Abstract
Benign prostatic hyperplasia (BPH) is a prevalent urological condition that predominantly affects the geriatric male population, resulting in lower urinary tract symptoms. Saw palmetto is a traditional Chinese medicine for treating BPH. This study aimed to investigate the potential therapeutic mechanisms of saw palmetto in BPH treatment. The active ingredients and potential targets of saw palmetto were obtained through the TCMSP database. BPH-related targets were retrieved from the GeneCards database. PPI, GO, and KEEG analyses were performed to predict the potential therapeutic mechanism. The active ingredient-common target and common target-pathway networks were constructed by Cytoscape software. Molecular docking and cellular experiments were carried out to further validate the potential mechanism. We obtained 13 active components in saw palmetto and 56 common targets in BPH treatment. KEEG analysis showed that the estrogen signaling pathway was the most enriched and exhibited a close association with BPH. PPI analysis, along with ingredient-target and target-pathway network analyses, indicated that stigmasterol was the core ingredient and PGR, NCOA1, and NCOA2 were identified as the hub genes mediating the effects of saw palmetto against BPH. In addition, molecular docking showed that stigmasterol had strong binding to PGR, NCOA1, and NCOA2. Cellular experiments revealed that stigmasterol significantly increased the percentage of BPH-1 cells in the G0/G1 phase and inhibited cell viability and division. Furthermore, it notably reduced the expression of PGR, NCOA1, and NCOA2. Saw palmetto might inhibit cell viability and division by suppressing the expression of PGR, NCOA1, and NCOA2, thereby playing a therapeutic role in treating BPH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benign prostatic hyperplasia (BPH), a prevalent urological condition predominantly affecting the geriatric male population, is characterized by the non-malignant proliferation of prostatic cells. This condition primarily impacts the periurethral region, resulting in clinical manifestations such as urethral obstruction and painful urination (Miernik and Gratzke 2020). Although the pathogenesis of BPH remains unclear, it is known to be related to age, hormonal fluctuations, and genetic factors (Devlin et al. 2021). BPH significantly affects the quality of life of patients, manifesting in symptoms such as frequent micturition, urgent micturition, and excessive urination at night. Even worse, it may lead to urinary retention and renal function damage (Launer et al. 2021). Currently, the treatment strategies for BPH mainly include drug treatment, minimally invasive surgery, and traditional surgery (Miernik and Gratzke 2020). Drug treatment mainly aims to relieve symptoms and shrink the prostate through α receptor antagonists and 5α-reductase inhibitors. However, this approach has certain limitations, such as slow onset of effect, long-term use, and possible side effects (Joseph et al. 2022). Alternatively, surgery can effectively alleviate symptoms but carries the risk of complications like postoperative sexual dysfunction and urinary incontinence (Khooblall et al. 2023). With the aging of the global population, the prevalence rate of BPH is expected to continue to rise, posing a huge pressure on the public health system (Launer et al. 2021). Therefore, it is of great practical significance to explore novel treatment strategies and drugs for BPH, which will not only improve the quality of life of elderly men but also reduce the burden on the medical system.
In the theory of traditional Chinese medicine (TCM), BPH is usually regarded as a symptom of “dysuria” or “spermatorrhea,” associated with deficiencies in kidney qi, stagnation of qi and blood, and internal accumulation of damp heat (Wen et al. 2024). The treatment of BPH in TCM primarily focuses on harmonizing qi and blood, replenishing kidney energy, and clearing heat and dampness, to alleviate symptoms and improve the quality of life (Huang et al. 2020). TCM pays attention not only to the relief of symptoms but also to the eradication of etiology and the restoration of the body’s overall balance of the body (Li et al. 2023a). In addition, Chinese medicine can make personalized treatment plans to address individual differences. At present, TCM has shown certain potential in the treatment of BPH and can achieve significant therapeutic effects with high efficacy and low toxicity (Zhao et al. 2021; Jin et al. 2019; Tran et al. 2022). Hence, the exploration and application of TCM can provide invaluable clues for developing new therapeutic drugs for BPH.
