O’Dell and Parsons 2004; Parsons et al. 1999). A down-regulation of these receptors would, therefore, be expected to inhibit the acquisition of MDMA self-administration (Sari 2004).
A wealth of data indicate a role of these receptor subtypes in the maintenance of self-administration (Fletcher et al. 2002; Neisewander et al. 2014; Parsons et al. 1998; Peltier and Schenk 1993; Przegaliñski et al. 2007), but the role in the acquisition of self-administration has received far less attention. Our hypothesis is that the latency to acquisition of MDMA self-administration reflects individual variation in 5-HT1A and/or 5-HT1B receptor mechanisms. If so, it should be possible to manipulate receptor mechanisms via repeated agonist or antagonist exposure and to determine the effect on acquisition of MDMA self-administration.
Repeated exposure to the 5-HT1B/1A agonist, RU 24969, resulted in tolerance to the hyperactive response (Oberlander et al. 1987). We have recently shown that RU 24969-produced hyperactivity in rats is due to activation of 5-HT1A, but not 5-HT1B, receptors (Aronsen et al. 2014), suggesting that behavioral tolerance reflects a down-regulation of this receptor subtype. The effect of RU 24969 pretreatment on 5-HT1B receptor mechanisms has not been specifically measured, but we have shown that RU 24969-produced adipsia is a 5-HT1B-mediated response (Aronsen et al. 2014). Therefore, in the present study, we determined the effect of repeated exposure to RU 24969 on the acquisition of MDMA self-administration and on RU 24969-produced adipsia. In order to assess the effect on 5-HT1A receptor mechanisms, we also measured hyperactivity in response to the selective 5-HT1A agonist, 8-OH-DPAT.
Method
Subjects
Male Sprague-Dawley rats (300–330 g) were bred in the vivarium at Victoria University of Wellington. They were housed in polycarbonate cages in groups of four until 3 days before the start of testing, after which they were housed individually. The colony was temperature (21 °C)—and humidity (55 %)—controlled and maintained on a 12:12-h light/dark cycle with lights on at 07:00 h. Food and water were available ad libitum except during testing. All tests were carried out during the light cycle.
Surgery
For rats that underwent self-administration testing, a silastic catheter was implanted into the left jugular vein under deep anesthesia produced by i.p. injection of ketamine (90 mg/kg) and xylazine (9 mg/kg). The distal end of the catheter was passed subcutaneously to an exposed part of the skull, attached to a 3-cm piece of 22-gauge stainless steel tubing, and fixed in place with screws embedded in dental acrylic. Following surgery, an analgesic (Carporfen®, 5.0 mg/kg, subcutaneous (s.c.)) and electrolyte replacement (Hartman’s solution, 12 ml, s.c.) were administered. Carprofen was also administered on each of 2 days following the surgery. RU 24969 pretreatment began once presurgery weight had been attained, generally within 4–6 days.
Apparatus
Self-administration was conducted in operant chambers (Med Associates ENV-001) equipped with two levers. Depression of the active lever resulted in a 12-s activation of a syringe pump (Razell, model A, 1 rpm), resulting in an intravenous infusion of 0.1 ml of MDMA (1.0 mg/kg). Depression of the inactive lever had no programmed consequence.
Locomotor activity was assessed in clear Plexiglas chambers (Med Associates Inc., USA; model ENV-515) measuring 42 × 42 × 30 cm, set in sound-attenuating boxes. Forward locomotion was measured with two sets of 16 infrared beams and sensors spaced evenly along the sides of the chambers producing squares measuring 25 × 25 mm. The interruption of three adjacent beams was recorded as one activity count. A white noise generator was used during experiments to mask any outside noise, and chambers were washed with Virkon “S” disinfectant (Southern Veterinary Supplies, NZ) after testing to control for olfactory confounds. Experiments were run in a dark room, except for a red light that was used to illuminate the room during drug administrations.
RU 24969 pretreatment
RU 24969 (3.0 mg/kg, s.c.), or saline vehicle, was administered in the home cage daily at 09:00 and 16:00 h, for three consecutive days. This protocol was adapted from that used in earlier studies (Callaway and Geyer 1992; Oberlander et al. 1987) and utilizes a dose of RU 24969 that we have previously shown to produce hyperactivity and adipsia (Aronsen et al. 2014).
