Abstract
Nicotine has been shown to affect cortical excitability measured using transcranial magnetic stimulation in smoking and non-smoking subjects in different ways. In tobacco-deprived smokers, administration of nicotine restores compromised cortical facilitation while in non-smokers, it enhances cortical inhibition. As cortical excitability and activity are closely linked to cognitive processes, we aimed to explore whether nicotine-induced physiological alterations in non-smokers and smokers are associated with cognitive changes. Specifically, we assessed the impact of nicotine on working memory performance (n-back letter task) and on attentional processes (Stroop interference test) in healthy smokers and non-smokers. Both tasks have been shown to rely on prefrontal areas, and nicotinic receptors are relevantly involved in prefrontal function. Sixteen smoking and 16 non-smoking subjects participated in the 3-back letter task and 21 smoking and 21 non-smoking subjects in the Stroop test after the respective application of placebo or nicotine patches. The results show that working memory and attentional processes are compromised in nicotine-deprived smokers compared to non-smoking individuals. After administration of nicotine, working memory performance in smokers improved, while non-smoking subjects displayed decreased accuracy with increased number of errors. The effects have been shown to be more apparent for working memory performance than attentional processes. In summary, cognitive functions can be restored by nicotine in deprived smokers, whereas non-smokers do not gain additional benefit. The respective changes are in accordance with related effects of nicotine on cortical excitability in both groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nicotine is the main psychoactive component of tobacco and driving agent of tobacco dependency. Despite well-documented and well-known evidence of the severe health consequences, one billion people smoke worldwide (World Health Organization). Nicotine is known to improve cognitive functions, including sustained attention, vigilance, visuospatial selective attention, spatial working memory, and associative memory (Lawrence et al. 2002; Meinke et al. 2006; Holmes et al. 2008), both in animal models and humans. It can ameliorate neurocognitive deficits in patients with schizophrenia and Alzheimer’s disease (Barr et al. 2008; Wilson et al. 1995; White and Levin 1999). Acute abstinence from smoking by smokers has deleterious effects on neurocognitive functions, including sustained attention, working memory, and response inhibition (Ashare et al. 2014; Snyder and Henningfield 1989) that can be partly compensated by nicotine replacement therapy or the nicotinic receptor agonist varenicline (Atzori et al. 2008; Ashare and McKee 2012). In non-smokers, results are inconsistent. Both improvement and impairment of cognitive and attentional performance after administration of a nicotine patch have been described (Wignall and De Wit 2011; Foulds et al. 1996). Neurophysiologically, cortical excitability and plasticity have been linked as possible biomarkers for cognitive function (Miniussi and Ruzzoli 2013). Decreased intracortical facilitation in deprived smokers measured by transcranial magnetic stimulation is compensated by administration of a nicotine patch (Grundey et al. 2013; Lang et al. 2008). These results suggest a restitutional effect of nicotine on cortical excitability in smokers, which might explain its performance-improving properties.
The aforementioned study by our group leads to the question of whether results regarding cortical excitability can be transferred to cognitive performance and function in both groups and, precisely, if similar restitutional effects on cognition in smokers can be found. On this account, we aimed to directly compare the impact of nicotine on cognitive functions in smokers and non-smokers that were matched in terms of age, gender, smoking status, and education. Since enhanced intracortical facilitation, e.g., by anodal direct current stimulation of the dorsolateral prefrontal cortex improves working memory performance (Brunoni and Vanderhasselt 2014) and nicotinic receptors have been shown to be involved relevantly in prefrontal functions (Poorthuis and Mansvelder 2013), we chose working memory performance as the benchmark of frontal cognitive function. An experimental paradigm used to investigate working memory is the n-back letter task (Baddeley 1992). Here, the 3-back letter condition is most sensitive to abstinence and medication effects (Loughead et al. 2009). Studies suggest that smokers’ verbal working memory capacity is lower than that of non-smokers (Greenstein and Kassel 2009). Furthermore, nicotine abstinence in smokers is associated with further reductions in the efficiency of working memory neurocircuitries (Jacobsen et al. 2007). To evaluate prefrontal attentional functions, we choose the Stroop color/word interference paradigm. Stroop interference occurs when naming the color of a word printed in a color different than that denoted in the word (e.g., the word “green” printed in red) and is measured by the reaction time in this situation compared to the reaction time required to name the colors of words printed in the congruent color (Jensen and Rohwer 1966). For deprived smokers, both impaired and unchanged performances have been described (Azizian et al. 2010; Pomerleau et al. 1994), while the comparison of non-deprived smokers and never-smokers revealed no differences (Wagner et al. 2013). The heterogeneous results can be partly explained by the differences in nicotine dosages used in the various studies and by the different ways these were administered, which might produce rapid or slow increases in systemic nicotine and thus result in different effects on cognitive performance. Diversity of age might have further influenced the results (Evans and Drobes 2009).