Saw palmetto, also known as Serenoa repens, is a common herbal plant native to the southeastern region of North America. The fruit of the saw palmetto is utilized in the production of herbal supplements and is also widely employed in TCM for the treatment of specific conditions such as BPH and urinary tract issues (Marks and Tyler 1999). Saw palmetto contains numerous components, such as fatty acids, phytosterols, and polyphenols, which play a pharmacological role in anti-inflammation, antiandrogenic activity, and enhancement of urodynamics (Gong and Gerber 2004). Saw palmetto has been widely used in the treatment of BPH, which can inhibit the enzyme 5α-reductase, leading to a reduction in the production of dihydrotestosterone, thereby slowing down the proliferation of prostate cells (Kwon 2019). Several studies have demonstrated the potential of saw palmetto in treating BPH. For instance, Marks and Tyler had discussed saw palmetto extract as a new (and oldest) alternative therapy for symptomatic BPH in their 1999 review (Marks and Tyler 1999). Kwon indicated that saw palmetto extract had a positive effect on BPH (Kwon 2019). Sudeep et al. revealed that saw palmetto oil, abundant in phytosterols, had similar efficacy in alleviating BPH and androgen deficiency when compared to traditional saw palmetto oil (Sudeep et al. 2020). Moreover, Serenoa repens has demonstrated good tolerability and has shown efficacy similar to α-blockers in improving voiding and storage symptoms, increasing urinary flow rate, and reducing prostate volume in men with BPH (Blair 2022). Nevertheless, the specific therapeutic mechanisms of saw palmetto in BPH treatment remain uncertain.
Network pharmacology emerges as a new interdisciplinary realm, integrating the theories and methods of systems biology, pharmacology, informatics, and network science. It is devoted to studying the intricate interaction between drugs and diseases (Nogales et al. 2022). Unlike traditional single-drug target research, network pharmacology offers a holistic analysis of network-based relationships among drug components, targets, and diseases, unveiling the overall and systematic characteristics of drug action. This comprehensive approach provides a fresh perspective and tool for studying the molecular mechanisms of TCM against diseases (Jiashuo et al. 2022). Network pharmacology helps us to deeply understand the complex mechanism of action of TCM by constructing a network model that interconnects drug components, targets, and diseases. Furthermore, the results obtained from this approach can serve as a robust foundation for subsequent experimental endeavors (Zhou et al. 2020).
In this study, we applied network pharmacology to screen the core active ingredients, core targets, and relevant signaling pathways of saw palmetto in BPH treatment. Subsequently, the interactions between these core ingredients and related targets were verified through molecular docking analysis. The potential therapeutic mechanism of saw palmetto against BPH was further validated by cellular experiments. The flowchart of this study is depicted in Fig. 1.
Materials and methods
Screening the active ingredients and targets of saw palmetto
The active components of saw palmetto were obtained from the publications. Subsequently, all active components were input into the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (https://old.tcmsp-e.com/index.php) to predict the targets associated with the potential active ingredients of saw palmetto. These targets were then converted into corresponding gene names using the UniProt database (https://www.uniprot.org/).
BPH target prediction
We screened the targets related to BPH through the GeneCards database (https://www.genecards.org/) with “Benign prostatic hyperplasia” as the keyword, and these targets were then standardized through the UniProt database to obtain their gene names and identifiers.
Screening the potential therapeutic targets of saw palmetto in BPH treatment and constructing a PPI network
To obtain the potential therapeutic targets of saw palmetto against BPH, we imported saw palmetto and disease targets into the Venny 2.1 online tool (http://liuxiaoyuyuan.cn/). The overlapping targets were the potential therapeutic targets of saw palmetto in BPH treatment. These common targets were then imported into the STRING database (http://string-db.org/) for protein–protein interaction (PPI) analysis. Cytoscape 3.10.0 was performed to determine the key therapeutic targets according to the degree value.
GO and KEEG analyses
To explore the therapeutic mechanism of saw palmetto in BPH treatment, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses of these common targets were conducted through the Hiplot database (http://hiplot.com.cn/). The GO enrichment analysis revealed genes associated with biological processes (BP), cellular compositions (CC), and molecular functions (MF), whereas the KEGG enrichment analysis primarily focused on identifying pathway-associated information.
Construction of herb-active ingredient-common target-disease and target-signaling pathway networks
The Cytoscape 3.9.1 software was employed to construct the networks that demonstrate the associations between the herb, active ingredients, targets, and pathways. In these networks, nodes represent active ingredients, targets, or pathways, while edges represent their interactions. The degree value of a node indicates the number of edges connected with the node, and the core component or path of the node is determined by the highest degree value.
