Abstract
Multiple sclerosis (MS) is an autoimmune neurodegenerative disease that targets myelin, leading to inflammation and neuron death. Monoclonal antibodies (MAb) have long been used to control the progression and exacerbations of this disorder, which may induce secondary autoimmune disease as a rare adverse event. This systematic review aimed to gather data of case reports around this subject and to explain the mechanism behind their occurrence. PubMed, Scopus, and Google scholar were searched for published case reports until February 21st 2024. The Joanna Briggs Institute (JBI) critical appraisal checklist was used to assess the quality of the included studies. In total, 20 articles met the inclusion criteria and were reviewed by the authors. The most autoimmune disorders were thyroiditis and as expected induced by alemtuzumab. Ocrelizumab had one secondary autoimmune complication reported. MAbs used in MS immunotherapy have shown to induce secondary autoimmune disorders including endocrine complications, which have been reported in many case reports. It is recommended to use these agents with caution and monitor patients for symptoms of the aforementioned conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by inflammation, demyelination, and neurodegeneration within the central nervous system (CNS), characterized by demyelination, axonal damage, and progressive neurological disability. MS typically manifests between the ages of 20 and 40, with a higher prevalence in women, and is recognized as the leading cause of non-traumatic disability among young adults (Ghiasian et al. 2021). While the exact etiology of MS remains elusive, it is widely accepted that genetic and environmental factors contribute to disease susceptibility and progression. Among the environmental factors, vitamin D deficiency, viral infections, and smoking have been implicated in the pathogenesis of MS (Abdollahzadeh et al. 2016; Poorolajal et al. 2017).
The efficacy and safety of disease-modifying therapies (DMTs) have garnered significant attention in MS management. While DMTs have revolutionized the treatment landscape for MS, their use is not without risk. Moreover, the efficacy of DMTs varies among individuals, and a substantial proportion of patients continue to experience disease progression despite treatment (Ghiasian et al. 2022). Recent studies have reported new adverse effects associated with therapies, underscoring the importance of post-marketing pharmacovigilance in optimizing patient care (Sahraian et al. 2022; Simbrich et al. 2021).
Among the various biological therapies used in MS management, alemtuzumab has drawn attention to its efficacy in reducing disease activity. However, it is associated with a spectrum of thyroid-related adverse events, including Graves’ disease and orbitopathy, which can have significant clinical implications (Scappaticcio et al. 2020). Additionally, studies have identified risk factors and clinical characteristics associated with alemtuzumab-induced Graves’ disease, providing insights into personalized risk stratification and preventive measures (Ueland et al. 2023).
Given these challenges, there is an urgent need for more personalized therapeutic approaches and a deeper understanding of the disease mechanisms at the individual level. Although extensive research has been conducted on MS, critical gaps remain, particularly in identifying biomarkers for predicting treatment response and understanding the long-term effects of current therapies.
In light of the multifaceted nature of MS pathogenesis and management, this study aims to investigate the potential role of biological therapy in inducing autoimmune endocrine diseases in MS patients. By doing so, it seeks to contribute to developing of more tailored treatment strategies, ultimately improving outcomes for individuals living with MS.
Method
Data sources
This systematic review aimed to identify and analyze autoimmune endocrine disorders associated with DMTs used to manage MS attacks and progression. The review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist guidelines. Searches were conducted in Google Scholar, PubMed, and Scopus databases for studies published up to February 2024.
Search strategy
The search strategy included the following terms: “multiple sclerosis,” and (Natalizumab or Ocrelizumab or Rituximab or Alemtuzumab or Ofatumumab or Ublituximab), and (case report) and all MeSH terms in Pubmed Database. In Scopus, each monoclonal antibody AND “multiple sclerosis” was searched separately in Abstract/Title section and the total was added to the records. Google Scholar was searched for each monoclonal antibody AND “multiple sclerosis” separately; the total was then added. The selection of biologic drugs was based on therapies recommended by Wolters Kluwer’s UpToDate®.