The current study sought to address these latter issues by comparing working memory performance and Stroop interference in gender-, age-, and education-matched smokers and non-smokers, during placebo medication and after nicotine patch administration. We matched in terms of education to minimize the variability and possibility of a systematic impact of a priori cognitive differences on our results. Additionally, most former studies focused on the effects of nicotine on cognition in subjects with psychiatric disorders (e.g., schizophrenia) or smokers and non-smokers alone. Hence, the experimental setup of a placebo-controlled study in a homogeneous group of smoking and non-smoking participants might provide clearer results with regard to the impact of nicotine on the respective cognitive processes. Based on the physiological effects of nicotine in smokers and non-smokers (Grundey et al. 2013) and on other studies showing improvement of working memory performance by enhancement of cortical excitability (Martin et al. 2014), we hypothesized that smokers exhibit reduced cognitive performance under nicotine withdrawal, which should however be restituted by nicotine-induced cortical facilitation. For non-smoking subjects, we hypothesized a deterioration in cognitive performance after nicotine administration.
Methods and material
Subjects
32 subjects participated in the 3-back letter task (16 smoking and 16 non-smoking subjects) and 42 subjects in the Stroop interference test (21 smokers and 21 non-smokers). Thirty-two subjects took part in both tests. Subjects (age- and gender-matched) were recruited from a student population and thus had identical educational background. Exclusion criteria were age younger than 18 years and older than 50 years, current history of neurological and psychiatric diseases, current or previous drug (other than nicotine) abuse, alcohol abuse, bronchial asthma, and allergies to components of the nicotine patch. We defined non-smoking subjects as participants without any history of chronic nicotine intake or without occasional nicotine consumption during the past 5 years. Chronic smokers had to have smoked a minimum of 5 years with at least 10 cigarettes a day prior to the experiments. Smokers were asked to avoid smoking at least 6 h prior to the experiments to have a low nicotine level and minimize the side effects of further nicotine administration. The Fagerstrøm scale determined level of addiction. The study was approved by the ethics committee of the University of Göttingen and was conducted in accordance with the Declaration of Helsinki. All subjects gave written informed consent prior to participation (see also Table 1).
3-Back letter task
Based on the evidence that the 3-back letter task is most sensitive to abstinence and medication effects (Loughead et al. 2009), we focused on this task. We used the 3-back letter working memory paradigm as used elsewhere (Mull and Seyal 2001). Subjects were presented with a pseudo-random set of 10 letters (A–J). The stimuli were generated using presentation version 0.71. Each letter was displayed on a computer monitor (14.1 in. for 300 ms. A different letter was displayed every 2 s. Black letters were presented on a white background and subtended 2.4 cm (when viewed at 50 cm). Subjects were required to respond (key press) if the presented letter was the same as the letter presented three stimuli previously or not to respond if the letter was different. Altogether, 143 letters were presented, and a total of 30 to 32 correct responses were possible, depending on the version of the test. We applied different versions of the test to avoid learning effects on performance. Subjects were allowed to practice the task for 20 min or until they obtained an accuracy of 50 % before the start of the experiment.
