Abstract
Cannabidiol (CBD) is a non-psychoactive substance that exerts numerous pharmacological benefits, including anti-inflammatory and antioxidant properties. It has received attention as a useful substance for the treatment of intractable pain, seizures, and anxiety, and related clinical trials have continued. However, the CBD pharmacokinetic results between reports are highly variable, making it difficult to clearly identify the pharmacokinetic properties of CBD. The main purpose of this study was to identify CBD clinical pharmacokinetic properties through meta-analysis. In particular, we sought to derive valid, interpretable independent variables and interpret their pharmacokinetic parameter correlations in relation to the large inter-individual and inter-study variability in CBD pharmacokinetics. For this study, CBD-related clinical trial reports were extensively screened and intercomparisons were performed between internal data sets through systematic classification and extraction of pharmacokinetic parameter values. The candidate independent variables associated with interpretation of CBD pharmacokinetic diversity established and explored in this study were as follows: diet, tetrahydrocannabinol (THC) combination, sample matrix type, liver and renal function, exposure route, dosage form, CBD exposure dose, cannabis smoking frequency, multiple exposure. The results of this study showed that CBD pharmacokinetics were influenced (increased plasma exposure by approximately 2–5 times) by diet immediately before or during CBD exposure, and that THC was not expected to have an antagonistic effect on the CBD absorption. The influence of changes in liver function would be significant in CBD pharmacokinetic diversity. Due to decreased liver function, the plasma exposure of CBD increased 2.57–5.15 times compared to healthy adults, and the half-life and clearance showed a 2.58-fold increase and a 5.15-fold decrease, respectively. CBD can be rapidly absorbed into the body (time to reach maximum concentration within 3.18 h) by oral, transdermal, and inhalation exposures, and lipid emulsification and nanoformulation of CBD will greatly improve CBD bioavailability (up to approximately 2 times). The pharmacokinetics of CBD generally follow linear kinetic characteristics. The importance of this study is that it suggests key factors that should be considered in terms of pharmacokinetics in further clinical trials and formulations of CBD in the future.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis sativa contains over hundreds of cannabinoids (Radwan et al. 2021). Among these numerous plant cannabinoids, there are two major components that are receiving clinical attention: cannabidiol (CBD) and tetrahydrocannabinol (THC). This is because they are ingredients related to the medicinal effects and side effects of cannabis (Richter et al. 2021). Moreover, CBD and THC are two of the most well-known compounds isolated from the Cannabis sativa plant (Richter et al. 2021). CBD is a non-psychoactive cannabinoid and is reported to have various pharmacological effects such as analgesic, anti-convulsant, anti-spasmodic, anti-emetic, anti-inflammatory, anti-oxidant, and neuroprotective (Kumar et al. 2021). On the other hand, THC is a psychoactive cannabinoid and is considered the main side effect of cannabis use causing euphoria and hallucination (Kumar et al. 2021). The pharmacological mechanisms of CBD and THC are very complex, and reports to date indicate that cannabinoid (CB)-1 and CB-2 receptors distributed in the body are closely related (Kumar et al. 2021; Richter et al. 2021). CB-1 receptors are mainly expressed in the central nervous system (CNS), including the brain, and THC is known to act as a very strong agonist for CB-1 receptors, causing various CNS side effects (Howlett and Abood 2017). However, CBD is known to have a relatively weak binding affinity to CB-1 receptors and to exert pharmacological effects by interacting with CB-2 receptors, which are mainly present in peripheral and immune tissues (Howlett and Abood 2017).
CBD is reported to be highly effective in treating intractable epilepsy disorders (which are difficult to treat with existing anti-convulsants) such as Lennox-Gastaut and Dravet syndromes (Golub and Reddy 2021). Therefore, in clinical practice, attempts to utilize CBD as medicine have continued, focusing on its excellent medicinal effects. For example, a clinical trial of a CBD oral capsule formulation was attempted on a group of patients with multiple sclerosis and symptoms of neuropathic pain and spasticity (Hansen et al. 2024), and a phase 1 clinical trial of a nanoparticle CBD formulation as an anti-inflammatory was attempted (Vitetta et al. 2021). In addition, the development of CBD dry-powder inhaler (Devinsky et al. 2021) and sublingual wafer (Hosseini et al. 2021) formulations and the results of phase 1 clinical trials for these were reported. In terms of safety, the content of THC in the formulation and its combined effects have also received attention. As mentioned above, unlike CBD, THC is a psychoactive cannabinoid and has been known to be the main cause of side effects such as acute memory impairments and psychotic symptoms in infrequent users (Englund et al. 2023). Therefore, the mixing ratio of CBD and THC in cannabis formulations has been raised as an important point in reducing side effects from cannabis exposure (Englund et al. 2023). The 2018 Farm Bill defined hemp as Cannabis sativa plant parts containing concentrations of THC of no more than 0.3% of dry weight (Johnson et al. 2022). And based on this, it has been implied that it would be better for CBD formulations to be applied as medicine to contain less than 0.3% THC or not include it at all. However, one recent report (Englund et al. 2023) suggested that the mixing ratio of CBD and THC in cannabis formulations may not have a significant impact on the side effects caused by cannabis. There is still ongoing debate as to whether the combination of CBD and THC in cannabis formulations offers an advantage or disadvantage in terms of clinical effectiveness and safety over CBD alone (Pennypacker and Romero-Sandoval 2020).
Even in recent years, as many countries (such as Canada, Uruguay, Thailand) have legalized the use of cannabis for recreational and general medical purposes, human exposure to CBD is increasing globally (Kumar et al. 2021). Despite the increasing exposure to CBD in daily life and clinical practice, the clarity of CBD’s clinical pharmacokinetic information is still unclear, and the dosage regimens of CBD in clinical applications are dependent on empirical aspects. The difficulty in determining the pharmacokinetic properties of CBD may be related to several reasons: there is considerable inter-individual pharmacokinetic variability; clear standardization into specific formulations by exposure route has not been achieved; there are practical difficulties (including cost and trial permission) in conducting large-scale clinical trials; exposures to CBD are often combined with multiple concomitants, such as THC, rather than as a stand-alone ingredient. The reason for the significant inter-individual pharmacokinetic variability of CBD may be that large differences in bioavailability between individuals occur due to formulations that are not standardized along with the physicochemical properties (especially highly fat soluble [log P value of approximately 6.5]) of CBD (Stasiłowicz-Krzemień et al. 2023). There are even large differences in results between clinical trial reports of CBD, which poses a major obstacle to understanding CBD pharmacokinetic properties and is a factor that adds to the confusion in clinical application.
