Abstract
Silibinin, or silybin, is a polyphenolic flavonoid and the main active component of silymarin, isolated from the seeds of the milk thistle plant (Silybum marianum). It has been shown to have antioxidant, antineoplastic, hepatoprotective, neuroprotective, anti-inflammatory, antimicrobial, and antidiabetic effects. In this systematic review, a literature search was conducted from inception until January 2024 on major electronic databases (PubMed, Scopus, Web of Science, and Google Scholar) to identify studies assessing the effects of silibinin on diabetes and its associated complications in different molecular, cellular, animal, and clinical studies. Silibinin has been shown to improve diabetic conditions through a variety of mechanisms, including reducing insulin resistance (IR), lowering reactive oxygen species (ROS) levels, and affecting glycolysis, gluconeogenesis, and glycogenolysis. Silibinin treatment reduced blood glucose (BG) levels, oxidative stress markers, and inflammatory cytokines while increasing glycosylated hemoglobin (HbA1C) and antioxidative marker levels in various cellular and animal models of diabetes. It also ameliorated levels of triglyceride (TG), cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL). Furthermore, silibinin has been identified as an effective treatment for diabetic complications, including hepatic damage, endothelial dysfunction, neuropathy, nephropathy, retinopathy, and osteoporosis. The promising anti-inflammatory, antioxidant, antidiabetic, and insulin-sensitizing activities of silibinin were also supported in clinical studies. The administration of silibinin could possess multiple protective impacts in improving DM and its complications. Nevertheless, further well-designed investigations are necessary to better understand its mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metabolic disorder of diabetes mellitus (DM), which is marked by hyperglycemia, is a rapid expansion global issue along with huge health, economic, and social consequences (Kaul et al. 2013). Based on a systematic analysis in 2021, almost 529 million people were living with DM worldwide. Noteworthy, total DM prevalence, particularly in the elderly, primarily represents type two diabetes mellitus (T2DM), which accounted for 96% (95.1–96.8) of diabetes cases and 95.4% (94.9–95.9) of diabetes disability-adjusted life-years (DALYs) globally. It was estimated that more than 1.31 billion (1.22–1.39) people have DM by 2050 (Mirzaei et al. 2023; Ong et al. 2023). Accordingly, insulin resistance (IR) is the main pathogenic mechanism of T2DM. It is specified as a mitigated responsiveness of insulin-targeting tissues to physiological insulin levels (Ghadiri et al. 2021; Lee et al. 2022).
Chronic hyperglycemia is responsible for diabetic complications, including retinopathy, nephropathy, neuropathy, periodontal disease, and impairment of the cardiovascular system (Kaul et al. 2013; Akhlaghipour et al. 2023). Diabetic liver damage is associated with ischemia/reperfusion mechanism as well as increased oxidative stress and apoptosis (Wang et al. 2022a). Moreover, there is a substantial association between IR and non-alcoholic fatty liver disease (NAFLD), as confirmed by a five times greater prevalence of NAFLD in individuals with T2DM in comparison to those without T2DM (Fujii et al. 2020; Roohbakhsh et al. 2020).
Diabetic osteoporosis (DO), a primary reason for fragility fractures in diabetic patients, is a chronic musculoskeletal complication associated with diabetes DM. Based on current studies, oxidative stress induced by high glucose levels may be correlated with DO pathogenesis (Baradaran Rahimi et al. 2019; Ying et al. 2020). In addition, Shackelford and coworkers have supported the idea that induced oxidative stress due to high glucose could inhibit osteoblast differentiation (Shackelford et al. 2000). It has been shown that DM-induced endothelial dysfunction, leading to atherosclerosis, plaque formation, and thrombogenesis, is a key pathogenetic event in cardiovascular diseases (CVDs) (Gholoobi et al. 2021). Accordingly, individuals with diabetes have an elevated risk of cardiovascular events, poor outcomes associated with CVD, and an increased mortality rate (Nesto 2004; Dastani et al. 2023).
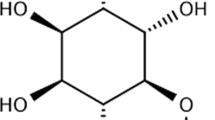
Among silymarin’s active ingredients is silibinin (silybin, Fig. 1), a flavonolignan originated from the milk thistle plant or Silybum marianum. In this regard, a wide range of therapeutic impacts have been demonstrated for silymarin, possibly due to its antioxidant and membrane-stabilizing activities (von Schönfeld et al. 1997), acting as an antineoplastic (Wing Ying Cheung et al. 2010), hepatoprotective (Detaille et al. 2008), neuroprotective (Marrazzo et al. 2011), anti-inflammatory (Aziz et al. 2014), antimicrobial agent (Rakelly de Oliveira et al. 2015), and anti-DM compound (Jain et al. 2016). Silibinin was reported to ameliorate diabetic conditions through several mechanisms, namely decreasing IR, ROS levels, glycolysis, gluconeogenesis, and glycogenolysis (Guigas et al. 2007; Bouderba et al. 2014). Additionally, silibinin was suggested to be beneficial for diabetic complications containing hepatic damage, endothelial dysfunction, neuropathy, nephropathy, retinopathy, and osteoporosis (Li Volti et al. 2011; Zhang et al. 2014; Ying et al. 2015). In this systematic review, we will assess the beneficial and harmful properties of silibinin on DM as well as DM-related complications. We will also summarize recent studies in this field regarding the pharmacological mechanisms engaged.
Methods
This systematic review was performed to answer the following issue: What are the potential positive and negative effects of silibinin on diabetes mellitus and its common complications?
Search strategy
From the beginning until January 2024, Scopus, PubMed, Web of Sciences, and Google Scholar were comprehensively searched. As a result, all English language-published studies were identified and included in the present research utilizing the subsequent keywords:
“Silibinin” OR “Silybin” AND “Diabetes” OR “Diabetic” OR “Diabetes Complications” OR “Complications of Diabetes Mellitus” OR “Diabetes Mellitus, Experimental” OR “Diabetes Mellitus, Lipoatrophic” OR “Fetal Macrosomia” OR “Diabetes Mellitus, Type 1” OR “Diabetes Mellitus, Type 2” OR “Diabetes, Gestational” OR “Diet, Diabetic” OR “Diabetic Angiopathies” OR “Diabetic Cardiomyopathies” OR “Diabetic Coma” OR “Diabetic Foot” OR “Diabetic Ketoacidosis” OR “Diabetic Nephropathies” OR “Diabetic Neuropathies” OR “Diabetic Retinopathy” OR “Donohue Syndrome” OR “Gastroparesis” OR “Glycation End Products, Advanced” OR “Advanced Glycation End Products” OR “Hyperglycemia” OR “Hyperglycemic Hyperosmolar Nonketotic Coma” OR “Latent Autoimmune Diabetes in Adults” OR “Insulin Resistance” OR “Prediabetic State” OR “Glucose intolerance” OR “Prediabetic.”