The Stroop color-word test
The Stroop task is a neuropsychological test that measures cognitive flexibility, selective attention, cognitive inhibition, and information processing speed (Bryan and Luszcz 2000; Peña-Casanova et al. 2009). The test includes three different sections in which subjects are asked to perform the task as quickly as possible. We used a computerized Stroop test (Assef et al. 2007) using Presentation® software (Version 0.70, www.neurobs.com) similar to the Victoria version. Stimuli were presented on a laptop (screen 14.4 in. Acer TravelMate 220) on a black background. The size of stimuli was 1.4 cm at approximately 50 cm distance. The first section was the Stroop word. Here, subjects were presented with four different words (red, blue, green, and yellow) written in black ink. A keyboard with only four keys, colored in red, blue, yellow, and green, was placed in front of the subjects. All other keys were removed. The participants were asked to press the appropriate response key (word: green; green key, etc.) as fast as possible. The second section was the Stroop color task. Here, some XXXs were presented in red, green, yellow, and blue ink, and again, the subjects were asked to press the corresponding key on the keyboard. The third and last section was the Stroop color-word task. In this section, the color of the ink in which the word was displayed was different from the meaning of the word (for example, the word “red” was written in blue). Subjects had to press the corresponding key of the color in which the word was written. The resulting increase in reaction time is called the color-word interference effect or Stroop effect. The three sections were repeated three times with 15 words/xxx, altogether 45 stimuli per section.
Pharmacological intervention
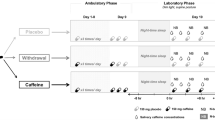
Each subject participated in two sessions in randomized order. Thirty cm2 transdermal nicotine patches, each containing nicotine 0.83 mg/cm2 releasing 15 mg over 16 h, were administered to all subjects and compared to a placebo patch condition. Physiologically and behaviorally relevant plasma levels are maintained with this dosage of nicotine patch (Thirugnanasambandam et al. 2011; Tønnesen et al. 1991). Six hours after application of the nicotine or placebo patch, the Stroop color-word test or 3-back letter task were performed in randomized order. This is the approximate time for the plasma level to reach its maximum following application of the patch (Nørregaard et al. 1992). Pharmacological intervention and task performance were conducted in randomized order among subjects, with an interval between sessions of 1 week to avoid any interference. To avoid nicotinic side effects (dizziness, vomiting, diarrhoea, etc.), non-smokers received additionally 20 mg domperidone. It is assumed that the substance does not relevantly cross the blood brain barrier (Barone 1999). Rare side effects (seizures; extrapyramidal symptoms) on the central nervous system suggest a subtle subclinical effect on cortical excitability in some patients (Spirt et al. 1992). In one of our last papers, we therefore measured the effect of domperidone on cortical excitability and found no differences in both groups; thus, physiological effects on the central nervous systems are improbable (Grundey et al. 2013). Subjects received domperidone together with the nicotine patch and again after 6 h or placebo with the placebo patch. All side effects were documented qualitatively.
Data analysis and statistics
For the 3-back letter task, the primary outcomes were hits, misses, correct rejections, false alarms, and respond time. Furthermore, the sensitivity index d′ was calculated for both groups and conditions (Haatveit et al. 2010). The index d′ derives from signal detection theory and reflects participants’ ability to discriminate target from non-target. d′ was calculated with the following formula: Z(hit rate) − Z(false alarm rate); where Z represents the z-scores of both rates (Macmillan and Creelman 1991). Perfect scores were adjusted using these formulas: 1–1 / (2n) for perfect (e.g., hit rate 1) and 1 / (2n) for zero false alarms. For each participant, an individual mean was calculated for all these variables. In the Stroop color-word test, a mean for the corresponding reaction times, percentage, and errors were calculated for each section (word, color, color-word) and subject. All calculations were conducted for the placebo and the nicotine condition. Then, repeated measures analyses of variance (ANOVA) were calculated for all variables. Group (smoker vs. non-smoker) served as between-subject factor, while within-subject factors were “drug” (placebo vs. nicotine patch) and “sequence” (word, color, and color-word) for the Stroop color-word test. For the 3-back letter task, drug (placebo and nicotine patch) served as within-subject factor and group as between-subject factor. Dependent variables were reaction time, hits, misses, correct rejections, false alarms, and d′. The Mauchly test was performed to test for sphericity and the Greenhouse–Geisser correction applied when necessary. Conditional on significant results of the ANOVA, paired-sample two-tailed t tests (smokers or non-smoking subjects with placebo or nicotine patch) or independent two-sample t test (smokers versus non-smokers) were performed for post hoc analysis. A p value <0.05 was considered significant for all statistical analysis. Post hoc tests were not corrected for multiple comparisons. Based on the results of the respective t test, Cohens’ d and effect size r were calculated (see Table 4). Between-group differences in demographic factors were tested by one-way ANOVA and chi-squared for sex. All data are expressed as mean ± standard of error of means (SEM). Analyses were performed with IBM® SPSS Statistics Version 21 for Mac.