Acquisition of MDMA self-administration
Self-administration sessions began the day after the last administration of RU 24969. Self-administration was conducted during 2-h daily sessions, 6 days per week. Each self-administration session began with an experimenter-delivered infusion of drug. Thereafter, depression of the active lever produced an infusion of MDMA according to an FR1 schedule. Responses on the active and inactive levers were recorded. Every seventh day, catheters were infused with sodium pentobarbital (20.0 mg/kg, i.v.). Failure to demonstrate an immediate loss of the righting reflex suggested a loss of catheter patency, and the rat was excluded from the study. Catheter patency was lost in four rats (three RU 24969 pretreated and one saline pretreated), resulting in final sample sizes of 9 and 8 for the RU 24969 and saline pretreated groups, respectively. Self-administration testing continued for each rat until a total of 90 infusions (90.0 mg/kg) had been self-administered or 25 days, whichever came first. This acquisition criterion is the same as has been used previously in our laboratory (Bradbury et al. 2014; Oakly et al. 2014).
Water consumption
Separate groups of rats were tested to determine the effects of RU 24969 pretreatment on RU 24969-produced adipsia. The day following the last administration of RU 24969, water bottles were removed from the home cages and were not returned for 24 h. Fifteen minutes before water bottles were reintroduced, RU 24969 (0, 1.0, 3.0 mg/kg, s.c., n = 6–8 per group) was administered. These doses were chosen based on our previous study (Aronsen et al. 2014) that suggested that adipsia following administration of these doses of RU2 4969 was due to 5-HT1B receptor activation. Water bottles were weighed before, and after 30 min of access, to determine water consumption.
Locomotor activity
Separate groups of rats were tested to determine the effect of RU 24969 pretreatment on 5-HT1A receptor mechanisms. Effects of the selective 5-HT1A receptor agonist, 8-OH-DPAT, on locomotor activity was assessed 2 days after the last administration of RU 24969, in order to match the delay between pretreatment and the test for RU 24969-induced adipsia. Rats were placed in the testing chamber for 30 min, followed by an injection of 8-OH-DPAT (0.0, 0.1, 0.3 mg/kg, s.c., n = 4–7 per group), and activity was measured for 60 min post-injection. Locomotor activity counts were recorded in 5-min intervals during the 30 min prior to, and 60 min following, the 8-OH-DPAT injection.
8-OH-DPAT is a selective 5-HT1A agonist but also has appreciable affinity for 5-HT7 receptors (Bard et al. 1993; Lovenberg et al. 1993). To determine whether 8-OH-DAT-produced hyperactivity was due to 5-HT1A activation, we determined the effect of the selective 5-HT1A antagonist, WAY 100635, on 8-OH-DPAT-produced hyperactivity. Rats were placed in the testing chamber and 15 min later were injected with WAY 100635 (0, 0.003, 0.3 mg/kg, s.c., n = 4–5 per group). Following a further 15 min, 8-OH-DPAT (0.3 mg/kg, s.c.) was injected, and activity was measured for an additional 60 min.
Data analysis
Acquisition of self-administration was compared between pretreatment groups with a survival analysis, using the log-rank test to compare Kaplan-Meier survival estimates (Kaplan and Meier 1958). Right censoring was applied to data from rats that did not acquire within the 25-day cutoff period.
RU 24969-produced adipsia was analyzed with a 2 (pretreatment) × 3 (dose of RU 24969) analysis of variance (ANOVA).
Effects of each dose of 8-OH-DPAT on locomotor activity were analyzed by individual 2 (pretreatment) × 12 (time after injection) mixed model ANOVAs with time as the within subject factor. Total activity counts as a function of dose of 8-OH-DPAT and RU 24969 pretreatment were analyzed using a 3 (8-OH-DPAT dose) × 2 (pretreatment) ANOVA. Data for 8-OH-DPAT-produced hyperactivity after administration of WAY 100635 were analyzed using a 3 (dose) × 12 (time after injection) mixed model ANOVA with time as the within subject factor.
Where appropriate, post hoc analysis was conducted using the Tukey HSD method with alpha set at 0.05.
