Abstract
Rationale
In humans, novelty/sensation seeking is seen as a personality trait with a positive relationship with addiction vulnerability. In animal studies, one of the standard procedures to model novelty seeking is the “response to novelty,” i.e., the levels of locomotor activity in a new environment. In rodents, a positive correlation was demonstrated between the response to novelty and several effects of drugs, especially the locomotor stimulant effects of cocaine.
Objectives
The present study was designed to test in mice whether the response to novelty is stable across environments and whether its relationship with the stimulant effects of cocaine is altered by environmental changes. Experiment 1 assessed the responses to novelty of the same mice in two different novel environments. Experiment 2 tested the correlation between response to novelty and acute stimulant effects of cocaine recorded in two distinct environments.
Results
The results show a weak correlation only during the first 5 min of the session between the responses to novelty measured in two distinct environments. Experiment 2 demonstrates that novelty responses and stimulant effects of cocaine are positively correlated only when both behavioral responses are measured in the same environment. In contrast, the relationship between response to novelty and acute stimulant effects of cocaine is completely lost when the behavioral responses are recorded in two different environments.
Conclusions
The present results question the usual interpretation of the correlation between the response to novelty and the stimulant effects of cocaine as reflecting a relationship between two underlying individual stable characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Novelty seeking, defined as “an enhanced specific exploration of novel situations, unknown objects or stimuli” (Redolat et al. 2009), is a widely used term in neuroscience. In particular, an impressive number of studies report a significant relationship between the level of novelty seeking and the vulnerability to drugs of abuse. In laboratory rodents, different experimental paradigms have been used to model novelty seeking (Kliethermes and Crabbe 2006). However, most experiments on the relationship between novelty seeking and the addictive effects of drugs have recorded the locomotor response to novelty which usually refers to the level of horizontal locomotion rodents display in a new environment (Blanchard et al. 2009; Davis et al. 2008; Gong et al. 1996; Kabbaj 2006; Kabbaj et al. 2000; Kalinichev et al. 2004; Pawlak et al. 2008; Shimosato and Watanabe 2003; Suto et al. 2001).
Compared to those with a low response to novelty, rats with a high response to novelty show a greater sensitivity to the acute locomotor stimulant effects of many psychoactive drugs (Arias et al. 2009) such as amphetamine (Hooks et al. 1992; Hooks et al. 1994; Nowak et al. 2000), dexamphetamine (Cools and Gingras 1998), cocaine (Brabant et al. 2005; Gong et al. 1996; Kosten and Miserendino 1998), morphine (Kalinichev et al. 2004), apomorphine (Hooks et al. 1994), and caffeine (Hooks et al. 1992). In mice tested in our laboratory, we have found a positive correlation between the locomotor response to novelty and the stimulant effects of 12 mg/kg cocaine (Brabant et al. 2005), and Shimosato and Watanabe (2003) showed a similar effect with other cocaine doses (5, 10, and 20 mg/kg). To demonstrate the positive relationship between the response to novelty and the effects of drugs, several studies used a median split analysis in which the metric locomotor response to novelty is converted into a dichotomous variable with animals defined as high responders (HR) or low responders (LR) to novelty. In these studies, animals were typically tested for their locomotor activity (total distance traveled) in an open field and then divided into two groups depending on whether their locomotor performance was above (HR) or below (LR) the median locomotor activity of all animals (Blanchard et al. 2009; Kabbaj 2006; Pawlak et al. 2008; Piazza et al. 1989; Shimosato and Watanabe 2003; Suto et al. 2001).
According to the theory underlying those studies, the response to novelty is a stable personality trait which is often modeled in rodents by the locomotor response to a novel environment. Some stability across environments is therefore expected. In other words, HR animals should have higher locomotor activity levels than LR animals whatever the physical characteristics of the novel environment in which they are tested. However, some previous studies suggested that the response to novelty might fluctuate in different environments. Marinelli (2005) reported that the pattern of response to novelty is dependent upon the testing environment (shape, size, illumination, etc.) and upon the duration of the test session. In addition, Kalueff et al. (2006) compared mice exploratory behaviors in various novel open fields varying in size and shape. They showed a stability of novelty exploration (changes in the size of open fields did not affect the pattern of exploration) only for vertical activity but not for horizontal locomotor activity (distance traveled) (Kalueff et al. 2006).