Therefore, attempts to more comprehensively interpret and analyze the pharmacokinetic properties of CBD were required. In particular, it was suggested that the exploration of factors related to CBD pharmacokinetic diversity and the integrated analysis of their influence degree would be useful in greatly reducing the gaps in existing knowledge about the pharmacokinetic properties of CBD. In this study, screening and integrated interpretation of CBD pharmacokinetic properties in vivo were performed based on meta-analysis of CBD clinical reports, considering research necessity, clinical importance, and increasing social interest. Interpretations based on meta-analysis will be able to present results on CBD’s pharmacokinetic properties and diversity-related factors. The results of this study are expected to be useful data in designing additional prospective clinical trials of CBD in the future. In addition, it is expected to be a useful reference in accelerating the establishment of CBD’s scientific clinical regimens and standardizing and developing formulations for each CBD exposure route.
Methods
Data collection
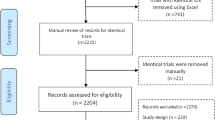
In this study, an extensive meta-analysis of CBD-related pharmacokinetic reports was first performed with reference to PRISMA guidelines and approaches (Sarkis-Onofre et al. 2021). Figure 1 shows the PRISMA flow diagram. Scientific search engines such as Google Scholar, Medline, PubMed, Embase, and Web of Science were used in the meta-analysis. And the related past reports were collected by applying the core keywords of this study, such as CBD, pharmacokinetics, and clinical trials, and this was the primary data collection process. The data collection period was from the start of the study until February 15, 2024, and officially published reports from the past to the present were the subject of this study. As a secondary data collection process, among the primary collected data, data in which specific pharmacokinetic parameter values of CBD were not presented, data in which the demographic information of the subjects was not clearly confirmed, and data in which information such as CBD administration formulation, exposure route, and dose were not clearly confirmed were excluded. As a result, comprehensive pharmacokinetic analysis and interpretation of CBD were conducted based on refined secondary data. Appendix shows the PRISMA checklist results. In addition, a schematic diagram (expanded by presenting a summary of PRISMA step details) of the extensive data collection and analysis strategies attempted to conduct this study is presented in Fig. S1.
Pharmacokinetic parameters
In this study, the following pharmacokinetic parameters were set as target-dependent variables for CBD pharmacokinetic analysis: maximum CBD concentration (Cmax) in sample matrix, time taken to reach maximum concentration of CBD (Tmax) in sample matrix, area under the profile curve to observed CBD concentration points (AUCall) according to CBD exposure time, area under the profile curve to extrapolated CBD concentration points (AUCinf) according to CBD exposure time, CBD elimination rate constant (Ke) in the sample matrix, CBD elimination half-life (T1/2) from the sample matrix, CBD clearance (CL) in the body, CBD distribution volume (V) in the body. In extracting pharmacokinetic parameter values, parameter units that were different for each report needed to be unified to facilitate overall comparison. As a result, the units of Cmax, Tmax, AUCall, AUCinf, Ke, T1/2, CL, and V are unified as ng/mL, h, h×ng/mL, h×ng/mL, 1/h, h, L/h, and L, respectively. Pharmacokinetic parameter values presented in each report were mostly presented as mean values, with a mixture of arithmetic and geometric means. In general, with the assumption that there will be no significant numerical difference between the arithmetic and the geometric mean values, compared to other independent variable elements, the type of mean value will have a relatively negligible impact on CBD pharmacokinetic variability, so the arithmetic and geometric means were reflected as the values presented in the report without separate unification. The values presented along with the mean, standard deviation, standard error, or range were different for each report, but these were also reflected as the values presented in the corresponding report without separate unification. Because complete raw data cannot be secured, it was judged that arbitrary changes in values would cause misinterpretation of the reported results.
Setting of independent variables
In the pharmacokinetic analysis of CBD, categorical independent variables were required to be compared between internal data sets. The independent variable items set were as follows: effect of diet on CBD pharmacokinetics, impact of THC combination on CBD pharmacokinetics, impact of sample matrix on CBD pharmacokinetics, influence of hepatic and renal functions on CBD pharmacokinetics, effect of exposure route on CBD pharmacokinetics, effect of formulation on CBD pharmacokinetics, CBD pharmacokinetic properties according to CBD exposure dose, impact of cannabis smoking pattern on CBD pharmacokinetics, effect of multiple exposures on CBD pharmacokinetics. Within each set of independent variables, exposure routes and formulation factors were classified into each large category factor. This was to facilitate comparison between factors within the set independent variables, as the CBD exposure routes and formulations in each report were very diverse and not standardized. Routes of exposure were classified as intravenous, inhalational, transdermal, and oral based on the information presented in the report. The transdermal classification primarily refers to applications of CBD through the oral mucosa (including sublingual and buccal). Formulation elements were classified based on the main forms of final human exposure to CBD, taking comprehensive consideration of the formulation and exposure route information presented in the report. Intravenous exposure formulation categories included ethanolic solutions and ethanolic solutions containing Tween 80. Inhalation exposure formulation categories included aerosol, dry powder, inhalation solutions, nebulizer, smoking cigarettes, spray, and vaporized. The transdermal exposure formulation categories included spray, wafer, lipid droplet, and nanoparticle. Oral exposure dosage forms included capsule, food, solution, lipid droplet, nanoemulsion, powder, emulsified powder, emulsified solution, lipid emulsion, syrup, and nanocapsule. In the oral exposure formulation classifications, emulsion or emulsified, it was implied that the addition of a surfactant or physical homogenization was performed to increase the solubilization of fat-soluble CBD in the formulation.
Results and discussion
Table S1 presents a summary of pharmacokinetic results from CBD clinical trial reports. A total of 47 reports were included in this study. Comparison of CBD pharmacokinetic results was mainly possible through comparison of independent variables in intra-study. Comparison of pharmacokinetic results in inter-study had great practical difficulties in controlling factors other than the independent variables being compared. In other words, there were differences between each report in terms of CBD exposure dose, administration route, use of THC in combination, and dietary status. In particular, CBD exposure doses varied widely (0.42 to 6000 mg) among reported pharmacokinetic studies, administration routes varied (such as oral, intravenous, inhalation, and transdermal), and the formulations attempted were also diverse (such as solution, capsule, nanoformulations, syrup, wafer). In addition, a significant number of reports have conducted studies on the pharmacokinetics of CBD using extracts of Cannabis sativa, and the content of THC incorporated in addition to CBD varies widely.