Molecular docking
Molecular docking, based on structural analysis, is a crucial technique for drug design and screening. It enables the prediction of binding affinity and mode between ligand and receptor molecules, making it a common method in drug research (Pinzi and Rastelli 2019). In the present study, we performed molecular docking to preliminarily validate the core active ingredients and therapeutic targets identified through network pharmacology. The three-dimensional protein structures were retrieved from the RCSB Protein Data Bank database (PDB, http://www.rcsb.org/), while the molecular structures of the active ingredients were sourced and downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The molecular docking analysis was conducted by Molecular Operating Environment (MOE) software. In this analysis, target-ligand pairs with a selection value of ≤ − 5 kcal/mol were deemed to possess moderate to strong binding affinity (Wang et al. 2022; Guo et al. 2023). Notably a lower binding energy indicates a higher binding efficacy between the molecules.
In vitro experimental validation
Preparation of stigmasterol solution
Stigmasterol (HPLC ≥ 98%), purchased from MedChemExpress, was dissolved in DMSO to achieve a final concentration of 10 mM for further applications.
Cell culture
Human BPH-derived prostate epithelial cell lines (BPH-1) were purchased from Otwo Biotech (Otwo, Shenzhen, China). The cells were cultured in DMEM (PromoCell, Wuhan, China) medium supplemented with high-quality fetal bovine serum (Sigma, F8313) and 1% penicillin/streptomycin (PromoCell, Wuhan, China) at 37 °C with 5% CO2 and 95% air.
Cell viability assay
The viability of BPH-1 cells was assessed by Cell Counting Kit-8 (CCK8) (Beyotime, Hangzhou, China) following the manufacturer’s protocol. Initially, the cells were plated in a 96-well microplate at a concentration of 1 × 104 cells per well and cultured in an incubator at 37 °C with 5% CO2 for 24 h. Once the cells had fully attached, they were treated with stigmasterol at concentrations of 0, 10, 25, 100, 200, and 500 µM for 24 h. After pre-incubation in a cell culture incubator, the medium was aspirated, and each well was replenished with 100 µL of the prepared CCK8 working solution. The plate was then returned to the incubator at 37 °C for further incubation until the color change to orange was observed. Subsequently, the absorbance was quantified at 450 nm using a microplate reader. The cell proliferation rate was determined using the following formula: \(({OD}_{Experimental\;Group}-{OD}_{Control\;Group})/({OD}_{Control\;Group}-{OD}_{Control\;Group})\:\times\:100\%\).
Flow cytometry analysis of apoptosis
BPH-1 cells were inoculated into a 6-well plate at a density of 1 × 105 cells/well and cultured for 24 h in a 37 °C environment with 5% CO2. After washing with PBS, the cells were collected and centrifuged at 2000 rpm for 5 min. The supernatant was discarded, and the cells were washed again with PBS, followed by another centrifugation at the same speed and duration. The supernatant was then discarded, and 100 µL of a 1% BSA solution was added. Subsequently, 100 µL of cell suspension, adjusted to a concentration of 2 × 105 to 1 × 106 cells/mL, was combined with 100 µL of Muse Annexin V & Dead Cell reagent (Luminex, USA) in a 1.5-mL centrifuge tube. After incubation in the dark at room temperature for 20 min, the stained samples were filtered and analyzed using flow cytometry (Muse, USA).
Cell cycle assay
Cells were plated and treated as described above. At 24 h post-treatment, they were harvested, rinsed with PBS, and trypsinized with 0.25% trypsin for 2 min. Fetal bovine serum was added to neutralize the trypsin, and the cells were collected by centrifugation at 1000 rpm for 5 min. After discarding the supernatant and washing with PBS, the cells were fixed in 70% ethanol and stored at − 4 °C. For analysis, cells were centrifuged again at 2000 rpm for 5 min, the ethanol was removed, and the cells were washed with PBS. They were then stained with DAPI in the dark for 20 min, followed by a final wash and resuspension in PBS. Samples were analyzed through flow cytometry.
qRT-PCR analysis
Total RNA was isolated from cell samples using the TRIzol reagent (AgBio, Hunan, China), following the manufacturer’s instructions. RNA concentration and integrity were assessed using a NanoDrop 2000 spectrophotometer. Subsequently, the RNA of sufficient quality was subjected to reverse transcription with the NovoScript® Plus 1st Strand cDNA Synthesis SuperMix. qRT-PCR was performed using the SYBR High-Sensitivity qPCR SuperMix (Novoprotein, Suzhou, China) on an ABI 7500 Real-Time PCR system. GADPH served as the endogenous control for normalization. The primer sequences utilized in the qRT-PCR reactions are detailed in Table 1.