Criteria for selection
Studies meeting the following criteria were inserted: (1) original in vitro, in vivo, and clinical papers focusing on the impacts of silibinin on DM or its complications and (2) studies published in English. Furthermore, excluded studies had the following characteristics: (1) absence of original data; (2) inaccessible full texts, unpublished manuscripts or letters to the editor; (3) not written in English; and (4) not meeting the study’s inclusion criteria.
Extraction of data
The search strategy stated above was used by two authors (P.Z. and S.A.) to search databases. All duplicate papers were eliminated using EndNote 21, followed by additional manual screening. In the event that a paper did not meet our selection criteria, it was removed from the review. Figure 2 illustrates the steps of the search process. Finally, 45 articles were included in this review based on our selection criteria.
Results
The major mechanisms responsible for the antidiabetic impacts of silibinin are depicted in Fig. 3.
Diabetes mellitus: in vitro models
The promising effects of silibinin on different in vitro diabetes models are summarized in Table 1. Silibinin was found to inhibit gluconeogenesis and decrease glucose-6-phosphate (Glu-6-P) hydrolysis in a dose-dependent manner in hepatocytes of starved rats with sub-saturating concentrations of various exogenous substrates in a perifusion system. It affected Glu-6-P transport rather than the catalytic activity of the enzyme. Silibinin decreased the maximal velocity of Glu-6-P without significantly affecting its Km. It also decreased gluconeogenesis and glycogenolysis which are induced by glucagon stimulation. In addition, silibinin was shown to inhibit Glu-6-P in rat liver microsomes (Guigas et al. 2007). Similarly, Zhan et al. discovered that silibinin reduces both basal and insulin-stimulated glucose uptake in 3T3-L1 fibroblast cells by directly decreasing glucose binding to glucose transporter type 4 (GLUT4) since silibinin and glucose share the same binding site on GLUT4. However, silibinin did not inhibit the insulin-induced translocation of GLUT4 to the plasma membrane of 3T3-L1 cells (Zhan et al. 2011).
In another study, silibinin decreased glycolysis dose-dependently by inhibiting pyruvate kinase activity in a hepatic cell perifusion system. Also, it affected oxidative phosphorylation, proved by a decrease in adenosine triphosphate (ATP)/adenosine diphosphate (ADP) ratio while enhancing the lactate-to-pyruvate ratio. Silibinin reduced ROS production in mitochondria by inhibiting oxidative phosphorylation and affecting the electron transport chain activity in perifused rat hepatocytes (Detaille et al. 2008).
Silibinin also inhibited the formation of amyloid deposits of human islet amyloid polypeptide (hIAPP) and improved the survival of INS-1 pancreatic β cells. At molar concentrations of 0.5–5 of silibinin, fibrillization of hIAPP was inhibited by diminishing the toxic hIAPP oligomerization. Also, silibinin delayed the structural transition of hIAPP (Cheng et al. 2012). Another experiment investigated how silibinin may protect the SH-SY5Y human neuroblastoma cells treated with insulin fibrils from necrosis/apoptosis changes caused by insulin amyloid fibrils. Accordingly, silibinin concentration-dependently suppressed insulin fibrillation. Furthermore, silibinin attenuated insulin fibril-induced neuronal toxicity through increased cell viability and reduced lactate dehydrogenase (LDH), apoptotic cell death, and intracellular ROS. Additionally, insulin fibrillation products were ineffective in damaging rat brains’ mitochondria in the presence of silibinin (Katebi et al. 2018).
Another study noticed that silibinin (50–300 μM) suppressed gluconeogenesis in rats’ liver cells via reduced mitochondrial energy and pyruvate carrier inhibition. Inhibition of gluconeogenesis from lactate and pyruvate might be the probable cause of the inhibitory effect of silibinin on pyruvate carboxylation. Furthermore, silibinin strongly inhibited oxygen consumption in the liver as an indication of impaired mitochondrial ATP production, which is critical for gluconeogenesis. Additionally, silibinin was capable of oxidizing nicotinamide adenine dinucleotide hydrogen (NADH) in vitro following peroxidase and hydrogen peroxide (H2O2). Moreover, it inhibited the NADH supply necessary for mitochondria and gluconeogenesis in liver cells (Colturato et al. 2012).
Palomino and coworkers investigated the administration of S. marianum and its main component, silibinin, on EA.hy 926 following 30 mM glucose for 24 h. The results of silibinin administration (1, 5, or 10 µM) showed complete cell viability protection and recovery of reduced glutathione and antioxidant enzymes in only the highest dose of silibinin (Palomino et al. 2017).
Since inflammation-induced pancreatic β cell injury possesses a critical role in the progression of DM, another study discussed the impacts of silibinin on the physiological function and viability of the INS-1 cells treated with tumor necrosis factor-α (TNFα) or interleukin-1β (IL-1β). Silibinin pretreatment markedly increased INS-1 cell viability by upregulating phosphatidylinositol 3-kinase and protein kinase B (PI3K-Akt) signalings. In addition, pre-incubation with silibinin remarkably enhanced intracellular insulin levels. The study revealed that silibinin-induced autophagy via maintenance of expression of estrogen receptors alpha (ERα) and estrogen receptors beta (ERβ), as well as activation of either ERα or ERβ, has a protective role in cell survival. However, the mechanistic target of the rapamycin (mTOR) cascade, which is reported to inhibit autophagy, was kept inactivated by silibinin (Yang et al. 2018).
Extracellular matrix (ECM) components, including collagen fibrils and molecules, influence pancreatic cell proliferation and viability via stimulating collagen receptors, integrin, and others. Yang et al. determined that silibinin pretreatment (20 μM) enhanced the INS-1 cell proliferation on collagen I- and V-coated dishes. However, high silibinin concentrations (40–80 μM) prevented cell growth in both collagen-coated and not-coated dishes. On the other hand, no changes were observed in insulin secretion of cells on collagen I- or V-coating. Noteworthy, silibinin increased collagen I expression while decreasing collagen V expression on both collagen I- and V-coated dishes. Moreover, silibinin increased nuclear translocation of β-catenin on both collagen-type dishes, promoting proliferation (Yang et al. 2019).
Increased levels of free fatty acids (FFAs) induce mitochondrial stress, leading to apoptosis and β cell dysfunction. Silibinin therapy (10 μM for 48 h) attenuated cytotoxicity and apoptotic protein expression in palmitic acid-treated INS-1 cells. Also, palmitic acid-induced mitochondrial dysfunction was ameliorated by silibinin treatment. Results indicated that the mentioned effects of silibinin pretreatment correlate with the ERα upregulation (Sun et al. 2019).
Similarly, Chu and coworkers stated that silibinin (5 or 10 μM) upregulated ERα, nuclear factor erythroid 2-related factor 2 (Nrf2), and heme oxygenase-1 (HO-1) expression in pancreatic β cells. The protective effects of silibinin were diminished when ERα expression was silenced or when ERα antagonists were used. These results supported that silibinin positively impacts pancreatic β cells by activating ERα and Nrf2 antioxidative pathways. Moreover, silibinin showed a remarkable reduction of intracellular ROS production in INS-1 and NIT-1 cells in the presence of high glucose and palmitic acid. It also increased cell viability and insulin secretion in these cells (Chu et al. 2020).
