Abstract
Rationale
Repeated methamphetamine (MA) use leads to increases in the incentive motivational properties of the drug as well as cognitive impairments. These behavioral alterations persist for some time following abstinence, and neuroadaptations in the structure and function of the prefrontal cortex (PFC) are particularly important for their expression. However, there is a weak understanding of the changes in neural firing and oscillatory activity in the PFC evoked by repeated drug use, thus complicating the development of novel treatment strategies for addiction.
Objectives
The purpose of the current study was to assess changes in cognitive and brain function following MA sensitization.
Methods
Sensitization was induced in rats, then temporal and recognition memory were assessed after 1 or 30 days of abstinence. Electrophysiological recordings from the medial PFC were also acquired from rats whereupon simultaneous measures of oscillatory and spiking activity were examined.
Results
Impaired temporal memory was observed after 1 and 30 days of abstinence. However, recognition memory was only impaired after 1 day of abstinence. An injection of MA profoundly decreased neuronal firing rate and the anesthesia-induced slow oscillation (SO) in both sensitized (SENS) and control (CTRL) rats. Strong correlations were observed between the SO and gamma band power, which was altered in SENS animals. A decrease in the number of neurons phase-locked to the gamma oscillation was also observed in SENS animals.
Conclusions
The changes observed in PFC function may play an integral role in the expression of the altered behavioral phenotype evoked by MA sensitization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repeated methamphetamine (MA) use is associated with progressive increases in drug craving and seeking behavior (Tolliver et al. 2010). MA abusers also exhibit impairments in a range of cognitive functions including decision-making (Paulus et al. 2003), attention (Salo et al. 2009), and episodic memory (Simon et al. 2004). Following abstinence, enhanced craving and seeking are observed in parallel with impairments in executive function, which may persist for over a year (Salo et al. 2011; Sax and Strakowski 2001). However, performance on other forms of cognitive function including episodic memory, may improve after ∼6 months of abstinence (Simon et al. 2004). Given the well-defined role of the prefrontal cortex (PFC) in drug craving (George and Koob 2010) and advanced forms of cognitive function (Euston et al. 2012), impairments in neural processing in the PFC may be among the most enduring and debilitating features of repeated MA use.
Similar to MA users, MA-sensitized rodents exhibit craving and seeking behaviors in parallel with cognitive abnormalities during abstinence (Dalley et al. 2005). Sensitized behavioral responses to drugs are thought to be associated with increases in the attribution of incentive salience to drug-paired stimuli that are likely mediated by changes in structure and function of neural circuits necessary to encode reward-related information (Steketee and Kalivas 2011).
Deficits in cognitive flexibility (Kosheleff et al. 2012; Simon et al. 2000), in addition to temporal and episodic memory (Cheng et al. 2007; Wittmann et al. 2007; Belcher et al. 2006; Simon et al. 2004) are observed in both humans and animals following repeated MA, which may play a role in the inability to abstain from drug taking. It has been argued that deficits in episodic or temporal memory may increase the difficulty for an addicted individual to follow a treatment plan and apply it in the future (Pitel et al. 2007; Bechara 2005). Impairments in time perception are also observed in MA abusers (Williamson et al. 2008), which could contribute to their propensity for impulsive decision-making and increase the probability of relapse (Kurti et al. 2013; Hoffman et al. 2006). Furthermore, MA abusers have impairments in episodic memory during initiation, retrieval, and execution of previously experienced and encoded memories (Iudicello et al. 2011).
Temporal and episodic memory are commonly measured in rodents using temporal order recognition memory (TORM) and novel object recognition (NOR) tasks, respectively. TORM examines an organism’s ability to sequence events in time and is disrupted by transient inactivation of the PFC (Hannesson et al. 2004). However, no studies to date have examined the effects of repeated MA on this behavior. Recognition memory is impaired following transient inactivation of the hippocampus (HC) (Broadbent et al. 2010) and the perirhinal cortex (Hannesson et al. 2004), specifically during memory encoding, retrieval, and consolidation (Winters and Bussey 2005). Furthermore, rodents receiving repeated MA exhibit impairments in NOR (Belcher et al. 2006; Camarasa et al. 2010; Herring et al. 2008; Lu et al. 2010; Mizoguchi et al. 2008; Reichel et al. 2010, 2012). As such, the goal of this study was to induce sensitization in rats, and then use TORM and NOR as indices of frontal and temporal lobe function to determine how repeated MA effects cognitive functions after short-term and extended abstinence.
Persistent changes in neural activity that last beyond the acute pharmacological effects of MA are likely critical for the expression of sensitization and the impaired cognitive phenotype observed following repeated MA use. For example, individuals using or recently abstinent from MA display increases in EEG power in delta and theta frequencies, which correlates with poor episodic memory and information processing compared with controls (Newton et al. 2004). Repeated psychostimulant administration has also been shown to change firing and oscillatory activity in the rodent PFC, which supports the hypothesis that altered neural processing in this structure may contribute to impaired cognitive function (Homayoun and Moghaddam 2006; Lapish et al. 2012).
Considerable evidence points to synchronous activity among neural oscillations and spiking as a mechanism for encoding information (Ward 2003). In reduced states of vigilance, such as sleep and anesthesia, transient states of synchronous activity are observed in a number of brain regions (Steriade 1997). During these states, a <1.5-Hz slow oscillation (SO) is commonly observed that is associated with intense bouts of synaptic activity resulting in bursts of action potentials and increased oscillatory power (Steriade et al. 1993). A number of physiological phenomena, from replay to increased gamma power, are observed during the SO (Steriade 2006). These phenomena are driven, in part, by increases in synaptic drive resulting in large positive deflections (upstates) of local field potentials (LFP) and likely play a critical role in plasticity and neural encoding (Masquelier et al. 2009). SOs remain intact following urethane anesthesia (Pirch et al. 1985), thus motivating its use in this study to examine how repeated MA use alters PFC firing and oscillatory activity evoked by the SO.