Eligibility criteria
The inclusion criteria for this study were as follows: (a) case reports or case series, (b) addressed the autoimmune endocrine complications of MS immunotherapy, (c) had a full text available. Records were excluded if they were (a) non-English or (b) complications other than endocrine disorders, (c) the review and meta-analysis studies, and letters to editors, clinical trials, and qualitative studies.
The authors agreed to refer to the Global Autoimmune Institute’s Autoimmune Disease List (2024) to standardize disease categorization.
Data extraction
Endnote® 7 (Clarivate Analytics) facilitated screening and data extraction. Two researchers independently extracted data based on predefined criteria, with results reviewed by the remaining authors.
Quality assessment
The methodological quality of case report studies was assessed using the Joanna Briggs Institute (JBI) critical appraisal checklist, which includes eight Likert questions evaluating crucial aspects of case reports (Munn et al. 2020). These criteria assess the accuracy of patient demographic information, documentation of patient history, description of current clinical conditions, clarity of diagnostic and therapeutic measures, elucidation of post-therapeutic intervention outcomes, and identification and explanation of reported side effects. The overall utility of case reports was evaluated accordingly. See t 2 for detailed assessment criteria.
Results
A comprehensive search of the aforementioned databases up to February 21st, 2024, yielded 85 articles. After removing 58 duplicate articles, 27 unique articles underwent independent review by two researchers. Screening of titles and abstracts led to excluding additional six articles. Subsequently, the remaining articles underwent full-text assessment, resulting in the exclusion of no articles (see Fig. 1). Ultimately, 21 articles met the eligibility criteria.
These 20 articles provided data on 38 patients, with records primarily originating from Italy, Germany, and the UK, in descending order of prevalence. Additional records were sourced from Canada, Greece, Australia, Ireland, the USA, and Portugal. The summarized findings of these studies are presented in Table 1.
Of the eligible studies, 17 investigated autoimmune dermatologic complications associated with Alemtuzumab in MS patients, while two studies focused on Natalizumab and one on Ocrelizumab. Consequently, 38 patients across 20 studies examining three different biological therapies for MS were identified.
Alemtuzumab
Alemtuzumab, a monoclonal antibody that targets CD52, a protein on surface of B, T lymphocytes, monocytes and dendritic cells, has been utilized for chronic lymphocytic leukemia (CLL) and other lymphoid neoplasms (Katsavos and Coles 2018). Research has confirmed its effectiveness in decreasing relapse rates of multiple sclerosis (MS) and disability progression, as well as its beneficial effects on radiological disease outcomes (Willis and Robertson 2016). However, its complications such as secondary autoimmune disease, necessitate careful monitoring and management (Cossburn et al. 2011).
Autoimmune complications
Alemtuzumab has been associated with many autoimmune complications, e.g., immune thrombocytopenia, hemolytic anemia, hepatitis, encephalitis, myasthenia gravis, Lambert–Eaton myasthenia, sarcoidosis, vitiligo, alopecia, myositis, and type 1 diabetes, but most prevalent reported is autoimmune thyroiditis by 33%, of which 63% were GD and 15% Hashimoto’s. (Yue et al. 2023).
Research indicates that alemtuzumab can lead to prolonged lymphopenia and trigger secondary autoimmunity during the reconstitution of the lymphocyte repertoire. Cytokine signaling, e.g., increased Interleukin 21 and decreased IFN, also has been proposed to play a role (Conway 2022). Patients with high baseline levels of Interleukin21 are at a higher risk of developing autoimmune conditions post-treatment with alemtuzumab (Ruck et al. 2022; Costelloe et al. 2012). The incidence of thyroid dysfunction in the first 3 years rises each year (Alamo et al. 2019). This typically tends to resolve on its own in approximately 30% of cases, possibly attributed to a higher frequency of neutralizing or blocking TRAb. (Moli et al. 2021).