Past studies on the relationship between the response to novelty and stimulant effects of drugs used the same environment to measure the response to novelty and the stimulant effects of drugs. However, the question arises as to whether the positive correlation between the response to novelty and the effects of drugs is affected by environmental changes. The present study was designed to test whether the response to novelty (measured by horizontal locomotor response to a novel environment) is stable across environments and whether its relationship with the stimulant effects of cocaine is altered by environmental changes. A first experiment tested whether there is a significant correlation between the locomotor responses to novelty of the same mice in two different environments. Another experiment then attempted to replicate the results obtained in previously published studies about the correlation between the response to novelty and the acute stimulant effects of cocaine, but using two distinct environments. Additionally, the relationship between the response to novelty and the stimulant effects of cocaine was assessed using the two most popular analytic techniques with either the continuous metric variables (correlations) or the categorization of mice into HR and LR mice on the basis of a median split analysis. Such a procedure allows for comparison with previously published studies.
Materials and methods
Subjects
For the whole study, 305 adult (age 12 weeks at the beginning of the test) female Swiss mice were randomly selected from the breeding of our laboratory. These mice were bred from progenitors purchased from Janvier Laboratories (Le Genest-Saint-Isle, France). They were housed two per cage 1 week before the beginning of the experiments and maintained at constant temperature (22 ± 1 °C) on a 12-h light/dark cycle (lights on at 6:00 a.m.; lights off at 6:00 p.m.). The mice had unlimited access to food (standard pellets, Carfil Quality BVDA, Oud-Turnhout, Belgium) and water. Experiments were always conducted during the light phase of the cycle, between 1 p.m. and 4:30 p.m. All procedures and animal maintenance were reviewed by the University of Liege Animal Care and Experimentation Committee, which gave its approval according to the Belgian implementation of the animal welfare guidelines laid down by the European Union (“Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes”).
Drug
Cocaine hydrochloride (Belgopia, Louvain-La-Neuve, Belgium) was dissolved in an isotonic saline solution (0.9 % NaCl vehicle) and administered at a dose of 15 mg/kg and at a volume of 0.01 ml/g. The control treatment (isotonic saline solution) was administered in the same volume. Substances were injected intraperitoneally (i.p.).
Apparatuses
For the present study, the mice were tested in two experimental devices that were used in previously published studies on the stimulant effects of drugs (e.g., Brabant et al. 2009; Didone et al. 2014). Both of them consisted of an open field designed to measure horizontal locomotor activity in mice.
The first open field is an automated activity monitor (A) that comprises a square enclosure made from 0.5-cm clear acrylglas panels without a base (20.5 cm × 20.5 cm × 28.5 cm height). The apparatus is fully described in Brabant et al. (2009). Briefly, locomotor activity is recorded by a pair of infrared light-beam sensors located on each side of the enclosure. A mouse has to cross the full distance (at least 6.5 cm) between two parallel beams for each locomotor count.
The second open field is a videotracking (VT) system from Viewpoint (Lyon, France). The apparatus is fully described in Didone et al. (2014). The open field consisted of a cube (40 cm × 40 cm × 40 cm) and locomotor activity is recorded by a video camera located above the open field. Total distance traveled by each mouse is extracted from the videotracking software.
It is important to note that both experimental devices record horizontal locomotor activity in very similar ways. We have conducted an experiment demonstrating highly significant positive correlations between locomotor counts assessed with the automated activity monitor and distance traveled assessed with the videotracking system when locomotor activity was recorded simultaneously with both devices. The correlations obtained on 17 female adult Swiss mice were statistically significant both for the first 5 min of the session (r = 0.76; p < 0.0001) and for an entire 30-min test session (r = 0.78; p < 0.0001).
Experiment 1: stability of the response to novelty across environments
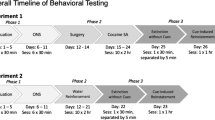
The first experiment was designed to study the stability of the response to novelty across environments. The mice were tested for their response to novelty in two different environments.
Ninety-six female adult Swiss mice were divided into two groups (group 1 and group 2) of 48. Each group was tested twice (once per week) to record the locomotor response to novelty in the two environments, but in the opposite order. During the first week, group 1 was tested in the automated activity monitor and group 2 in the videotracking system. During the second week, the reverse procedure was used: group 1 was tested in the videotracking system and group 2 in the automated activity monitor.
Mice were simply placed into the open field without any injection to assess their locomotor activity for 30 min. The open fields were thoroughly cleaned between each test session.
Experiment 2: relationship between the response to novelty and cocaine stimulant effects
The second experiment was designed to investigate whether there is a positive correlation between the response to novelty and the acute stimulant effects of cocaine when these two behavioral measures are assessed in different environments.