Gamma oscillations (30–100 Hz) are considered important for temporal encoding (Buzsaki and Chrobak 1995), storage and recall of information (Lisman and Idiart 1995), and are modulated by SOs (Isomura et al. 2006). Phase locking of spike activity to the gamma oscillation is hypothesized to play an important role in the formation of localized cell assemblies and information transfer between them (Sirota et al. 2008). Moreover, disturbances in gamma are observed in a number of neuropsychiatric disorders, including addiction (Liu et al. 2005) and schizophrenia (Williams and Boksa 2010), and these disturbances may represent an aspect of neural processing that is critically altered in these clinical populations. The current study provides the first examination of the persistent changes in neural firing and ongoing oscillatory activity evoked by repeated MA administration, to better understand the changes in brain function brought about by drugs of abuse.
Materials and methods
Subjects
Seventy-one adult male (PND 64 at beginning of experiments) Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing 250–300 g were individually housed and maintained on a reverse light/dark schedule with ad libitum access to food and water. All procedures were approved by the Purdue School of Science Institutional Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (2003).
Drug treatments
Rats were administered either methamphetamine hydrochloride (MA; Sigma Chemical Co., St Louis, Mo., USA) or 0.9 % saline (SAL) via intraperitoneal (i.p.) injection. MA was dissolved in sterile SAL and injected at a dose of 0.5 or 5.0 mg/kg in a final volume of 1.0 ml/kg. For surgeries, rats received an i.p. injection of urethane (Sigma Chemical Co., St Louis, Mo., USA) ∼4 h before surgery at a dose of 1.5 g/kg dissolved in sterile water in a volume of 0.1 ml/kg. Rats were administered additional urethane if necessary at a dose of 0.75 g/kg.
Behavioral measures
Behavioral sensitization
Animals were acclimated to the locomotor chamber (54.61-cm diameter × 41.91-cm height) for 60 min, then were injected, and placed back into the chamber for 60 min. Animals received 5.0 mg/kg of MA (SENS) or SAL (CTRL) every other day for 13 days (induction phase), and then were undisturbed for 7 days in their home cage. Following this period, rats were injected on Day 21 to provide an initial assessment of sensitization prior to cognitive testing. After all cognitive testing, SENS and CTRL groups were split into groups receiving one of two doses of MA (0.5 or 5.0 mg/kg) and SAL, counterbalanced over two consecutive days to confirm sensitization (MA challenge, days 54–55). In each experiment, behavior was acquired via video camera mounted above the chambers, recorded in ANY-maze (Wood Dale, IL), and scored offline by two experimenters that were blind to animal treatment. Distance traveled (m) and stereotypy were the dependent variables used to assess sensitization. Cronbach’s alpha was used to assess the internal consistency of the 126 stereotypy values between the two raters. The scores between the two raters were highly reliable (α = 0.82).
Cognitive testing
Rats were tested on TORM and NOR after 1 and 30 days of abstinence from repeated MA injections (Online Resource 1 Fig. S1 for timeline). For these experiments, an open field chamber (86.36 × 93.98 × 31.24 cm) was used that contained a strip of Velcro on two opposite sides of the chamber, approximately 16.5 cm from the corner of the box. The objects included two rubber ducks (8.1 × 6.35 cm), Rubik’s cubes (5.8 × 5.8 cm), or circular white cups (7.62 × 4.57 cm). The order that animals experienced the objects was randomized.
On testing days, animals were placed in the chamber and were allowed to explore two identical objects for 4 min (trial 1). In trial 2, after a 60-min inter-trial interval, animals were placed in the chamber for 4 min with two identical objects different than the ones experienced previously. To assess temporal memory, rats were exposed to two objects (one from each previous trial) for another 4 min after a 45-min inter-trial interval (trial 3). NOR testing took place in the same chamber after a 45-min inter-trial interval. Animals in the 1- and 30-day abstinence groups were placed in the chamber with the most recent object (from trial 2) encountered along with a novel object for 4 min (trial 4). The amount of time (seconds) that an animal spent interacting with each object was the dependent variable.
Surgical procedures
Simultaneous spike train and LFP recordings were acquired from the PFC of anesthetized rats that went through an identical SENS procedure as described above. Following SAL or MA injection on day 21, one animal per day over 9 days underwent electrophysiological procedures. This period was chosen based on observations of TORM impairments in animals repeatedly treated with MA. Animals were anesthetized with urethane and placed into a Kopf stereotaxic frame for surgery. An incision was made, the skull was exposed, and a craniotomy was made over the PFC. After removal of the dura, a microelectrode array was lowered into the right medial PFC (AP, +2.70; ML, +0.50; DV, −1.5: relative to bregma).
To make the electrode arrays, 4 × 16 μm stablohm wires (California Fine Wire; Grover Beach, CA) were twisted into tetrodes and fed through 75 μm polyamide tubing (Small Parts, Logansport, IN). These were then fed through 34-gauge microfil tubing arrays (World Precision Instruments, Sarasota, FL). The array was fed through a 200-μl pipet tip, and tetrodes were wired to EIB-27 micro (Neuralynx; Bozeman, MT) and secured with dental acrylic.
LFP and single-unit recordings
Probes were lowered and cells were located before the start of recording (Online Resource 1 Fig. S2 for timeline). At least one wire was referenced to a ground screw over the cerebellum (for LFPs) while the remaining wires were referenced locally (for single-unit recording). LFPs and spikes were acquired using a Neuralynx recording system. LFPs were sampled at 32,556 Hz then to 1017 Hz prior to analysis. After recordings, animals were euthanized using CO2 and brains were extracted to assess placements (Fig. 6). The shape and size of the probe are illustrated in the +3.20-mm section.