Results
All subjects completed the entire study. Only three non-smoking subjects complained about mild nausea, which was well controlled by administration of domperidone. Groups were gender-, age-, and education-matched. Statistical analysis however revealed a minor but significant age difference between smoking and non-smoking subjects (23.9 versus 25.8 years) for the 3-back letter task (see also Table 1). However, the mean age difference is 1.9 years (varying from 21 to 30 years), which is irrelevant in terms of cognitive function and performance (Brehmer et al. 2012). Chi-squared revealed no significant difference with regard to gender.
Working memory performance in smoking and non-smoking subjects
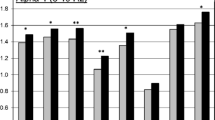
Sixteen smoking and 16 non-smoking subjects participated in the working memory task. The repeated measurement ANOVA for each dependent variable (hits, misses, respond times, correct rejections, false alarms, d′ 3-back) yielded significant results for the interaction between drug × group (see also Table 2). Post hoc analysis (independent two-sample t test) revealed that deprived smokers perform worse in terms of hits (df = 30; t = 2.676; p = 0.012), misses (df = 30; t = −2.490; p = 0.019), reaction time (df = 30; t = 2.758; p = 0.010), and d′ (df = 30; t = 2.869; p = 0.007) compared to non-smoking subjects under placebo conditions. The number of false alarms and correct rejections did not differ between the two groups. After administration of the nicotine patch, the cognitive performance of smokers improved significantly in terms of reaction time (paired-sample, two-tailed t test, df = 15; t = 2.274; p = 0.038) but not in terms of hits (p = 0.100), missed responses (p = 0.151), false responses (p = 0.386), correct rejections (p = 0.498), and d′ (p = 0.093). For non-smoking subjects, results are divergent. Nicotine patches led to impaired working memory performance with a trend toward increased reaction time (from 493 to 560 ms) (t = −1.760; p = 0.099) and significantly decreased the number of hits (df = 15; t = 3.176; p = 0.006), while increasing the number of missed responses (df = 15; t = −3.576; p = 0.003), false alarms (df = 15; t = −2.293; p = 0.037), and d′ (df = 15; t = 3.736; p = 0.002). Compared to smoking subjects with nicotine patch, the rate of hits was lower and the rate of missed responses higher (df = 30; t = −2.080; p = 0.046 (hits); df = 30; t = 2.232; p = 0.033 (misses)). Reaction time of both groups converged, due to a slight impairment of non-smokers and a significant improvement of smokers (see also Fig. 1 and Tables 3 and 4).
In Fig. 1, you find the results of the 3-back letter task in smoking and non-smoking subjects for averaged correct hits (a), averaged misses (b), d′ (c), and reactiocn time in ms (d) after placebo and nicotine administration. Deprived smokers display prolonged reactions times (d), less hits (a), more missed responses (b) and a lower d′ compared to non-smokers. After nicotine application, reaction time in smokers decrease significantly while the number of missed responses in non-smokers increases and the number of correct hits decreases. Vertical bars depict standard error of mean (SEM). *Marks significant differences (Student’s t test; p > 0.05). RT reaction time, n° numbers, plc placebo
Stroop effect in smoking and non-smoking subjects
Twenty-one smoking and 21 non-smoking subjects participated in the Stroop-interference test. The repeated measurement ANOVA for the different variables (percentage, errors, and reaction times) revealed significant results for the main factor sequence (in all three variables) and the interaction sequence × group for the variable reaction time (see also Table 2). The post hoc t tests (independent, two-sample t test) show that deprived smokers performed worse in terms of number of errors (df = 40; t = −2.388; p = 0.022) and reaction time in Stroop-interference (df = 40; t = −2.794; p = 0.008) compared to non-smokers. After administration of nicotine, a non-significant trend toward improved accuracy and reaction time in smoking subjects occurred. Performance of non-smoking subjects worsened under nicotine in terms of reaction time for the color condition (df = 20; t = −2.452; p = 0.024) but also tended to do so for other sequences (word; color-word). Moreover, accuracy tended to decrease with nicotine (p = 0.074; see also Fig. 2).