A total of 192 female adult Swiss mice were divided into 4 groups of 48. Each group underwent a two-phase experiment: in the first phase, the locomotor response to novelty was recorded, and in the second phase, the acute stimulant effects of cocaine were measured. In the first phase (first day of the experiment), the mice were simply placed in a novel open field for 30 min without injection. The second phase lasted for 2 days and was identical to most of the studies measuring the acute locomotor stimulant effects of cocaine. First, the mice were habituated to the open field in which they received a cocaine injection the next day. The habituation session took place on the second day of the experiment. The mice were injected with saline and placed into the open field for 30 min. On the third day of the experiment, the mice were tested for their locomotor response to cocaine. Half of the groups of mice were injected with 15 mg/kg cocaine, whereas the other half (control groups) was injected with saline. The mice were then placed into the open field and their activity was recorded for 30 min.
The four groups of the second experiment differed only by the environments (automated activity monitor vs videotracking system) used to assess their locomotor activity during the two phases of the experiment. The first group (A-A) was tested in the automated activity monitors at both phases. In the second group (VT-A), the first phase occurred in the videotracking system and the second phase in the automated activity monitors. In the third group (A-VT), the first phase occurred in the automated activity monitors and the second phase in the videotracking system. The fourth group (VT-VT) was tested in the videotracking system at both phases. The open fields were thoroughly cleaned between each test session.
Statistical analyses
In experiment 1, the effect of the order of the testing sessions on locomotor activity was tested with Student t tests for independent samples to compare mean locomotor activity between groups 1 and 2. Student t tests were computed separately for locomotor activity in the videotracking system and the automated activity monitors, as well as for the first 5 min and for the entire 30-min test session. As the order of testing had no significant effects on locomotor activity (see “Results” section), groups 1 and 2 were pooled together for the subsequent analyses.
In order to test for the stability of the novelty response across environments, Pearson’s correlations were computed between the locomotor scores assessed with the automated activity monitors and with the videotracking system. Correlations were performed on locomotor activity for the first 5 min of the session and for the entire 30-min test session in order to cover similar session lengths as in previously published studies.
To further analyze the data of the first experiment, the time courses of habituation in the automated activity monitors and in the videotracking system were compared using 5-min intervals. To make the comparison possible between locomotor scores measured with two different recording systems, the raw locomotor scores were converted into percentages of locomotor activity scores of the first 5 min of the test in a given open field (the first 5 min therefore corresponds to a 100 % value for each mouse). The curves of habituation were then analyzed with a mixed-model two-way ANOVA with the environment (automated activity monitors vs videotracking system, two levels) defined as a between-subject factor and the time course of the session (5-min intervals, five levels) as a within-subject factor. The first time interval (5 min) was not included in the analysis as all mice had a 100 % score for this interval and there was therefore no variance between the scores at that interval.
A cluster analysis based on the hierarchic classification method was then used to further explore the patterns of locomotor activity in both open fields. Indeed, the different sizes of the open fields might lead to exploration behaviors of shorter or longer durations, such that a comparison of the same time periods in the two environments might not be optimal. The cluster analysis allows identifying clusters of behavioral activity. On the basis of such an analysis, a new set of correlations was calculated on unprocessed raw data to determine the pattern of correlations between locomotor activities in both environments, taking into account the defined clusters.
In experiment 2, the stimulant effects of cocaine measured either in the automated activity monitors or in the videotracking system were analyzed separately using two-way mixed-model ANOVAs. In these analyses, the drug treatment (saline vs cocaine, two levels) was defined as a between-subject factor and the time course of the session (5-min intervals, six levels) as a within-subject factor. Correlations were then computed to test for the relationship between the response to novelty and the stimulant effects of cocaine. Correlations were performed using the first 5 min of the session and the entire 30-min session of the response to novelty. However, in order to avoid computing too many correlations and because the stimulant effects of cocaine last longer than the response to novelty (see also “Results” section), only the locomotor activity for the entire 30-min cocaine test session was used for the correlations. Similarly to experiment 1, a cluster analysis based on the hierarchic classification method was used to further explore the patterns of locomotor activity in both open fields during the response to novelty session. On the basis of such an analysis, the identified clusters were used in correlation analyses to test for correlations between these clusters and the locomotor stimulant effects of cocaine.
The results of experiments 1 and 2 were reanalyzed using the classification of mice into “high responders” (HR) and “low responders” (LR) to novelty instead of using correlations on raw locomotor activity. The classification procedure and the results are provided in a supplementary file.
Where necessary, square root transformations normalized raw data prior to ANOVA, more nearly meeting the assumption of homogeneity of variances (following a significant Leven’s test). For the sake of clarity, means of the raw values are presented in graphs. Relevant between-mean differences were assessed with Newman-Keuls post hoc tests. Significance was always set at p < 0.05.