Data analysis
Locomotor activity was analyzed using repeated measures (RM) analysis of variance (ANOVA) with treatment as a between-subject factor and days as a within-subject factor. Stereotypy was scored using a 1–9 rating scale (Ellinwood and Balster 1974) and was analyzed using Kruskal–Wallis comparing day as a factor between and within treatments. Bonferroni and Dunn’s multiple comparisons were used for post-hoc testing locomotor and stereotypy, respectively. For the MA challenge data, stereotypy was examined via Mann–Whitney to assess between-group differences. Time-series analysis using the first 25 min was conducted for locomotor activity and stereotypy on the final challenge day (5.0 mg/kg). For locomotor activity, a two-way RM ANOVA was conducted with treatment as the between-subject factor and time as the within-subject factor. For stereotypy, a Friedman test for CTRL and SENS groups were performed separately with bin as the repeated measure. Mann–Whitney U tests (corrected to p < 0.01) were conducted to compare between-group differences for each bin. Total time spent with each object for TORM and NOR were assessed via Bonferroni corrected planned comparisons within each experiment. Total exploration time for TORM and NOR was assessed using unpaired t tests to compare group differences between CTRL and SENS groups. Significance thresholds for planned comparisons (alpha) were adjusted for the number of comparisons and are stated in the results, whereas p values for Bonferroni post-hoc tests are reported as already corrected for number of comparisons by this statistic.
Spike trains were manually identified and sorted into individual cell clusters based on the features of the waveform in Spike Sort 3D (Neuralynx, Bozeman, MT). After cell sorting, duplicate timestamps and inter-spike intervals <3 ms were removed from spike trains. Only spike trains containing >150 spikes were analyzed.
All electrophysiological data were analyzed in MATLAB (MathWorks, Natick, MA). To detect the SO, LFPs were subsampled to 10 Hz to remove transient voltage deflections (Online Resource 1 Fig. S4). A SO was detected as an increase in voltage of >2000 μV lasting >200 ms. The duration was measured as the time voltage increased beyond 2000 μV until it dropped below 500 μV. The peak was determined as the maximum voltage in the 10-Hz subsampled signal observed during a SO. The frequency was derived as the time between the beginning of two consecutive SOs.
To assess spike-LFP coupling, all spike trains from a recording session were binned at 1 ms resulting in a n × p spike train matrix where n = spike train number and p = time (1 ms bins). The spike train matrix was summed over time resulting in a 1 × p vector, which was then convolved with a Gaussian kernel (1 ms step, 200 ms window). Synchrony between the spike train vector and the LFP was then assessed via magnitude squared coherence.
To assess chance levels of spike train-LFP synchrony, a Monte Carlo approach was employed. The ISIs of each spike train matrix were shuffled and then a spike train vector was created from the shuffled data as described above (Online Resource 1 Fig. S3). Synchrony between the LPF and the convolved vector constructed from shuffled spike trains was also assessed via magnitude-squared coherence as described above. This process was repeated (500 bootstraps) and for each bootstrap coherence was quantified by integrating the spectrum in the <1-Hz range. The resulting scalar values were then used to compute the mean and standard deviation of <1 Hz coherence in the shuffled data.
To assess coupling between slow and gamma oscillations, LFPs were digitally filtered with a first-order Chebyshev lowpass filter (<5 Hz) and a Chebyshev bandpass filter (30–50 Hz) to isolate the slow and gamma oscillation, respectively. To quantify the relationship between these oscillations, the analytic phase and amplitude of the gamma isolated signal was derived via Hilbert transform. From the analytic amplitude, the amplitude envelope of gamma was derived for the entire function and extracted for ±1.0 s surrounding the initiation of the SO. Only recordings with clear up/down state transitions were analyzed in order to control for possible differences in synaptic activity induced by urethane anesthesia.
The relationship between the size of the SO and gamma power was determined by computing power in the 30–50-Hz band from the gamma-filtered signal during each SO. Gamma power was quantified by integrating the power in the 30–50-Hz range, which was computed via Thompsons multitaper method. Gamma power was then stratified by the corresponding size of the SO.
Phase locking to gamma was determined by extracting the phase of the gamma oscillation from the entire gamma-filtered time series. Then, the corresponding phase occurring with each spike was extracted and used to compute the probability density function (PDF) for each cell with phase increments of 20°. PDFs were then subjected to Rayleigh’s test of uniformity at alpha = 0.01. All LFPs were acquired from different tetrodes than those used to acquire spike train data. For phase-locked cells, κ was estimated as the inverse of the sample variance. To determine the extent that cells would be phase-locked by chance, Monte Carlo analysis was performed on each spike train (500 bootstraps). The ISIs of each spike train were shuffled in time and then phase locking to an unshuffled LFP was assessed as described previously.
Results
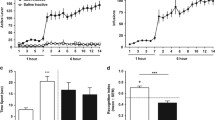
Analysis of locomotor activity revealed progressive decreases over days in the SENS group (treatment × day interaction, F(7, 238) = 3.18, p < 0.005; Fig. 1a), which were paralleled by increases in stereotypy, depicted by a score of 6 or above on the rating scale indicating repetitive stereotypic movements (dashed line) (Fig. 1b; χ 2 = 22.89, p < 0.005). This inverse relationship likely reflects the transition from enhanced locomotor output to stereotypy.