Drugs
±8-OH-DPAT (Tocris, New Zealand; Abcam, New Zealand), RU 24969 (Tocris, New Zealand), and WAY 100635 (Tocris, New Zealand) were dissolved in sterile physiological saline. ±MDMA-HCl (ESR, Porirua, New Zealand) for self-administration was dissolved in a sterilized solution of heparinized saline (3 IU heparin/ml and 0.9 % NaCl). All doses refer to salt weights.
Results
Figure 1 shows the survival curves for the acquisition of self-administration for saline- or RU 24969-treated groups. RU 24969 pretreatment produced a significant increase in the probability of acquiring MDMA self-administration (χ 2 (1) = 12.21, p < 0.01). Of the control group that met the acquisition criterion, 50 % met the criterion within 17 sessions, whereas 50 % of RU 24969 pretreatment group met the acquisition criterion within 10 sessions. It is noteworthy that three rats in the RU 24969 pretreatment group self-administered lethal doses of MDMA (>20 mg/kg) during the first self-administration session, and therefore, additional data from these rats could not be obtained. The high intake during the first self-administration session for these three rats supports the other data, suggesting that RU 24969 pretreatment enhanced the initial reinforcing effects of MDMA.
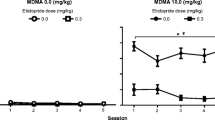
Figure 2 (left panel) shows the effect of RU 24969 pretreatment on RU 24969-produced adipsia. There was a significant interaction between pretreatment and dose (F (2, 37) = 7.85, p = 0.01, ɳ p 2 = 0.30) and a significant effect of dose (F (2, 37) = 53.55, p < 0.01, ɳ p 2 = 0.74). Post hoc tests confirmed a significant difference in the adipsic response between the RU 24969 and saline pretreatment groups following 0.0 and 3.0 mg/kg RU 24969. Since there was a decrease in basal water consumption produced by repeated RU 24969 treatment, the data were further analyzed by expressing drug effects as a percentage of baseline. These data are presented in Fig. 2 (right panel). A two-way ANOVA (pretreatment × dose) revealed a significant effect of pretreatment (F (1, 27) = 20.40, p < 0.01, ɳ p 2 = 0.43).
Locomotor activity produced by the various doses of 8-OH-DPAT as a function of RU 24969 pretreatment is shown in Fig. 3. There were no differences between groups following the 0 mg/kg 8-OH-DPAT dose. The data from 0.1-mg/kg 8-OH-DPAT dose produced a time × pretreatment interaction (F (11, 110) = 4.06, p < 0.01, ɳ p 2 = 0.29) and main effects of time (F (11, 110) = 19.8, p < 0.01, ɳ p 2 = 0.66) and pretreatment (F (1, 10) = 17.6, p < 0.01, ɳ p 2 = 0.64). Post hoc tests revealed significant decreases in activity during time = 10 and 15 min following the injection. There was a significant time × pretreatment interaction (F (11, 121) = 2.77, p < 0.01, ɳ p 2 = 0.20) and main effects of time (F (11, 121) = 62.5, p < 0.01, ɳ p 2 = 0.85) and pretreatment (F (1, 11) = 7.45, p < 0.05, ɳ p 2 = 0.40) for the 0.3-mg/kg 8-OH-DPAT groups. Post hoc tests revealed a significant decrease in activity at time = 25 min. Analysis of total activity counts as a function of dose and pretreatment showed a main effect of 8-OH-DPAT dose (F (2, 27) = 46.0, p < 0.01, ɳ p 2 = 0.77) and a main effect of pretreatment (F (1, 27) = 19.5, p < 0.01, ɳ p 2 = 0.42).
Figure 4 (left panel) shows the time course of the effects of WAY 100635 on 8-OH-DPAT-produced hyperactivity. ANOVA showed a significant interaction between time after injection and dose (F (22, 121) = 7.66, p < 0.01, ɳ p 2 = 0.58) and significant main effects of time (F (11, 121) = 31.1, p < 0.01, ɳ p 2 = 0.74) and dose (F (2, 11) = 21.5, p < 0.01, ɳ p 2 = 0.80). Post hoc tests revealed a significant decrease in 8-OH-DPAT-produced hyperactivity at time = 5, 10, and 15 min following administration of 0.3 mg/kg WAY 100635. The effect of dose is further illustrated in Fig. 4 (right panel). Post hoc analysis showed a significant decrease in 8-OH-DPAT-produced hyperactivity after the 0.3-mg/kg dose of WAY 100635.