Results
Experiment 1: stability of the response to novelty across environments
Because of a technical malfunction of the automated activity monitor system, the scores of five mice were lost. These mice were removed from all analyses.
The order of testing sessions had no significant effects on mean locomotor activity, as shown by the Student t tests comparing locomotor activity in groups 1 and 2 (all p > 0.05).
As shown on Fig. 1, locomotor scores in the automated activity monitors and in the videotracking system were significantly correlated only during the first 5 min (r = 0.25; p = 0.016). However, the correlation was of low magnitude with a determination coefficient (r 2) of only 0.063. For the entire 30-min test session, there was no significant correlation between the locomotor scores in the two open fields (r = 0.07; p = 0.53).
The mixed-model two-way ANOVA (open field × time course of the session) used to compare the curves of habituation in the automated activity monitors and in the videotracking system revealed a significant main effect of the open field (F (1,89) = 130.33; p < 0.0001), a significant main effect of the session (F (4,356) = 82.28; p < 0.0001), and a significant interaction between these factors (F (4,356) = 2.80; p < 0.05). These results indicate that habituation to the environment occurred differently in the automated activity monitors and in the videotracking system (Fig. 2). Post hoc tests showed that the percentages of activity differed between the two open fields at the second, third, fourth, fifth, and last time interval, indicating that habituation occurred faster and more efficiently in the automated activity monitors than in the videotracking system.
Comparison of the curves of habituation to novelty in the automated activity monitor (white circles) and in the videotracking system (black circles). For each 5-min time interval, locomotor activity is expressed as a mean percentage (±SEM) of the locomotor activity during the first 5 min of the session
Figure 3 shows the results of the cluster analysis used to identify correlated and uncorrelated patterns of locomotor activity in both open fields. Three clusters were identified for the videotracking system but only two for the automated activity monitor. Post hoc tests indicate that the first 5 min of testing (cluster A1 and cluster VT1) was different from the rest of the testing session in both open fields. There was a significant correlation between clusters VT1 and A1 (r = 0.25; p = 0.016). In the automated activity monitor, locomotor activity quickly dropped after the first 5 min and then remained low until the end of the session (cluster A2). In the videotracking system, locomotion decreased slowly over the session during 15 min (cluster VT2) and remained more stable during the last 10 min (cluster VT3). Except for clusters VT1 and A1, there were no significant correlations between the clusters identified in the two open fields (r VT1.A2 = 0.15, p = 0.17; r VT2.A1 = −0.014, p = 0.90; r VT2.A2 = 0.055, p = 0.60; r VT3.A1 = 0.006, p = 0.95; r VT3.A2 = 0.055, p = 0.60).
Results of the cluster analyses computed on the locomotor activity in the automated activity monitor (white circles) and in the videotracking system (black circles). In order to show the results on a single figure, the clusters are shown on the percentage of activity relative to the first 5 min of the session. Each bubble represents a cluster of behavioral activity
Experiment 2: relationship between the response to novelty and cocaine stimulant effects
Figure 4 shows that cocaine strongly increased locomotor activity both in the automated activity monitor (Fig. 4a) and in the videotracking system (Fig. 4b). The two-way ANOVA computed on locomotor scores in the automated activity monitor showed a significant main effect of the cocaine treatment (F (1,94) = 171.79, p < 0.0001) and a significant main effect of the time course (F (5,470) = 74.21, p < 0.0001), together with a significant interaction treatment × time (F (5,470) = 8.81, p < 0.0001). The two-way ANOVA computed on the locomotor activity in the videotracking system showed similar results with a significant main effect of the cocaine treatment (F (1,94) = 85.22, p < 0.0001), a significant main effect of the time course (F (5,470) = 41.93, p < 0.0001), and a significant interaction treatment × time (F (5,470) = 9.91, p < 0.0001).
Locomotor activity of mice injected with either cocaine (black circles) or saline (white circles) in the automated activity monitor (a) and in the videotracking system (b). Locomotion is expressed as mean (±SEM) number of locomotor counts in the automated activity monitors and in mean distance traveled (cm/5 min) in the videotracking system. a, b Time course of locomotion in 5-min intervals
Table 1 shows the correlations (r) between the response to novelty and the acute stimulant effects of cocaine using either the automated activity monitors or the videotracking system. After cocaine injections, notable positive correlations between the activity on the response to novelty session and the cocaine test session were only found when both tests occurred in the same environment. When both tests occurred in the videotracking system, there was a significant positive correlation for both the first 5 min of the session (r = 0.47) and for the entire 30-min test session (r = 0.49). When both tests occurred in the automated activity monitors, there was a significant positive correlation for the entire 30-min test session (r = 0.78), whereas the correlation for the first 5 min only approached statistical significance (r = 0.40). In contrast, when the environment changed between the response to novelty session and the cocaine session, correlation coefficients were very close to zero (r = −0.12 and r = 0.07, respectively, for 5 and 30 min when the mice were switched from the activity monitors to the videotracking and r = −0.04 and r = 0.02 when the mice were switched from the videotracking to the activity monitors).
