Abstract
Rationale
L-type Ca2+ channels (LTCC) and GABAB receptors are both possible targets in the development of new pharmacological compounds for cocaine addiction. Drugs that target either receptor attenuate a wide range of cocaine-seeking behaviors in the rat. However, there is no current human-approved pharmacotherapeutic intervention for psychostimulant addiction.
Objectives
This study examined the effects of a human-approved LTCC blocker, fendiline, on cocaine-taking and cocaine-seeking behavior in rats. The effects of combining fendiline with the GABAB receptor agonist baclofen on cocaine self-administration were also tested.
Methods
Male Wistar rats were trained to self-administer cocaine, and the effects of fendiline pretreatment (vehicle, 1.78, 3.16, 5.62 mg/kg, intraperitoneal (IP)) were tested on progressive ratio responding and cue- and drug-induced reinstatement. The effects of baclofen (vehicle, 0.56, 1.78, 3.16, 5.62 mg/kg, IP) combined with fendiline (5.62 mg/kg, IP) were tested on progressive ratio responding. Control experiments measured locomotor activity and lever pressing for food in rats that received both baclofen and fendiline prior to the test session.
Results
Acute injections of fendiline prior to cue- or drug-induced reinstatement significantly attenuated lever-pressing behavior (p < 0.05). Fendiline and baclofen, but not fendiline alone, not only significantly attenuated breakpoints, but also impaired general motor behavior and naturalistic reinforcement (p < 0.05).
Conclusion
These data suggest that the LTCC blocker fendiline may represent a novel pharmacotherapeutic intervention to prevent reinstatement to cocaine seeking. Also, co-administration of fendiline and baclofen not only can attenuate the motivation to take cocaine, but also impairs general motor behavior and naturalistic reinforcement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repeated psychostimulant use can lead to maladaptive changes in the neurocircuitry that controls motivation and reinforcement in the brain. Detrimental adaptations within reward/motivation circuits drive a cycle of behaviors through drug use, abstinence, and relapse (Koob and Volkow 2010). These behaviors can be modeled in animals using an intravenous self-administration (IVSA) paradigm (Lynch et al. 2010). Pharmacotherapeutic compounds can be tested for anti-addictive properties by measuring drug taking (self-administration) and/or relapse to drug taking (cue- or drug-induced reinstatement).
A defining characteristic of all drugs of abuse is their propensity to increase dopamine efflux in the cortico-limbic regions of the brain (Wise 1996); however, attempts to treat addiction with compounds that target the dopamine receptor directly have showed little clinical significance (Berger et al. 1996; Haney et al. 1999). Accordingly, current research is focusing on indirect mechanisms to inhibit dopaminergic activity as a means to attenuate behaviors related to addiction (Bailey and Husbands 2014). Recent research suggests that an FDA-approved medication, fendiline, may be useful in this regard. Specifically, Voigt et al. (2014) reported that fendiline inhibited the maintenance and expression of methamphetamine-induced conditioned place preference (CPP). Furthermore, the effects of fendiline were produced in the absence of aversive or rewarding properties and without significant impairment on motivated motor behavior (Voigt et al. 2014).
Fendiline was initially developed as an L-type Ca2+ channel blocker for cardiac smooth muscle and approved for human use to treat angina, hypertension, and cardiac arrhythmias (Bayer and Mannhold 1986). L-type Ca2+ channels found in the cortico-limbic regions are proposed to mediate drug-seeking activities. They regulate calcium entry into cells and depolarize the cell membrane when activated (Berger and Bartsch 2014) and change expression in response to repeated exposure to psychostimulants (Ford et al. 2009). L-type Ca2+ channel surface expression and total protein levels are upregulated in the medial prefrontal cortex (mPFC) compared to the motor cortex following repeated cocaine injection (Ford et al. 2009). Chronic amphetamine injection in rats increases messenger RNA (mRNA) and protein expression of L-type Ca2+ channels in the ventral tegmental area (VTA), the location of dopaminergic cell bodies that project to cortico-limbic structures (Rajadhyaksha et al. 2004). Licata and Pierce (2003) proposed that intracellular Ca2+-mediated pathways in the VTA underlie stimulant-induced behavioral sensitization; additionally, Ford et al. (2009) propose that abnormal L-type Ca2+ channel expression in the mPFC can enhance activation of the cortico-limbic circuit in response to drugs, and to drug cues, and contribute to drug-seeking behavior.
In agreement with these hypotheses, three daily microinfusions of the L-type Ca2+ agonist BayK 8644 into the VTA augment behavioral sensitization to cocaine when tested 2 weeks after the microinfusions (Licata et al. 2000). Blockade of L-type Ca2+ channels with nimodipine attenuated cocaine self-administration, whereas BayK 8644 sensitized cocaine self-administration (Kuzmin et al. 1996). Other L-type Ca2+ channel antagonists block the induction of nicotine CPP, attenuate the induction and expression of nicotine-induced locomotor sensitization (Biala 2002), block the induction of methamphetamine and cocaine CPP (Suzuki et al. 1992), and attenuate drug-induced reinstatement of nicotine CPP (Biala and Budzynska 2008). These findings suggest that fendiline may attenuate behaviors associated with addiction via its antagonist action at L-type Ca2+ channels.