Discussion
Pretreatment with RU 24969 decreased the latency to acquisition of MDMA self-administration and increased the proportion of rats that acquired MDMA self-administration. The leftward shift in the acquisition curve for self-administration might reflect a sensitized reinforcing effect since higher doses of drug have also been shown to decrease the latency to acquisition of self-administration (Carroll and Lac 1997; Schenk and Partridge 2000).
A remarkable consequence of pretreatment with RU 24969 was the substantial increase in the proportion of rats that met the criterion for acquisition of MDMA self-administration. As we have previously reported (Bradbury et al. 2014; Schenk et al. 2012), 50 % of control rats meet the criterion within the 25-day cutoff period, as was also observed in the saline-pretreated group in the present study. Thus, some rats appear to be inherently more or less sensitive to the reinforcing effects of MDMA. Following RU 24969 pretreatment, however, all of the rats met the criterion for acquisition of MDMA self-administration within the limits of the study (25 test sessions). We have suggested that the initial resistance to self-administration can be overcome by limiting the impact of 5-HT, since a similar increase in the percentage of subjects that acquired MDMA self-administration was produced following neurotoxic 5,7 DHT lesions in rats (Bradbury et al. 2014) and in 5-HT transporter knockout rats (Oakly et al. 2014).
In order to assess the impact of more specific 5-HT mechanisms on the acquisition of MDMA self-administration, the present study repeatedly administered the 5-HT1B/1A agonist, RU 24969, as a pretreatment in an attempt to down-regulate 5-HT1A and 5-HT1B receptors. We determined effects of the pretreatment by measuring behavioral responses that have been attributed to either 5-HT1B (RU 24969-produced adipsia (Aronsen et al. 2014)) or 5-HT1A (8-OH-DPAT-produced hyperactivity (Hillegaart et al. 1996)) mechanisms.
As previously reported (Aronsen et al. 2014), RU 24969 produced dose-dependent adipsia. The dose-response curve for this response is relatively narrow; minimal effects were produced following administration of 0.3 mg/kg, and maximal effects were produced following administration of 3.0 mg/kg (Aronsen et al. 2014). RU 24969 pretreatment decreased basal water consumption, and when this was accounted for, RU 24969 pretreatment decreased the subsequent RU 24969-produced adipsic response. These findings are consistent with a rightward shift in the dose-response curve and suggest a down-regulation of 5-HT1B receptors. 5-HT1B receptor down-regulation has previously been evidenced by decreased messenger RNA (mRNA) levels (Chennaoui et al. 2001; Hiroi and Neumaier 2009) or decreased binding density (Kindlundh et al. 2003; Suzuki et al. 2010), both of which could explain the present behavioral data.
RU 24969 pretreatment also shifted the dose-response curve for 8-OH-DPAT-produced hyperactivity to the right; the most pronounced effect of pretreatment was on hyperactivity produced by the lowest doses of 8-OH-DPAT tested. This might explain why a similar pretreatment with RU 24969 failed to alter hyperactivity produced by a higher dose of 1.25 mg/kg 8-OH-DPAT (Oberlander et al. 1987).
Because 8-OH-DPAT has appreciable affinity for the 5-HT7 receptor (Bard et al. 1993; Lovenberg et al. 1993), it was important to ensure that the effects observed were due to 5-HT1A activation. Hyperactivity produced by 8-OH-DPAT was attenuated by the selective receptor antagonist, WAY 100635, confirming a 5-HT1A receptor mechanism. Of interest, a similar RU 24969 pretreatment regimen also reduced the locomotor response to RU 24969 (Callaway and Geyer 1992), a behavioral response that we have attributed to 5-HT1A receptor activation (Aronsen et al. 2014). Therefore, these findings are consistent with a down-regulation of 5-HT1A receptors following RU 24969 pretreatment. 5-HT1A down-regulation has been shown via decreased agonist-stimulated binding of [35S]GTPγS to G proteins (Fuss et al. 2013; Hensler et al. 2010), decreased receptor-binding densities or immunoreactivity (Fuss et al. 2013; Gui et al. 2011), decreased 5-HT1A mRNA (Wang et al. 2009), and decreased protein levels (Iyo et al. 2009; Wang et al. 2009). It would be of great interest to determine which, if any, of these mechanisms can explain the present data.