Statistical analysis
In this research, data analysis was conducted using GraphPad Prism version 9.0. Results were presented as mean ± standard deviation (SD). Statistical comparisons across and within groups were performed using one-way analysis of variance (ANOVA). A p-value of less than 0.05 was considered statistical significance.
Results
Potential active ingredients and targets of saw palmetto
In this study, we screened 13 active components of saw palmetto by consulting relevant literature (Table 2). These included stigmasterol, linolenic acid, lauric acid, oleic acid, linoleic, palmitic acid, beta-sitosterol, myristic acid, stearic acid, campesterol, caprylic acid, palmitoleic acid, and capric acid. After excluding overlapping targets, we ultimately obtained 72 targets for these active ingredients through the TCSMP database.
Therapeutic target prediction for saw palmetto in the treatment of BPH
We obtained 4280 potential BPH-related targets by searching the GeneCards database. A Venn diagram of disease-related targets and saw palmetto targets was subsequently constructed, and 56 intersecting targets were selected as potential therapeutic targets for saw palmetto in BPH treatment (Fig. 2A). To obtain the key therapeutic targets, the PPI network was constructed based on the common targets, which consisted of 56 nodes and 596 edges. As shown in Fig. 2B, PGR, NCOA1, NCOA2, ADRB1, and ADRB2 might be regarded as the key therapeutic targets with the highest degree value in the PPI network.
GO and KEEG enrichment analyses
GO and KEEG analyses were conducted to determine the therapeutic mechanism of saw palmetto against BPH based on the common targets. The top 20 GO terms and KEEG pathways are presented in the bubble diagram (Fig. 3A–D). Figure 3A illustrates that targets associated with BP are mainly involved in response to steroid hormone, regulation of tube diameter, response to xenobiotic stimulus, response to oxidative stress, and response to extracellular stimulus. In terms of MF (Fig. 3B), these targets are mainly associated with steroid hormone receptor activity, nuclear receptor activity, DNA-binding transcription factor binding, RNA polymerase II–specific DNA-binding transcription factor binding, and ligand-activated transcription factor activity. At the CC level (Fig. 3C), they are mainly related to the transcription regulator complex, organelle outer membrane, outer membrane, membrane raft, and membrane microdomain.
The KEGG pathway analysis highlighted the top 20 most significantly enriched signaling pathways as depicted in Fig. 3D. Among these, the estrogen signaling pathway, which has a significant association with BPH, was chosen for further exploration.
Construction and analysis of ingredient-target and target-signaling pathway networks
To determine the key active ingredient of saw palmetto against BPH, we constructed a network based on disease, herb, active ingredients, and common targets, which consisted of a total of 71 nodes (Fig. 4A). As shown in Table 2, stigmasterol, with the highest degree value (degree = 20) among the active ingredients of saw palmetto, plays a crucial role in treating BPH. In addition, we further constructed a network including stigmasterol and its corresponding targets. As depicted in Fig. 4B, PGR, NCOA1, NCOA2, ADRB1, and ADRB2 exhibited a higher degree value than the other targets. Figure 4C demonstrates that the estrogen signaling pathway contains seven targets, namely PGR, NCOA1, NCOA2, SP1, BCL2, JUN, and CTSD.
By integrating the PPI analysis that revealed high degree values for PGR, NCOA1, NCOA2, ADRB1, and ADRB2, the ingredient-target network that identified stigmasterol as the core ingredient, the KEGG results highlighting the estrogen signaling pathway as the main enrichment pathway related to BPH, and the target-pathway network demonstrating the involvement of PGR, NCOA1, and NCOA2 in the estrogen signaling pathway, it can be inferred that saw palmetto, particularly its ingredient stigmasterol, may influence the estrogen signaling pathway by modulating the expression of PGR, NCOA1, and NCOA2, thereby potentially playing a role in BPH treatment.
Molecular docking
To validate the authenticity of molecular interactions with their targets and determine precise binding mechanisms, we recognized stigmasterol as the key compound, focusing on PGR, NCOA1, and NCOA2 based on the above results. The docking results, presented in Table 3, indicated strong binding affinities for stigmasterol interaction with PGR (binding energy = − 6.1 kcal/mol), NCOA1 (binding energy = − 6.3 kcal/mol), and NCOA2 (binding energy = − 7.6 kcal/mol), defined by a threshold of − 5 kcal/mol or lower.
In Fig. 5A–C, the visualization presents the optimal docking images, where the receptors and ligands with high binding energy have been optimally aligned.