Results of the Stroop test in smoking and non-smoking subjects for reaction time in ms (a) and averaged number of errors (b) after placebo and nicotine administration. Compared to non-smokers, deprived smokers perform worse in terms of reaction time and number or errors after placebo medication. After nicotine administration, reaction times tend to improve. Attentional processes of non-smokers are impaired after nicotine administration with regard to reaction time (significant for color). Number of errors also tend to increase (p = 0.074; from 1.57 to 0.48). Vertical bars depict standard error of mean (SEM). *Marks significant differences (Student’s t test; p > 0.05)
Discussion
The main results of this study show that working memory and attentional processes are compromised in smokers under nicotine withdrawal, as compared to non-smoking individuals, and that nicotine has different effects on performance in smokers and non-smokers: At the dosage applied in the present experiments, it improves performance in smokers but worsens it in non-smokers. These effects seem to be clearer for working memory performance, as compared to attentional processes.
Proposed mechanism of restitutional nicotinergic effects on working memory performance in smokers and its deterioration in non-smokers
N-methyl-D-aspartate glutamate receptors (NMDA-Rs) and the glutamate system are critically involved in working memory performance (Driesen et al. 2013), chronic nicotine exposure, and withdrawal in smokers (Li et al. 2014). Withdrawal after chronic nicotine use leads to downregulation of glutamate receptor function, which helps to explain cognitive performance deterioration in smokers. Neurophysiologically, deprived smokers display diminished intracortical facilitation (ICF) compared to non-smokers (Grundey et al. 2013). ICF is also known to be primarily controlled by the glutamatergic system (Ziemann et al. 1998), which further supports the concept that downregulation of nicotinic receptors by chronic nicotine intake (Alkondon et al. 2000), and consequently reduced presynaptic glutamate release during nicotine withdrawal, might explain the differences in working memory performance between smokers and non-smokers in the placebo condition. These neurophysiologic mechanisms provide a possible explanation for the respective performance restitution after nicotine administration in deprived smokers, still other mechanisms cannot be ruled out.
In non-smoking subjects, the results of the present study indicate a deterioration in working memory performance, with a decreased rate of hits, increased rate of missed responses, false alarms, and reaction times (alerting attention) after nicotine patch administration. Neurophysiological data from our former study, obtained with identical nicotine dosages in non-smokers, suggest that working memory deficits in non-smokers after nicotine administration might be connected to increased intracortical inhibition, which mainly depends on cholinergic and GABAergic effects (Grundey et al. 2013). Since baclofen, a GABA-B-agonist, has been shown to impair working memory performance (Stackman and Walsh 1994), impaired performance might also be caused by a direct GABA-ergic effect. However, likewise other transmitters, or complex interactions between different neurotransmitters, are involved in these effects.
Differences in Stroop interference between deprived smokers and non-smokers might be explained by decreased intracortical excitability in smokers
Regarding Stroop task performance, an indicator of selective attention, our study has shown that nicotine-deprived smoking participants perform worse than to non-smokers (reaction time in all sections and errors). After administration of nicotine, the performance of smokers showed a tendency to improve (errors and reaction times). Non-smokers did not profit from nicotine and displayed slowed reaction times for all sections (word, color, and color-word) after nicotine patch administration. The Stroop task can be employed as a “marker” for an unspecific excitatory/inhibitory upregulation and downregulation of cognitive processes (Klein et al. 2013). Similar to working memory performance, Stroop interference improves with increased cortical excitation, especially of the dorsolateral prefrontal cortex (Adleman et al. 2002). Accordingly, excitability-enhancing anodal transcranial direct current stimulation (tDCS) of the left, partially also the right, prefrontal cortex leads to significant performance improvements in the Stroop test (Jeon and Han 2012). Impaired Stroop performance in deprived smokers might thus be connected to reduced cortical excitability, while impaired performance in non-smokers after administration of nicotine might be connected to increased inhibition, as suggested by the respective TMS measurements described above (Grundey et al. 2013). Administration of nicotine in deprived smokers would then restitute Stroop performance via enhanced facilitatory excitability.