The available literature is consistent with the idea that MDMA self-administration, like self-administration of other drugs of abuse, progresses as a result of sensitized DA and desensitized 5-HT responses. Thus, repeated exposure to MDMA increased DA (Colussi-Mas et al. 2010; Kalivas et al. 1998) and decreased 5-HT (Baumann et al. 2008; Reveron et al. 2010; Shankaran and Gudelsky 1999) synaptic output, as measured by in vivo microdialysis, DA antagonists reduced MDMA self-administration (Brennan et al. 2009; Daniela et al. 2004), and DA, but not 5-HT, agonists potentiated drug-seeking following extinction of MDMA self-administration (Schenk et al. 2011).
MDMA preferentially releases 5-HT and the ensuing activation of post-synaptic receptors impacts DA release, providing potential mechanisms for the enhanced DA response. In this study, both 5-HT1B and 5-HT1A receptor mechanisms were down-regulated, as measured by behavioral assays. Given the selectivity of RU 24969 for 5-HT1A/1B receptors, it is unlikely that alterations in a different receptor mechanism underlie the facilitated acquisition of self-administration found in the present study.
Activation of 5-HT1B receptors enhanced DA neurotransmission (Alex and Pehek 2007), so the down-regulation of these receptor mechanisms, which would be expected to decrease MDMA-produced DA, cannot easily explain the facilitated self-administration. On the other hand, a wealth of data suggest that activation of 5-HT1A receptors is inhibitory to cocaine self-administration (Müller et al. 2007), possibly via inhibition of DA release (Ichikawa and Meltzer 2000). Therefore, a down-regulation of this receptor subtype might be expected to disinhibit MDMA-produced DA, leading to more rapid acquisition of self-administration due to increased reinforcing effects. This might also explain the facilitated acquisition of MDMA self-administration in serotonin transporter knockout rats (Oakly et al. 2014), since this manipulation also desensitized 5-HT1A receptor mechanisms (Homberg et al. 2008).
5-HT1A receptors are widely localized in brain and are well positioned to modulate activity in a large number of brain systems (Aznar et al. 2003). Of importance, these receptors are localized on tyrosine hydroxylase-immunoreactive cells in the VTA (Doherty and Pickel 2001) and also in DA terminal regions in the NAc (Alex and Pehek 2007). Systemic administration of 8-OH-DPAT inhibited amphetamine-produced DA release in the NAc (Ichikawa et al. 1995). The down-regulation produced by RU 24969 pretreatment would, therefore, be expected to disinhibit stimulated DA. Similar studies have not been conducted using MDMA, but this mechanism would explain the facilitated acquisition of self-administration.
The acquisition of self-administration is also influenced by factors in addition to the initial reinforcing effects of the drug, and some of these factors are modified by 5-HT1A receptor mechanisms. For example, reliable self-administration is often facilitated via Pavlovian conditioning processes by pairing delivery of the drug reinforcer with a discrete, discriminative stimulus, like a light, as was done in the present study (Di Ciano and Everitt 2004). The strengthening of stimulus/reward associations is markedly inhibited by administration of 5-HT1A agonists (Blair et al. 2004; Winsauer et al. 1999; Frick et al. 2015). These findings raise the possibility that activation of post-synaptic 5-HT1A receptors pursuant to MDMA-stimulated 5-HT release limits the acquisition of MDMA self-administration, in some subjects, by interfering with associative learning. If so, our data suggest that this effect is mitigated by exposure to a regimen of RU 24969 pretreatment that down-regulated these receptor mechanisms, thereby facilitating MDMA self-administration as indicated by both a leftward and upward shift in the self-administration acquisition curves.