The cluster analysis computed on the response to novelty session provided identical results to the cluster analysis in experiment 1. The same three clusters were found for the videotracking system, whereas there were only two clusters for the automated activity monitor (see Fig. 3). These clusters were then tested in correlations with the acute locomotor stimulant effects of cocaine. The results of these correlations are shown in Table 2 and confirm the results of the correlation analyses in Table 1. There were significant correlations between the locomotor response to novelty and the stimulant effects of cocaine only when both responses were measured in the same environment. There was a significant positive correlation between the clusters VT1/VT2/VT3 and the stimulant effects of cocaine in the group VT-VT/cocaine (Table 2). There were also positive correlations between clusters A1 and A2 and the stimulant effects of cocaine in group A-A, although cluster A1 was only close to statistical significance (r = 0.40; p = 0.051). When there was a change in the environment between the response to novelty session and the cocaine session, none of the correlations were statistically significant.
Discussion
The present study investigated the relationship between the locomotor response to novelty and the acute stimulant effects of cocaine in mice tested in two different environments. A number of previous studies in rodents reported significant positive correlations between locomotor activity in a novel environment, usually interpreted as a locomotor response to novelty, and the stimulant effects of cocaine tested in the same environment (e.g., Kosten and Miserendino 1998; Sell et al. 2005). Most often, these results are interpreted in the theoretical framework of a relationship between novelty seeking and the vulnerability to drug addiction. The results of these studies are often viewed as an animal model of the human relationship between a personality trait of novelty/sensation seeking and an individual vulnerability to drug addiction. This theoretical framework involves some implicit assumptions that have never been formally tested to our knowledge. First, it is assumed that the locomotor response to a novel environment in rodents is related to some stable traits, usually assumed to be genetically determined. As a consequence, a relative stability of this locomotor response is expected across environments. Individuals with a higher locomotor response to novelty in one environment are expected to also show a higher response to novelty in another environment. Secondly, the relationship between the locomotor response to novelty and the stimulant effects of cocaine is also assumed to be relatively stable across environments. Indeed, it is not expected by the underlying theory that a significant relationship should be observed only when the effects of cocaine are tested in the same environment as the response to novelty. The aim of the present study was to explicitly test these assumptions using two different environments similar to those used in previous studies that assessed the stimulant effects of cocaine.
A striking observation of the present study is that there is only a weak correlation during the first 5 min of the session between the locomotor activities tested in two different novel environments. For the whole 30-min session, the correlation is close to zero. In similar previous studies in rodents, a wide variety of open fields were used to assess the response to novelty. In mouse studies, these open fields strongly differed in size (from 14 × 20 to 62.5 × 31 cm; e.g., Adriani and Laviola 2002; Vidal-Infer et al. 2012), in shape (rectangular, circular, or square; e.g., Adriani and Laviola 2002; Fukushiro et al. 2010; Parkitna et al. 2013; Pastor et al. 2005), and color (black, white, or transparent walls; e.g., Adriani and Laviola 2002; Davis et al. 2008; Fernández-Teruel et al. 2002). Although the level of lighting in the open fields is likely to affect the behavior of mice, this information is usually missing in previous studies. Clearly, the two open fields selected for the present study have physical properties (size, shape, and color) that are in the range of those used in previously published studies. The lack of correlation between the responses recorded in the two different environments in the present study suggests that those previous studies did not necessarily record a similar stable characteristic of novelty response. Additionally, when using the usual classification of mice as HR and LR, there is no statistically significant relationship between being classified as a HR/LR mouse in the automated activity monitors and being classified as a HR/LR mouse in the videotracking system (see supplementary material). In fact, when using the first 5 min of the session for the classification, 39 % of the mice switched from one classification (HR or LR) to the other when moved from one environment to the other. Some previous studies classified HR and LR mice on the basis of the higher and lower quartiles (i.e., the upper and lower 25 % of the sample) instead of the median. We have also tested such an extreme group classification of mice (data not shown) with the same conclusions. When mice are classified as LR or HR mice either relative to the median or as the lower and higher quartiles, the results show a lack of relationship between mouse classifications in the two environments. Together, these results indicate that the locomotor response to such novel environments is more affected by the specific physical characteristics of these environments than by stable individual traits related to the reactivity to novelty in a broad sense.