Natalizumab
Natalizumab is a monoclonal antibody used to treat RRMS (Hutchinson 2007). Natalizumab inhibits the interaction between the integrin alpha subunit of VLA-4 on leukocytes and VCAM-1, which is vital in regulating immune and inflammatory responses. Research indicates that the VLA-4 and VCAM-1 pathways may be significant in the autoimmune response seen in autoimmune thyroid disorders (Marazuela et al. 1994; Nakashima et al. 1994).
Autoimmune complications
Natalizumab has been associated with the development of autoimmune hepatitis, immune thrombocytopenic purpura, and rheumatoid arthritis (Lisotti et al. 2012; Stosic et al. 2011; Su et al. 2020). Some theories suggest that Immune Reconstitution Syndrome (IRS) plays a role (Carcelén-Gadea et al. 2013; Metz et al. 2012) and also inducing a shift towards a Th17-mediated inflammatory response while blocking Th1 cell entry can be an explanation (Su et al. 2020). However, additional research is necessary to fully understand the mechanisms and potential risk factors for these complications.
Ocrelizumab
Ocrelizumab, a second-generation humanized antibody that targets the CD20 antigen expressed by a vast range of B cells and about 5% of T cells, has been approved for the treatment of RRMS and primary progressive MS (Duarte et al. 2021; Lünemann et al. 2020). Main complications of ocrelizumab-therapy have been infusion-related reactions and infections (Montalban et al. 2017).
Autoimmune complications
Increased risk of developing psoriasis and inflammatory bowel disease has been reported by ocrelizumab administration (Lamb 2022). Also, glomerulosclerosis and Graves’ disease have been reported in some case reports (Duarte et al. 2021; Greve et al. 2023). Some hypotheses state there may be an association with B cell depletion immune system dysregulation (Lee et al. 2020) (Table 2).
Discussion
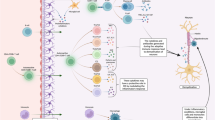
This study aims to explore the mechanisms underlying biological therapy-induced autoimmune endocrine diseases in MS patients as is summarized in Table 3. By elucidating the underlying pathophysiology, pictured in Fig. 2 and identifying at-risk patient populations, this research seeks to inform personalized treatment strategies and enhance the safety and efficacy of MS management.
To our knowledge, this is the first systematic review of case reports on secondary autoimmune endocrine disorders induced by multiple sclerosis immunotherapy monoclonal antibodies.
We reviewed 20 articles that met the inclusion criteria (3 America, 16 Europe, and 1 Australia). Seventeen studies involved Alemtuzumab with a total of 34 patients who developed autoimmune endocrine complications; two studies included Natalizumab with 3 patients suffering from its endocrine adverse effects. One study contributed to Ocrelizumab endocrine complications, with one patient experiencing the side effects of this drug. A total of 38 patients had experienced induced autoimmune endocrine side effects.
A systematic review and meta-analysis highlight the prevalence and clinical characteristics of alemtuzumab-induced thyroid events in MS patients, emphasizing the importance of vigilant monitoring and early intervention (Scappaticcio et al. 2020). This aligns with findings by other studies, which have consistently demonstrated the necessity for proactive management of thyroid dysfunction in this patient population due to the autoimmune risks associated with immune reconstitution therapies (IRT). Additionally, it was hypothesized in the same article that there is an antigen preference of TRAb in MS patients treated with alemtuzumab, suggesting a specific immunological shift linked to the therapy.
Interferons, which have been used as a pharmacotherapy for MS, have long been associated with thyroid complications and one of the stated theories proposes their indirect immune-mediated action, pro-inflammatory cytokines increase, inhibition of regulator T cells and switching to immunological Th1 pattern (Tomer and Menconi 2009). This mechanistic insight is crucial for understanding the broader context of immune responses in MS therapies. Development of immune reconstitution therapies, including antibody-based cell depletion therapies, targeting CD52 + (alemtuzumab) or CD20 + (ocrelizumab) leukocytes (Kazakou et al. 2023), through short-term intense immunosuppression or immune cell depletion, rebuilds an immune system with freshly built immune tolerance (Lünemann et al. 2020).