References
Alex K, Pehek E (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113:296–320
Arborelius L, Chergui K, Murase S, Nomikos GG, Höök BB, Chouvet G, Hacksell U, Svensson TH (1993) The 5-HT1A receptor selective ligands, (R)-8-OH-DPAT and (S)-UH-301, differentially affect the activity of midbrain dopamine neurons. Naunyn Schmiedebergs Arch Pharmacol 347:353–362
Aronsen D, Webster J, Schenk S (2014) RU 24969-produced adipsia and hyperlocomotion: differential role of 5HT1A and 5HT1B receptor mechanisms. Pharmacol Biochem Behav 124:1–4
Aznar S, Qian Z, Shah R, Rahbek B, Knudsen GM (2003) The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res 959:58–67
Ball KT, Walsh KM, Rebec GV (2007) Reinstatement of MDMA (ecstasy) seeking by exposure to discrete drug-conditioned cues. Pharmacol Biochem Behav 87:420–425
Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL (1993) Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem 268:23422–23426
Baumann MH, Clark RD, Franken FH, Rutter JJ, Rothman RB (2008) Tolerance to 3,4-methylenedioxymethamphetamine in rats exposed to single high-dose binges. Neuroscience 152:773–784
Blair C, Bonardi C, Hall G (2004) Differential effects of 8-OH-DPAT on two forms of appetitive Pavlovian conditioning in the rat. Behav Neurosci 118:1439
Boulenguez P, Rawlins J, Chauveau J, Joseph M, Mitchell S, Gray J (1996) Modulation of dopamine release in the nucleus accumbens by 5-HT1B agonists: involvement of the hippocampo-accumbens pathway. Neuropharmacology 35:1521–1529
Bradbury S, Bird J, Colussi-Mas J, Mueller M, Ricaurte G, Schenk S (2014) Acquisition of MDMA self-administration: pharmacokinetic factors and MDMA-induced serotonin release. Addict Biol 19:874–884
Brennan KA, Carati C, Lea RA, Fitzmaurice PS, Schenk S (2009) Effect of D1-like and D2-like receptor antagonists on methamphetamine and 3,4-methylenedioxymethamphetamine self-administration in rats. Behav Pharmacol 20:688–694
Callaway CW, Geyer MA (1992) Tolerance and cross-tolerance to the activating effects of 3,4-methylenedioxymethamphetamine and a 5-hydroxytryptamine1B agonist. J Pharmacol Exp Ther 263:318–326
Carroll ME, Lac ST (1997) Acquisition of iv amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology (Berl) 129:206–214
Chennaoui M, Drogou C, Gomez-Merino D, Grimaldi B, Fillion G, Guezennec C (2001) Endurance training effects on 5-HT1B receptors mRNA expression in cerebellum, striatum, frontal cortex and hippocampus of rats. Neurosci Lett 307:33–36
Colussi-Mas J, Wise RJ, Howard A, Schenk S (2010) Drug seeking in response to a priming injection of MDMA in rats: relationship to initial sensitivity to self-administered MDMA and dorsal striatal dopamine. Int J Neuropsychopharmacol 13:1315–1327
Cottler LB, Womack SB, Compton WM, Ben-Abdallah A (2001) Ecstasy abuse and dependence among adolescents and young adults: applicability and reliability of DSM-IV criteria. Human Psychopharmacol 16:599–606
Daniela E, Brennan K, Gittings D, Hely L, Schenk S (2004) Effect of SCH 23390 on (±)-3,4-methylenedioxymethamphetamine hyperactivity and self-administration in rats. Pharmacol Biochem Behav 77:745–750
Degenhardt L, Barker B, Topp L (2004) Patterns of ecstasy use in Australia: findings from a national household survey. Addiction 99:187–195
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci 85:5274–5278
Di Ciano P, Everitt BJ (2004) Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology 47(Suppl 1):202–13
Doherty MD, Pickel VM (2001) Targeting of serotonin 1A receptors to dopaminergic neurons within the parabrachial subdivision of the ventral tegmental area in rat brain. J Comp Neurol 433:390–400
Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G (2002) 3,4-Methylenedioxymethamphetamine (MDMA,“ ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology (Berl) 161:356–364
Fletcher PJ, Azampanah A, Korth KM (2002) Activation of 5-HT1B receptors in the nucleus accumbens reduces self-administration of amphetamine on a progressive ratio schedule. Pharmacol Biochem Behav 71:717–725
Frick LR, Bernardez-Vidal M, Hocht C, Zanutto BS, Rapanelli M (2015) Dual role of serotonin in the acquisition and extinction of reward-driven learning: involvement of 5-HT1A, 5-HT2A and 5-HT3 receptors. Behav Brain Res 277:193–203