Several possible alternative explanations must be considered when trying to interpret this lack of a strong correlation between locomotor responses in two different novel environments. First, it is possible that the time frame to properly record the novelty response is not the same in both environments, especially because they have different sizes and therefore different durations for a full exploration of the open field. A cluster analysis was computed in order to answer this question. In such an analysis, clusters of behavioral activity are extracted from the correlation matrix of the 5-min raw locomotor scores. Correlations can then be computed on the extracted clusters in each environment without a predefined time frame. In the present study, three clusters of activity were identified in the videotracking system for only two clusters in the automated activity monitors. In both open fields, a cluster was identified for the first 5 min that probably better reflects a purer component of the response to novelty. This observation is in agreement with the results of Crawley (2007) who concluded that the first 5 min in a new open field provides the best index to assess the response to novelty. After the first 5 min, other behavioral mechanisms occur and are probably related to the habituation to the new environment. However, in most previous studies in rats and mice, locomotor response to novelty is usually assessed with longer time intervals, usually 20–30 min (Brabant et al. 2005; Gong et al. 1996; Kalinichev et al. 2004; Kosten and Miserendino 1998), suggesting that the correlations reported in those studies might be contaminated with components of locomotor activity other than the response to novelty. Additionally, except for this “first 5-min” cluster, the extracted clusters from the two environments did not show significant correlations. This indicates that differences in the time frame do not explain the lack of strong correlations between the novelty responses in the two different environments. As the mice were tested twice, once in each environment, a transfer of habituation might also have occurred from the first to the second environment, compromising the measurement of the novelty response in the second environment. However, counterbalanced experimental groups were used and showed similar levels of within-session habituation with no indication of a transfer of habituation from one context to the other. It is therefore very unlikely that this explains the lack of correlation between the responses to novelty measured in the different environments.
It might be argued that both environments differing in size, color, and lighting are not equally suitable to record a locomotor response to novelty. For example, the slightly stronger illumination in the automated activity monitors might produce aversive reactions in the mice that would prevent the proper recording of the novelty response. In that case, the lack of correlation between locomotor activities recorded in both environments would simply result from the fact that one environment properly records the novelty response, whereas the other records other forms of behaviors (anxiety, stress). However, such an explanation is unlikely for several reasons. First, similar correlations were obtained in the present study between the novelty response and cocaine stimulant effects in both environments, provided that both measures were carried out in the same environment. It seems unlikely that these similar correlations involved a relationship between cocaine effects and the novelty response in one environment and between cocaine effects and some other behavioral components, anxiety for example, in the other environment. Secondly, this would not explain why the locomotor response to novelty in any environment did not correlate with the stimulant effects of cocaine when tested in the alternative environment. Finally, such an explanation would raise another disturbing question. Indeed, both open fields of the present study are quite similar to the material used in previously published studies on the relationship between the novelty response and drug effects. This would therefore mean that some of these previous studies did not really investigate the novelty response, but instead obtained correlations between drug effects and some other behavioral components.
A significant correlation between the locomotor response to a novel environment and the stimulant effects of cocaine was reported in a number of different environments in previous studies (e.g., Kosten and Miserendino 1998; Sell et al. 2005). However, to our knowledge, all these previous studies tested the locomotor response to novelty and the stimulant effects of cocaine in the same environment (Kosten and Miserendino 1998; Sell et al. 2005). It is therefore possible that the reported significant correlations reflect similar behavioral activations produced by novelty and cocaine, interacting with the properties of the environment, rather than a relationship between two underlying stable individual characteristics (i.e., the propensity to respond to novelty and the vulnerability to the stimulant effects of cocaine). It was therefore interesting to test whether the correlation between the locomotor response to novelty and the stimulant effects of cocaine is preserved when both effects are tested in different environments. The present results show that the correlation is totally abolished when these behaviors are tested in distinct environments. This suggests an alternative explanation to the usual theoretical framework. The present results are more consistent with the idea that the specific properties of the environment similarly stimulate the locomotor activity of mice when they are under cocaine or under “novelty.” Another way of seeing these results would be to suggest that cocaine reactivates some “novelty” properties of the environment, as previously proposed in another experimental context (Carey and Damianopoulos 2006). This conclusion also fits with the role of brain dopamine. Indeed, dopamine is known to be involved in the stimulant effects of cocaine (Whishaw 2001), while dopamine neurons were also reported to react to novel stimuli (Bardo et al. 1996). At least, the present results indicate that the theoretical framework underlying the experiments on the relationship between the response to novelty and the stimulant effects of cocaine should be cautiously reexamined in future studies. It is noteworthy, however, that this conclusion does not necessarily pertain to studies focusing on the relationship between the response to novelty and cocaine self-administration (e.g., Belin et al. 2008), as a different behavioral variable is recorded. In most intravenous self-administration experiments, the environment in which the novelty response is assessed is usually different from the environment in which cocaine self-administration takes place (Davis et al. 2008; Dickson et al. 2015; Grimm and See 1997; Mitchell et al. 2005). Interestingly, there was a positive correlation between the locomotor novelty response measured in open fields and the reinforcing effects of cocaine measured in operant chambers in only two of these intravenous self-administration studies (Davis et al. 2008; Grimm and See 1997). Our results suggest that the specific properties of the environment in which the response to novelty is recorded should also be carefully considered in future studies on cocaine self-administration.