Locomotor activity and stereotypy during the induction phase and MA challenge. (a) Mean locomotor activity immediately following injections for each day of the induction phase (SENS, n = 20; CTRL, n = 17). (Bonferroni correction, # p < 0.05; ## p < 0.005; ### p < 0.0005—significantly lower within-group locomotor activity compared with day 1 in SENS animals). (b) Mean stereotypy during the induction phase (SENS, n = 9; CTRL, n = 8). (Dunn’s, # p < 0.05; ### p < 0.0005—significantly greater within-group stereotypy compared with day 1 in SENS animals). (c) Locomotor activity on days 54 and 55 following the 0.5 mg/kg MA dose (left; SENS, n = 12; CTRL, n = 9) and 5.0 mg/kg dose (right; SENS, n = 8; CTRL, n = 9). (Bonferroni correction: *p < 0.05; **p < 0.005; ***p < 0.0005—significantly different than rats that received a SAL injection). (Bonferroni correction: ### p < 0.0005—significantly different than rats that received a MA injection). (d) Stereotypy on days 54 and 55 following treatment with 0.5 mg/kg MA (left; SENS, n = 12; CTRL, n = 9) and 5.0 mg/kg dose (right; SENS, n = 8; CTRL, n = 6); (Mann–Whitney, *p < 0.05—significantly different than CTRL rats that received a MA injection); (Mann–Whitney, *p < 0.005—significantly different than rats that received a SAL injection). (e) Locomotor activity post-injection on the 5.0 mg/kg challenge day (SENS, n = 8; CTRL, n = 6). (Bonferroni correction, *p < 0.01; **p < 0.001—significantly different than SENS animals that received a MA injection). (f) Stereotypy post-injection on the 5.0 mg/kg challenge day (SENS, n = 8; CTRL, n = 6). (Mann–Whitney, *p < 0.01). All data are depicted as mean ± SEM
Increased locomotor activity was observed on days 54–55 in both CTRL and SENS groups following the 0.5 mg/kg MA challenge (Fig. 1c, left, main effect of treatment, F(1, 42) = 25.67, p < 0.0001). Increased locomotor activity was observed only in the CTRL group following the 5.0 mg/kg dose (Fig. 1c, right, treatment x injection interaction F(1, 26) = 8.99, p < 0.05). No differences in stereotypy between groups were observed following the 0.5 mg/kg MA challenge (Fig. 1d, left). However, increased stereotypy was observed following the higher 5.0 mg/kg dose (SENS, U = 2.00, p < 0.005; CTRL, U = 0.0, p < 0.005; Fig. 1d, right). A significant increase in behavioral stereotypies were also observed between CTRL and SENS animals treated with MA (directional Mann–Whitney U = 9.00, p < 0.04)
For locomotor activity, time-series analyses on the challenge day (5.0 mg/kg) shows a main effect of time (F(4, 96) = 6.68, p < 0.0001), treatment (F(3, 24) = 18.95, p < 0.0001), and a time by treatment interaction (F(12, 96) = 7.38, p < 0.0001) (Fig. 1e). Bonferroni post-hocs revealed a significant difference in locomotor activity between CTRL animals that received MA vs. SENS animals that received MA 15-min (p < 0.001) and 20-min (p < 0.002) post-injection. For stereotypy, we observed a significant difference between bins in the SENS group (χ 2(4) = 24.89, p < 0.0001) and in the CTRL group (χ 2(4) = 16.93, p = 0.0020; Fig. 1f). Mann–Whitney U tests found significant differences between CTRL and SENS animals that received MA 15-min (U = 1.5, p = 0.0037) and 20-min (U = 2.5, p = 0.0057) post-injection. Collectively, these data support that there is an augmented psychomotor response, indicative of behavioral sensitization, brought about by repeated injections of MA that persisted 41–42 days following induction.
After 1 day of abstinence, neither TORM (Fig. 2(a1)) nor NOR (Fig. 2(a2)) was observed in the SENS animals. The CTRL group exhibited both TORM (t(7) = 2.67, p < 0.02; Fig. 2(a1)) and NOR (t(8) = 3.02, p < 0.01; Fig. 2(a2)) after this time point. After 30 days of abstinence, TORM (Fig. 2(b1)) was observed in the CTRL group (t(9) = 4.19, p < 0.005) but remained impaired in SENS animals. In contrast to TORM, no deficit in NOR (Fig. 2(b2)) was observed after 30 days of abstinence in SENS (t(9) = 3.73, p < 0.005) or CTRL animals (t(8) = 2.72, p < 0.02). Bonferroni planned comparisons were corrected to an alpha level of p < 0.025. There were no differences after 1 or 30 days of abstinence between groups in total interaction time for TORM or NOR.
(a1) TORM and (a2) NOR 1 day following the last injection of the induction phase (TORM: SENS, n = 9; CTRL, n = 8; NOR: SENS, n = 10, CTRL, n = 9), and after 30 days of abstinence (TORM (b1): SENS, n = 10; CTRL, n = 10; NOR (b2): SENS, n = 10; CTRL, n = 9). (Bonferroni corrected planned comparison, *p < 0.02; **p < 0.01; ***p < 0.005—significantly different than other object)
Twenty LFPs and 74 neurons were recorded from CTRL (n = 7), and 14 LFPs and 65 neurons were recorded from SENS (n = 6) animals (see Fig. 6 for placements). Spiking activity (top) and concomitant LFP activity (bottom) from a single animal throughout recording are illustrated in Fig. 3a. Power spectrum analysis of LFPs in CTRL and SENS animals prior to and following MA treatment found main effects of background (F(1, 3520) = 4.92, p = 0.0266), treatment (F(1, 3520) = 46.29, p < 0.001), and frequency (F(54, 3520) = 130.57, p < 0.001). Decreases in power in both CTRL and SENS animals were observed following MA injection (Fig. 3b). Black lines above each power spectrum denote where 95 % confidence intervals for each frequency diverge.