Fendiline also potentiates GABAB receptor-mediated hyperpolarization in vitro and baclofen-induced depression of VTA dopaminergic activity (Kerr et al. 2002; Chen et al. 2005). The GABAB receptor has been identified as a target for addiction pharmacology (Brebner et al. 2002), as the GABAB agonist, baclofen, inhibits several behaviors that are used to model addiction in rats, including the self-administration of the psychostimulants methamphetamine (Ranaldi and Poeggel 2002), d-amphetamine (Brebner et al. 2005), and cocaine (Brebner et al. 2000a; Filip et al. 2007; Filip and Frankowska 2007; Roberts et al. 1996), as well as cocaine-induced reinstatement (Campbell et al. 1999). However, GABAB agonists like baclofen were subsequently shown to induce sedative and motor impairments, thus precluding their use in the treatment of drug addiction in humans (Cryan et al. 2004; Shoptaw et al. 2003).
Despite these intriguing observations, the effects of fendiline on the self-administration of cocaine and cue- and drug-induced reinstatement of cocaine-seeking behavior have yet to be examined. In addition, the combined effect of fendiline and baclofen may provide a combined treatment that effectively attenuates self-administration without the side effects normally associated with baclofen alone. The present study examined these issues using models of cocaine addiction.
Materials and methods
Animals
Subjects were male Wistar rats (N = 85, Charles River, Quebec) that weighed 230–250 g at the start of experiments and were quarantined in the vivarium (12-h reverse photo-cycle, lights on 3 p.m.–3 a.m.) for 7 days upon arrival. Rats were housed according to standards of the Canadian Council on Animal Care and given an ad libitum diet of standard rat chow and fresh tap water. Daily handling occurred prior to each experiment.
Drugs
Cocaine hydrochloride (Medisca, Montreal, QC) was dissolved in 0.9 % saline. Fendiline [N-(3,3-diphenylpropyl)-a-methylbenzylamine] (Sigma-Aldrich) was dissolved in 25 % ethanol, and (±) baclofen (Sigma-Aldrich) was dissolved in 0.9 % saline. For experiments where both fendiline and baclofen were administered together, the vehicle control treatments (25 % ethanol and 0.9 % saline, respectively) were administered by body weight (ml/kg). Doses for both fendiline and baclofen followed a logarithmic increase and were chosen based on previous investigation that identified the minimum dose necessary for an effect and the maximal dose that did not overtly induce motor slowing (Voigt et al. 2014).
Procedure
Behavioral training
Rats were implanted with a chronically indwelling catheter into the right jugular vein that extended subcutaneously and then sutured to the dorsal surface between the scapulae (Roberts and Goeders 1989). Daily flushing with heparinized saline was used to maintain cannula patency. Animals began daily self-administration conditioning 5 days post-surgery. Operant conditioning chambers (Med Associates Inc.) were equipped with an active lever and inactive lever, a white (100 mAmp) cue light, and a house light. The house light signaled the start and end of each session, and the cue light above the active lever flashed on and off, coincident with the delivery of the cocaine. Responses on the active lever delivered cocaine injections (0.75 or 1.5 mg/kg, IV, per injection in 0.1 ml of saline over 5 s, 20 s post-infusion time-out) on a fixed ratio 1 (FR1) schedule of reinforcement. Two doses of cocaine were used to measure effects of treatment on different levels of reinforcement. Responses on the inactive lever had no consequences. Daily self-administration sessions started with one priming injection and lasted 3 h for experiments 1 and 2 and 2 h for experiments 3 and 4 or until a maximum of 40 IV injections was achieved. Once a stable pattern of cocaine intake (>15 injections per session with regular post-infusion time-out of 5–8 min) was established, rats were randomly assigned to an experimental group.
Cocaine self-administration
Experiment 1: Rats were trained to self-administer cocaine (0.75 mg/kg (n = 6) or 1.5 mg/kg (n = 6) per IV injection) under a progressive ratio (PR) schedule of reinforcement. Cocaine infusions were contingent on increased responding per drug infusion through the following progression: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32,…1110, 1378, 1706 (Arnold and Roberts 1997). A breakpoint was reached when the rat failed to reach the next response requirement within 1 h; at this point, the session was terminated. Fendiline treatment began after 3 days of PR responding, with the proviso that breakpoints were stable within two increments across 3 days. Fendiline pretreatment days (vehicle, 1.78, 3.16, or 5.62 mg/kg, intraperitoneal (IP), immediately prior to session) were separated by at least three consecutive days over which breakpoints varied by less than two increments. The order of treatments was set up according to the Latin square design with four possible conditions. Individual rats were randomly assigned a condition with the proviso that each condition had at least one rat.