Effects of CBD pharmacokinetics with and without diet
Table 1 shows a summary of reported results (Nadulski et al. 2005; Stott et al. 2013a; Taylor et al. 2019; Crockett et al. 2020; Sholler et al. 2022) to assess the effects of diet on CBD pharmacokinetics. Regarding oral exposure to CBD, the mean absorption rate of CBD into the blood stream through the gastrointestinal tract under fasted and fed conditions was inconsistent and varied between reports. In a report by Taylor et al. (Taylor et al. 2018), the mean Tmax of CBD tended to be smaller in the fed state (3.00 h) than in the fasted state (3.50–4.00 h). According to a report by Crockett et al. (Crockett et al. 2020), when a high fat diet was combined with oral administration of CBD, the mean Tmax(3.38 h) was lower than that of the fasted diet (3.75 h), but when it was combined with low fat (5.26 h), milk (5.88 h), and alcohol (5.76 h), the mean Tmax values increased. There was also a difference between fasted and fed states in the rate of CBD absorption through the transdermal (oromucosal), with mean Tmax lower in fasted (1.39 h) than fed (4.00 h) (Stott et al. 2013a). However, considering that the Tmax mean values in both fasted and fed states ranged from 1.39 to 5.88 h, and that the mean difference between fasted and fed states in the intra-study comparison was within approximately 2.5 h, it was suggested that the effect of diet on Tmax of CBD is unlikely to be very significant. In common with both oral and transdermal exposure of CBD, the Cmax, AUCall, and AUCinf values of CBD were significantly increased in the fed state rather than the fasted state, and the extent was approximately 2- to 5-fold. This suggested that the absorption of highly fat-soluble CBD into the blood stream through the gastrointestinal tract was greatly enhanced with diet. Interestingly, it was suggested that transdermal absorption also increased with diet, which was interpreted to be an indirect effect due to increased overall body activity and blood flow rates after diet. The degree of absorption of CBD differed depending on the type of diet, and both milk and alcohol increased the absorption of CBD compared to fasted, but it was confirmed that the absorption of CBD increased significantly when accompanied by a diet with a high fat content. Both oral and transdermal exposure to CBD generally had higher systemic CL in the fasted state compared to the fed state, ranging from approximately 1.5 to 5 times. This implied that in vivo loss of exposed CBD would be significantly higher in the fasted state than in the fed state. The phenomenon of differences in CBD CL depending on dietary status may be related to the fact that the absorption of CBD administered in the fasted state into the blood stream was significantly lower both oral and transdermal. The estimated V values for CBD oral exposure were higher in the fasted states than in the fed states, which may be related to significantly higher systemic CL (higher levels of CBD elimination from the blood) in the fasted states. Proceeding with a diet (1 h) following oral administration of CBD is unlikely to have a significant effect on the pharmacokinetics of CBD. According to a report by Nadulski et al. (2005), there was no significant difference in dietary progress after oral administration of CBD compared to CBD Tmax, Cmax, and AUCall values in the fasted state, and the difference in mean values was within 10%. This suggests that the influence of diet on CBD’s pharmacokinetics (particularly absorption in the body) is likely to be determined by diet immediately prior to CBD exposure. As a result, in order to increase the absorption of CBD into the body, medication guidance such as taking it with or after a meal will be required. In a report on CBD pharmacokinetics using urine as a matrix, no significant differences were found in the Tmax and Cmax values of unchanged CBD excretion between fed and fasted states.
Impact of CBD pharmacokinetics with and without THC
It has been suggested that the combined use of THC and CBD may alter the pharmacokinetic properties of CBD by affecting the metabolism of CBD in the body. This is because THC and CBD have very similar chemical structures and are predicted to have some shared mechanisms in metabolic pathways (such as phases I and II). Additionally, both THC and CBD are reported to be significantly metabolized in the liver via common CYP enzymes (such as CYP3A4 and CYP2C19). To date, there are almost no prospective comparative studies on the pharmacokinetic results of CBD alone and CBD and THC combined in humans. In this study, we aimed to screen and explore the effects of the combined use of THC on CBD pharmacokinetics in humans through a retrospective pharmacokinetic meta-analysis. Table 2 shows a summary of reported results (Guy and Flint 2014; Bergeria et al. 2022; Sholler et al. 2022) to evaluate the effects of THC combination on CBD pharmacokinetics. In the Tmax comparison between the CBD alone and THC combination groups in inhalation exposure to CBD (Bergeria et al. 2022; Sholler et al. 2022), the mean Tmax (in urine and oral fluid excretion) increased when used in combination with THC, but no significant differences between groups were identified (p > 0.05). In a comparison of Tmax between the CBD alone and THC combination groups according to CBD transdermal exposure (Guy and Flint 2014), the mean Tmax (in plasma) increased with CBD alone (2.17 h), but no significant difference between groups was found (p > 0.05). In both inhalation and dermal exposure to CBD (Guy and Flint 2014; Bergeria et al. 2022; Sholler et al. 2022), the Cmax (for values in plasma as well as excretion in urine and oral fluid) and AUC (in plasma) of CBD were commonly increased when combined with THC, and the magnitude of the increase was approximately 1.26 to 1.46 times. For inhalation exposure to CBD, the T1/2 of CBD based on the urinary excretion profile with THC coadministration was increased by an average of 1.66-fold compared to CBD alone. As a result, it was suggested that CBD’s absorption into the body would, on average, be increased further with the combination of THC. And it was predicted that the combination of THC would increase the bioavailability of CBD and improve its retention in the body. The phenomenon of increased absorption (through inhalation and transdermal) of CBD in the body according to the combined use of THC was interpreted to be because the more lipophilic nature of THC mixes with the physicochemical properties of CBD, increasing transfer and absorption into fat-soluble tissues in the body. It has also been suggested that THC may not play an antagonistic role in the absorption of CBD into the body. Therefore, in order to relatively increase the degree of absorption of CBD into the body, it may be necessary to consider the combined use of THC at a dose that does not cause side effects.
Effect of sample matrix on CBD pharmacokinetic parameter estimation
CBD pharmacokinetic parameter values in the reports were derived based on various biomatrices such as plasma, oral fluid, and serum. Therefore, it was required to compare and interpret the influence of sample matrix types on the estimation of CBD pharmacokinetic parameter values. This is because it will be very important in selecting sample matrices and interpreting results between matrices in future CBD pharmacokinetic studies. Table 3 shows a summary of reported results (Schwope et al. 2011; Lee et al. 2012; Desrosiers et al. 2014; Newmeyer et al. 2014; Pichini et al. 2020; Busardò et al. 2021; Pérez-Acevedo et al. 2021) to assess the impact of sample matrix type on the estimation of CBD pharmacokinetic parameter values. The mean Tmax values of CBD between sample matrices were all within 3 h and there was no significant difference (p > 0.05). On the other hand, the mean values of Cmax and AUCall showed large differences between sample matrices despite exposure to the same dose of CBD, and in particular, the differences between plasma and oral fluid in the CBD inhalation route were significant. That is, in the case of Cmax, the difference ranged from 13.45 to 70 times, and in the case of AUCall, there was a 7.78-fold difference. In the case of oral exposure to CBD, the mean difference in Cmax and AUCall between serum and oral fluid was 1.33 to 8.71 times, and the difference between sample matrices was relatively small compared to the inhalation exposure route. In terms of estimated T1/2, the differences between serum and oral fluid according to oral exposure to CBD were not large, ranging from 1.16 to 3.11 times. Significant differences in CBD pharmacokinetic parameter values (particularly Cmax and AUCall) between sample matrices following inhalation exposure may be associated with the inhalation formulation, mechanical factors (such as propellant), and variable respiratory conditions between individuals. In other words, even if the CBD dose was the same, there would be large inter-individual differences in inhalation patterns and respiratory physiological conditions, which resulted in a very large difference in the concentration of CBD detected in the biological matrix. On the other hand, in the case of oral exposure, although there may be differences between formulations, the differences in CBD concentrations detected in biological matrices due to the relatively uniform and simple administration method of ingestion through the gastrointestinal tract would not be relatively large. This implied that the wide variability in pharmacokinetic parameter estimates due to the choice of administration route must be clearly considered in clinical practice, and that caution is especially needed in the interpretation of CBD pharmacokinetic results following inhalation exposure.