Fuss J, Vogt MA, Weber KJ, Burke TF, Gass P, Hensler JG (2013) Hippocampal serotonin-1A receptor function in a mouse model of anxiety induced by long-term voluntary wheel running. Synapse 67:648–655
Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI (2003) The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”). Pharmacol Rev 55:463–508
Gui Z, Zhang Q, Liu J, Zhang L, Ali U, Hou C, Fan L, Sun Y, Wu Z, Hui Y (2011) Unilateral lesion of the nigrostriatal pathway decreases the response of fast-spiking interneurons in the medial prefrontal cortex to 5-HT1A receptor agonist and expression of the receptor in parvalbumin-positive neurons in the rat. Neurochem Int 59:618–627
Hållbus M, Magnusson T, Magnusson O (1997) Influence of 5-HT1B/1D receptors on dopamine release in the guinea pig nucleus accumbens: a microdialysis study. Neurosci Lett 225:57–60
Hensler JG, Vogt MA, Gass P (2010) Regulation of cortical and hippocampal 5-HT 1A receptor function by corticosterone in GR+/− mice. Psychoneuroendocrinology 35:469–474
Hillegaart V, Estival A, Ahlenius S (1996) Evidence for specific involvement of 5-HT1A and 5-HT2A/C receptors in the expression of patterns of spontaneous motor activity of the rat. Eur J Pharmacol 295:155–161
Hiroi R, Neumaier JF (2009) Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience 158:456–464
Homberg JR, De Boer SF, Raasø HS, Olivier JD, Verheul M, Ronken E, Cools AR, Ellenbroek BA, Schoffelmeer AN, Vanderschuren LJ (2008) Adaptations in pre-and postsynaptic 5-HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology (Berl) 200:367–380
Ichikawa J, Kuroki T, Kitchen MT, Meltzer HY (1995) R (+)-8-OH-DPAT, a 5-HT1A receptor agonist, inhibits amphetamine-induced dopamine release in rat striatum and nucleus accumbens. Eur J Pharmacol 287:179–184
Ichikawa J, Meltzer HY (2000) The effect of serotonin1A receptor agonism on antipsychotic drug-induced dopamine release in rat striatum and nucleus accumbens. Brain Res 858:252–263
Iyo AH, Kieran N, Chandran A, Albert PR, Wicks I, Bissette G, Austin MC (2009) Differential regulation of the serotonin 1 A transcriptional modulators five prime repressor element under dual repression-1 and nuclear-deformed epidermal autoregulatory factor by chronic stress. Neuroscience 163:1119–1127
Kalivas PW, Duffy P, White SR (1998) MDMA elicits behavioral and neurochemical sensitization in rats. Neuropsychopharmacology 18:469–479
Kalivas PW, Sorg B, Hooks M (1993) The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmacol 4:315–334
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Kindlundh A, Lindblom J, Bergström L, Nyberg F (2003) The anabolic–androgenic steroid nandrolone induces alterations in the density of serotonergic 5HT1B and 5HT2 receptors in the male rat brain. Neuroscience 119:113–120
Koob GF (1992) Neural mechanisms of drug reinforcement. Ann N Y Acad Sci 654:171–191
Lile JA, Ross JT, Nader MA (2005) A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend 78:135–140
Loh EA, Roberts DC (1990) Break-points on a progressive ratio schedule reinforced by intravenous cocaine increase following depletion of forebrain serotonin. Psychopharmacology (Berl) 101:262–266
Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11:449–458
Müller CP, Carey RJ, Huston JP, Silva MADS (2007) Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol 81:133–178
Neisewander JL, Cheung TH, Pentkowski NS (2014) Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: implications for medications development. Neuropharmacology 76:301–319
O’Dell LE, Parsons LH (2004) Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nucleus accumbens dopamine levels. J Pharmacol Exp Ther 311:711–719
Oakly A, Brox B, Schenk S, Ellenbroek B (2014) A genetic deletion of the serotonin transporter greatly enhances the reinforcing properties of MDMA in rats. Mol Psychiatry 19:534–535
Oberlander C, Demassey Y, Verdu A, Van de Velde D, Bardelay C (1987) Tolerance to the serotonin 5-HT1 agonist RU 24969 and effects on dopaminergic behaviour. Eur J Pharmacol 139:205–214
Parsons LH, Koob GF, Weiss F (1999) RU 24969, a 5-HT1B/1A receptor agonist, potentiates cocaine-induced increases in nucleus accumbens dopamine. Synapse 32:132–135