Experiment 2: Rats (n = 7) followed the same training protocol and stability criteria as experiment 1, except only one dose of cocaine was used (1.5 mg/kg/injection). On test days, rats were given a combined pretreatment of baclofen (vehicle, 0.56, 1.78, 3.16, 5.62 mg/kg, IP) administered 30 min prior to test sessions and fendiline (5.62 mg/kg, IP), which was given immediately prior to the session. The vehicle control group received the vehicle of both baclofen (0.9 % saline) and fendiline (25 % ethanol) The order of baclofen doses was set up in a similar manner as explained in experiment 1. All rats received all doses of baclofen in combination with fendiline, and the same assessment criteria as described in experiment 1 separated treatment days.
Cue- and drug-induced reinstatement
Experiment 3: Rats were trained to self-administer cocaine (1.5 mg/kg/injection) under a FR1 schedule as described in the “Behavioral training” section above, with the addition of a cue tone (4000 Hz, 65 dB, 5 sec) presented contingently with each active lever press. After stable responding (>15 injections per session with regular post-infusion time-outs) on the FR1 schedule was achieved, rats were switched to an FR5 schedule of reinforcement for 10 days. In extinction trials, which were initiated on day 11, rats could still press the levers, but responses on the previously drug-paired lever no longer resulted in an infusion of cocaine; additionally, no drug cues were presented. Extinction continued for ten consecutive days at which point, all rats showed reduced responding for the active lever (≤15 active lever responses). Cue-induced reinstatement was tested on the day after the final extinction trial. The 2-h session started with a non-contingent presentation of the drug-associated cues (tone and light). Presses on the active lever during reinstatement sessions resulted in presentation of the light and tone cues, but no cocaine was delivered. To assess fendiline’s effect on cue-induced reinstatement, each rat was randomly assigned to receive fendiline (vehicle n = 10, 3.16 n = 10, or 5.62 mg/kg n = 15, IP) immediately prior to the reinstatement session.
Experiment 4: In this experiment, fendiline pretreatment was tested on cocaine-induced reinstatement. Rats (n = 15) progressed through the same conditioning, training, extinction, and reinstatement stages as described above. For reinstatement in this experiment, however, no cue tone was presented and a non-contingent injection of cocaine (10 mg/kg, IP) was given at the start of the reinstatement session. Lever-pressing behavior during the reinstatement session was recorded but failed to result in an infusion of cocaine. Rats were pretreated with fendiline (vehicle, 3.16, 5.62 mg/kg, IP) immediately prior to the start of the reinstatement session. All rats in experiment 4 received vehicle and both doses of fendiline, with at least 3 days (range of 3–8) of extinguished responding (≤15 active lever responses) separating treatment days; treatments were administered in a random order.
Locomotor activity
Experiment 5: In an effort to reduce the number of animals used in this study, rats that completed the self-administration (experiment 2) or reinstatement (experiment 4) studies were used to assess the effects of the combination of fendiline and baclofen on locomotor activity. One week after the self-administration or reinstatement experiment ended, rats were habituated to an open field environment for 30 min the day before testing began. Eight black Plexiglas boxes (40 cm × 40 cm × 40 cm) were illuminated with red LED lights during recording. Rats were randomly assigned to one of four groups: Vehicle (0.9 % saline and 25 % ethanol, n = 5), 1.78 mg/kg baclofen and 5.62 mg/kg fendiline (n = 9), 3.16 mg/kg baclofen and 5.62 mg/kg fendiline (n = 5), or 5.62 mg/kg baclofen and 5.62 mg/kg fendiline (n = 5). Baclofen was administered 30 min prior to testing and fendiline immediately prior to testing. Activity was recorded for 2 h and tracked with EthoVision XT (Noldus Information Technology, Wageningen, The Netherlands). Groups were randomized to include rats from both experiments 2 and 4 in each locomotor activity group.
Naturalistic reinforcement
Experiment 6: Rats (n = 12) did not receive surgery and were food deprived for 16 h prior to the first training session. The operant conditioning chambers were set up as described under “Behavioral training” section with the addition of a food hopper between the active and inactive levers. There were no cues associated with the delivery of food reinforcers. Responses on the active lever delivered one sweetened food pellet (Bio Serve) on a FR1 schedule of reinforcement. Daily sessions started with one priming pellet and lasted 2 h. Once a stable pattern of food responding (>100 pellets per session with regular post-delivery time-outs) was established, rats were switched to an FR5 schedule of reinforcement for the remainder of the experiment. Drug treatments were administered once responding on the FR5 schedule of reinforcement was stable (±10 pellets received) across 3 days. This experiment followed a repeated measures design where all rats received all treatment combinations, separated by at least 3 days of stable responding (±10 pellets received) between each treatment. Treatment doses were as follows: 0.9 % saline and 25 % ethanol, 1.78 mg/kg baclofen and 5.62 mg/kg fendiline, 3.16 mg/kg baclofen and 5.62 mg/kg fendiline, and 5.62 mg/kg baclofen and 5.62 mg/kg fendiline. The order of treatment doses followed the same procedure outlined in experiment 1.