Influence of liver and renal functions on CBD pharmacokinetics
CBD is reported to be metabolized in the body primarily in the liver and excreted in feces and urine via the kidneys. Therefore, the possibility that liver and renal functions are significantly related to the pharmacokinetics of CBD has been continuously raised. In theory, considering the basic pharmacokinetic properties of CBD, hepatic and renal functions could each affect the metabolism and excretion of CBD in the body. Table 4 shows a summary of reported results (Taylor et al. 2019; Tayo et al. 2020) to assess the effects of hepatic and renal functions on CBD pharmacokinetics. Compared to the healthy adult group, the mean Tmax values in the patient group with impaired liver function ranged from 2.0 to 2.8 h, with no significant difference between the groups (p > 0.05). On the other hand, the mean values of CBD’s Cmax, AUCall, and AUCinf were increased in the patient group with impaired liver function compared to the healthy adult group, and the degree of increase increased as the degree of liver function decline increased. Cmax increased 2.57 times in the severe hepatic impairment patient group (381 ng/mL) compared to the healthy adult group (148 ng/mL), and AUCall and AUCinf increased from 4.13 to 5.15 times. The mean T1/2 and CL values of CBD in plasma increased and decreased, respectively, in the patient group with impaired liver function compared to the healthy adult group, and the degree of increase and decrease increased as the degree of liver function decline increased. The mean T1/2 and CL values of CBD increased by 2.58 times and decreased by 5.15 times, respectively, in patients with severe hepatic impairment compared to healthy adults. This suggests that the degree of liver function has an important effect on the pharmacokinetics of CBD, and that as liver function declines, the exposure in the body can greatly increase due to decreased metabolism of CBD. Therefore, in the clinical application of CBD, it will be very important to establish a dosage that takes into account the liver functions of the patient group. In addition, it has been indirectly suggested that the combined use with drugs that significantly inhibit or induce liver metabolism may be a factor that changes CBD pharmacokinetic properties.
Compared to the healthy adult group, the mean Tmax values in the patient group with reduced renal function were 2.0 to 2.5 h, with no significant difference between groups (p > 0.05). Compared to the healthy adult group, the mean values of Cmax, AUCall, and AUCinf in the patient group with impaired renal function were slightly increased, but the magnitude was not significant (p > 0.05). And above all, as the degree of renal function decline increases, there has been no confirmed trend in which CBD exposure in plasma increases or decreases. Compared to the healthy adult group, the mean T1/2 and CL in plasma in the patient group with impaired renal function were slightly increased and decreased, respectively, but the magnitudes were not significant (p > 0.05). As with Cmax, AUCall, and AUCinf, certain trends were not identified in which CBD loss from plasma increases or decreases as the degree of renal function decline increases. As a result, it was implied that changes in CBD pharmacokinetic parameter values depending on renal function would not be significant. The relatively small influence of renal function on CBD pharmacokinetics may be related to the rapid and extensive metabolism in the liver following body exposure to CBD, and subsequent excretion through the kidneys mainly in the form of metabolites. Under the assumption that the pharmacological activity of CBD metabolites is largely lower than that of the parent form and that side effects are negligible, consideration of renal function in the clinical application of CBD will not be as primary and important as liver function. To date, studies on the pharmacological activity of CBD metabolites are very lacking, and in the future, integrated pharmacokinetic analysis and pharmacological studies focusing on metabolites will need to be performed.
Effect of route of exposure on CBD pharmacokinetics
Reported possible routes of CBD exposure in humans vary, including oral, transdermal, inhalation, and intravenous. This may be related to the fact that CBD is used not only for medical and therapeutic purposes but also for recreational purposes, and that there are no clear scientific dosing regimens and standardized administration guides. Depending on the route of exposure, CBD pharmacokinetic properties may be significantly affected, resulting in variations in pharmacological effects. Therefore, comparing and confirming CBD pharmacokinetic properties according to exposure route will be very important in the development of future CBD formulations and in selecting the optimal administration route (to reach the target efficacy). Table 5 shows a summary of reported results (Ohlsson et al. 1986; Guy and Flint 2014; Guy and Robson 2014; Cherniakov et al. 2017; Swortwood et al. 2017; Atsmon et al. 2018a, b; Meyer et al. 2018; Bergeria et al. 2022; Sholler et al. 2022) to evaluate the effects of CBD exposure routes on CBD pharmacokinetics. During CBD inhalation exposure, the mean plasma Tmax values ranged from 0.05 to 3.18 h, showing relatively rapid absorption into the blood stream. The mean Tmax values in oral fluid during CBD inhalation exposure were all very low, within 0.29 h. Upon exposure to CBD through oral ingestion, the mean Tmax values in plasma ranged from 1 to 1.64 h, showing rapid absorption of CBD into the blood stream through the gastrointestinal tract. During the oral intake of CBD, the mean Tmax values in the oral fluid were low, from 0.47 to 3.30 h, suggesting that CBD is absorbed rapidly and extensively through the gastrointestinal tract. Upon transdermal exposure to CBD, mean plasma Tmax values were 1.63 to 3 h, indicating rapid absorption of CBD into the blood stream through the oral mucosa. Based on intra-study results of intravenous and inhaled CBD exposure, estimated CBD bioavailability by inhalation route was 15.16–29.09% (calculated based on the ratio of accessible AUCall values). The mean values of Cmax, AUCall, and AUCinf in plasma did not differ largely between oral, inhalation, and transdermal. The differences in mean Cmax, AUCall, and AUCinf values between oral and transdermal CBD exposure ranged from 1.01 to 4.2 times. The differences in mean Cmax, AUCall, and AUCinf values between oral and inhaled CBD exposure ranged from 1.34 to 1.43 times. The differences in mean Cmax, AUCall, and AUCinf values between inhalation and transdermal CBD exposures ranged from 1.25 to 3.68 times. The mean plasma elimination rate constants for oral, inhaled, and transdermal CBD exposures ranged from 0.1 to 0.98 1/h. Inhalation exposure showed large variability in CBD T1/2 in plasma, from 0.18 to 31 h, which was interpreted to be related to erratic CBD pharmacokinetic profiles in the elimination phase (related to variable patterns of CBD absorption by inhalation exposure route). On the other hand, the T1/2 values in plasma following transdermal and oral CBD exposures had relatively low variability, ranging from 1.09 to 3.21 h. The mean values of CL and V according to CBD venous exposure were high at 74.4 L/h and 2520 L, respectively, suggesting widespread distribution and significant elimination of CBD in the body. The mean Tmax values for urinary excretion following inhalation and oral CBD exposures ranged from 1.28 to 5.33 h, suggesting rapid excretion through the kidneys following CBD exposure.