Wang and colleagues showed that silibinin (50 μM) activated the antioxidant pathway in PA-exposed intestinal L cell line GLUTag cells by elevating the expression levels of Nrf2, HO-1, and SOD2, which are involved in antioxidant defense. Noteworthy, it reduced ROS production in L cells, leading to an improvement in cell survival and propagation in glucagon-like peptide-1 (GLP-1) production. The protective effect of silibinin was found to be mediated through ER, and blocking these receptors reversed the benefits of silibinin. Additionally, proteomic analysis revealed the different regulation of proteins by silibinin and palmitate, a fatty acid associated with cellular damage (Wang et al. 2022b).
Another experiment investigated the probable silibinin protection of GLUTag cells against palmitic acid (PA)-induced injury. PA caused apoptosis as well as endoplasmic reticulum stress in GLUTag cells, while silibinin (50 μM) reduced PA-induced lipotoxicity and endoplasmic reticulum stress (Shi et al. 2023).
The protective impact of silibinin was evaluated on rat INS-1 cells exposed to PA and high glucose (HG), which resembled the conditions of glucolipotoxicity in T2DM. Silibinin demonstrated a dose-dependent protection, with the most significant effect observed at a concentration of 140 μM. However, higher concentrations of silibinin restricted cell growth. Silibinin prevented cell loss and maintained the level of key proteins participating in the metabolism of glucose and fatty acid, namely GLUT4 and carnitine palmitoyltransferase 1 (CPT1). Moreover, silibinin improved ATP generation and mitochondrial function, prevented the loss of mitochondrial membrane potential (MMP), as well as reduced the ROS induced by PA and HG treatment. Silibinin protects INS-1 cells from ferroptosis induced by PA and HG treatment by preventing lipid peroxidation, iron accumulation, as well as downregulation of glutathione peroxidase 4 (GPX4), glutathione (GSH), ferroptosis suppressor protein 1 (FSP1), and cyclooxygenase-2 (COX-2) expression. It also enhanced mitophagy and increased PTEN-induced kinase 1 (PINK1) and parkin levels. Elimination of PINK1 attenuated the protective effects of silibinin (Du et al. 2023).
Liposomal silibinin improved glucose consumption and cell viability in hepatocytes stimulated with high glucose and PA. Additionally, it was observed that liposomal silibinin enhanced adenosine-monophosphate (AMP)-activated protein kinase (AMPK) phosphorylation while decreasing the transforming growth factor-beta 1 (TGF-β1), collagen I, collagen III, as well as α-smooth muscle actin (α-SMA) expression to a greater extent than free silibinin. These results suggested that liposomal silibinin may potentially ameliorate lipid and glucose metabolism through the AMPK/TGF-β1/Smad signaling cascade (Cai et al. 2023).
In a molecular study, silibinin (50, 75, 100, and 200 µg) reduced fructosamine levels in albumin in the presence of 75 mM glucose. It also decreased the absorbance of hyperchromic albumin, indicating the promising role of silibinin against glucose-stimulated glycation, possibly due to its strong binding to albumin. Also, the reduction of fluorescence intensity due to glucose-induced quenching was gradually reversed by silibinin addition. Moreover, silibinin increased protein stability by increasing α-helix content, which was found to decrease due to glycation. Additionally, glucose caused high carbonyl content and decreased free sulfhydryl groups in albumin. However, adding silibinin decreased carbonyl content and increased free sulfhydryl groups, indicating a potential antioxidative role of silibinin. Silibinin also mitigated the percentage of glycation of lysine and arginine residues, which were enhanced through glucose incubation (Neelofar et al. 2019).
Another study compared nanotized silibinin and its antioxidant, antidiabetic, and antiglycation properties with its bulk form through an in vitro approach. The results showed better antioxidant status in nanoformulation against 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azinobis (3-ethylbenzthiazoline-6-sulphonic acid) disodium salt (ABTS) radicals. Also, it exhibited more potent suppression of α-amylase and α-glucosidase in an antidiabetic assay. In addition, silibinin nanoparticles illustrated more attenuation of the fructosamine and human serum albumin (HSA)’s advanced glycation end products (AGEs), indicating a lower HSA glycation level than bulk form. Accordingly, the reduction of free lysine content during glycation was diminished by silibinin, and this effect was greater with the nano form. Both forms of silibinin decreased the oxidative damage in HSA dose-dependently, while the nanoparticle was more protective (Khalid and Naseem 2023).
Protective impacts of silibinin on insulin resistance (IR)
Von Schönfeld and colleagues evaluated the protective role of silibinin on the exocrine pancreas against cyclosporin A (CiA) toxicity. Silibinin therapy for 8 days (50–200 mg/kg/day, intraperitoneally (IP)) did not affect glucose concentration, CiA concentration, and body weight in male Wistar rats. However, an in vitro study on isolated perfused pancreas showed that treatment with silibinin can reduce insulin secretion. Also, the highest dose of silibinin restored amylase secretion to normal levels (von Schönfeld et al. 1997).
Treatment with silibinin (100 mg/kg/day; oral administration for eight weeks) diminished body weight, insulin, and glucose levels in diabetic Psammomys obesus diabetic rst. It also improved lipid profile and corrected IR in rats. Liver transaminases, namely aspartate transaminase (AST) and alanine transaminase (ALT), were mitigated after administration of silibinin. Histological evaluation revealed that silibinin therapy results in a significant alleviation of microvesicular steatosis. However, minor signs of initial cell degeneration were reported locally, containing some sinusoidal dilatation (Bouderba et al. 2014).
Similarly, silibinin improved the viability of pancreatic β cells by regulating the Insig-1/SREBP-1c cascade. Additionally, it stimulated the insulin secretion-related genes insulin receptor substrate 2 (IRS-2), pancreatic and duodenal homeobox 1 (PDX-1), as well as insulin mRNA expression while attenuating the uncoupling protein-2 (UCP-2) expression. Also, silibinin partially restored insulin secretion and inhibited lipid accumulation and FFA synthesis (Chen et al. 2014).
Another investigation showed the silibinin effects on PA-stimulated IR in myoblast C2C12 cells. They revealed that silibinin (16–100 μM/mL) inhibited the decrease of insulin-stimulated 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG) uptake as well as downregulation of GLUT4 translocation in C2C12 myotubes dose-dependently. It was achieved through downregulating the insulin receptor substrate 1(IRS-1)/phosphatidylinositol3-kinase(PI3K)/protein kinase B (Akt) pathway inhibition (Li et al. 2015).