Statistical analysis
Repeated-measures ANOVAs followed by post hoc analyses were used to determine the effect of drug treatment in experiments 1, 2, 4 (two-way repeated measures), and 6. Two- and one-way ANOVAs followed by post hoc analyses were used to determine the effect of drug treatment in experiments 3 and 5, respectively. Statistical outliers (n = 3 out of 85 rats), defined as responding two standard deviations above or below the mean (FR, PR, reinstatement), and any rats that did not receive all doses in repeated measures design experiments were removed prior to analysis (one rat from experiment 2). Data are presented as group means plus or minus the standard error of the mean (SEM) with an alpha value set at 0.05.
Results
Experiment 1: effect of fendiline on cocaine PR IVSA
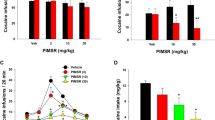
Although there was a trend toward attenuation of breakpoints at high doses of fendiline (5.62 mg/kg, IP) on low-dose cocaine (0.75 mg/kg/injection), there was no overall significant effect of fendiline on responding for either high or low doses of cocaine (Fig. 1).
Experiment 2: effect of fendiline and baclofen on PR IVSA
Figure 2 shows the effects of concurrent pretreatment of fendiline and baclofen on cocaine self-administration on a PR schedule of reinforcement. Repeated-measures ANOVA followed by Newman-Keuls post hoc revealed that concurrent fendiline (5.62 mg/kg, IP) and baclofen (1.78, 3.16, 5.62 mg/kg, IP) pretreatment significantly attenuated breakpoints compared to both baseline and vehicle treatment (F(5,30) = 11.57, p < 0.002). There was no significant difference between baseline and vehicle pretreatment.
Dose-response effect of baclofen combined with fendiline on cocaine self-administration under a PR schedule of reinforcement. Baclofen combined with fendiline caused a dose-dependent decrease in breakpoints compared to baseline and the vehicle control, and there was no difference between baseline and vehicle. Newman-Keuls test; *p < 0.05. Symbols represent group means ± SEM
Experiment 3: effect of fendiline on cue-induced reinstatement
Figure 3 shows the effect of fendiline pretreatment on cue-induced reinstatement after 10 days of extinction training. Two-way ANOVA followed by Newman-Keuls post hoc revealed that acute injection of the high dose of fendiline (5.62 mg/kg, IP) attenuated cue-induced reinstatement of active lever pressing (F(4,96) = 3.02, p < 0.022). Figure 3 also includes active lever pressing averaged across the last 3 days of extinction and responses on the inactive lever during the 2-h reinstatement session. There was no difference between the extinction (left bar) responding across the three groups, and active lever pressing in all three groups significantly increased after reexposed to the cues associated with drug delivery (middle bar). Moreover, inactive lever pressing (right bar), an indirect measure of non-specific motor behavior, did not differ between the three groups.
Effect of fendiline on cue-induced reinstatement to cocaine seeking. Left-most bar in each cluster represents the averaged lever presses on the active lever across the last 3 days of extinction. The middle and right-most bars in each cluster represent lever presses on the active and inactive levers, respectively, during the cue-induced reinstatement session. Only high doses of fendiline significantly decreased lever pressing maintained by presentation of the drug cue compared to the vehicle group. There were no significant differences between groups based on the active lever during extinction or the inactive lever during reinstatement. Newman-Keuls post hoc; *p < 0.05. Bars represent group means + SEM
Experiment 4: effect of fendiline on cocaine-induced reinstatement
Pretreatment with fendiline prior to a non-contingent injection of cocaine is shown in Fig. 4. Two-way repeated-measures ANOVA followed by Newman-Keuls post hoc revealed that both experimental treatments (3.16 or 5.62 mg/kg fendiline IP) significantly reduced lever pressing compared to pretreatment with a vehicle control (F(4,56) = 4.82, p < 0.003). Similar to experiment 3, Fig. 4 includes measures of average active lever pressing (left bar) during the last 3 days of extinction and the active (middle bar) and inactive lever (right bar) during cocaine-induced reinstatement. Extinction was not significantly different before each treatment, and there was no difference in responding on the inactive lever across treatment groups. Active lever pressing during extinction compared to reinstatement was only significant in the vehicle group.
Effect of fendiline on cocaine-induced reinstatement to cocaine seeking. Left-most bar in each cluster represents the averaged lever presses on the active lever across the last 3 days of extinction. The middle and right-most bars in each cluster represent lever presses on the active and inactive levers, respectively, during the cocaine-induced reinstatement session. Fendiline pretreatment significantly decreased lever pressing on the cocaine-associated lever compared to vehicle pretreatment. There were no group differences in active lever pressing during extinction or inactive lever pressing during reinstatement. Repeated-measures design with Newman-Keuls post hoc; *p < 0.05. Bars represent group means + SEM
Experiment 5: effect of fendiline and baclofen on locomotor activity
The effect of concurrent fendiline and baclofen pretreatment on spontaneous locomotor activity is presented in Fig. 5. Compared to control rats that received the vehicle for both drugs (25 % ethanol and 0.9 % saline), all doses of baclofen (1.78, 3.16, 5.62 mg/kg, IP) combined with fendiline (5.62 mg/kg, IP) caused a significant decrease in horizontal movement (one-way ANOVA, Newman-Keuls post hoc, F(3,20) = 6.78, p < 0.003).