Influence of formulation on CBD pharmacokinetics
Reported CBD formulations for human application are diverse, ranging from simple lipid droplets and solutions to nanoformulations. As mentioned earlier, this may be related to the various possible routes of CBD exposure in the humans, including oral, transdermal, inhalation, and intravenous. It may also be related to the fact that CBD’s high lipophilic property limits its absorption and solubilization in the body, and many formulation attempts (focusing on improving bioavailability) are continuing to overcome these limitations. As with other chemical drugs, even at the same dose and exposure route, the pharmacokinetic properties of CBD can vary greatly depending on the formulation. Therefore, comparing and confirming CBD pharmacokinetic properties according to formulations for each exposure route will be a very important reference for commercialization and standardization research of CBD formulations. Table 6 shows a summary of reported results (Guy and Flint 2014; Swortwood et al. 2017; Knaub et al. 2019; Hobbs et al. 2020; Izgelov et al. 2020; De Prá et al. 2021; Hosseini et al. 2021; Williams et al. 2021; Abbotts et al. 2022; Bergeria et al. 2022; Berl et al. 2022; Sholler et al. 2022) to evaluate the effects of CBD formulations on CBD pharmacokinetics. CBD inhalation exposure showed approximately 3.92 times lower plasma Tmax and 1.73–3.65 times higher Cmax and AUCall using nebulizer than aerosol. This suggests that using a nebulizer for CBD inhalation exposure can result in rapid body absorption and high bioavailability. Regarding CBD inhalation exposure through smoking cigarettes and vaporization, the Tmax values in oral fluid were the same, but the mean Cmax values tended to be higher for smoking cigarettes. This was interpreted to be related to the increase in CBD concentration in oral fluid due to various concomitants contained in smoking cigarettes. In CBD transdermal exposure, CBD Tmax in plasma was the same between wafer (simple drug carrier matrix with amorphous holes to encapsulate CBD) and spray formulations at 4.5 h, and the Cmax, AUCall, and AUCinf values were all within 1.98 times and there was no large difference. In terms of CL, the mean difference between wafer and spray formulations was small at 1.08 times. In CBD transdermal exposure, the CBD Tmax, Cmax, AUCall, and AUCinf values in plasma between the wafer and the lipid droplet (phytocomplex extract as a solution in medium-chain triglyceride oil) were all within 1.27 times, with no large difference. In terms of CL, the mean difference between wafer and lipid droplet formulations was small at 1.12 times, and no significant difference was identified (p > 0.05). The results suggest that differences in CBD pharmacokinetics between wafer, spray, and lipid droplet formulations for CBD transdermal exposure are not large. The lack of significant differences in CBD pharmacokinetic results between transdermal exposure formulations may be related to the physiological environment of the oral mucosa. That is, the oral mucosa may not have acted as a major rate-limiting step in CBD absorption (even if there were differences in formulation) due to its relatively high permeability to fat-soluble substances and its abundant vascular and lymphatic tissues. In oral exposure to CBD, the mean Tmax was relatively lower in nanoemulsion than in simple solution, powder, syrup, or capsule, and Cmax, AUCall, and AUCinf were higher. This may be related to the fact that CBD, which is fat-soluble, is well emulsified in nano size, making it easily absorbed into the body through the gastrointestinal tract upon oral exposure. When lipid emulsification progressed relatively well in CBD oral exposure formulations, the mean Tmax was low and Cmax, AUCall, and AUCinf were increased. This suggested that the solubilization process of highly fat-soluble CBD is very important for systemic absorption of CBD through the gastrointestinal tract. Interestingly, differences in the absorption of CBD through the gastrointestinal tract according to its lipid component were identified. Lipid droplets composed of glyceryl monolinoleate (GML) had Tmax 2.68 times lower and Cmax and AUCall 1.30–1.85 times higher than lipid droplets composed of medium-chain triglycerides (MCT). This may be related to the fact that GML, with its partially hydrated long fatty acid chains, worked more effectively in emulsifying CBD than MCT, which is composed of relatively short- and medium-length fatty acid chains. Therefore, the types and content ratios of lipids and surfactants used in CBD formulation may have a significant impact on CBD pharmacokinetics, so the development of a standardized oral dosage form is urgently needed.
Dose linearity in CBD pharmacokinetics
CBD’s reported human application formulations and possible routes of exposure are diverse. And CBD still lacks clearly standardized formulations and dosage regimen settings. Therefore, CBD exposure doses to the human are not standardized and are being attempted in a variety of arbitrary ways. Comparison of pharmacokinetic parameters according to CBD exposure dose will be a very important reference for future dose extrapolation and development of new dosages by determining whether CBD has dose linearity. Table 7 shows a summary of reported results (Karschner et al. 2011; Stott et al. 2013b; Manini et al. 2015; Atsmon et al. 2018b; Devinsky et al. 2018; Schoedel et al. 2018; Taylor et al. 2018; Patrician et al. 2019; Perkins et al. 2020; Hosseini et al. 2021; Vitetta et al. 2021; Hansen et al. 2024) for performing comparison of parameter values across CBD exposure doses in CBD pharmacokinetics. In the case of transdermal application of CBD, the spray formulation showed no large change in Tmax mean values of 1.0–4.0 h regardless of CBD dosage, and no trends in increase or decrease in CL and V were confirmed. In common, Cmax, AUCall, and AUCinf values increased as CBD dose increased. However, the increases were not exactly proportional to administered doses and were somewhat irregular. In the transdermal application of CBD, wafer and nanoparticle formulations had no significant difference in Tmax between CBD exposure doses (p > 0.05), similar to the spray formulation, and the mean values of Cmax, AUCall, and AUCinf increased as the dose increased. However, the increases were not exactly proportional to the dose of CBD exposure. As a result, the pharmacokinetics of CBD transdermal formulations could be interpreted as there is relatively no significant change in plasma CBD absorption and elimination rates as CBD dose increases, but exposure levels increase in a pattern that is not directly proportional. In the case of oral CBD application, there was no large difference in plasma Tmax and T1/2 between CBD exposure doses in the capsule formulation, and the mean Cmax, AUCall, and AUCinf values increased as CBD exposure dose increased. However, the degree of increase was not exactly proportional to the dose, as in transdermal formulations, and was irregular. When applying CBD orally, the solution formulations showed no large change in Tmax mean values of 3.67–6.13 h regardless of CBD doses, and no significant differences between dose groups were identified in CL and V (p > 0.05). On the other hand, the mean values of Cmax, AUCall, and AUCinf increased as the CBD dose increased, and the degree of increase was not exactly proportional to the CBD exposure dose. As a result, the pharmacokinetics of CBD transdermal as well as oral formulations could be interpreted as having pharmacokinetic properties close to linear kinetics. In the future, along with the development of standardized CBD formulations and by conducting prospective large-scale clinical studies under tight control conditions of other factors excluding CBD exposure dose, the dose-linear pharmacokinetic properties of CBD will be able to be confirmed more clearly.