Accordingly, silibinin (20–80 mg/kg for 28 days) protected the pancreas from oxidative stress-induced damage resulting through stimulating the antioxidant enzyme activity namely catalase and SOD, neutralizing ROS, and reducing oxidative stress. Additionally, silibinin enhanced the levels of endogenous antioxidants, such as glutathiones, contributing to the restoration of antioxidant defense in the pancreas. The study indicated that silibinin treatment significantly improves parameters in diabetic rats, including body weight, lipid profile, blood glucose levels, insulin, HbA1c, and IR index, namely homeostatic model assessment β cell function (HOMA β cell function). Although homeostatic model assessment IR (HOMA IR), another insulin resistance index, was reduced following silibinin treatment (Jain et al. 2016).
Diabetes mellitus: in vivo models
The antidiabetic impacts of silibinin against several animal models are reviewed in Table 2.
Silibinin against streptozocin (STZ)-induced DM
Silibinin (25–200 mg/kg/day for eight weeks) has been reported to suppress increased blood glucose, HbA1C, serum TG and cholesterol levels, and HDL in diabetic mice. Additionally, blood glucose level in the glucose tolerance test (GTT) was decreased after intraperitoneal glucose loading. Also, silibinin protected pancreatic β cells through silent information regulator 1 (Sirt-1) against STZ-induced injury. Moreover, silibinin attenuated the upregulation of apoptosis levels and STZ-induced activation of caspase-3 in pancreatic β cells (Wang et al. 2012).
As a solution for the therapeutic inadequacy of silibinin, a nanoparticle form of silibinin in biocompatible polymers (CSbnp) was designed and compared to silibinin treatment alone (SbT). CSbnp treatment illustrated a highly significant downregulation of FBS, cholesterol, TG, AST, ALT, and ALP in STZ-induced diabetes in Wistar rats compared to only the SbT group. In addition, CSbnpT demonstrated a statistical reduction of MDA, AGEs, and HbA1c. Nanoparticle treatment played a more considerable role in the antioxidant enzyme improvement (SOD, GSH, and catalase) and glycogen content of the liver than only silibinin treatment. Serum insulin and body weight were significantly increased by CSbnp in comparison to SbT (Das et al. 2014).
Furthermore, histopathological analysis of pancreatic tissue specimens in a CSbnpT group showed significant improvement in structural integrity and the number of Langerhans’ islet cells. On the other hand, the SbT group ameliorated the histoarchitecture of islet cells. In liver histopathological analysis, CSbnp decreased fat droplets and sinusoidal abnormality, as well as silybin. However, the revival of cytoarchitecture was better in the CSbnpT group (Das et al. 2014).
Silibinin has shown the ability to restore the structure and function of pancreatic beta cells, mitochondria, and nuclei, demonstrating its potential antidiabetic effects in the pancreas of STZ-diabetic rats. Silibinin also exhibited antioxidant properties by increasing SOD activity, reducing mitochondrial ROS production. It also protected the cellular structures involved in insulin synthesis and secretion, including the Golgi apparatus, endoplasmic reticulum, and secretory granules. Additionally, silibinin prevented collagen deposition in β cells and improved mitochondrial function (El-Far et al. 2016).
Another study revealed that silibinin treatment (100 mg/kg/day) for 4 weeks mitigated fasting blood glucose (FBS) while increasing serum insulin levels in high-fat diet (HFD)/STZ-induced type two diabetic SD rats. Additionally, silibinin administration alleviated the TNF-α and IL-1β serum levels while elevating the expression of ERα, Nrf2, and HO-1 in pancreatic β cells. Furthermore, silibinin significantly increased the number of pancreatic β cells and ERα-positive pancreatic β cell percentages in diabetic pancreatic islets (Chu et al. 2020).
Toğay and coworkers evaluated DNA damage in rats with STZ-induced diabetes and the potential protective impacts of silibinin (100 and 200 mg/kg for 4 weeks). They showed that diabetes-enhanced DNA injury is caused by oxidative stress. However, silibinin reduced DNA damage level to that of the control group, suggesting a protective impact of silibinin treatment against DNA injury (Toğay et al. 2018).
In another study, silibinin was loaded into liposomes to improve its bioavailability. In rats with type two diabetes mellitus complicated with non-alcoholic fatty liver disease (T2DM-NAFLD), liposomal silibinin was compared to metformin (MET) as a therapeutic agent. Liposomal silibinin treatment (70 mg/kg/day; oral administration for 4 weeks) propagated body weight, reduced fasting blood glucose levels, improved sugar tolerance and insulin sensitivity compared to silibinin alone, and was more effective than silibinin in improving insulin resistance in T2DM-NAFLD rats. In addition, liposomal silibinin significantly ameliorated serum lipid disorder while inhibiting ALT and AST protease activity and levels of pro-inflammatory cytokines. It also rescued histopathological impairments, such as lipid accumulation, inflammation infiltration, fiber deposition, and impaired hepatic lobular structure. The protective impacts of liposomal silibinin on lipid metabolism and liver function were more significant than those of free silibinin (Cai et al. 2023).
Protective effect of silibinin against SHRSP.Z-Leprfa/Izm-Dmcr (SP•ZF) diabetic rat
Xu et al. noticed that silibinin (100 and 300 mg/kg/day; by gastric gavage) strikingly reduces FBS and body weight gain in SHRSP.Z-Leprfa/Izm-Dmcr (SP•ZF) diabetic rats without inducing hepatotoxicity or nephrotoxicity. Glycogen synthase kinase 3 β (GSK3β), as the factor suppressing glycogen synthesis, was remarkably decreased with both doses of silibinin treatment. Moreover, silibinin therapy exhibited increased glycogen deposition in the liver, skeletal muscle, and peripheral tissue. A high dose of silibinin reduced gluconeogenesis by a notable decrease in G6Pase and phosphoenolpyruvate carboxykinase (PEPCK) expression (Xu et al. 2018).
They also showed lower duodenal expression of glucagon-like peptide-1 receptor (GLP1R) as an upstream marker in the gut-brain-liver axis by administration of silibinin in diabetic SP•ZF rats. Although expression of phospho-T197 PKA (p-PKA), which is important in triggering the axis, was unchanged after silibinin administration. The correlation of the brain and duodenum through the afferent intestinal vagus possibly caused the activation of the nucleus of the solitary tract (NTS) neurons after silibinin treatment. In addition, hepatic vagotomy in HFD/STZ-stimulated diabetic SD rats reversed the hypoglycemic effect of silibinin and confirmed the role of the gut-brain-liver axis after silibinin treatment (Xu et al. 2018).
Hyperglycemia-induced diabetes in zebrafish
It was stated that there is an increase in villus height in the intestine after birth, which is closely linked to the capacity for intestinal absorption in diabetes. Mohammadi et al. observed that the zebrafish receiving silibinin (10 drops, 10 mM concentration) demonstrated higher villus diameter and width in the intact intestine of diabetic zebrafish compared to other groups. Silibinin plays a role in stimulating blood supply by the healing of intestinal mucosa and restoring mucosal adhesion to its previous state. Furthermore, the group treated with glucose-containing silibinin showed the highest muscle diameter and number of Passgoblet cells, which was significantly different from other groups (Mohammadi et al. 2020).