Most of previous studies on the relationship between the response to novelty and the locomotor stimulant effects of cocaine were conducted in male rats, whereas the present study used female mice. It is therefore possible that the present results do not generalize to male rats. However, it is noteworthy that similar positive correlations between the response to novelty and the behavioral effects of cocaine were reported in female mice and rats (e.g., Davis et al. 2008; Grimm and See 1997; Sell et al. 2005). In addition, the present study replicated the positive correlation between the novelty response and cocaine-induced hyperactivity in female mice when both behavioral responses were assessed in the same environment. Further studies are needed to determine whether the relationship between the novelty response and drug effects is stable across environments in male rats.
References
Adriani W, Laviola G (2002) Spontaneous novelty seeking and amphetamine-induced conditioning and sensitization in adult mice: evidence of dissociation as a function of age at weaning. Neuropsychopharmacology 27(2):225–236
Arias C, Mlewski EC, Miller S, Molina JC, Spear NE (2009) Novelty modulates the stimulating motor effects of ethanol in preweanling rats. Pharmacol Biochem Behav 92:448–456
Bardo MT, Donohew RL, Harrington NG (1996) Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res 77:23–43
Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine taking. Science 320(5881):1352–1355
Blanchard MM, Mendelsohn D, Stamp JA (2009) The HR/LR model: further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev 33(7):1145–1154
Brabant C, Quertemont E, Tirelli E (2005) Evidence that the relations between novelty-induced activity, locomotor stimulation and place preference induced by cocaine qualitatively depend upon the dose: a multiple regression analysis in inbred C57BL/6J mice. Behav Brain Res 158:201–210
Brabant C, Alleva L, Grisar T, Quertemont E, Lakaye B, Ohtsu H, Lin JS, Jatlow P, Picciotto MR, Tirelli E (2009) Effects of the H3 receptor inverse agonist thioperamide on cocaine-induced locomotion in mice: role of the histaminergic system and potential pharmacokinetic interactions. Psychopharmacology (Berl) 202(4):673–687
Carey RJ, Damianopoulos EN (2006) Cocaine conditioning and sensitization: the habituation factor. Pharmacol Biochem Behav 84(1):128–133
Cools AR, Gingras MA (1998) Nijmegen high and low responders to novelty: a new tool in the search after the neurobiology of drug abuse liability. Pharmacol Biochem Behav 60(1):151–159
Crawley JN (2007) What’s wrong with my mouse? Wiley-Interscience, Hoboken, NJ
Davis BA, Clinton SM, Akil H, Becker JB (2008) The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred high-responder and low-responder rats. Pharmacol Biochem Behav 90(3):331–338
Dickson PE, Ndukum J, Wilcox T et al (2015) Association of novelty-related behaviors and intravenous cocaine self-administration in diversity outbred mice. Psychopharmacology (Berl) 232:1011–1024
Didone V, Masson S, Quoilin C, Seutin V, Quertemont E (2014) Correlation between ethanol behavioral sensitization and midbrain dopamine neuron reactivity to ethanol. Addict Biol. doi:10.1111/adb.12216
Fernández-Teruel A, Driscoll P, Gil L, Aguilar R, Tobeña A, Escorihuela RM (2002) Enduring effects of environmental enrichment on novelty seeking, saccharin and ethanol intake in two rat lines (RHA/Verh and RLA/Verh) differing in incentive-seeking behavior. Pharmacol Biochem Behav 73:225–231
Fukushiro DF, Benetti LF, Josino FS et al (2010) Environmental novelty and illumination modify ethanol-induced open-field behavioral effects in mice. Pharmacol Biochem Behav 95:13–22
Gong W, Neill DB, Justice JB (1996) Locomotor response to novelty does not predict cocaine place preference conditioning in rats. Pharmacol Biochem Behav 53(1):191–196
Grimm JW, See RE (1997) Cocaine self-administration in ovariectomized rats is predicted by response to novelty, attenuated by 17-beta estradiol, and associated with abnormal vaginal cytology. Physiol Behav 61:755–761
Hooks MS, Jones GH, Liem BJ, Justice JB (1992) Sensitization and individual differences to IP amphetamine, cocaine, or caffeine following repeated intra-cranial amphetamine infusions. Pharmacol Biochem Behav 43(3):815–823