Impact of cannabis smoking pattern on CBD pharmacokinetics
Table 8 shows a summary of reported results (Desrosiers et al. 2014; Newmeyer et al. 2014; Swortwood et al. 2017) to assess the effects of cannabis smoking pattern on CBD pharmacokinetics. As demographic information, a common tendency was found to differ in CBD pharmacokinetics between frequent and occasional smoker groups, which differed in the frequency of smoking exposure to cannabis cigarettes before the clinical trial. That is, when exposed to the same dose of CBD through oral and inhalation, the mean values of Cmax and AUC in sample matrices (plasma and oral fluid) were all increased in the frequent smoker group compared to the occasional smoker group. The relative increase in CBD exposure in the frequent smoker group compared to the occasional smoker group may be related to the improvement of CBD transit in the body due to the periodic use of cannabis cigarettes. It may also be related to the inhibition of CBD metabolic enzymes and exporting pumps in the body due to the periodic use of cannabis cigarettes. Since cannabis cigarettes are mixed with various concomitants in addition to CBD and THC, the possibility that the pharmacokinetic effects are caused by ingredients other than CBD cannot be ruled out. In order to clarify the causal effects of cannabis cigarette smoking frequency on CBD pharmacokinetics, future studies focusing on quantitative analysis of genetic expression according to frequency of exposure to cannabis cigarettes will need to be conducted. Based on this retrospective analysis study, the level of exposure to CBD in the patient group with a relatively high frequency of smoking cannabis cigarettes will increase, so demographic information (such as smoking patterns) will need to be considered when setting CBD dosage.
Effect of multiple exposures on CBD pharmacokinetics
Table 9 shows a summary of reported results (Stott et al. 2013b; Hosseini et al. 2021) to evaluate the effects of CBD multiple exposures on CBD pharmacokinetics. No large differences were identified in the overall pharmacokinetic results of single and multiple exposures to CBD in a transdermal spray formulation. The mean Tmax values for single and multiple exposures to spray CBD formulation were 1.00–2.00 h, with no significant changes (p > 0.05) depending on the frequency of CBD exposure. Although the mean values of Cmax and AUCall increased by up to 1.96 times during multiple exposures to CBD compared to a single exposure, the increase was not large in terms of statistical significance. Similar to the spray formulation, no large differences were found in the overall pharmacokinetic results of single and multiple exposures to CBD in the transdermal wafer formulation. In other words, the mean values of Tmax, Cmax, and AUCall during single and multiple exposures to CBD in wafer formulations did not correspond to significant differences (p > 0.05) between exposure frequency groups. Even in the estimated CL values, the differences between CBD single and multiple exposure groups were not significant (p > 0.05), with differences within 2.43%. As a result, it was determined that there would be no large changes in pharmacokinetic properties (such as changes in body accumulation, metabolism, and absorption rates) during multiple exposures to CBD compared to single exposures. This was consistent with the linear kinetics of CBD pharmacokinetic properties previously estimated.
Exploring the influence of other factors on CBD pharmacokinetics
Additional factors that may cause inter-individual pharmacokinetic variability of CBD include gender and age. This is because, despite exposure to the same dose of CBD, the distribution and absorption of CBD in the body may vary significantly due to physiological factors that have clear differences between genders. For example, CBD is a highly fat-soluble substance, so it would prefer to move into fatty tissues in the body. In women with a relatively high fat percentage, CBD distribution in the body may be widespread and elimination may occur slowly. Additionally, there will be clear differences in the degree of liver function (comprehensively considering enzyme expression and liver tissue development) depending on age, and as a result, the degree of metabolism according to exposure to CBD may change significantly. This could be reasonably inferred from the fact that CBD exposure in plasma was significantly increased in the group of patients with impaired liver function compared to the normal healthy group. Most clinical studies of CBD were conducted on mixed populations of men and women, so it was limited to clearly compare CBD pharmacokinetic results by gender in this study. In addition, because the ages of the CBD clinical research target groups were mostly mixed up to old age, it was limited to compare CBD pharmacokinetic results by categorizing each age group. In future CBD clinical studies, prospective trials considering gender and age will need to be conducted.
Conclusion
The main purpose of this study was to more clearly interpret and understand the pharmacokinetic properties of CBD through integrated data analysis due to the high variability in CBD pharmacokinetics between previously presented tests. This was because it was a very important preliminary work in accelerating the clinical application of CBD and access to precision medicine.
The nine key conclusions related to CBD human pharmacokinetics drawn through this study are as follows: (1) Diet immediately before or during CBD exposure will increase the body’s exposure to CBD; (2) THC will not have an antagonistic effect on the absorption of CBD into the body; (3) the choice of sample matrix will be important in the estimation of CBD pharmacokinetic parameter values; (4) regarding the diversity of CBD pharmacokinetics, the effects of changes in liver function are likely to be relatively more significant than those of renal function; (5) CBD can be rapidly absorbed into the body through oral, transdermal, and inhalation exposure routes; (6) lipid emulsions and nanoformulations of CBD will greatly improve CBD bioavailability; (7) the pharmacokinetics of CBD generally follow linear kinetic properties; (8) a history of frequent exposure to cannabis cigarette smoking may contribute to increased body exposure to CBD; (9) there will not be much change in pharmacokinetic properties according to multiple exposure to CBD compared to single exposure.
Based on the results of this human pharmacokinetics review, the following clinical approaches could be proposed to improve the effectiveness and minimize side effects of CBD. When administering CBD, the effect of diet needs to be considered diet can cause increases in Cmax and AUC. In patients with liver disease, the CL of CBD may be reduced and the AUC may be significantly increased, so the degree of liver function of the individual needs to be considered when setting the dosage regimen. Even with the same dose of CBD, there are significant differences in pharmacokinetics between formulations, so changing to formulations other than those previously applied in clinical practice needs to be carefully considered. Since a history of frequent exposure to cannabis cigarette smoking may be related to increased Cmax and AUC of CBD, the results of screening for past and current cannabis cigarette smoking in the patient group before applying CBD need to be considered in setting the dosage regimen.