Stigmasterol could inhibit the viability of BPH-1 cells
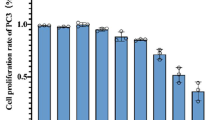
The CCK-8 assay demonstrated that BPH-1 cell viability was reduced with stigmasterol treatments at concentrations of 100, 200, and 500 µM for 24 h when compared to the control group (all p < 0.001), as depicted in Fig. 6A. Among these, the treatments with 100 and 200 µM concentrations for 24 h exhibited the most promising results and were therefore selected for subsequent experiments.
Stigmasterol inhibits cell viability and division and promotes apoptosis of BPH-1 cells. Analysis of BPH-1 cell viability (A), apoptosis (B), and cell cycle (C) after stigmasterol treatment. All data was presented as mean ± standard deviation. **p < 0.01, compared with the control group; ***p < 0.001, compared with the control group
Effect on cell apoptosis
The flow cytometry experiment was conducted to determine the effect of stigmasterol on cell apoptosis. As shown in Fig. 6B, treatment with stigmasterol could significantly accelerate the cell apoptosis rates of the BPH-1 cells (p < 0.001).
Effect on cell cycle
Figure 6C demonstrates that following a 24-h treatment with 100 µM and 200 µM stigmasterol, the experimental group exhibited a higher number of cells in the G0/G1 phase compared to the control group (all p < 0.001). Conversely, the percentage of cells in the G2/M phases was notably lower in the stigmasterol-treated group (p < 0.01 and p < 0.001, respectively). These findings suggest that the drug’s application effectively inhibited cell division, thereby controlling BPH-1 cell growth.
Effect of stigmasterol on the mRNA expressions of PGR, NCOA1, and NCOA2 in BPH-1 cells
The mRNA expressions of PGR, NCOA1, and NCOA2 were evaluated by qRT-PCR, with results shown in Fig. 7A–C. It was observed that the expression levels of PGR (p < 0.05), NCOA1 (p < 0.001), and NCOA2 (p < 0.05) significantly decreased after treatment with stigmasterol compared to the control group.
Analysis of PGR, NCOA1, and NCOA2 mRNA expression after stigmasterol treatment by qRT-PCR. A PGR, B NCOA1, and C NCOA2 mRNA expression. All data was presented as mean ± standard deviation. *p < 0.05, compared with the control group; **p < 0.01, compared with the control group; ***p < 0.001, compared with the control group
Discussion
Saw palmetto is recognized for its beneficial effects in treating BPH, yet the underlying molecular mechanisms remain largely undefined. In this study, we aimed to determine the potential therapeutic mechanism of saw palmetto in the treatment of BPH through network pharmacology and further validate this molecular mechanism through molecular docking and cellular experiments.
In this study, we identified 13 active components from saw palmetto. It is worth noting that stigmasterol, a key ingredient of saw palmetto, is found to be most closely associated with BPH-related targets through ingredient-target network analysis. This indicates that stigmasterol plays a pivotal role in the treatment of BPH. Stigmasterol, a phytosterol in saw palmetto, has anti-inflammatory and antioxidant pharmacological effects (Jie et al. 2022). Besides, stigmasterol showed significant biological activity in the regulation of cell function. For example, studies have indicated that stigmasterol can achieve anti-tumor effects by inhibiting the migration of gastric cancer cells, arresting cell cycle, promoting cell apoptosis, and blocking the JAK/STAT signaling pathway (Li et al. 2018). Lu et al. have revealed that stigmasterol inhibits the proliferation and promotes the apoptosis of fibroblast-like synovial cells through the PI3K/AKT signaling pathway, thereby having a positive effect on collagen-induced arthritis rat model (Lu et al. 2023). In addition, stigmasterol isolated from marine microalgae boat insert can induce apoptosis of human hepatocellular carcinoma HepG2 cells (Kim et al. 2014). These results show the potential application value of stigmasterol in treating human diseases.