The majority of immune reconstitution autoimmune diseases post-alemtuzumab treatment of MS have been theorized to be antibody-dependent; however, type 1 diabetes is the result of pancreas cell destruction by self-reactive T cells. Therefore, Malmetrom et al. suggest that post-alemtuzumab secondary autoimmune conditions are not confined to be mediated by autoantibody (Malmeström et al. 2014).
A study on cardiac transplantation indicated that induction therapy with alemtuzumab may result in a lower incidence of new onset diabetes, suggesting a complex relationship between alemtuzumab and diabetes across different medical contexts. However, it was suggested that lower need of corticosteroid post transplantation can be the possible etiology (Jones et al. 2010). This finding contrasts with the higher incidence of autoimmune conditions in MS patients, highlighting the need for disease-specific investigations into the drug's effects.
The associated thyroid dysfunction related to IRT has been characterized as delayed onset with transient dysfunction and spontaneously resolved. Also, although conventional GD is thought to be induced by humoral immunity, T cells are at function in this case (Muller et al. 2018). During immune reconstitution autoimmunity, Th2 profile cytokines are replenished to greater extent, making Th1 mediated disorders like Hashimoto’s thyroiditis less common (Weetman 2009). Interestingly, MS progression is also derived by Th1 phenotype (Conway 2022).
Although alemtuzumab causes depletion of B and T lymphocytes (including CD8 + and CD4 +); recovery of B cell counts occurs earlier (by 3 to 6 months) while T cell counts, especially CD4 + , remain low even after 12 months. Overpopulation of B cells (127% of their base level at 27th months (Roos et al. 2019)), in the absence of regulatory T cells, and overpopulation of naive B cells with prolonged depletion of memory B cells is the possible cause for the secondary autoimmunity of B cells that may occur with alemtuzumab treatment.
This pathophysiology requires the involvement of T cells. It is therefore less likely to occur until CD4 + T cell numbers reach a sufficient level, leading to a delay between hyperreactivity of B-lymphocytes and the development of autoimmunity. Furthermore, T cell reconstitution is primarily due to peripheral expansion (rather than thymic reconstitution), promoting self-reactive immune cell populations (Sellner and Rommer 2020).
Ocrelizumab, on the other hand, B cell and CD20 + T cell are depleted while CD4 + and CD8 + T cells levels do not change (Sellner and Rommer 2020). Repopulation of B cells is preferentially achieved by immature or transitional B cells and myeloid cells following anti-CD20 + treatment have an enhanced activation state and proinflammatory differentiation (Häusler et al. 2018). These differences in immune reconstitution mechanisms between alemtuzumab and ocrelizumab highlight the necessity of drug-specific monitoring protocols to mitigate the risk of secondary autoimmunity.
Secondary autoimmunity following treatment with alemtuzumab has been noted in individuals with MS, but it has not been observed in cancer or rheumatoid arthritis patients. It has been suggested that cytokine signaling or lymphocyte function specific to MS patients might enhance the development of autoimmunity after immune reconstitution. However, no such enhancing factors have been identified thus far (Conway 2022). The balance between Th1 and Th2 and the derivation from the original phenotype in each individual might be the main factor.
It has been suggested in another article (Carcelén-Gadea et al. 2013) that after discontinuation of natalizumab, a self-limited autoimmune thyroid disorder may emerge as a presentation of IRS in predisposed individuals who have a positive family history of thyroid disease or have had interferon therapy, as use of natalizumab appears to control the immune response against the thyroid, with implications for post-therapy monitoring strategies.