(a) Representative spike trains (top) and an LFP trace (bottom) from a single animal throughout an experiment. (b) The mean (solid line) ± SEM (shaded portion) of log spectral power of LFPs recorded from CTRL (left) versus SENS (right) animals pre- and post-injection. (c) Frequency of SO (d) and frequency of FR during the entire recording session; (Tukey’s post-hoc, **p < 0.005—significantly different than both groups during baseline)
No baseline differences in frequency of SOs (Fig. 3c) or firing rate (FR) (Fig. 3d) were observed between CTRL and SENS groups. No differences in the frequency of SOs (Fig. 3c) during baseline or following SAL injection were observed across CTRL and SENS groups. However, an injection of 5.0 mg/kg of MA decreased the frequency of the SO (mixed design factorial ANOVA, main effect of treatment, F(2, 72) = 12.34, p < 0.0001) and FR in both groups (mixed design factorial ANOVA, main effect of treatment, F(1, 5472) = 70.97, p < 0.0001) when compared with baseline. The mean FR (1/mean ISI) of neurons ranged from 0.003 to 1.5 Hz (CTRL) and 0.003 to 1.3 Hz (SENS). The majority of spike trains recorded during baseline exhibited irregular firing properties as indicated by median values of >1.0 for both coefficient of variation (Dayan and Abbott 2001); CTRL = 1.292, MA = 1.295) and Local Variation (Shinomoto et al. 2003); CTRL = 1.282, SENS = 1.237) (Online Resource 1 Table S1). Median values of burst fraction assessed during baseline (Van Elburg and Van Ooyen 2004) were comparable for CTRL = 0.162 and SENS = 0.193 animals. Additionally, approximately half of the spike trains in both CTRL (50 %) and SENS (55 %) met the criteria used in Parsegian et al. (2011) for burst neurons during baseline (see Online Resource 1 Table S1).
In order to assess changes in PFC function that might have contributed to the observed impairments in cognition (Fig. 2(a1 and a2)), and not those associated with the acute effects of MA administration, all subsequent analyses were performed during the baseline period prior to MA injection. During SOs (Fig. 4a, black), large increases in gamma oscillations (Fig. 4a, green) were observed, which are highlighted in a zoomed in representation (bottom) showing that the gamma burst occurred during the up-state of the SO. The power in the gamma band was not distributed equally throughout the SO. Rather, a large spike in gamma power was observed during the rise of the SO (Fig. 4b; CTRL (left): goodness of fit, χ 2(2034) = 8.41 × 108, p < 0.0001; SENS (right): χ 2(2034) = 5.01 × 108, p < 0.0001). However, when the power of the gamma oscillation was stratified by the size of the corresponding SO, a clear positive relationship was observed where increases in the size of the SO corresponded to increases in gamma power (Fig. 4c, mixed design factorial ANOVA, main effect of SO size, F(1, 1358) = 519.84, p < 0.0001). Furthermore, the SENS group had less gamma power for relatively small SOs, while increases in gamma power were observed for larger SOs (treatment × size of SO interaction, F(1, 1358) = 36.68, p < 0.0001).

Figure 5a shows a representative SO (top) and the same SO filtered in the gamma band (bottom) with the vertical black lines in each panel denoting an action potential. In Fig. 5b, a large proportion of spiking activity during the recording period was phase-locked to the gamma oscillations. In CTRL animals (white bar), 54 % of all cells were phase-locked to the gamma oscillation, which is consistent with previous observations (Senior et al. 2008; van Wingerden et al. 2010). However, a smaller proportion of cells (31 %) were phase-locked in the SENS group (gray bar; Fisher’s exact test, χ 2(1, N = 139) = 12.35, p < 0.001). The degree of phase locking to gamma oscillations was greater than chance levels in both groups (dashed lines) calculated via surrogate data. The inset shows a rose plot of a gamma phase-locked cell from a single animal during baseline recording. Lastly, Fig. 6 depicts the unilateral placement of probes relative to bregma, with examples of photomicrographs from one animal with a successful hit shown directly below.
(a) A representative example of a SO (top) and the same example bandpass filtered in the gamma band (bottom) during baseline from a single animal. The vertical black lines represent spiking activity of a single cell, notice they align with the peaks of the gamma oscillation. (b) Percentage of gamma phase-locked cells in the CTRL (white bar) and SENS (gray bar) groups with dashed lines reflecting chance levels of phase locking from surrogate data. (Fisher’s exact test, **p < 0.001—significantly different than SENS group). The inset shows a rose plot of a gamma phase-locked cell from a single animal during baseline recording
Three coronal sections (Paxinos and Watson 1997) from +3.20 to +2.20 mm depict the unilateral placement of probes (AP, +2.70; ML, +0.50; DV, −1.5) relative to bregma, with photomicrographs of a successful placement from one animal shown directly below. Open circles represent placements for CTRL animals (n = 7), and closed circles represent placements for SENS animals (n = 6)
Discussion
Low doses of MA are known to stimulate locomotor activity (Futamura et al. 2010; Zakharova et al. 2009), while moderate-to-high doses commonly evoke stereotypy, which has been demonstrated in rats using various doses ranging from 1.0 to 10.0 mg/kg (McGuire et al. 2011; Slamberova et al. 2011). In the current experiments, MA-induced hyperlocomotion progressively transitioned to behavioral stereotypy during the repeated 5.0 mg/kg dose. These alterations were also demonstrated to be long lasting. Relatively low locomotor output in SENS animals following the induction phase was attributable to increased stereotypies, which are together indicative of behavioral sensitization (Pierce and Kalivas 1997). Although the CTRL group exhibited increased stereotypy scores when they received 5.0 mg/kg of MA compared with SAL, these scores were <6 on the rating scale, and, as such, stereotypies were not detectable. Furthermore, this score is similar to that of the SENS group that received MA on Day 1, which is indicative of an acute MA response. Collectively, these data confirm that animals that received repeated MA developed behavioral sensitization that persisted at least 42 days following the induction phase.
To our knowledge, this is the first study to demonstrate impairments in TORM as a consequence of repeated MA administration in non-MA-intoxicated rodents. Deficits in NOR observed in SENS animals following 1 day of abstinence might complicate the interpretation of the TORM impairments observed on this day. It is possible that the inability of an animal to determine if it has seen an object precludes its ability to determine the order in which objects were observed. Thus, deficits in TORM may be attributable to an inability to detect novelty 1-day post induction. However, 30-day post-induction, NOR was intact while TORM was impaired, which establishes that deficits in TORM were likely not attributable to an inability to detect novelty at this time.