Experiment 6: effect of fendiline and baclofen on naturalistic reinforcement
Figure 6 shows the effect of combined fendiline and baclofen pretreatment on food-reinforced behavior. Compared to the vehicle control treatment (25 % ethanol and 0.9 % saline), fendiline (5.62 mg/kg, IP) combined with baclofen (1.78, 3.16, 5.62 mg/kg, IP) significantly decreased lever pressing for sweetened food pellets (Repeated-measures ANOVA, Newman-Keuls post hoc, F(4,44) = 20.49, p < 0.002).
Effect of fendiline and baclofen on lever pressing for sweetened food pellets on an FR5 schedule of reinforcement. All combinations of baclofen with fendiline significantly reduced lever pressing for the food-associated lever compared to baseline and the vehicle control. Repeated-measures with Newman-Keuls post hoc; *p < 0.05. Symbols represent group means ± SEM
Discussion
The present investigation revealed that fendiline alone does not attenuate cocaine-reinforced breakpoints; however, when rats were treated with both baclofen and fendiline prior to self-administration, there was a dose-dependent decrease in breakpoints. Combined doses that attenuated cocaine self-administration also impaired locomotor activity and responding for a naturalist reinforcer. Acute injection of fendiline alone was able to attenuate lever pressing in a relapse model involving preexposure to the cues associated with drug delivery, or to cocaine, after 10 days of extinction. This is the first study to evaluate the effects of the L-type Ca2+ channel blocker fendiline on self-administration of cocaine and on reinstatement of cocaine-seeking behavior. These results are consistent with other behavioral observations that fendiline attenuates methamphetamine CPP (Voigt et al. 2014).
Voigt et al. (2014) demonstrated that fendiline is not inherently aversive or rewarding, and at the doses used in this experiment, general motor activity and motivated motor behavior are not affected. The present investigation also shows that fendiline does not affect non-specific behavior, as evidenced by analysis of the inactive lever-pressing behavior in the reinstatement experiments. We therefore conclude that any effects of fendiline on self-administration behavior are not due to general motor slowing, but are a specific effect on cocaine-seeking behavior. Doses of fendiline chosen for this investigation are based on previous reports describing the best human equivalent dose that should be well tolerated as a chronic treatment (Voigt et al. 2014). Our results also showed that the vehicle for fendiline (25 % ethanol) had no effect on any of the behaviors tested. This is consistent with previous reports from Voigt et al. (2014).
Self-administration
Animal IVSA is a valid model of drug-taking behaviors observed in human populations (Lynch et al. 2010). A PR schedule of reinforcement allows researchers to measure an animal’s motivation to receive a drug (Richardson and Roberts 1996). Our data show that fendiline alone does not change cocaine-reinforced breakpoints, suggesting that it does not alter an animal’s motivation to take cocaine. Despite a trend for high doses of fendiline to attenuate breakpoints for the low dose of cocaine, there is little reason to test doses of fendiline higher than the 5.62 mg/kg dose used here. This is because higher doses induce motor slowing and would not be well tolerated as a chronic treatment (Voigt et al. 2014). It is possible that fendiline may significantly reduce breakpoints on a lower unit injection dose of cocaine (e.g., 0.5 mg/kg/injection); however, it is unlikely that a pharmacotherapy that only disrupts low-dose rodent cocaine self-administration would be transferable to a human population. Therefore, we conclude that fendiline alone would not be a likely candidate to treat cocaine addicts who currently abuse the drug.
In our study, there was no difference in baseline cocaine-reinforced breakpoints between the low-dose group (0.75 mg/kg/injection) and high-dose group (1.5 mg/kg/injection). Previous investigations have shown that cocaine-reinforced breakpoints for the low dose can range from 10 to 15 (Richardson and Roberts 1996; Roberts et al. 1996), whereas high-dose breakpoints can range from 13 to 18 (Brebner et al. 2000b; Roberts et al. 1996). Our baseline average for both the high and low-dose groups falls within the normative data from other literature as described above. Furthermore, our rats self-administered cocaine for 3 h on FR1 schedule of reinforcement prior to being switched to the PR schedule, whereas other studies had a much longer daily limit of 5 or 6 h on FR1 (Roberts et al. 1996; McGregor et al. 1996; Brebner et al. 2000b). Therefore, the similarity between the two cocaine doses in our study may reflect decreased access to cocaine during the FR phase. Irrespective of these differences, it is clear that fendiline alone is not attenuating a rat’s motivation to take cocaine.
Baclofen is a GABAB agonist and is consistently observed to attenuate psychostimulant self-administration in rats (Brebner et al. 2000a, 2005; Filip et al. 2007; Roberts et al. 1996). Previous investigation has demonstrated that the lowest dose of baclofen that significantly attenuates cocaine (1.5 mg/kg/injection) breakpoints is 2.5 mg/kg (Roberts et al. 1996). Our results show that when baclofen is combined with fendiline (5.62 mg/kg), even lower doses (1.78 mg/kg) of baclofen are able to attenuate cocaine-reinforced breakpoints compared to baseline. Considering that fendiline alone had no effect on breakpoints but showed significant reduction in breakpoints in combination with baclofen, it suggests that fendiline potentiates baclofen’s effect in vivo.