The results also revealed that the group treated with silibinin had lower blood glucose levels compared to the glucose-containing control. Additionally, essential enzymes for fish health, such as AST, ALT, and alkaline phosphatase (ALP), decreased in the silibinin than in the glucose-containing control group. Moreover, silibinin treatment exhibited the lowest expression rates of the interferon-gamma (IFN-γ) and TNF-α genes, which play a significant role in IR (Mohammadi et al. 2020).
Protective effects of silibinin against DM complications
These promising protective effects of silibinin against various DM complications are summarized in Table 3 and Fig. 4.
DM induced hepatic damage
Wang and colleagues investigated the effect of silibinin (50 mg/kg/day; IP for 4 weeks) alone and in combination with ginsenoside compound-Mc1 (GCM) (10 mg/kg/day, IP for 4 weeks) on the liver of diabetic aged rat underwent ischemia–reperfusion. Accordingly, silibinin did not change plasma levels of AST, ALT, and apoptotic proteins. Conversely, combination treatment notably decreased the expression of Bax and cleaved-caspase 3 as apoptotic proteins. Histopathological findings revealed improvement of intercellular edema, inflammatory cell infiltration, sarcoplasmic vacuolation, and eccentric nucleus with silibinin. However, the changes were remarkably ameliorated when it was combined with GCM. ROS and 8-isoprostane as the indicators of oxidative stress were diminished by both treatments, although these reductions in the combination therapy were significantly more significant. Also, the combination treatment decreased manganese-superoxide dismutase (MnSOD) levels and cleaved-caspase 3 expressions and propagated the phospho-AMPK expression. Inhibition of AMPK reversed the effects, suggesting the probable role of the AMPK-dependent mechanism in the hepato-protective effect of combined treatment (Wang et al. 2022a).
DM induced endothelial dysfunction and vascular inflammation
Based on the results, silibinin (20 mg/kg/day; IP for 4 weeks) positively affects endothelial dysfunction and asymmetric dimethylarginine (ADMA) amounts in obese diabetic mice. ADMA is an endothelial nitric oxide synthase (eNOS) inhibitor that rises in some pathological conditions associated with endothelial dysfunction. Specifically, the administration of silibinin reduced both plasma and aorta ADMA levels and improved endothelial dysfunction. Overall, the study suggested that silibinin may be a promising candidate in order to prevent or medicate cardiovascular diseases in diabetic patients (Li Volti et al. 2011).
Autophagy has been defined as the removal of organelles and damaged proteins. Inadequate autophagy is a reason for endothelial cell dysfunction and delays in diabetic ulcer recovery. To understand the silibinin effects on autophagic responses, Rezabakhsh and coworkers treated human umbilical vein endothelial cells (HUVECs) with silibinin (0.1–400 µM) in high glucose conditions. Silibinin (10 µM) significantly elevated cell viability and decreased LDH level. Silibinin therapy notably reduced nitric oxide (NO) levels and restored levels of GSH in normal and high glucose concentrations. However, as an oxidative stress marker, the amount of malondialdehyde (MDA) was enhanced in both sets of conditions. Also, dramatic ROS generation under high glucose conditions was significantly diminished. Silibinin-primed cells showed increased endothelial cell migration capacity, indicating silibinin’s wound-healing properties. Treatment with silibinin also increased autophagolysosomes and autophagic proteins (Rezabakhsh et al. 2018).
Diabetic encephalopathy
The findings showed that HO-1, an antioxidant defense protein, was expressed differently in multiple brain regions in diabetic mice than in lean mice. In this regard, silibinin treatment (20 mg/kg/day; IP for 4 weeks) increased HO-1 levels in the forebrain and cerebellum but reduced it in the brainstem. They suggested silibinin may activate the HO system and provide neuroprotection in specific brain regions. Furthermore, silibinin treatment decreased isoprostanes as lipid peroxidation markers and 8-OH deoxyguanosine as a hallmark of DNA damage in the forebrain and cerebellum (Marrazzo et al. 2011).
Additionally, silibinin (50 and 100 mg/kg/day, orally) remarkably elevated the percentage of spontaneous alternation in STZ-induced diabetic SD rats. Moreover, it ameliorated STZ-stimulated impairment of image memory. Furthermore, silibinin (25, 50, and 100 mg/kg) ameliorated memory deficits and spatial learning ability. At the same time, it showed no notable impact on autonomous activities in diabetic rats (Liu et al. 2020).
Diabetic nephropathy
Khazim and colleagues revealed that silibinin (100 mg/kg/day; IP for 6 weeks) has a protective effect against podocyte injury caused by high glucose levels. Silibinin was shown to block the increase in SOD generation, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, NADPH oxidase 4 (Nox4) expression, and apoptotic cell numbers in podocytes exposed to high glucose. Silibinin-treated mice exhibited reduced Nox4 expression, superoxide production, and podocyte dropout than the control mice. Moreover, the mice treated with silibinin showed less evident albuminuria (Khazim et al. 2013).
Similarly, treatment with silibinin in diabetic rats (40 and 80 mg/kg/day; orally) led to improvements in kidney function, reduction in oxidative stress, and reversal of histopathological changes, including enlargement of the glomeruli and thickening of the glomerular basement membrane. Moreover, treatment with silibinin remarkably decreased the blood glucose levels, total cholesterol, TG, blood urea nitrogen (BUN), and creatinine, which were increased in diabetic rats. Additionally, urinary albumin excretion rate (UAER) as well as creatinine clearance (Ccr) was dose-dependently mitigated, and serum albumin and total protein amounts were improved towards normal (Jain and Somani 2015).
When silibinin was administered to diabetic mice (15 and 30 mg/kg/day orally for 10 weeks), it improved their diabetic condition. It was evidenced by decreased body weight, HbA1c levels, and serum insulin levels. Additionally, BUN, serum creatinine (SCR), and urinary albumin levels were improved as hallmarks of renal function. Moreover, silibinin decreased the signs of kidney injury, such as the glomerular mesangial expansion as well as the tubulointerstitial damage index. It also reduced oxidative stress in diabetic mice by decreasing MDA levels and increasing SOD and GSH levels. Results showed that silibinin alleviates diabetic nephropathy via the protein kinase B (AKT) signaling cascade, which is known to propagate cell proliferation along with apoptosis inhibition (Liu et al. 2019b).
Histopathological study on human renal glomerular endothelial cells (HRGECs) incubated with 50 μM silibinin revealed that silibinin may inhibit TGFβ1-stimulated fibrosis of cells through suppression of the nuclear factor-kappa B (NF-κB) pathway, resulted by markedly decrease in upregulation of fibronectin, collagen I, and α-SMA induced by TGF-β1. An in vivo study on HFD-stimulated renal fibrosis in mice models revealed a significant reduction of kidney weight/body weight and microalbuminuria with 50 mg/kg/day silibinin. In contrast, silibinin did not impact fasting glucose and body weight in HFD mice. Finally, results reported a notable reduction of fibronectin, collagen I, and p-p65 expression in mice kidneys through suppression of the NF-κB cascade (Liu et al. 2019a).