Parsons LH, Weiss F, Koob GF (1998) Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci 18:10078–10089
Peltier R, Schenk S (1993) Effects of serotonergic manipulations on cocaine self-administration in rats. Psychopharmacology (Berl) 110:390–394
Przegaliñski E, Gołda A, Frankowska M, Zaniewska M, Filip M (2007) Effects of serotonin 5-HT1B receptor ligands on the cocaine- and food-maintained self-administration in rats. Eur J Pharmacol 559:165–172
Reveron ME, Maier EY, Duvauchelle CL (2010) Behavioral, thermal and neurochemical effects of acute and chronic 3,4-methylenedioxymethamphetamine (“ecstasy”) self-administration. Behav Brain Res 207:500–507
Ritz MC, Kuhar MJ (1989) Relationship between self-administration of amphetamine and monoamine receptors in brain: comparison with cocaine. J Pharmacol Exp Ther 248:1010–1017
Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, Roth BL, Baumann MH (2005) Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. J Pharmacol Exp Ther 313:1361–1369
Sari Y (2004) Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28:565–582
Schenk S, Colussi-Mas J, Do J, Bird J (2012) Profile of MDMA self-administration from a large cohort of rats: MDMA develops a profile of dependence with extended testing. J Drug Alcohol Res 1:1–6
Schenk S, Gittings D, Colussi-Mas J (2011) Dopaminergic mechanisms of reinstatement of MDMA-seeking behaviour in rats. Br J Pharmacol 162:1770–1780
Schenk S, Hely L, Lake B, Daniela E, Gittings D, Mash DC (2007) MDMA self-administration in rats: acquisition, progressive ratio responding and serotonin transporter binding. Eur J Neurosci 26:3229–3236
Schenk S, Partridge B (2000) Sensitization to cocaine’s reinforcing effects produced by various cocaine pretreatment regimens in rats. Pharmacol Biochem Behav 66:765–770
Shankaran M, Gudelsky GA (1999) A neurotoxic regimen of MDMA suppresses behavioral, thermal and neurochemical responses to subsequent MDMA administration. Psychopharmacology (Berl) 147:66–72
Suzuki H, Han SD, Lucas LR (2010) Chronic passive exposure to aggression decreases D2 and 5-HT1B receptor densities. Physiol Behav 99:562–570
Topp L, Hall W, Hando J (1997) Is there a dependence syndrome for ecstasy? NDARC Technical Report No. 51. National Drug and Alcohol Research Centre, University of NSW, Sydney, pp 1–36
United Nations Office on Drugs and Crime (2015) World drug report, 2015
Wang S-h, Zhang Z-j, Guo Y-j, Teng G-j, Chen B-a (2009) Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Res 170:245–251
Wang Z, Woolverton WL (2007) Estimating the relative reinforcing strength of (±)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology (Berl) 189:483–488
Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL (2005) Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther 313:848–854
Winsauer P, Rodriguez F, Cha A, Moerschbaecher J (1999) Full and partial 5-HT1A receptor agonists disrupt learning and performance in rats. J Pharmacol Exp Ther 288:335–347
Yan Q-S, Yan S-E (2001a) Activation of 5-HT1B/1D receptors in the mesolimbic dopamine system increases dopamine release from the nucleus accumbens: a microdialysis study. European Journal of Pharmacology 418: 55-64.
Yan Q-S, Yan S-E (2001b) Serotonin-1B receptor-mediated inhibition of [3H] GABA release from rat ventral tegmental area slices. Journal of Neurochemistry 79: 914-922.
Yan Q-S, Zheng S-Z, Yan S-E (2004) Involvement of 5-HT1B receptors within the ventral tegmental area in regulation of mesolimbic dopaminergic neuronal activity via GABA mechanisms: a study with dual-probe microdialysis. Brain Res 1021:82–91
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were approved by the Victoria University of Wellington Animal Ethics Committee.
Conflict of interest
The authors declare that they have no conflict of interest to disclose.
Rights and permissions
About this article
Cite this article
Aronsen, D., Bukholt, N. & Schenk, S. Repeated administration of the 5-HT1B/1A agonist, RU 24969, facilitates the acquisition of MDMA self-administration: role of 5-HT1A and 5-HT1B receptor mechanisms. Psychopharmacology 233, 1339–1347 (2016). https://doi.org/10.1007/s00213-016-4225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4225-x