The observed results for NOR are consistent with previous studies that have examined this behavior following brief periods (7 days or fewer) of abstinence (Camarasa et al. 2010; Herring et al. 2008; Lu et al. 2010; Mizoguchi et al. 2008; Reichel et al. 2010, 2012). Furthermore, the only study to have evaluated NOR following 30 days or more of abstinence from repeated MA found that NOR is not impaired with protracted abstinence (Clark et al. 2007), which is also consistent with the current results.
These differences in the duration of impairments in TORM and NOR following induction suggests some level of specificity in the neural systems that are altered following drug cessation. The PFC is critical for the expression for TORM in both rats (Hannesson et al. 2004) and monkeys (Petrides 1991), which supports that PFC-mediated behaviors, and the underlying neural processes facilitating them, are altered following abstinence. In support of this view, other PFC-mediated cognitive functions, such as behavioral flexibility and impulsivity, are also impaired in preclinical rodent models of MA addiction following abstinence (Dalley et al. 2007; Parsegian et al. 2011). However, we cannot rule out that repeated MA altered TORM and NOR via changes in the acuity of sensory systems (i.e., smell, vision). Importantly, the duration of TORM impairments suggests that this behavioral measure of PFC function was impaired when electrophysiological testing was performed, thus allowing us to explore MA-induced alterations in this brain region at a time point where PFC-mediated behavioral deficits likely exist. However, without having performed electrophysiological recordings 30-days after induction when TORM was still impaired, it is not possible to conclude that MA-induced electrophysiological changes in the PFC last as long as the 30-day impairment seen in TORM.
Interestingly, gamma band activity is elevated in both rodents and humans during recognition memory tasks. In one such study, awake-behaving mice exhibited elevated theta and gamma power in the dorsal subiculum, an area that receives projections from the CA1 of the HC. In the same study, increased coherence in gamma between dorsal subiculum and CA1 was observed when rodents were exploring the environment without exploring objects (Chang and Huerta 2012). In humans, gamma activity assessed via EEG is increased when a familiar object is presented. However, decreased gamma power and phase locking between electrode pairs occurs as the familiar object is repeatedly presented, supporting that gamma activity is important for memory encoding (Gruber and Müller 2005). Although not directly measured in these experiments, it is possible that animals receiving repeated MA have difficulty encoding the memory of the familiar object due to alterations in normal gamma band activity. Furthermore, the aforementioned studies do not assess oscillatory activity in the PFC. Future studies will be necessary to determine whether repeated MA alters gamma band power and phase synchrony in the HC as well as the PFC in animals performing TORM and NOR to causally link the changes in physiology and behavior.
The current study, to our knowledge, is the first to examine electrophysiological activity in the medial PFC using simultaneous measures of LFP and spiking activity in rodents following repeated MA. Initially, this structure was thought to be analogous to the primate dorsolateral PFC based on inputs from the medial dorsal thalamus (Rose and Woolsey 1947). However, modern views of this structure suggest homology with ventromedial PFC (Balleine and O’Doherty 2009) and anterior cingulate cortex (Seamans et al. 2008).
Extended access self-administration of MA was previously shown to persistently alter the baseline FR and burst properties of PFC neurons (Parsegian et al. 2011). Furthermore, a single MA injection evoked a robust decrease in neural firing, which is consistent with previous studies using high doses of psychostimulants in either anesthetized or awake-behaving animals (Gulley and Stanis 2010; Homayoun and Moghaddam 2006; Jang et al. 2007). In the current study, no differences in baseline firing following sensitization were observed, which may be attributable to less exposure to the drug or the non-contingent route of administration. Importantly, the data presented herein suggest that alterations in oscillatory activity may be an initial neuroadaptation that precedes changes in baseline firing.
During baseline, we observed increases in neural firing and large increases of gamma power in both groups during the SO, which reflects increases in synaptic activity during the upstate (Hoffman et al. 2007). When gamma power was stratified by the size of the SO, a clear positive relationship was observed, which demonstrates that gamma power scales with the size of the SO, and by extension, possibly the strength of synaptic inputs. The gamma oscillation plays an important role in information processing, including object recognition (Martinovic et al. 2007). It is generated by GABAergic interneurons that provide inhibition in cortical networks (Bartos et al. 2007; Buzsaki and Chrobak 1995; Sohal et al. 2009) and while evoked increases in gamma power might be thought to reduce neural firing, experimental data suggest a more complex relationship. Driving the gamma oscillation increases the slope of the function that relates the number of spikes evoked by progressively larger inputs (stimulated excitatory postsynaptic currents) and was ultimately shown to amplify signals (i.e., increase spiking) in neocortical circuits (Sohal et al. 2009). In the current study, the slope of the function relating gamma power to SO size was steeper in the SENS group compared with CTRL, which could also reflect amplified signals in these neural circuits. In the PFC especially, optimal signal to noise ratios are hypothesized to be important for the maintenance and selection of behavior (Durstewitz and Seamans 2008). This balance could be persistently compromised in SENS animals and thus contribute to the impairments in cognitive function observed. Moreover, it is possible that alterations in the relationship between gamma power and the size of synaptic inputs could also contribute to the increases in the incentive motivational properties attributed to drugs and drug-related stimuli following repeated exposure.
Phase locking of action potentials to oscillatory activity is hypothesized to be an important feature of neural networks engaged in encoding, maintenance, or transfer of information (Lee et al. 2005; O’Keefe and Recce 1993). Specifically, gamma band phase locking is thought to coordinate neuronal activity in local neural networks and facilitates processes such as replay and memory consolidation (Carr et al. 2012; Colgin 2011). It may also be critical for spike-time-dependent plasticity by strengthening connections between neurons and facilitating the formation of cell assemblies to transiently encode information (van Wingerden et al. 2010). Alterations in gamma band power and synchrony may be a critical long-term physiological change in individuals with mental illnesses, including addictions (Rømer Thomsen et al. 2013). The current study supports this view, as a larger portion of spike activity was phase-locked to the gamma oscillation in CTRL compared with the SENS group. This suggests that alterations in gamma band phase locking may be a physiological adaptation that persists following repeated MA administration, which also may negatively impact cognitive function. However, considering that these recordings were not collected in animals performing the cognitive tasks, it is not possible to confirm that changes seen in electrophysiology cause the observed deficits in cognition. To directly test this hypothesis, additional studies in awake-behaving animals will be required.