Homeostatic proliferation commonly refers to the expansion of peripheral T cells in situations of lymphopenia. In lymphoreplete settings, low-affinity interactions between the major histocompatibility (MHC)/peptide complex and T cell receptor (TCR) in the periphery provide continuous “survival signals” to naïve T cells without triggering proliferation. Conversely, in cases of lymphopenia, these signals prompt T cell proliferation, to the level of homeostatic proliferation is directly linked to the extent and duration of lymphopenia. Consequently, homeostatic proliferation selectively amplifies T cells with a heightened specificity and stronger avidity for self-antigens. This process may contribute to the development of autoimmunity triggered by lymphopenia, as observed with alemtuzumab and other depleting agents (Aranha et al. 2013).
Rituximab is an old generation of CD20 + targeting monoclonal antibodies which have been used in treatment of Graves’ orbitopathy but thyroid function levels have not been affected when using this drug (Salvi et al. 2015). The remaining non-CD20 + expressing plasma cells releasing autoantibodies can be the explanation (Duarte et al. 2021). Also, this agent, based on its mechanism of action, has been proposed to be administered as a protective agent, lowering the population of naïve B cell in reconstitution phase to lower the incidence of post-alemtuzumab GD autoimmunity (Conway 2022).
Smoking is one of the risk factors for developing thyroid complications post-alemtuzumab and sex distribution unlike dominant female prevalence in MS and GD is equal in this case (Nirmalan et al. 2023). Alemtuzumab dose, frequency, and interval have not appeared to be a risk factor (Trinh et al. 2016). In autoimmune thyroid disease, androgen-mediated reduction in thyroid cells expression of HLA-I and -II antigens have been suggested as a potential mechanism. Additionally, females are known to have an immune response with dominance of Th2, leading to enhanced B cell activation and production of autoantibodies (Tsourdi et al. 2015). Also it has been suggested that TSHRAb production by increased counts of memory-like T lymphocytes is more outstanding and more sustained in alemtuzumab induced thyroid disease than conventional cases (Tsourdi et al. 2015).
Also findings of Rolla et al. emphasize that alemtuzumab leads to a long-lasting reduction in CD4 + T cell counts, with Treg cell playing a crucial role in maintain long-term immune regulation and potentially mitigating some autoimmune responses (Rolla et al. 2022).
Conclusion
Multiple sclerosis (MS) presents significant management challenges due to its complex nature and the evolving landscape of treatment options. To optimize patient care, clinicians should consider enhancing clinical education, adopting a multidisciplinary approach, implementing routine monitoring, and developing personalized care plans.
For future research directions, we suggest conducting longitudinal studies to explore the long-term impact of novel MS treatments, risk factors and prognosis, and prioritizing patient-centered outcomes.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used ChatGPT 3.5 in order to enhance the grammar and presentation of this article. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the publication’s content.
Data availability
No datasets were generated or analysed during the current study.
References
Abdollahzadeh R, Fard MS, Rahmani F, Moloudi K, Azarnezhad A (2016) Predisposing role of vitamin D receptor (VDR) polymorphisms in the development of multiple sclerosis: A case-control study. J Neurol Sci 367:148–151
Alamo A, Condorelli RA, La Vignera S, Calogero AE (2019) Autoimmune thyroid disease following treatment with alemtuzumab for multiple sclerosis. Int J Immunopathol Pharmacol 33:205873841984369
Aranha AA, Amer S, Reda ES, Broadley SA, Davoren PM (2013) Autoimmune Thyroid Disease in the Use of Alemtuzumab for Multiple Sclerosis: A Review. Endocr Pract 19(5):821–828
Bianco A, Nicoletti T, Traini E, Del Giacomo P, Del Gatto V, Lucchini M et al (2020) Dominus effect: challenging complications of alemtuzumab-related thyroid autoimmunity. J Endocrinol Invest 43(8):1159–1161
Carcelén-Gadea M, Ferrer-Garcia JC, Cervelló DA (2013) Transient autoimmune hyperthyroidism following the withdrawal of Natalizumab in patients with multiple sclerosis. Neurol Sci 34(12):2259–2261
Conway JV (2022) Graves’ disease following commencement of alemtuzumab therapy: Case report discussing clinical considerations and possible pathophysiology. J Clin Transl Endocrinol Case Rep 25:100120
Cossburn M, Pace AA, Jones J, Ali R, Ingram G, Baker K et al (2011) Autoimmune disease after alemtuzumab treatment for multiple sclerosis in a multicenter cohort. Neurology 77(6):573–579
Costelloe L, Jones J, Coles A (2012) Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev Neurother 12(3):335–341
Daraki V, Eleni-Konstantina S, Mastorodemos V, Kontolaimaki K, Mytilinaiou M, Chrysoulaki M, Sfakiotaki M, Betsi G, Xekouki P (2021) Graves’ disease and concurrent immune thrombocytopenia in a patient with albinism following alemtuzumab treatment for multiple sclerosis: a rare case report. In: Endocrine Abstracts, vol 73. Bioscientifica.