This study suggests that careful consideration of multiple factors that may influence CBD pharmacokinetic diversity will be essential in clinical practice. In the future, based on the results of this study, consideration of various factors that may affect changes in CBD pharmacokinetics may be reflected in prospective clinical study design and formulation development processes.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CBD:
-
Cannabidiol
- THC:
-
Tetrahydrocannabinol
- CB:
-
Cannabinoid
- CNS:
-
Central nervous system
- CL:
-
Clearance
- GML:
-
Glyceryl monolinoleate
- MCT:
-
Medium-chain triglycerides
References
Abbotts KSS, Ewell TR, Butterklee HM, Bomar MC, Akagi N, Dooley GP, Bell C (2022) Cannabidiol and cannabidiol metabolites: pharmacokinetics, interaction with food, and influence on liver function. Nutrients 14:2152
Atsmon J, Cherniakov I, Izgelov D, Hoffman A, Domb AJ, Deutsch L, Deutsch F, Heffetz D, Sacks H (2018a) PTL401, a new formulation based on pro-nano dispersion technology, improves oral cannabinoids bioavailability in healthy volunteers. J Pharm Sci 107:1423–1429
Atsmon J, Heffetz D, Deutsch L, Deutsch F, Sacks H (2018b) Single-dose pharmacokinetics of oral cannabidiol following administration of PTL101: a new formulation based on gelatin matrix pellets technology. Clin Pharmacol Drug Dev 7:751–758
Bergeria CL, Spindle TR, Cone EJ, Sholler D, Goffi E, Mitchell JM, Winecker RE, Bigelow GE, Flegel R, Vandrey R (2022) Pharmacokinetic profile of ∆9-tetrahydrocannabinol, cannabidiol and metabolites in blood following vaporization and oral ingestion of cannabidiol products. J Anal Toxicol 46:583–591
Berl V, Hurd YL, Lipshutz BH, Roggen M, Mathur EJ, Evans M (2022) A randomized, triple-blind, comparator-controlled parallel study investigating the pharmacokinetics of cannabidiol and tetrahydrocannabinol in a novel delivery system, Solutech, in association with cannabis use history. Cannabis Cannabinoid Res 7:777–789
Busardò FP, Pérez-Acevedo AP, Pacifici R, Mannocchi G, Gottardi M, Papaseit E, Pérez-Mañá C, Martin S, Poyatos L, Pichini S (2021) Disposition of phytocannabinoids, their acidic precursors and their metabolites in biological matrices of healthy individuals treated with vaporized medical cannabis. Pharmaceuticals 14:59
Cherniakov I, Izgelov D, Barasch D, Davidson E, Domb AJ, Hoffman A (2017) Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J Control Release 266:1–7
Crockett J, Critchley D, Tayo B, Berwaerts J, Morrison G (2020) A phase 1, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia 61:267–277
De Prá MAA, Vardanega R, Loss CG (2021) Lipid-based formulations to increase cannabidiol bioavailability: in vitro digestion tests, pre-clinical assessment and clinical trial. Int J Pharm 609:121159
Desrosiers NA, Himes SK, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA (2014) Phase I and II cannabinoid disposition in blood and plasma of occasional and frequent smokers following controlled smoked cannabis. Clin Chem 60:631–643
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, Greenwood S, Morrison G, Sommerville K, Group GPAS (2018) Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 90:e1204–e1211
Devinsky O, Kraft K, Rusch L, Fein M, Leone-Bay A (2021) Improved bioavailability with dry powder cannabidiol Inhalation: a phase 1 clinical study. J Pharm Sci 110:3946–3952
Englund A, Oliver D, Chesney E, Chester L, Wilson J, Sovi S, De Micheli A, Hodsoll J, Fusar-Poli P, Strang J (2023) Does cannabidiol make cannabis safer? A randomised, double-blind, cross-over trial of cannabis with four different CBD: THC ratios. Neuropsychopharmacology 48:869–876
Golub V, Reddy DS (2021) Cannabidiol therapy for refractory epilepsy and seizure disorders. In: Murillo-Rodriguez E, Pandi-Perumal SR, Monti JM (eds) Cannabinoids and neuropsychiatric disorders. Springer International Publishing, Cham, pp 93–110
Guy GW, Flint ME (2004) A single centre, placebo-controlled, four period, crossover, tolerability study assessing, pharmacodynamic effects, pharmacokinetic characteristics and cognitive profiles of a single dose of three formulations of cannabis based medicine extracts (CBMEs) (GWPD9901), plus a two period tolerability study comparing pharmacodynamic effects and pharmacokinetic characteristics of a single dose of a cannabis based medicine extract given via two administration routes (GWPD9901 EXT). J Cannabis Ther 3:35–77. https://doi.org/10.1300/J175v03n03_03
Guy G, Robson P (2014) A Phase I, open label, four-way crossover study to compare the pharmacokinetic profiles of a single dose of 20 mg of a cannabis based medicine extract (CBME) administered on 3 different areas of the buccal mucosa and to investigate the pharmacokinetics of CBME per oral in healthy male and female volunteers (GWPK0112). Cannabis. Routledge, pp 79–120
Hansen JS, Boix F, Hasselstrøm JB, Sørensen Lambert K, Kjolby M, Gustavsen S, Hansen Rikke M, Petersen T, Sellebjerg F, Kasch H, Rasmussen PV, Finnerup NB, Sædder EA, Svendsen KB (2024) Pharmacokinetics and pharmacodynamics of cannabis-based medicine in a patient population included in a randomized, placebo-controlled, clinical trial. Clin Transl Sci 17:e13685
Hobbs JM, Vazquez AR, Remijan ND, Trotter RE, McMillan TV, Freedman KE, Wei Y, Woelfel KA, Arnold OR, Wolfe LM (2020) Evaluation of pharmacokinetics and acute anti-inflammatory potential of two oral cannabidiol preparations in healthy adults. Phytother Res 34:1696–1703
Hosseini A, McLachlan AJ, Lickliter JD (2021) A phase I trial of the safety, tolerability and pharmacokinetics of cannabidiol administered as single-dose oil solution and single and multiple doses of a sublingual wafer in healthy volunteers. Br J Clin Pharmacol 87:2070–2077
Howlett AC, Abood ME (2017) Chapter five - CB1 and CB2 receptor pharmacology. In: Kendall D, Alexander SPH (eds) Adv Pharmacol. Academic, pp 169–206
Izgelov D, Davidson E, Barasch D, Regev A, Domb AJ, Hoffman A (2020) Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. Eur J Pharm Biopharm 154:108–115
Johnson E, Kilgore M, Babalonis S (2022) Cannabidiol (CBD) product contamination: quantitative analysis of ∆9-tetrahydrocannabinol (∆9-THC) concentrations found in commercially available CBD products. Drug Alcohol Depend 237:109522
Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA (2011) Plasma cannabinoid pharmacokinetics following controlled oral ∆9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 57:66–75
Knaub K, Sartorius T, Dharsono T, Wacker R, Wilhelm M, Schön C (2019) A novel self-emulsifying drug delivery system (SEDDS) based on VESIsorb® formulation technology improving the oral bioavailability of cannabidiol in healthy subjects. Molecules 24:2967
Kumar P, Mahato DK, Kamle M, Borah R, Sharma B, Pandhi S, Tripathi V, Yadav HS, Devi S, Patil U, Xiao J, Mishra AK (2021) Pharmacological properties, therapeutic potential, and legal status of Cannabis sativa L.: an overview. Phytother Res 35:6010–6029