The current study demonstrates that repeated MA administration causes impairments in temporal and recognition memory, although the impairments in recognition memory may improve after extended abstinence. These impairments may be paralleled by alterations in the integrating and synchronous properties of gamma band activity in the PFC. Collectively, these results suggest that repeated MA exposure evokes large scale alterations in the physiological processes that mediate gamma band activity and possibly inhibitory drive in cortical networks. Further elucidation of these processes will inform our understanding of how information processing is altered and how this contributes to alterations in behavior in addicted individuals.
References
Balleine BW, O’Doherty JP (2009) Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35:48–69
Bartos M, Vida I, Jonas P (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56
Bechara A (2005) Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8:1458–1463
Belcher AM, O’Dell SJ, Marshall JF (2006) A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav Brain Res 170:167–172
Broadbent NJ, Gaskin S, Squire LR, Clark RE (2010) Object recognition memory and the rodent hippocampus. Learn Mem 17:5–11
Buzsaki G, Chrobak JJ (1995) Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol 5:504–510
Camarasa J, Rodrigo T, Pubill D, Escubedo E (2010) Memantine is a useful drug to prevent the spatial and non-spatial memory deficits induced by methamphetamine in rats. Pharmacol Res 62:450–456
Carr MF, Karlsson MP, Frank LM (2012) Transient slow gamma synchrony underlies hippocampal memory replay. Neuron 75:700–713
Chang EH-Y, Huerta PT (2012) Neurophysiological correlates of object recognition in the dorsal subiculum. Front Behav Neurosci 6
Cheng RK, Etchegaray M, Meck WH (2007) Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res 1186:255–266
Clark RE, Kuczenski R, Segal DS (2007) Escalating dose, multiple binge methamphetamine regimen does not impair recognition memory in rats. Synapse 61(7):515–522
Colgin LL (2011) Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol 21:467–474
Dalley JW, Theobald DE, Berry D, Milstein JA, Lääne K, Everitt BJ, Robbins TW (2005) Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology 30:525–537
Dalley JW, Laane K, Theobald DE, Pena Y, Bruce CC, Huszar AC, Wojcieszek M, Everitt BJ, Robbins TW (2007) Enduring deficits in sustained visual attention during withdrawal of intravenous methylenedioxymethamphetamine self-administration in rats: results from a comparative study with d-amphetamine and methamphetamine. Neuropsychopharmacology 32:1195–1206
Dayan P, Abbott LF (2001) Theoretical neuroscience. MIT Press, Cambridge
Durstewitz D, Seamans JK (2008) The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-O-methyltransferase genotypes and schizophrenia. Biol Psychiatry 64:739–749
Ellinwood EH Jr, Balster RL (1974) Rating the behavioral effects of amphetamine. Eur J Pharmacol 28:35–41
Euston DR, Gruber AJ, McNaughton BL (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76:1057–1070
Futamura T, Akiyama S, Sugino H, Forbes A, McQuade RD, Kikuchi T (2010) Aripiprazole attenuates established behavioral sensitization induced by methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry 34:1115–1119
George O, Koob GF (2010) Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev 35:232–247
Gruber T, Müller MM (2005) Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb Cortex 15:109–116
Gulley JM, Stanis JJ (2010) Adaptations in medial prefrontal cortex function associated with amphetamine-induced behavioral sensitization. Neuroscience 166:615–624
Hannesson DK, Howland JG, Phillips AG (2004) Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci 24:4596–4604
Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT (2008) Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology 199:637–650
Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology 188:162–170
Hoffman KL, Battaglia FP, Harris K, MacLean JN, Marshall L, Mehta MR (2007) The upshot of up states in the neocortex: from slow oscillations to memory formation. J Neurosci 27:11838–11841
Homayoun H, Moghaddam B (2006) Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci 26:8025–8039
Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsaki G (2006) Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron 52:871–882
Iudicello JE, Weber E, Grant I, Weinborn M, Woods SP, Group HNRC (2011) Misremembering future intentions in methamphetamine-dependent individuals. Clin Neuropsychol 25:269–286
Jang J, Ha HJ, Kim YB, Chung YK, Jung MW (2007) Effects of methamphetamine on single unit activity in rat medial prefrontal cortex in vivo. Neural Plast 2007:29821
Kosheleff AR, Rodriguez D, O’Dell SJ, Marshall JF, Izquierdo A (2012) Comparison of single-dose and extended methamphetamine administration on reversal learning in rats. Psychopharmacology (Berl) 224:459–467
Kurti A, Swanton D, Matell M (2013) The potential link between temporal averaging and drug-taking behavior. Subjective Time. MIT Press
Lapish CC, Chiang J, Wang JZ, Phillips AG (2012) Oscillatory power and synchrony in the rat forebrain are altered by a sensitizing regime of D-amphetamine. Neuroscience 203:108–121
Lee H, Simpson GV, Logothetis NK, Rainer G (2005) Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron 45:147–156
Lisman JE, Idiart M (1995) Storage of 7+/−2 short-term memories in oscillatory subcycles. Science 267:1512–1515
Liu N, Liu Y, Fan Y, Yu H, Wilson FA, Ma Y, Hu X (2005) EEG activities in the orbitofrontal cortex and dorsolateral prefrontal cortex during the development of morphine dependence, tolerance and withdrawal in rhesus monkeys. Brain Res 1053:137–145
Lu P, Mamiya T, Lu L, Mouri A, Niwa M, Kim H-C, Zou L-B, Nagai T, Yamada K, Ikejima T (2010) Silibinin attenuates cognitive deficits and decreases of dopamine and serotonin induced by repeated methamphetamine treatment. Behav Brain Res 207:387–393