We subsequently identified three key therapeutic targets (PGR, NCOA1, and NCOA2) that potentially mediate the protective effects of saw palmetto against BPH by integrating the PPI analysis (PGR, NCOA1, NCOA2, ADRB1, and ADRB2 had the highest degree values), the KEGG results (the estrogen signaling pathway was the main enrichment pathway and was closely related to BPH (Liu et al. 2023; Liu et al. 2024; Fan et al. 2020)), and the target-pathway network (demonstrating that PGR, NCOA1, and NCOA2 participate in the estrogen signaling pathway). PGR, a nuclear receptor widely expressed in the reproductive system, including the prostate, regulates gene expression by binding to progesterone and then affects the biological functions of cells such as growth, differentiation, and apoptosis (Chen et al. 2017). The expression level of PGR is significantly upregulated in BPH, suggesting a possible promotional role in the pathogenesis of BPH (Song et al. 2016). In addition, research by Li et al. established a correlation between PGR genetic variants and the risk of developing BPH (Li and Klein 2021). NCOA1 and NCOA2 are a kind of protein that can enhance the signal transduction of nuclear receptors. By binding to nuclear receptors, they promote the transcription activation of target genes, thus influencing various biological processes, such as cell growth, differentiation, and metabolism. For example, a study revealed that the accumulation of NCOA1, without HERC3, can promote the degradation of the extracellular matrix, which may be related to the invasion and metastasis of prostate cancer (Li et al. 2023b). Besides, the mRNA expression level of NCOA1 was found to be significantly decreased by treating with myrocin A in BPH (Khooblall et al. 2023; Kwon et al. 2024). Molecular docking analysis was further conducted to verify the interactions between a core component of saw palmetto and hub genes. Our study indicated strong binding affinities between stigmasterol and PGR, NCOA1, and NCOA2. These findings indicate that PGR, NCOA1, and NCOA2 play an important role in saw palmetto against BPH.
Cellular experiments were subsequently carried out to verify whether stigmasterol modulated the expression of PGR, NCOA1, and NCOA2, thereby potentially playing a role in the treatment of BPH. First, the therapeutic effect of stigmasterol was detected by cell function experiments, including cell viability, apoptosis, and cycle. It was shown that stigmasterol could significantly increase the percentage of BPH-1 cells in the G0/G1 phase and inhibit cell viability and division. Second, the effect of stigmasterol on the mRNA expression of PGR, NCOA1, and NCOA2 was evaluated by qRT-PCR analysis. We observed that stigmasterol could notably downregulate the expression of PGR, NCOA1, and NCOA2. Consistent with our results, Kang et al. indicated that phytosterol extracted from hull-less pumpkin can increase the expression of apoptosis-related factors in the BPH rat model (Kang et al. 2021). Rauwolfia vomitoria extract can significantly inhibit BPH epithelial cell viability by inducing apoptosis (Huang et al. 2022). Cheon et al. showed that oleanolic acid suppressed cell proliferation by reducing the expression of PCNA and cell cycle markers in the BPH animal model (Cheon et al. 2020). In addition, resveratrol suppressed the viability of WPMY-1 cells by inducing the G0/G1-phase cell cycle, which was a result of the downregulation of cyclin and cyclin-dependent kinase (Jang et al. 2021). Moreover, Wei et al. highlighted that cryptotanshinone may regulate the proliferation and apoptosis of BPH-1 cells by inhibiting the EGFR/STAT3 axis, thus inhibiting the progress of BPH (Wei et al. 2023). Taken together, we speculated that saw palmetto might influence the cell functions of BPH-1 cells by modulating the expression of PGR, NCOA1, and NCOA2, thereby playing a potential role in the treatment of BPH.
Our study has certain limitations. First, the current study did not determine the functions of PGR, NCOA1, and NCOA2 in the treatment of BPH. Multi-level experiments are needed to further explore the specific mechanisms underlying saw palmetto’s effects on these proteins in BPH treatment. Second, we have only verified the therapeutic effect of the active ingredients at the cellular level, and we will further verify it by constructing an animal model of BPH in the future. Third, the therapeutic mechanisms require further exploration and verification through in vivo experiments.
Conclusion
In this study, we integrated network pharmacology, molecular docking, and cellular experiments to explore the therapeutic mechanism of saw palmetto in BPH treatment. Our study indicated that saw palmetto, especially its stigmasterol component, might play a therapeutic role in BPH by suppressing the expression of PGR, NCOA1, and NCOA2, thereby inhibiting cell viability and division. This study enhances our understanding of TCM’s therapeutic mechanisms and presents a promising strategy for establishing the scientific basis and treatment approach for TCM in disease management.
Data availability
No datasets were generated or analysed during the current study.