Duarte DB, Silva AMD, Freitas C, Cardoso H (2021) Graves’ disease with spontaneous resolution following ocrelizumab in primary progressive multiple sclerosis. Endocr Regul 55(3):169–173
Garrahy A, Murphy NP, Byrne MM (2019) Letter to the Editor: “alemtuzumab-Induced Thyroid Dysfunction Exhibits Distinctive Clinical and Immunological Features.” J Clin Endocrinol Metab 104(9):3624–3625
Ghiasian M, Nafisi H, Ranjbar A, Mohammadi Y, Ataei S (2021) Antioxidative effects of Silymarin on the reduction of liver complications of fingolimod in patients with relapsing–remitting multiple sclerosis: a clinical trial study. J Biochem Mol Toxicol 35(8):e22800
Ghiasian M, Rashidizad SA, Mohammadi M, Mohammadi Y, Ataei S (2022) Comparison of the Total Side Effects of Injectable Disease-Modifying Drugs for the First-line Treatment of Relapsing-Remitting Multiple Sclerosis. Avicenna J Pharm Res 3(1):32–36
Greve AS, Prakash S, Krag S, Randers E (2023) Focal segmental glomerulosclerosis in a patient with multiple sclerosis treated with Teriflunomide and Ocrelizumab. J Nephrol 36(3):659–661
Häusler D, Häusser-Kinzel S, Feldmann L, Torke S, Lepennetier G (2018) Functional characterization of reappearing B cells after anti-CD20 treatment of CNS autoimmune disease. Proc Nat Acad Sci 115(39):9773–8
Hutchinson M (2007) Natalizumab: A new treatment for relapsing remitting multiple sclerosis. Ther Clin Risk Manag 3(2):259–268
Jones R, Shullo M, Zomak R, Newman C, Rebel M, Yost C et al (2010) 308: Lower Incidence of New Onset Diabetes after Cardiac Transplant with Alemtuzumab Induction. J Heart Lung Transplant 29(2):S104
Katsavos S, Coles A (2018) Alemtuzumab as treatment for multiple sclerosis. Cold Spring Harb Perspect Med 8(10):a032029
Kazakou P, Tzanetakos D, Vakrakou AG, Tzartos JS, Evangelopoulos Μ-E, Anagnostouli M et al (2023) Thyroid autoimmunity following alemtuzumab treatment in multiple sclerosis patients: a prospective study. Clin Exp Med 23(6):2885–2894
Lamb YN (2022) Ocrelizumab: A Review in Multiple Sclerosis. Drugs 82(3):323–334
Le Moli R, Russo M, Malandrino P, Vella V, Belfiore A, Frasca F (2021) Onset of Marine-Lenhart syndrome and Graves’ ophthalmopathy in a female patient treated with alemtuzumab for multiple sclerosis. Hormones (Athens) 20(1):161–165
Lee HH, Sritharan N, Bermingham D, Strey G (2020) Ocrelizumab-induced severe colitis. Case Rep Gastrointest Med 2020:8858378
Lisotti A, Azzaroli F, Brillanti S, Mazzella G (2012) Severe acute autoimmune hepatitis after natalizumab treatment. Dig Liver Dis 44(4):356–357
Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H (2020) Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol 16(1):56–62
Mahzari M, Arnaout A, Freedman MS (2015) Alemtuzumab Induced Thyroid Disease in Multiple Sclerosis: A Review and Approach to Management. Can J Neurol Sci 42(5):284–291
Malmeström C, Andersson BA, Lycke J (2014) First reported case of diabetes mellitus type 1 as a possible secondary autoimmune disease following alemtuzumab treatment in MS. J Neurol 261(10):2016–2018