Diabetic retinopathy
According to a study using a diabetic rat model, the administration of silibinin effectively prevented the destruction of retinal capillaries, a key characteristic of diabetic retinopathy. Furthermore, silibinin treatment was found to decrease leukostasis and intercellular adhesion molecule-1 (ICAM-1) expression, a notable factor in the adhesion of leukocytes to retinal blood vessels. These findings supported that silibinin may have a preventive impact on diabetic retinopathy by reducing vascular damage (Zhang et al. 2014).
DM induced osteoporosis
High glucose levels have been shown to suppress the osteogenic differentiation of human bone marrow stromal cells (hBMSCs) by decreasing osteogenic marker expression and increasing oxidative damage markers. Yang et al. determined that silibinin (10 and 100 μM) counteracts the suppressive impacts of high glucose on osteogenic differentiation by reducing oxidative damage and activating the PI3K/Akt signaling cascade. It also enhanced the osteogenic marker expression and calcium levels, which are essential for bone development and skeletal remodeling (Ying et al. 2015).
Similarly, supplementation of silibinin in diabetic rats prevented bone loss and improved bone characteristics. The femur’s length, width, and height drastically increased in the group treated with 100 mg/kg/day of silibinin compared to the diabetic group. The femoral bone mineral density (BMD) also increased in the silibinin-treated group. Microarchitectural parameters also improved in the 100 mg silibinin-treated group. Furthermore, cancellous bones in the metaphyseal area were increased in the silibinin-treated diabetic group. Silibinin also suppressed serum osteocalcin levels. Finally, the study demonstrated a reduction in both oxidative and inflammatory stress with silibinin treatment (Wang et al. 2017).
AGEs have been reported to induce osteoblast apoptosis and have a crucial role in the development of diabetic osteoporosis. This mechanism is highly associated with the receptor of AGEs (RAGE). Moreover, mitochondrial abnormalities are also related to osteoblast dysfunction. Mao et al. examined the potential protective effect of silibinin against AGE-induced apoptosis in osteoblastic MC3T3-E1 cells. Treatment with silibinin at a concentration of 100 μM for 24 h dose-dependently increased the viability of MC3T3-E1 cells. Silibinin also directly reduced the RAGE expression. Silibinin modulated RAGE-mediated mitochondrial events by improving mitochondrial ROS generation, membrane potential, and ATP production. Eventually, the study supported the idea that silibinin protects osteoblastic cells from apoptosis through the RAGE-dependent mitochondrial pathway (Mao et al. 2018).
In a hyperglycemic environment, ALP and calcification capacity expression decreased in the MC3T3-E1 osteoblast precursor cell line. However, silibinin therapy can increase these values, as shown by the greater mineralized nodules, mineralized area, ALP activity, and ALP gray value. Furthermore, a hyperglycemic condition may attenuate the cell viability and forkhead transcription factor-1 (FoxO1), SIRT1, GPX1, and SOD2 expression in MC3T3-E1 cells while increasing human catalase (CAT) levels, which are reversed by silibinin treatment. The quantification of cell counting kit-8 (CCK-8), FoxO1, SIRT1, GPX1, SOD2, and CAT confirms these findings. Additionally, silibinin can reverse the inhibitory effects of a hyperglycemic condition on MC3T3-E1 cells by increasing SIRT1 and SOD2 expression while decreasing ROS levels. These results suggest that silibinin enhances cells’ ability to diminish oxidative stress through initiating the SIRT1/SOD2 cascade (Tao et al. 2022).
In addition, silibinin treatment (0.12 g, 0.25 mmol, silibinin-modified hydroxyapatite-coated titanium rods (SHA-TR) were inserted in the femoral cavity) significantly improved bone tissue contact and increased bone mass around titanium rods. Local therapy with silibinin also promoted bone tissue formation and improved SIRT1 and SOD2 protein expression in diabetic conditions. Moreover, silibinin treatment enhanced the stability of the titanium implant in the marrow cavity of diabetic rats and reversed the imbalance of bone metabolism and oxidative stress (Tao et al. 2022).
Clinical investigations
In addition to cellular and animal investigations, the antidiabetic properties of silibinin were also supported by clinical examinations (Table 4). Silibinin treatment (231 mg/day for 4 weeks) significantly reduced the elevated sorbitol levels in 14 patients with a diagnosis of non-insulin-dependent diabetes mellitus (NIDDM). Patients included five males and nine females, with an average age of 58.2 years and a duration of DM of more than 1 year (average 4.3 years). Patients continued to receive their routine medication for 1 week before hospitalization until their BG was brought under control. Although no remarkable change in blood glucose levels was observed, the treatment showed improved nerve conduction velocities and alleviated numbness and peripheral neuralgia in NIDDM patients (Zhang et al. 1995).
Another study was conducted on 30 patients with end-stage diabetic type 2 nephropathy (ESDN) undergoing dialysis treatment. Dietzmann et al. compared these patients to age-matched healthy controls. The flavonoid silibinin was administered intravenously as a single dose of 350 mg. The study observed that individuals with ESDN exhibit reduced levels of thiols in their peripheral blood mononuclear cells compared to healthy individuals. This deficiency in thiols is linked to impaired activation of T cells and an increase in the production of the pro-inflammatory cytokine TNF-α. However, the administration of flavonoids, including silibinin and silymarin, resulted in the restoration of thiol levels, improved activation of T cells, and decreased TNF-α release (Dietzmann et al. 2002).
Lieussi and coworkers conducted an investigation on the impacts of silibinin-β-cyclodextrin (IBI/S) treatment on 42 outpatients with chronic alcoholic liver disease and non-insulin-dependent diabetes mellitus (NIDDM) over a 6-month period. The participants were divided into two groups: receiving IBI/S (135 mg/day of silibinin orally) and receiving a placebo in a three-center, double-blind, randomized trial. The findings showed that patients who received IBI/S experienced notable mitigation in fasting blood glucose levels in comparison with those who received a placebo. The IBI/S group also showed positive changes in mean daily blood glucose levels, HbA1c, and HOMA-IR (which estimates insulin sensitivity), although these differences were not statistically significant. Furthermore, the IBI/S group had lower TG levels and decreased MDA as an oxidative stress index than the placebo group. However, neither group showed remarkable changes in cholesterol levels or liver function tests, and no side effects were reported (Lirussi et al. 2002).
Conclusion and future perspectives
Diabetes mellitus is a widespread metabolic disorder, with almost 529 million people living with it in 2021 and an estimated 1.31 billion by 2050. Diabetic complications include retinopathy, nephropathy, neuropathy, periodontal disease, and cardiovascular impairments, leading to increased mortality among individuals with diabetes. Silibinin is the major active component of silymarin, a polyphenolic flavonoid derived from the seeds of the milk thistle plant (S. marianum). Oxidative stress induced by free radicals is one of the primary processes for the development of diabetes.