References
Blair HA (2022) Hexanic extract of Serenoa repens (Permixon(®)): a review in symptomatic benign prostatic hyperplasia. Drugs Aging 39(3):235–243
Chen R, Yu Y, Dong X (2017) Progesterone receptor in the prostate: a potential suppressor for benign prostatic hyperplasia and prostate cancer. J Steroid Biochem Mol Biol 166:91–96
Cheon SY, Jin BR, Kim HJ, An HJ (2020) Oleanolic acid ameliorates benign prostatic hyperplasia by regulating PCNA-dependent cell cycle progression in vivo and in vitro. J Nat Prod 83(4):1183–1189
Devlin CM, Simms MS, Maitland NJ (2021) Benign prostatic hyperplasia - what do we know? BJU Int 127(4):389–399
Fan Y, Song TR, Wei Q, Yang L, Lin T, Feng XB et al (2020) Modulatory effect of aquaporin 5 on estrogen-induced epithelial-mesenchymal transition in prostate epithelial cells. Chin Med J 134(4):448–455
Gong EM, Gerber GS (2004) Saw palmetto and benign prostatic hyperplasia. Am J Chin Med 32(3):331–338
Guo R, Yi Z, Wang Y, Wang L (2023) Network pharmacology and experimental validation to explore the potential mechanism of Sanjie Zhentong Capsule in endometriosis treatment. Front Endocrinol 14:1110995
Huang Y, Li J, Yang S, Yuan D, Wang S (2020) Efficacy and safety of transurethral split of prostate for benign prostatic hyperplasia: a meta-analysis. BMC Urol 20(1):141
Huang G, He X, Xue Z, Long Y, Liu J, Cai J et al (2022) Rauwolfia vomitoria extract suppresses benign prostatic hyperplasia by inducing autophagic apoptosis through endoplasmic reticulum stress. BMC Complement Med Ther 22(1):125
Jang J, Song J, Lee J, Moon SK, Moon B (2021) Resveratrol attenuates the proliferation of prostatic stromal cells in benign prostatic hyperplasia by regulating cell cycle progression, apoptosis, signaling pathways, BPH markers, and NF- κB activity. Int J Mol Sci. 22(11):5969
Jiashuo WU, Fangqing Z, Zhuangzhuang LI, Weiyi J, Yue S (2022) Integration strategy of network pharmacology in traditional Chinese medicine: a narrative review. J Tradit Chin Med. 42(3):479–86
Jie F, Yang X, Yang B, Liu Y, Wu L, Lu B (2022) Stigmasterol attenuates inflammatory response of microglia via NF-κB and NLRP3 signaling by AMPK activation. Biomed Pharmacother 153:113317
Jin BR, Chung KS, Kim HJ, An HJ (2019) Chinese skullcap (Scutellaria baicalensis Georgi) inhibits inflammation and proliferation on benign prostatic hyperplasia in rats. J Ethnopharmacol 235:481–488
Joseph DB, Henry GH, Malewska A, Reese JC, Mauck RJ, Gahan JC et al (2022) 5-Alpha reductase inhibitors induce a prostate luminal to club cell transition in human benign prostatic hyperplasia. J Pathol 256(4):427–441
Kang XC, Chen T, Zhou JL, Shen PY, Dai SH, Gao CQ et al (2021) Phytosterols in hull-less pumpkin seed oil, rich in ∆(7)-phytosterols, ameliorate benign prostatic hyperplasia by lowing 5α-reductase and regulating balance between cell proliferation and apoptosis in rats. Food Nutr Res. 65. https://doi.org/10.29219/fnr.v65.7537
Khooblall P, Bole R, Leelani N, Lundy S, Bajic P (2023) A scoping review of ejaculatory dysfunction due to surgical treatments for benign prostatic hyperplasia: limitations of available tools for assessment and reporting. Sex Med Rev 11(4):375–383
Kim YS, Li XF, Kang KH, Ryu B, Kim SK (2014) Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB Rep 47(8):433–438
Kwon Y (2019) Use of saw palmetto (Serenoa repens) extract for benign prostatic hyperplasia. Food Sci Biotechnol 28(6):1599–1606
Kwon H, Jin BR, Kim HJ, Kwon J, Park K, Kim C et al (2024) New pimarane diterpenoids isolated from EtOAc-extract of Apiospora arundinis culture medium show antibenign prostatic hyperplasia potential. ACS Omega 9(5):5616–5623
Launer BM, McVary KT, Ricke WA, Lloyd GL (2021) The rising worldwide impact of benign prostatic hyperplasia. BJU Int 127(6):722–728
Li W, Klein RJ (2021) Genome-wide association study identifies a role for the progesterone receptor in benign prostatic hyperplasia risk. Prostate Cancer Prostatic Dis 24(2):492–498
Li K, Yuan D, Yan R, Meng L, Zhang Y, Zhu K (2018) Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J BUON 23(5):1420–1425