The mechanism by which fendiline and baclofen interact to produce a synergistic effect is unknown. It has been suggested that fendiline is a positive allosteric modulator (PAM) at the GABAB receptor due to a potentiated effect on baclofen-induced inhibition of dopaminergic neurons in the VTA (Chen et al. 2005). Moreover, fendiline is able to potentiate baclofen-induced hyperpolarization of cortical neurons (Kerr et al. 2002). However, conflicting evidence reports that fendiline does not bind to the GABAB receptor and therefore has another mechanism of action (Urwyler et al. 2004). It is possible that the effects of fendiline on baclofen are mediated via its action at the L-type Ca2+ channel (Bayer and Mannhold 1986). How L-type Ca2+ channels interact with GABAB receptors is unclear and requires further study.
This investigation shows that fendiline may be useful in lowering the dose of baclofen needed to attenuate cocaine use. The purpose of this combined treatment was to assess whether a lower dose of baclofen than used in other investigations could be used to attenuate cocaine self-administration without inducing side effects that would preclude the treatment in humans. Fendiline was an ideal choice to test this hypothesis because it shows an effect in vivo, does not cause non-specific motor slowing, and is not aversive or rewarding (Voigt et al. 2014). Indeed, based on available literature, we report here the lowest dose of baclofen (1.78 mg/kg) that can significantly attenuate self-administration of a high dose of cocaine on a PR schedule of reinforcement. One limitation of this interpretation is that our study did not include a baclofen-only dose-response curve to compare against the combined effect of baclofen and fendline. In this regard, our observations on the potentiating effects of fendiline are based on comparisons to numerous publications that have tested baclofen on cocaine-reinforced breakpoints.
All the rats used in the locomotor activity experiment had previous exposure to cocaine in either PR or reinstatement experiments. These rats were used in order to reduce the number of animals required to complete the study, and since there was no cocaine exposure during the locomotor testing, there was no reason to believe that their previous drug history would affect the results. Analysis of locomotor behavior shows that all combinations of baclofen and fendiline that attenuated cocaine self-administration also significantly reduced lateral locomotor movement. Further, the same doses also attenuated lever pressing for a naturalistic reinforcer (sweetened food pellets). Although locomotor activity and lever pressing for food were decreased compared to controls, the rats were still active during the 2-h locomotor activity test and still pressed the food-associated lever nearly 1000 times in 2 h when pretreated with 1.78 mg/kg baclofen and 5.62 mg/kg fendiline. Indeed, when even higher doses of baclofen were administered in combination with fendiline, the rats were still able to press the active lever over 500 times during their test sessions. One goal of this investigation was to propose a combination of medications that may be effective at attenuating cocaine use while reducing the impact of side effects like sedation and motor impairment, which have precluded widespread clinical treatment with baclofen (Cryan et al. 2004; Shoptaw et al. 2003).
Reinstatement
Certain human relapse behaviors can be modeled in rodents using cue- and drug-induced reinstatement. As observed in humans, rats will relapse to cocaine-seeking behavior when reexposed to cues (light and tone) previously associated with cocaine delivery or to the drug that was self-administered during acquisition. We found that a single acute injection of fendiline prior to reinstatement was sufficient to attenuate lever-pressing behavior in both experiments. In contrast, previous investigations with fendiline on methamphetamine-induced CPP suggest that repeated chronic fendiline treatment is necessary (Voigt et al. 2014). The difference between chronic and acute fendiline treatment may reflect the nature of the behavior being measured, differences between methamphetamine and cocaine, or differences in the strength of associations formed between the drug and context in the two models of addiction.
The method by which fendiline alone is able to attenuate reinstatement is likely related to its ability to block L-type Ca2+ channels. Rats that receive five daily IP injections of cocaine, followed by a withdrawal period, show increased cell surface expression and total protein expression of L-type Ca2+ channels in the mPFC compared to the motor cortex (Ford et al. 2009). Increased expression of this channel may cause increased excitability of mPFC neurons and therefore contribute to the hyperresponsiveness that is characteristic of craving and relapse (Ford et al. 2009). It has also been observed that chronic amphetamine injection can increase mRNA and protein levels of L-type Ca2+ channels in VTA dopamine neurons (Rajadhyaksha et al. 2004). Repeated pharmacological stimulation of the L-type Ca2+ channel in the VTA can augment the behavioral response to cocaine 2 weeks after the last injection of the L-type Ca2+ channel agonist (Licata et al. 2000). Therefore, fendiline may have a mechanism of action in both the mPFC and the VTA. It is possible that fendiline blocks L-type Ca2+ channels on mPFC neurons and therefore reduces the hyperexcitability of the neurons that contribute to craving and relapse. Further, fendiline may reduce the excitability of VTA dopamine neurons during drug- and cue-induced reinstatement.