Hooks MS, Jones GH, Holzman SG, Juncos JL, Kalivas PW, Justice BJ (1994) Individual differences in behavior following amphetamine GBR-12909, or apomorphine but not SKF-38393 or quinpirole. Psychopharmacology (Berl) 116:217–225
Kabbaj M (2006) Individual differences in vulnerability to drug abuse: the high responders/low responders model. CNS Neurol Disord Drug Targets 5(5):513–520
Kabbaj M, Devine DP, Savage VR, Akil H (2000) Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci 20(18):6983–6988
Kalinichev M, White DA, Holtzman SG (2004) Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. I. Expression of morphine-induced locomotor sensitization. Psychopharmacology (Berl) 177(1-2):61–67
Kalueff AV, Keisala T, Minasyan A, Kuuslahti M, Tuohimaa P (2006) Temporal stability of novelty exploration in mice exposed to different open field tests. Behav Processes 72(1):104–112
Kliethermes CL, Crabbe JC (2006) Genetic independence of mouse measures of some aspects of novelty seeking. Proc Natl Acad Sci U S A 103(13):5018–5023
Kosten TA, Miserendino MJD (1998) Dissociation of novelty- and cocaine-conditioned locomotor activity from cocaine place conditioning. Pharmacol Biochem Behav 60(4):785–791
Marinelli M (2005) The many facets of the locomotor response to a novel environment test: theoretical comment on Mitchell, Cunningham, and Mark. Behav Neurosci 119(4):1144–1151
Mitchell JM, Cunningham CL, Mark GP (2005) Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behav Neurosci 119:464–472
Nowak KL, Ingraham CM, McKinzie DL et al (2000) An assessment of novelty-seeking behavior in alcohol-preferring and non-preferring rats. Pharmacol Biochem Behav 66(1):113–121
Parkitna JR, Sikora M, Gołda S et al (2013) Novelty-seeking behaviors and the escalation of alcohol drinking after abstinence in mice are controlled by metabotropic glutamate receptor 5 on neurons expressing dopamine D1 receptors. Biol Psychiatry 73(3):263–270
Pastor R, Miquel M, Aragon CMG (2005) Habituation to test procedure modulates the involvement of dopamine D2- but not D1-receptors in ethanol-induced locomotor stimulation in mice. Psychopharmacology (Berl) 182(3):436–446
Pawlak C, Ho Y, Schwarting R (2008) Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci Biobehav Rev 32(8):1544–1568
Piazza PV, Deminière JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245:1511–1513
Redolat R, Pérez-Martínez A, Carrasco MC, Mesa P (2009) Individual differences in novelty-seeking and behavioral responses to nicotine: a review of animal studies. Curr Drug Abuse Rev 2:230–242
Sell SL, Dillon AM, Cunningham KA, Thomas ML (2005) Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats. Behav Brain Res 161:69–74
Shimosato K, Watanabe S (2003) Concurrent evaluation of locomotor response to novelty and propensity toward cocaine conditioned place preference in mice. J Neurosci Methods 128:103–110
Suto N, Austin J, Vezina P (2001) Locomotor response to novelty predicts a rat’s propensity to self-administer nicotine. Psychopharmacology (Berl) 158(2):175–180
Vidal-Infer A, Arenas MC, Daza-Losada M, Aguilar MA, Miñarro J, Rodríguez-Arias M (2012) High novelty-seeking predicts greater sensitivity to the conditioned rewarding effects of cocaine. Pharmacol Biochem Behav 102(1):124–132
Whishaw IQ (2001) Comment les substances pharmacologiques (drogues) et les hormones influencent-elles le comportement? In: Worth Publishers (ed) Cerveau & comportement. De Boeck & Larcier, Paris
Acknowledgments
The present research was supported by grants obtained by Etienne Quertemont from the Fonds Spéciaux pour la Recherche (FSR) from the University of Liège and the Belgian National Fund for Scientific Research (FNRS, Convention n 2.4529.09). Laura Nyssen is a PhD candidate under contract with the FNRS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nyssen, L., Brabant, C., Didone, V. et al. Response to novelty and cocaine stimulant effects: lack of stability across environments in female Swiss mice. Psychopharmacology 233, 691–700 (2016). https://doi.org/10.1007/s00213-015-4146-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4146-0