Martinovic J, Gruber T, Muller MM (2007) Induced gamma band responses predict recognition delays during object identification. J Cogn Neurosci 19:921–934
Masquelier T, Hugues E, Deco G, Thorpe SJ (2009) Oscillations, phase-of-firing coding, and spike timing-dependent plasticity: an efficient learning scheme. J Neurosci 29:13484–13493
McGuire BA, Baladi MG, France CP (2011) Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. Eur J Pharmacol 658:156–159
Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim H-C, Yamada K (2008) Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology 196:233–241
Newton TF, Kalechstein AD, Hardy DJ, Cook IA, Nestor L, Ling W, Leuchter AF (2004) Association between quantitative EEG and neurocognition in methamphetamine-dependent volunteers. Clin Neurophysiol 115:194–198
O’Keefe J, Recce ML (1993) Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3:317–330
Parsegian A, Glen WB Jr, Lavin A, See RE (2011) Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry 69:253–259
Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA (2003) Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry 53:65–74
Petrides M (1991) Functional specialization within the dorsolateral frontal cortex for serial order memory. Proc R Soc London, Ser B 246:299–306
Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216
Pirch J, Corbus M, Ebenezer I (1985) Conditioned cortical slow potential responses in urethane anesthetized rats. Int J Neurosci 25:207–218
Pitel AL, Witkowski T, Vabret F, Guillery-Girard B, Desgranges B, Eustache F, Beaunieux H (2007) Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcohol Clin Exp Res 31:238–248
Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2010) Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36:782–792
Reichel CM, Chan CH, Ghee SM, See RE (2012) Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology 223:371–380
Rømer Thomsen K, Joensson M, Lou HC, Møller A, Gross J, Kringelbach ML, Changeux J-P (2013) Altered paralimbic interaction in behavioral addiction. Proc Natl Acad Sci 110:4744–4749
Rose JE, Woolsey CN (1947) The orbitofrontal cortex and its connections with the mediodorsal nucleus in rabbit, sheep and cat. Res Publ Assoc Res Nerv Ment Dis 27:210–232
Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH (2009) Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat 37:292–297
Salo R, Gabay S, Fassbender C, Henik A (2011) Distributed attentional deficits in chronic methamphetamine abusers: evidence from the Attentional Network Task (ANT). Brain Cogn 77:446–452
Sax KW, Strakowski SM (2001) Behavioral sensitization in humans. J Addict Dis 20:55–65
Seamans JK, Lapish CC, Durstewitz D (2008) Comparing the prefrontal cortex of rats and primates: insights from electrophysiology. Neurotox Res 14:249–262
Senior TJ, Huxter JR, Allen K, O’Neill J, Csicsvari J (2008) Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci 28:2274–2286
Shinomoto S, Shima K, Tanji J (2003) Differences in spiking patterns among cortical neurons. Neural Comput 15:2823–2842
Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W (2000) Cognitive impairment in individuals currently using methamphetamine. Am J Addict 9:222–231
Simon SL, Dacey J, Glynn S, Rawson R, Ling W (2004) The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 27:59–66
Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G (2008) Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60:683–697
Slamberova R, Mikulecka A, Pometlova M, Schutova B, Hruba L, Deykun K (2011) Sex differences in social interaction of methamphetamine-treated rats. Behav Pharmacol 22:617–623
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459:698–702
Staff NRC (2003) Guidelines for the care and use of mammals in neuroscience and behavioral research. National Academies Press
Steketee JD, Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63:348–365
Steriade M (1997) Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex 7:583–604
Steriade M (2006) Grouping of brain rhythms in corticothalamic systems. Neuroscience 137:1087–1106
Steriade M, Nuñez A, Amzica F (1993) Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci 13:3266–3283
Tolliver BK, McRae-Clark AL, Saladin M, Price KL, Simpson AN, DeSantis SM, Baker NL, Brady KT (2010) Determinants of cue-elicited craving and physiologic reactivity in methamphetamine-dependent subjects in the laboratory. Am J Drug Alcohol Abuse 36:106–113
Van Elburg RA, Van Ooyen A (2004) A new measure for bursting. Neurocomputing 58:497–502
van Wingerden M, Vinck M, Lankelma JV, Pennartz CM (2010) Learning-associated gamma-band phase-locking of action-outcome selective neurons in orbitofrontal cortex. J Neurosci 30:10025–10038
Ward LM (2003) Synchronous neural oscillations and cognitive processes. Trends Cogn Sci 7:553–559
Williams S, Boksa P (2010) Gamma oscillations and schizophrenia. J Psychiatry Neurosci 35:75–77
Williamson LL, Cheng R-K, Etchegaray M, Meck WH (2008) “Speed” warps time: methamphetamine’s interactive roles in drug abuse, habit formation, and the biological clocks of circadian and interval timing. Curr Drug Abuse Rev 1:203–212
Winters BD, Bussey TJ (2005) Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. J Neurosci 25:52–61
Wittmann M, Leland DS, Churan J, Paulus MP (2007) Impaired time perception and motor timing in stimulant-dependent subjects. Drug Alcohol Depend 90:183–192
Zakharova E, Leoni G, Kichko I, Izenwasser S (2009) Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res 198:45–50
Acknowledgments
This work was supported by grant nos. AA022821 (CCL), AA022268 (DNL), and ABMRF (CCL).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1086 kb)
Rights and permissions
About this article
Cite this article
Janetsian, S.S., Linsenbardt, D.N. & Lapish, C.C. Memory impairment and alterations in prefrontal cortex gamma band activity following methamphetamine sensitization. Psychopharmacology 232, 2083–2095 (2015). https://doi.org/10.1007/s00213-014-3840-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-014-3840-7