Conclusion
In the present study, we examined cognitive performance of nicotine-deprived smokers and non-smokers before and after nicotine patch administration. Impaired cognitive performance in deprived smokers might be linked to reduced intracortical facilitation, while impaired performance in non-smokers after nicotine patch administration might be linked to increased intracortical inhibition. Both cognitive performance and neurophysiological parameters recover after nicotine administration in smokers. This study supports the deficit-compensating theory of chronic nicotine consumption. Moreover, respective changes in cognitive performance might be relevant for nicotine addiction and the probability of relapse. At present, it remains unclear whether a priori differences in cognitive performance in smokers and non-smokers result in nicotine addiction or if respective performance differences are a result of nicotine addiction.
Some limitations of the present study should be taken into account. First of all, nicotine patches were administered in a single blind manner, so the person conducting the experimental session knew about the condition. Secondly, TMS measurements and cognitive performance were not conducted in the same groups of subjects; thus, statements about causality are limited. However, similar demographic characteristics of the groups explored in the physiological and cognitive studies allow preliminary conclusions to be drawn. Moreover, results of our study partly differ from those of former cognitive studies performed in smokers and non-smokers. Kumari and colleagues found improvement of working memory performance after nicotine administration in male non-smokers (Kumari et al. 2003); others described no effect of nicotine on working memory performance in non-smokers (Ernst et al. 2001). Opposing results might be based on different nicotine dosages, forms of administration (subcutaneous, intranasal, etc.), and various differences between groups of participants. Non-linear effects of nicotine cannot be ruled out and were not explicitly surveyed so far but are known for neuromodulators like dopamine (Fresnoza et al. 2014; Monte-Silva et al. 2011). Another limitation of our study is the fact that we did not obtain blood levels or test breath for CO concentration to verify compliance of smoking subjects. However, since experiments began in the morning and smoking subjects were only moderately dependent on the Fagerstrøm scale, it was realistic to rely on the statements of the subjects. Only smokers with a light nicotine dependency participated in the current study; heavily dependent smokers may show different results. A last limitation of the study is the fact that we used education as matching factor even though this is only a vague factor of cognitive performance.
References
Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL (2002) A developmental fMRI study of the Stroop color-word task. Neuroimage 16(1):61–75
Alkondon M, Braga MF, Pereira EF, Maelicke A, Albuquerque EX (2000) Alpha7 nicotinic acetylcholine receptors and modulation of gabaergic synaptic transmission in the hippocampus. Eur J Pharmacol 393(1–3):59–67
Ashare RL, Falcone M, Lerman C (2014) Cognitive function during nicotine withdrawal: implications for nicotine dependence treatment. Neuropharmacology 76(Pt B):581–591
Ashare RL, McKee SA (2012) Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Exp Clin Psychopharmacol 20(1):63–70
Assef EC, Capovilla AG, Capovilla FC (2007) Computerized stroop test to assess selective attention in children with attention deficit hyperactivity disorder. Span J Psychol 10(1):33–40
Atzori G, Lemmonds CA, Kotler ML, Durcan MJ, Boyle J (2008) Efficacy of a nicotine (4 mg)-containing lozenge on the cognitive impairment of nicotine withdrawal. J Clin Psychopharmacol 28(6):667–674
Azizian A, Nestor LJ, Payer D, Monterosso JR, Brody AL, London ED (2010) Smoking reduces conflict-related anterior cingulate activity in abstinent cigarette smokers performing a Stroop task. Neuropsychopharmacology 35(3):775–782