Li J, Li D, Chen Y, Chen W, Xu J, Gao L (2023a) Gut microbiota and aging: traditional Chinese medicine and modern medicine. Clin Interv Aging 18:963–986
Li X, Wang X, Chen C, Zhang E, Zhang Y, Li H et al (2023b) Accumulation of NCOA1 dependent on HERC3 deficiency transactivates matrix metallopeptidases and promotes extracellular matrix degradation in intervertebral disc degeneration. Life Sci 320:121555
Liu Y, Shao R, Suo T, Zhu J, Liu E, Wang Y et al (2023) Traditional Chinese medicine Danzhi qing’e decoction inhibits inflammation-associated prostatic hyperplasia via inactivation of ERK1/2 signal pathway. J Ethnopharmacol 309:116354
Liu Z, Li S, Chen S, Sheng J, Li Z, Lv T et al (2024) YAP-mediated GPER signaling impedes proliferation and survival of prostate epithelium in benign prostatic hyperplasia. iScience 27(3):109125
Lu D, Yang Y, Ma W, Yao X, Ling Y, Huang Y et al (2023) Stigmasterol depresses the proliferation and facilitates the apoptosis of fibroblast-like synoviocytes via the PI3K/AKT signaling pathway in collagen-induced arthritis rats. Altern Ther Health Med 8:AT9044
Marks LS, Tyler VE (1999) Saw palmetto extract: newest (and oldest) treatment alternative for men with symptomatic benign prostatic hyperplasia. Urology 53(3):457–461
Miernik A, Gratzke C (2020) Current treatment for benign prostatic hyperplasia. Dtsch Arztebl Int 117(49):843–854
Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt H (2022) Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci 43(2):136–150
Pinzi L, Rastelli G (2019) Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci 20(18):4331
Song L, Shen W, Zhang H, Wang Q, Wang Y, Zhou Z (2016) Differential expression of androgen, estrogen, and progesterone receptors in benign prostatic hyperplasia. Bosn J Basic Med Sci 16(3):201–208
Sudeep HV, Thomas JV, Shyamprasad K (2020) A double blind, placebo-controlled randomized comparative study on the efficacy of phytosterol-enriched and conventional saw palmetto oil in mitigating benign prostate hyperplasia and androgen deficiency. BMC Urol 20(1):86
Tran DNH, Yeh HF, Huang WJ, Wu PW, Liao YJ, Hwang SJ et al (2022) Efficacy evaluation of Chinese herbal medicine, VGH-BPH1, for patients with benign prostatic hyperplasia: a randomized, double-blind, placebo-controlled, and crossover study. J Chin Med Assoc 85(5):639–646
Wang T, Jiang X, Ruan Y, Zhuang J, Yin Y (2022) Based on network pharmacology and in vitro experiments to prove the effective inhibition of myocardial fibrosis by Buyang Huanwu decoction. Bioengineered 13(5):13767–13783
Wei P, Lin D, Zhang M, Luo C, Wu X, Deng B et al (2023) Cryptotanshinone modulates proliferation, apoptosis, and fibrosis through inhibiting AR and EGFR/STAT3 axis to ameliorate benign prostatic hyperplasia progression. Eur J Pharmacol 938:175434
Wen L, Qiu S, Fan C (2024) Discussion on treatment of benign prostatic hyperplasia based on collateral disease theory. New Chin Med 56(09):202–205
Zhao Y, Zhang Y, Li Y, Yang M, Yuan J, Cao Y et al (2021) Yohimbine hydrochloride inhibits benign prostatic hyperplasia by downregulating steroid 5α-reductase type 2. Eur J Pharmacol 908:174334
Zhou Z, Chen B, Chen S, Lin M, Chen Y, Jin S et al (2020) Applications of network pharmacology in traditional Chinese medicine research. Evid Based Complement Alternat Med 2020:1646905
Funding
No funding was received for this research.
Author information
Authors and Affiliations
Contributions
Bo Zhang conceptualized, wrote, and reviewed the manuscript. Yiying Wang contributed to the network pharmacology analysis. Kunping Yan conducted the experiments and data analysis. Jiangang Yang contributed to the experiments and interpretation. All authors contributed to the article and approved the submitted version. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, B., Wang, Y., Yan, K. et al. Network pharmacology and experimental validation to explore the pharmacological mechanism of saw palmetto and its core ingredients in benign prostatic hyperplasia treatment. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03289-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03289-z