Lee D, Schwope DM, Milman G, Barnes AJ, Gorelick DA, Huestis MA (2012) Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clin Chem 58:748–756
Manini AF, Yiannoulos G, Bergamaschi MM, Hernandez S, Olmedo R, Barnes AJ, Winkel G, Sinha R, Jutras-Aswad D, Huestis MA, Hurd YL (2015) Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med 9:204–210
Meyer P, Langos M, Brenneisen R (2018) Human pharmacokinetics and adverse effects of pulmonary and intravenous THC-CBD formulations. Med Cannabis Cannabinoids 1:36–43
Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk E-M, Stadelmann AM (2005) Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of ∆9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit 27:799–810
Newmeyer MN, Desrosiers NA, Lee D, Mendu DR, Barnes AJ, Gorelick DA, Huestis MA (2014) Cannabinoid disposition in oral fluid after controlled cannabis smoking in frequent and occasional smokers. Drug Test Anal 6:1002–1010
Ohlsson A, Lindgren JE, Andersson S, Agurell S, Gillespie H, Hollister LE (1986) Single-dose kinetics of deuterium‐labelled cannabidiol in man after smoking and intravenous administration. Biomed Environ Mass Spectrom 13:77–83
Patrician A, Versic-Bratincevic M, Mijacika T, Banic I, Marendic M, Sutlović D, Dujić Ž, Ainslie PN (2019) Examination of a new delivery approach for oral cannabidiol in healthy subjects: a randomized, double-blinded, placebo-controlled pharmacokinetics study. Adv Ther 36:3196–3210
Pennypacker SD, Romero-Sandoval EA (2020) CBD and THC: do they complement each other like Yin and Yang? Pharmacotherapy 40:1152–1165
Pérez-Acevedo AP, Pacifici R, Mannocchi G, Gottardi M, Poyatos L, Papaseit E, Pérez‐Mañá C, Martin S, Busardò FP, Pichini S (2021) Disposition of cannabinoids and their metabolites in serum, oral fluid, sweat patch and urine from healthy individuals treated with pharmaceutical preparations of medical cannabis. Phytother Res 35:1646–1657
Perkins D, Butler J, Ong K, Nguyen T-H, Cox S, Francis B, Mcintosh M, Lilley B (2020) A phase 1, randomised, placebo-controlled, dose escalation study to investigate the safety, tolerability and pharmacokinetics of cannabidiol in fed healthy volunteers. Eur J Drug Metab Pharmacokinet 45:575–586
Pichini S, Mannocchi G, Gottardi M, Pérez-Acevedo AP, Poyatos L, Papaseit E, Pérez-Mañá C, Farré M, Pacifici R, Busardò FP (2020) Fast and sensitive UHPLC-MS/MS analysis of cannabinoids and their acid precursors in pharmaceutical preparations of medical cannabis and their metabolites in conventional and non-conventional biological matrices of treated individual. Talanta 209:120537
Radwan MM, Chandra S, Gul S, ElSohly MA (2021) Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 26:2774
Richter G, Hazzah T, Hartsel JA, Eades J, Hickory B, Makriyannis A (2021) Chap. 38 - Cannabis sativa: an overview. In: Gupta RC, Lall R, Srivastava A (eds) Nutraceuticals (Second Edition). Academic, pp 603–624
Sarkis-Onofre R, Catalá-López F, Aromataris E, Lockwood C (2021) How to properly use the PRISMA Statement. Syst Rev 10:1–3
Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, Etges T, Sommerville K (2018) Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav 88:162–171
Schwope DM, Karschner EL, Gorelick DA, Huestis MA (2011) Identification of recent cannabis use: whole-blood and plasma free and glucuronidated cannabinoid pharmacokinetics following controlled smoked cannabis administration. Clin Chem 57:1406–1414
Sholler DJ, Spindle TR, Cone EJ, Goffi E, Kuntz D, Mitchell JM, Winecker RE, Bigelow GE, Flegel RR, Vandrey R (2022) Urinary pharmacokinetic profile of cannabidiol (CBD), ∆9-tetrahydrocannabinol (THC) and their metabolites following oral and vaporized CBD and vaporized CBD-dominant cannabis administration. J Anal Toxicol 46:494–503
Stasiłowicz-Krzemień A, Szulc P, Cielecka-Piontek J (2023) Co-dispersion delivery systems with solubilizing carriers improving the solubility and permeability of cannabinoids (cannabidiol, cannabidiolic acid, and cannabichromene) from Cannabis sativa (Henola variety) inflorescences. Pharmaceutics 15:2280
Stott C, White L, Wright S, Wilbraham D, Guy G (2013a) A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur J Clin Pharmacol 69:825–834
Stott C, White L, Wright S, Wilbraham D, Guy G (2013b) A phase I study to assess the single and multiple dose pharmacokinetics of THC/CBD oromucosal spray. Eur J Clin Pharmacol 69:1135–1147
Swortwood MJ, Newmeyer MN, Andersson M, Abulseoud OA, Scheidweiler KB, Huestis MA (2017) Cannabinoid disposition in oral fluid after controlled smoked, vaporized, and oral cannabis administration. Drug Test Anal 9:905–915
Taylor L, Gidal B, Blakey G, Tayo B, Morrison G (2018) A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 32:1053–1067
Taylor L, Crockett J, Tayo B, Morrison G (2019) A phase 1, open-label, parallel‐group, single‐dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol 59:1110–1119
Tayo B, Taylor L, Sahebkar F, Morrison G (2020) A phase I, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet 59:747–755
Vitetta L, Butcher B, Henson JD, Rutolo D, Hall S (2021) A pilot safety, tolerability and pharmacokinetic study of an oro-buccal administered cannabidiol-dominant anti-inflammatory formulation in healthy individuals: a randomized placebo-controlled single-blinded study. Inflammopharmacology 29:1361–1370
Williams NNB, Ewell TR, Abbotts KSS, Harms KJ, Woelfel KA, Dooley GP, Weir TL, Bell C (2021) Comparison of five oral cannabidiol preparations in adult humans: pharmacokinetics, body composition, and heart rate variability. Pharmaceuticals 14:35
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2023-00245453).
Author information
Authors and Affiliations
Contributions
Ji-Hun Jang: Conceptualization, Investigation, Methodology, Funding acquisition, Formal analysis, Data curation, Software, Writing - Original draft preparation, Reviewing and Editing; Ju-Hwan Jeong: Investigation, Methodology, Data curation; Seung-Hyun Jeong: Conceptualization, Investigation, Methodology, Project administration, Funding acquisition, Formal analysis, Supervision, Data curation, Software, Writing - Original draft preparation, Reviewing and Editing. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Research involving human or animal participants
This article does not contain any studies with human and animal subjects performed by any of the authors.
Competing interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jang, JH., Jeong, JH. & Jeong, SH. Quantitative summary on the human pharmacokinetic properties of cannabidiol to accelerate scientific clinical application of cannabis. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03185-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03185-6