Marazuela M, Postigo AA, Acevedo A, Díaz-González F, Sanchez-Madrid F, de Landázuri MO (1994) Adhesion molecules from the LFA-1/ICAM-1,3 and VLA-4/VCAM-1 pathways on T lymphocytes and vascular endothelium in Graves’ and Hashimoto’s thyroid glands. Eur J Immunol 24(10):2483–2490
Metz I, Radue EW, Oterino A, Kümpfel T, Wiendl H, Schippling S et al (2012) Pathology of immune reconstitution inflammatory syndrome in multiple sclerosis with natalizumab-associated progressive multifocal leukoencephalopathy. Acta Neuropathol 123(2):235–245
Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G et al (2017) Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 376(3):209–220
Muller I, Willis M, Healy S, Nasser T, Loveless S, Butterworth S et al (2018) Longitudinal Characterization of Autoantibodies to the Thyrotropin Receptor (TRAb) During Alemtuzumab Therapy: Evidence that TRAb May Precede Thyroid Dysfunction by Many Years. Thyroid 28(12):1682–1693
Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A et al (2020) Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth 18(10):2127–2133
Nakashima M, Eguchi K, Ida H, Yamashita I, Sakai M, Origuchi T et al (1994) The expression of adhesion molecules in thyroid glands from patients with Graves’ disease. Thyroid 4(1):19–25
Nirmalan A, Blecher N, Hyder S, Couch SM, Godfrey KJ, Stan MN et al (2023) Alemtuzumab-Induced Thyroid Eye Disease: A Comprehensive Case Series and Review of the Literature. Ophthalmic Plast Reconstr Surg 39(5):470–474
Obermann M, Ruck T, Pfeuffer S, Baum J, Wiendl H, Meuth SG (2016) Simultaneous early-onset immune thrombocytopenia and autoimmune thyroid disease following alemtuzumab treatment in relapsing-remitting multiple sclerosis. Mult Scler (Houndmills, Basingstoke, England) 22(9):1235–1241
Oddo S, Laroni A, Uccelli A, Giusti M (2014) A case of thyroiditis during natalizumab therapy for multiple sclerosis. J Endocrinol Invest 34(5):408–409
Poorolajal J, Bahrami M, Karami M, Hooshmand E (2017) Effect of smoking on multiple sclerosis: a meta-analysis. J Public Health 39(2):312–320
Richter S, Wagner B, Celius EG (2019) Two cases of diabetes mellitus type 1 after alemtuzumab treatment for multiple sclerosis: another probable secondary autoimmune disease. J Neurol 266(5):1270–1
Rolla S, De Mercanti SF, Bardina V, Maglione A, Taverna D, Novelli F et al (2022) Long-term effects of alemtuzumab on CD4+ lymphocytes in multiple sclerosis patients: a 72-month follow-up. Front Immunol 13:818325
Roos JCP, Moran C, Chatterjee VK, Jones J, Coles A, Murthy R (2019) Immune reconstitution after alemtuzumab therapy for multiple sclerosis triggering Graves’ orbitopathy: a case series. Eye (Lond) 33(2):223–229
Ruck T, Barman S, Schulte-Mecklenbeck A (2022) Alemtuzumab-induced immune phenotype and repertoire changes: implications for secondary autoimmunity. Brain 145(5):1711–1725
Sahraian MA, Salehi AM, Jenabi E, Esfahani ME, Ataei S (2022) Post marketing new adverse effects of oral therapies in multiple sclerosis: A systematic review. Mult Scler Relat Disord 68:104157
Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D et al (2015) Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 100(2):422–431