Silibinin acts as an antioxidant by activating the antioxidant markers and propagating the expression of antioxidant enzymes, namely HO-1, SOD, GSH, and CAT. Silibinin, conversely, mitigates DM and its complications by attenuating the levels of IL-6, MDA, IL-1β, TNF-α, and other factors of oxidative stress and inflammation. Silibinin influences signaling pathways associated with carbohydrate and lipid metabolism. It inhibits gluconeogenesis and glycogenolysis in vivo and in vitro, resulting in reduced FBS and HbA1C levels. It also improves the amount of TG, cholesterol, LDL, and HDL.
On the other hand, silibinin has beneficial effects as a neuroprotective, renoprotective, hepatoprotective, anti-inflammatory, and antidiabetic agent. Furthermore, silibinin demonstrated significant effectiveness in both in vivo and in vitro experiments without notable adverse effects. The promising antioxidant, anti-inflammatory, antidiabetic, and insulin-sensitizing activities of silibinin were also supported in clinical studies. Overall, silibinin has demonstrated promising impacts in the treatment of diabetes and its complications. Noteworthy, further in vivo studies are necessary to understand silibinin’s mechanisms in diabetes and its related complications. Additionally, a comprehensive understanding of the metabolic pathway of silibinin post-oral administration requires essential pharmacokinetic studies. Moreover, additional human clinical trials are warranted to investigate silibinin’s pharmacological potential in diabetic individuals.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- 2-NBDG:
-
(2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose)
- ABTS:
-
2,2′-Azinobis (3-ethylbenzthiazoline-6-sulphonic acid) disodium salt
- ADMA:
-
Asymmetric dimethylarginine
- ADP:
-
Adenosine diphosphate
- AGEs:
-
Advanced glycation end products
- AKT:
-
Protein kinase B
- Akt:
-
Protein kinase B
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine transaminase
- AMPK:
-
Adenosine 5′-monophosphate (AMP)-activated protein kinase
- AST:
-
Aspartate transaminase
- ATP:
-
Adenosine triphosphate
- BG:
-
Blood glucose
- BMD:
-
Bone mineral density
- BUN:
-
Blood urea nitrogen
- CAT:
-
Catalase
- CCK-8:
-
Cell Counting Kit-8
- Ccr:
-
Creatinine clearance
- CiA:
-
Cyclosporin A
- COX-2:
-
Cyclooxygenase-2
- CPT1:
-
Carnitine palmitoyl transferase 1
- CSbnp:
-
Chitosan embossed silybin nanoparticles
- CSbnpT:
-
Chitosan embossed silybin nanoparticles treated
- DM:
-
Diabetes mellitus
- DO:
-
Diabetic osteoporosis
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ECM:
-
Extracellular matrix
- eNOS:
-
Endothelial nitric oxide synthase
- ER:
-
Estrogen receptors
- ESDN:
-
End-stage diabetic nephropathy
- FBS:
-
Fasting blood glucose
- FFA:
-
Free fatty acid
- FoxO1:
-
Forkhead transcription factor-1
- FSP1:
-
Ferroptosis suppressor protein 1
- G6Pase:
-
Glucose-6 phosphatase
- GCM:
-
Ginsenoside compound-Mc1
- GLP-1:
-
Glucagon-like peptide-1
- GLP1R:
-
Glucagon-like peptide-1 receptor
- GLUT4:
-
Glucose transporter type 4
- GPX:
-
Glutathione peroxidase
- GSH:
-
Glutathione
- GSK3β:
-
Glycogen synthase kinase 3 β
- GTT:
-
Glucose tolerance test
- H2O2 :
-
Hydrogen peroxide
- HAS:
-
Human serum albumin
- HbA1c:
-
Glycosylated hemoglobin
- hBMSCs:
-
Human bone marrow stromal cells
- HDL:
-
High-density lipoprotein
- HFD:
-
High-fat diet
- HG:
-
High glucose
- hIAPP:
-
Human islet amyloid polypeptide
- hIAPP:
-
Human islet amyloid polypeptide
- HO-1:
-
Heme oxygenase-1
- HOMA IR:
-
Homeostatic model assessment for insulin resistance
- HOMA β cell function:
-
Homeostatic model assessment β-cell function
- HRGECs:
-
Human renal glomerular endothelial cells
- HUVECs:
-
Human umbilical vein endothelial cells
- HUVECs:
-
Human umbilical vein endothelial cells
- IBI/S:
-
Silibinin-β-cyclodextrin
- ICAM-1:
-
Intercellular adhesion molecule-1
- IFN-γ:
-
Interferon-gamma
- IL-1β:
-
Interleukin-1β
- IP:
-
Intraperitoneal
- IRS:
-
Insulin receptor substrate
- LDH:
-
Lactate dehydrogenase
- MDA:
-
Malondialdehyde
- MET:
-
Metformin
- MMP:
-
Mitochondrial membrane potential
- MnSOD:
-
Manganese-superoxide dismutase
- mRNA:
-
Messenger RNA
- mTOR:
-
Mechanistic target of rapamycin
- NADH:
-
Nicotinamide adenine dinucleotide hydrogen
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NAFLD:
-
Non-alcoholic fatty liver disease
- NF-κB:
-
Nuclear factor kappa beta
- NIDDM:
-
Non-insulin dependent diabetes mellitus
- NO:
-
Nitric oxide
- Nox4:
-
NADPH oxidase 4
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NTS:
-
Nucleus of solitary tract
- PA:
-
Palmitic acid
- PDX-1:
-
Pancreatic and duodenal homeobox 1
- PEPCK:
-
Phosphoenolpyruvate carboxy kinase
- PI3K:
-
Phosphoinositide 3-kinase
- PINK1:
-
PTEN induced kinase1
- p-PKA:
-
Phospho-T197 PKA
- RAGE:
-
Receptor of AGEs
- ROS:
-
Reactive oxygen species
- SbT:
-
Silybin treated
- SCR:
-
Serum creatinine
- SD:
-
Sprague-Dawley
- SHA-TR:
-
Silibinin-modified hydroxyapatite-coated titanium rods
- Sirt-1:
-
Silent information regulator 1
- SOD:
-
Superoxide dismutase
- SP•ZF:
-
SHRSP.Z-Leprfa/Izm-Dmcr
- STZ:
-
Streptozotocin
- T2DM:
-
Type 2 diabetes mellitus
- TG:
-
Triglyceride
- TGF-β1:
-
Transforming growth factor beta 1
- TNFα:
-
Tumor necrosis factor-α
- UAER:
-
Urinary albumin excretion rate
- UCP-2:
-
Uncoupling protein-2
- α-SMA:
-
α-Smooth muscle actin
References
Akhlaghipour I, Nasimi Shad A, Askari VR, Maharati A, Baradaran Rahimi V (2023) How caffeic acid and its derivatives combat diabetes and its complications: a systematic review. Journal of Functional Foods 110:105862
Aziz TA, Marouf BH, Ahmed ZA, Hussain SA (2014) Anti-inflammatory activity of silibinin in animal models of chronic inflammation. Am J Pharmacol Sci 2:7–11