Baddeley A (1992) Working memory. Science 255(5044):556–559, Review
Barone JA (1999) Domperidone: a peripherally acting dopamine2-receptor antagonist. Ann Pharmacother 33(4):429–440, Review
Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP et al (2008) The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology 33(3):480–490
Brehmer Y, Westerberg H, Bäckman L (2012) Working-memory training in younger and older adults: training gains, transfer, and maintenance. Front Hum Neurosci 6:63
Brunoni AR, Vanderhasselt MA (2014) Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn 86:1–9
Bryan J, Luszcz MA (2000) Measures of fluency as predictors of incidental memory among older adults. Psychol Aging 15(3):483–489
Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D'Souza DC et al (2013) The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology 38(13):2613–2622
Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE et al (2001) Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci 98(8):4728–4733
Evans DE, Drobes DJ (2009) Nicotine self-medication of cognitive-attentional processing. Addict Biol 14(1):32–42
Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA (1996) Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacol (Berl) 127(1):31–38
Fresnoza S, Paulus W, Nitsche MA, Kuo MF (2014) Nonlinear dose-dependent impact of D1 receptor activation on motor cortex plasticity in humans. J Neurosci 34(7):2744–2753
Greenstein JE, Kassel JD (2009) The effects of smoking and smoking abstinence on verbal and visuospatial working memory capacity. Exp Clin Psychopharmacol 17(2):78–90
Grundey J, Freznosa S, Klinker F, Lang N, Paulus W, Nitsche MA (2013) Cortical excitability in smoking and not smoking individuals with and without nicotine. Psychopharmacol (Berl) 229(4):653–664
Haatveit BC, Sundet K, Hugdahl K, Ueland T, Melle I, Andreassen OA (2010) The validity of d prime as a working memory index: results from the "Bergen n-back task". J Clin Exp Neuropsychol 32(8):871–880
Holmes AD, Chenery HJ, Copland DA (2008) Transdermal nicotine modulates strategy-based attentional semantic processing in non-smokers. Int J Neuropsychopharmacol 11(3):389–399
Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR (2007) Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacol (Berl) 193(4):557–566
Jensen AR, Rohwer WD Jr (1966) Stroop color-word test: a review. Acta Psychol (Amst) 25(1):36–93, Review. No abstract available
Jeon SY, Han SJ (2012) Improvement of the working memory and naming by transcranial direct current stimulation. Ann Rehabil Med 36(5):585–595
Klein E, Mann A, Huber S, Bloechle J, Willmes K, Karim AA et al (2013) Bilateral bi-cephalic tDCS with two active electrodes of the same polarity modulates bilateral cognitive processes differentially. PLoS One 8:8(8)
Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das M, Zachariah E et al (2003) Cognitive effects of nicotine in humans: an fMRI study. Neuroimage 19(3):1002–1013
Lang N, Hasan A, Sueske E, Paulus W, Nitsche MA (2008) Cortical hypoexcitability in chronic smokers? A transcranial magnetic stimulation study. Neuropsychopharmacology 33(10):2517–2523
Lawrence NS, Ross TJ, Stein EA (2002) Cognitive mechanisms of nicotine on visual attention. Neuron 36(3):539–548
Li X, Semenova S, D'Souza MS, Stoker AK (2014) Markou A (2014): Involvement of glutamatergic and GABAergic systems in nicotine dependence: implications for novel pharmacotherapies for smoking cessation. Neuropharmacology 554–65
Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C (2009) Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatry 14(8):820–826
Martin DM, Liu R, Alonzo A, Green M, Loo CK (2014) Use of transcranial direct current stimulation (tDCS) to enhance cognitive training: effect of timing of stimulation. Exp Brain Res 232(10):3345–3351
Macmillan NA, Creelman CD (1991) Detection theory: a user’s guide. Cambridge University Press, Cambridge
Meinke A, Thiel CM, Fink GR (2006) Effects of nicotine on visuo-spatial selective attention as indexed by event-related potentials. Neuroscience 141(1):201–212