Scappaticcio L, Castellana M, Virili C, Bellastella G, Centanni M, Cannavò S et al (2020) Alemtuzumab-induced thyroid events in multiple sclerosis: a systematic review and meta-analysis. J Endocrinol Invest 43:219–229
Sellner J, Rommer PS (2020) Immunological consequences of “immune reconstitution therapy” in multiple sclerosis: A systematic review. Autoimmun Rev 19(4):102492
Simbrich A, Thibaut J, Khil L, Maximov S, Wiendl H, Berger K et al (2021) Chances and Challenges of Registry-Based Pharmacovigilance in Multiple Sclerosis: Lessons Learnt from the Implementation of the Multicenter REGIMS Registry. Drug Saf 44(1):7–15
Stosic M, De Jesus P, McCarthy J, Hutton G, Rivera V (2011) Immune thrombocytopenic purpura in a patient with multiple sclerosis treated with natalizumab. Neurology 77(5):505–507
Su E, Novic J, Han MH (2020) Emergence of rheumatoid arthritis following exposure to natalizumab. Mult Scler Relat Dis 40:101936
Thakar R, Gajewska-Knapik K, Ogilvy-Stuart AL, Chatterjee K, Moran C (2019) Response to Letter to the Editor: “alemtuzumab-Induced Thyroid Dysfunction Exhibits Distinctive Clinical and Immunological Features.” J Clin Endocrinol Metab 104(9):3626–3627
Tomer Y, Menconi F (2009) Interferon induced thyroiditis. Best Pract Res Clin Endocrinol Metab 23(6):703–712
Trinh T, Haridas AS, Sullivan TJ (2016) Ocular findings in alemtuzumab (Campath-1H)-induced thyroid eye disease. Ophthalmic Plast Reconstr Surg 32(6):e128–e129
Tsourdi E, Gruber M, Rauner M, Blankenburg J, Ziemssen T, Hofbauer LC (2015) Graves’ disease after treatment with alemtuzumab for multiple sclerosis. Hormones (Athens) 14(1):148–153
Ueland GS, Ueland HO, Stokland A-EM, Bhan A, Schønberg A, Sollid ST et al (2023) Prevalence, Risk Factors, and Clinical and Biochemical Characteristics of Alemtuzumab-Induced Graves Disease. J Clin Endocrinol Metab 109(2):344–50
Weetman A (2009) Immune reconstitution syndrome and the thyroid. Best Pract Res Clin Endocrinol Metab 23(6):693–702
Willis MD, Robertson NP (2016) Alemtuzumab for multiple sclerosis. Curr Neurol Neurosci Rep 16(9):84
Yue H, Shah SB, Modzelewski KL, Knobel M, Copeli F, Kao L (2023) A Grave Set of Diagnoses: A Case of Mania with Comorbid Autoimmune Thyroiditis Precipitated by Multiple Sclerosis Treatment. Harv Rev Psychiatry 31(5):242–247
Acknowledgements
Authors like to thank Vice Chancellor of research in Hamadan University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
A.S. conceptualized the subject of the manuscript, supervised the process and revised the manuscript. S.E. searched the database and was a major contributor in writing the manuscript. SA constructed the methodology, gathered and analyzed the chosen articles. NG prepared the original draft. All authors read and approved the final manuscript. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahraian, M.A., Emami, S., Ataei, S. et al. Exploring autoimmune endocrine diseases induced by monoclonal antibodies used as multiple sclerosis pharmacotherapy: a systematic review. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03386-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03386-z