The anti-addictive properties of L-type Ca2+ antagonists are supported by experiments on a variety of psychostimulants. L-type Ca2+ channel blockers attenuate the initiation of cocaine self-administration (Kuzmin et al. 1996), attenuate the induction and expression of nicotine-induced locomotor sensitization, block the induction of nicotine CPP and inhibit drug-induced reinstatement of nicotine CPP (Biala 2002; Biala and Budzynska 2008, respectively), and inhibit methamphetamine and cocaine CPP (Suzuki et al. 1992). Furthermore, L-type Ca2+ channels regulate normal dopaminergic activity in the VTA (Liu et al. 2014), L-type Ca2+ channel agonists can induce strong VTA bust firing (Zhang et al. 2005), and microinjection of an L-type Ca2+ channel antagonist into the VTA can impair the induction of cocaine-induced behavioral sensitization (Licata et al. 2004). Moreover, fendiline is likely to better tolerated in humans, compared to treatments like baclofen, because it has no effect on general or motivated motor behavior and shows no tolerance with repeated administration (Voigt et al. 2014).
Summary
In this study, fendiline was assessed for anti-addictive properties using rat cocaine self-administration models of human drug use and relapse to drug use. Fendiline is most effective as a pretreatment prior to reinstatement or when used in combination with a GABAB agonist like baclofen for self-administration. These data suggest that in humans, fendiline may be best utilized as a chronic daily treatment for cocaine addicts who wish to reduce their risk of relapse. Fendiline is ideally suited for this role because it is already approved for human use, shows no non-specific effects on behavior (shown here and in Voigt et al. 2014), does not show tolerance, and is not inherently aversive or reinforcing (Voigt et al. 2014). Based on the available evidence, a combined treatment of fendiline and baclofen would be best instituted in situations where addicts have managed to stop using cocaine and are motivated to remain drug free. It should be mentioned that drug addiction is often characterized by poly-drug use, and fendiline has, so far, only been examined using psychostimulants. Therefore, future studies should examine the effect of fendiline in animal models of addiction to opioids and alcohol.
While some research suggests that fendiline may possess pharmacological characteristics similar to GABAB PAMs (Kerr et al. 2002; Chen et al. 2005), inconclusive evidence exists for the classification of fendiline as a GABAB PAM (Urwyler et al. 2004). Therefore, we suggest that fendiline exerts its anti-addictive effects via L-type Ca2+ channel blockade. Irrespective of the specific mechanism of action, we have shown that fendiline alone cannot reduce the motivation to take cocaine, but it does have anti-addictive properties in relapse models of addiction. Further, our evidence indicates that fendiline may possess the unique ability to potentiate the anti-addictive properties of FDA-approved GABAB agonists like baclofen. Future investigations with fendiline alone and in combined treatments may provide novel, well-tolerated pharmacotherapies for psychostimulant addicts. Importantly, this study contributes to a body of literature examining the feasibility of human-approved medications to treat psychostimulant addiction.
References
Arnold JM, Roberts D (1997) A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Be 57:441–447
Bailey CP, Husbands SM (2014) Novel approaches for the treatment of psychostimulant and opioid abuse-focus on opioid receptor-based therapies. Expert Opin Drug Dis 9:1333–1344
Bayer R, Mannhold R (1986) Fendiline: a review of its basic pharmacological and clinical properties. Pharmatherapeutica 5:103–136
Berger SM, Bartsch D (2014) The role of L-type voltage-gated calcium channels Cav1.2 and Cav1.3 in normal and pathological brain function. Cell Tissue Res 357:463–476
Berger SP, Reid MS, Delucchi K et al (1996) Haloperidol antagonism of cue-elicited cocaine craving. Lancet 347:504–508
Biala G (2002) Calcium channel antagonists suppress nicotine-induced place preference and locomotor sensitization in rodents. Pol J Pharmacol 55:327–335
Biala G, Budzynska B (2008) Calcium-dependent mechanisms of the reinstatement of nicotine-conditioned place preference by drug priming in rats. Pharmacol Biochem Be 89:116–125
Brebner K, Phelan R, Roberts DC (2000a) Effect of baclofen on cocaine self-administration in rats reinforced under fixed-ratio 1 and progressive-ratio schedules. Psychopharmacology 148:314–321
Brebner K, Phelan R, Roberts DC (2000b) Intra-VTA baclofen attenuates cocaine self-administration on a progressive ratio schedule of reinforcement. Pharmacol Biochem Be 66:857–862
Brebner K, Childress AR, Roberts DC (2002) A potential role for GABAB agonists in the treatment of psychostimulant addiction. Alcohol Alcoholism 37:478–484
Brebner K, Ahn S, Phillips AG (2005) Attenuation of d-amphetamine self-administration by baclofen in the rat: behavioral and neurochemical correlates. Psychopharmacology 177:409–417
Campbell U, Lac S, Carroll M (1999) Effects of baclofen on maintenance and reinstatement of intravenous cocaine self-administration in rats. Psychopharmacology 143:209–214