Miniussi C, Ruzzoli M (2013) Handb Clin Neurol 116:739–750
Monte-Silva K, Liebetanz D, Grundey J, Paulus W, Nitsche MA (2011) Dosage-dependent non-linear effect of L-dopa on human motor cortex plasticity. J Physiol 588(Pt 18):3415–3424
Mull BR, Seyal M (2001) Transcranial magnetic stimulation of left prefrontal cortex impairs working memory. Clin Neurophysiol 112:1672–1675
Nørregaard J, Tønnesen P, Simonsen K, Säwe U (1992) Long-term nicotine substitution after application of a 16-hour nicotine patch in smoking cessation. Eur J Clin Pharmacol 43(1):57–60
Peña-Casanova J, Quiñones-Ubeda S, Gramunt-Fombuena N, Quintana M, Aguilar M, Molinuevo JL et al (2009) NEURONORMA Study Team. Spanish Multicenter Normative Studies (NEURONORMA Project): norms for the Stroop color-word interference test and the Tower of London-Drexel. Arch Clin Neuropsychol 24(4):413–429
Pomerleau CS, Teuscher F, Goeters S, Pomerleau OF (1994) Effects of nicotine abstinence and menstrual phase on task performance. Addict Behav 19(4):357–362
Poorthuis RB, Mansvelder HD (2013) Nicotinic acetylcholine receptors controlling attention: behavior, circuits and sensitivity to disruption by nicotine. Biochem Pharmacol 86(8):1089–1098
Snyder FR, Henningfield JE (1989) Effects of nicotine administration following 12 h of tobacco deprivation: assessment on computerized performance tasks. Psychopharmacol (Berl) 97(1):17–22
Spirt MJ, Chan W, Thieberg M, Sachar DB (1992) Neurolepticmalignant syndrome induced by domperidone. Dig Dis Sci 37(6):946–948
Stackman RW, Walsh TJ (1994) Baclofen produces dose-related working memory impairments after intraseptal injection. Behav Neural Biol 61(2):181–185
Thirugnanasambandam N, Grundey J, Adam K, Drees A, Skwirba AC, Lang N et al (2011) Nicotinergic impact on focal and non-focal neuroplasticity induced by non-invasive brain stimulation in non-smoking humans. Neuropsychopharmacology 36(4):879–886
Tønnesen P, Nørregaard J, Simonsen K, Säwe U (1991) A double-blind trial of a 16-hour transdermal nicotine patch in smoking cessation. N Engl J Med 325(5):311–315
Wagner M, Schulze-Rauschenbach S, Petrovsky N, Brinkmeyer J, von der Goltz C, Gründer G, Spreckelmeyer KN et al (2013) Neurocognitive impairments in non-deprived smokers—results from a population-based multi-center study on smoking-related behavior. Addict Biol 18(4):752–761
White HK, Levin ED (1999) Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer's disease. Psychopharmacol (Berl) 143(2):158–165
Wignall ND, de Wit H (2011) Effects of nicotine on attention and inhibitory control in healthy nonsmokers. Exp Clin Psychopharmacol 19(3):183–191
Wilson AL, Langley LK, Monley J, Bauer T, Rottunda S, McFalls E et al (1995) Nicotine patches in Alzheimer's disease: pilot study on learning, memory, and safety. Pharmacol Biochem Behav 51(2–3):509–514
Ziemann U, Chen R, Cohen LG, Hallett M (1998) Dextromethorphan decreases the excitability of the human motor cortex. Neurology 51(5):1320–1324
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (DFG grant NI 683/4-1) ‘Towards risk prediction of nicotine dependency by exploring individual limits of cortical neuroplasticity in humans’ and ‘Impact of the nicotinergic alpha7 receptor on cortical plasticity in smokers and non-smokers’ (NI683/4-2) within the DFG priority program ‘Nicotine: Molecular and Physiological Effects in Central Nervous System’.
Conflict of interest
JG, RA, GA, and GB received no financial support, and no compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. WP is member of the Advisory Boards of GSK, UCB, Desitin. MAN is member of the Advisory Boards of UCB, Eisai, GSK, and Neuroelectronics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grundey, J., Amu, R., Ambrus, G.G. et al. Double dissociation of working memory and attentional processes in smokers and non-smokers with and without nicotine. Psychopharmacology 232, 2491–2501 (2015). https://doi.org/10.1007/s00213-015-3880-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-3880-7