Chen Y, Phillips K, Minton G, Sher E (2005) GABAB receptor modulators potentiate baclofen-induced depression of dopamine neuron activity in the rat ventral tegmental area. Brit J Pharmacol 144:926–932
Cryan JF, Kelly PH, Chaperon F et al (2004) Behavioral characterization of the novel GABAB receptor-positive modulator GS39783 (N, N′-dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4, 6-diamine): anxiolytic-like activity without side effects associated with baclofen or benzodiazepines. J Pharmacol Exp Ther 310:952–963
Filip M, Frankowska M (2007) Effects of GABAB receptor agents on cocaine priming, discrete contextual cue and food induced relapses. Eur J Pharmacol 571:166–173
Filip M, Frankowska M, Przegaliński E (2007) Effects of GABAB receptor antagonist, agonists and allosteric positive modulator on the cocaine-induced self-administration and drug discrimination. Eur J Pharmacol 574:148–157
Ford KA, Wolf ME, Hu XT (2009) Plasticity of L-type Ca2+ channels after cocaine withdrawal. Synapse 63:690–697
Haney M, Collins ED, Ward AS, Foltin RW, Fischman MW (1999) Effect of a selective dopamine D1 agonist (ABT-431) on smoked cocaine self-administration in humans. Psychopharmacology 143:102–110
Kerr DI, Ong J, Puspawati NM, Prager RH (2002) Arylalkylamines are a novel class of positive allosteric modulators at GABAB receptors in rat neocortex. Eur J Pharmacol 451:69–77
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacol 35:217–238
Kuzmin A, Semenova S, Ramsey N, Zvartau E, Ree J (1996) Modulation of cocaine intravenous self-administration in drug-naive animals by dihydropyridine Ca2+ channel modulators. Eur J Pharmacol 295:19–25
Licata SC, Pierce RC (2003) The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J Neurochem 85:14–22
Licata SC, Freeman AY, Pierce-Bancroft AF, Pierce RC (2000) Repeated stimulation of L-type calcium channels in the rat ventral tegmental area mimics the initiation of behavioral sensitization to cocaine. Psychopharmacology 152:110–118
Licata SC, Schmidt HD, Pierce RC (2004) Suppressing calcium/calmodulin-dependent protein kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine-induced behavioural sensitization in rats. Eur J Neurosci 19:405–414
Liu Y, Harding M, Pittman A et al (2014) Cav1. 2 and Cav1. 3 L-type calcium channels regulate dopaminergic firing activity in the mouse ventral tegmental area. J Neurophysiolo 112:1119–1130
Lynch WJ, Nicholson KL, Dance ME, Morgan RW, Foley PL (2010) Animal models of substance abuse and addiction: implications for science, animal welfare, and society. Comparative Med 60:177–188
McGregor A, Baker G, Roberts DCS (1996) Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on intravenous cocaine self-administration under a progressive ratio schedule of reinforcement. Pharmacol Biochem Be 53:5–9
Rajadhyaksha AM, Husson I, Satpute SS et al (2004) L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase ½ phosphorylation in the ventral tegmental area after chronic amphetamine treatment. J Neurosci 24:7464–7476
Ranaldi R, Poeggel K (2002) Baclofen decreases methamphetamine self-administration in rats. Neuroreport 13:1107–1110
Richardson NR, Roberts DCS (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Meth 66:1–11
Roberts DCS, Goeders N (1989) Drug self-administration: experimental methods and determinants. In: Boulton AA, Baker GB, Greenshaw AJ (eds) Neuromethods: psychopharmacology. Humana Press Inc., Clifton NJ, pp 439–398
Roberts DCS, Andrews MM, Vickers GJ (1996) Baclofen attenuates the reinforcing effects of cocaine in rats. Neuropsychopharmacol 15:417–424
Shoptaw S, Yang X, Rotheram-Fuller EJ et al (2003) Randomized placebo-controlled trial of baclofen for cocaine dependence: preliminary effects for individuals with chronic patterns of cocaine use. J Clin Psychiat 64:1440–1448
Suzuki T, Shiozaki Y, Masukawa Y, Misawa M (1992) Effects of calcium antagonists on the cocaine-and methamphetamine-induced conditioned place preference. Arukoru Kenkyuto Yakubutsu Ison 27:81–90
Urwyler S, Gjoni T, Kaupmann K, Pozza MF, Mosbacher J (2004) Selected amino acids, dipeptides and arylalkylamine derivatives do not act as allosteric modulators at GABAB receptors. Eur J Pharmacol 483:147–153
Voigt RM, Riddle JL, Napier TC (2014) Effect of fendiline on the maintenance and expression of methamphetamine-induced conditioned place preference in Sprague–Dawley rats. Psychopharmacology 231:2019–2029
Wise RA (1996) Neurobiology of addiction. Curr Opin Neurobiol 6:243–251
Zhang L, Liu Y, Chen X (2005) Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L type channels in the rat. J Physiol Lond 568:469–481
Acknowledgments
The authors would like to thank Anthony Phillips for his comments and suggestions in the preparation of this article. This research was supported by CFI/NSRIT #17561, NSHRF MED-Project-2008-4695, and St FX UCR #91642.
Conflict of interest
There are no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cunningham, J.J., Orr, E., Lothian, B.C. et al. Effects of fendiline on cocaine-seeking behavior in the rat. Psychopharmacology 232, 4401–4410 (2015). https://doi.org/10.1007/s00213-015-4061-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4061-4