Baradaran Rahimi V, Askari VR, Hosseini M, Yousefsani BS, Sadeghnia HR (2019) Anticonvulsant activity of viola tricolor against seizures induced by pentylenetetrazol and maximal electroshock in mice. Iran J Med Sci 44:220–226
Bouderba S, Sanchez-Martin C, Villanueva GR, Detaille D, Koceïr EA (2014) Beneficial effects of silibinin against the progression of metabolic syndrome, increased oxidative stress, and liver steatosis in Psammomys obesus, a relevant animal model of human obesity and diabetes. J Diabetes 6:184–192
Cai JL, Zhu YL, Li XP, Deng GM, Han YS, Yuan FY, Yi GQ, Xia XH (2023) Liposomal Silybin improves glucose and lipid metabolisms in type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease via AMPK/TGF-β1/Smad signaling. Tohoku J Exp Med 261:257–265
Chen K, Zhao L, He H, Wan X, Wang F, Mo Z (2014) Silibinin protects β cells from glucotoxicity through regulation of the Insig-1/SREBP-1c pathway. Int J Mol Med 34:1073–1080
Cheng BA, Gong H, Li XC, Sun Y, Zhang X, Chen H, Liu XR, Zheng L, Huang K (2012) Silibinin inhibits the toxic aggregation of human islet amyloid polypeptide. Biochem Biophys Res Commun 419:495–499
Chu C, Gao X, Li X, Zhang XY, Ma RX, Jia Y, Li DH, Wang DK, Xu FX (2020) Involvement of estrogen receptor-alpha in the activation of Nrf2-antioxidative signaling pathways by silibinin in pancreatic beta-cells. Biomol Ther 28:163–171
Colturato CP, Constantin RP, Maeda AS Jr, Constantin RP, Yamamoto NS, Bracht A, Ishii-Iwamoto EL, Constantin J (2012) Metabolic effects of silibinin in the rat liver. Chem Biol Interact 195:119–132
Das S, Roy P, Pal R, Auddy RG, Chakraborti AS, Mukherjee A (2014) Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS ONE 9:e101818
Dastani M, Rahimi HR, Askari VR, Jaafari MR, Jarahi L, Yadollahi A, Rahimi VB (2023) Three months of combination therapy with nano-curcumin reduces the inflammation and lipoprotein (a) in type 2 diabetic patients with mild to moderate coronary artery disease: evidence of a randomized, double-blinded, placebo-controlled clinical trial. BioFactors 49:108–118
Detaille D, Sanchez C, Sanz N, Lopez-Novoa JM, Leverve X, El-Mir MY (2008) Interrelation between the inhibition of glycolytic flux by silibinin and the lowering of mitochondrial ROS production in perifused rat hepatocytes. Life Sci 82:1070–1076
Dietzmann J, Thiel U, Ansorge S, Neumann KH, Täger M (2002) Thiol-inducing and immunoregulatory effects of flavonoids in peripheral blood mononuclear cells from patients with end-stage diabetic nephropathy. Free Radic Biol Med 33:1347–1354
Du Q, Wu X, Ma K, Liu W, Liu P, Hayashi T, Mizuno K, Hattori S, Fujisaki H, Ikejima T (2023) Silibinin alleviates ferroptosis of rat islet β cell INS-1 induced by the treatment with palmitic acid and high glucose through enhancing PINK1/parkin-mediated mitophagy. Arch Biochem Biophys 743:109644
El-Far M, Negm AMR, El-Azim AA, Wahdan M (2016) Antioxidant therapeutic actions of medicinal phytochemicals, silymarin, and silibinin, on streptozotocin diabetic rats: first novel comparative assessment of structural recoveries of histological and ultrastructural changes on islets of langerhans, beta cells, mitochondria and nucleus. Int J Pharm Pharm Sci 8:69–76
Fujii H, Kawada N, Nafld JSGO (2020) The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci 21:3863
Ghadiri M, Baradaran Rahimi V, Moradi E, Hasanpour M, Clark CCT, Iranshahi M, Rakhshandeh H, Askari VR (2021) Standardised pomegranate peel extract lavage prevents postoperative peritoneal adhesion by regulating TGF-β and VEGF levels. Inflammopharmacology 29:855–868
Gholoobi A, Askari VR, Naghedinia H, Ahmadi M, Vakili V, Baradaran Rahimi V (2021) Colchicine effectively attenuates inflammatory biomarker high-sensitivity C-reactive protein (hs-CRP) in patients with non-ST-segment elevation myocardial infarction: a randomised, double-blind, placebo-controlled clinical trial. Inflammopharmacology 29:1379–1387
Guigas B, Naboulsi R, Villanueva GR, Taleux N, Lopez-Novoa JM, Leverve XM, El-Mir MY (2007) The flavonoid silibinin decreases glucose-6-phosphate hydrolysis in perifused rat hepatocytes by an inhibitory effect on glucose-6-phosphatase. Cell Physiol Biochem 20:925–934
Jain D, Somani R, Gilhotra R (2016) Silibinin ameliorates hyperglycaemia, hyperlipidemia and prevent oxidative stress in streptozotocin induced diabetes in Sprague Dawley rats. Int J Pharm Res All Sci 5:136–144
Jain D, Somani R (2015) Silibinin, a bioactive flavanone, prevents the progression of early diabetic nephropathy in experimental type-2 diabetic rats. Int J Green Pharm 9:118–124
Katebi B, Mahdavimehr M, Meratan AA, Ghasemi A, Nemat-Gorgani M (2018) Protective effects of silibinin on insulin amyloid fibrillation, cytotoxicity and mitochondrial membrane damage. Arch Biochem Biophys 659:22–32
Kaul K, Tarr JM, Ahmad SI, Kohner EM, Chibber R (2013) Introduction to diabetes mellitus. Diabetes: an old disease, a new insight, vol 771. pp 1–11
Khalid A, Naseem I (2023) Increased therapeutic effect of nanotized silibinin against glycation and diabetes: An in vitro and in silico-based approach. Biochim Biophys Acta - General Subj 1867:130364
Khazim K, Gorin Y, Cavaglieri RC, Abboud HE, Fanti P (2013) The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol - Renal Physiol 305:F691–F700
Lee S-H, Park S-Y, Choi CS (2022) Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J 46:15–37
Li H, Yang Y, Mo Z, Ding Y, Jiang W (2015) Silibinin improves palmitate-induced insulin resistance in C2C12 myotubes by attenuating IRS-1/PI3K/Akt pathway inhibition. Braz J Med Biol Res 48:440–446
Li Volti G, Salomone S, Sorrenti V, Mangiameli A, Urso V, Siarkos I, Galvano F, Salamone F (2011) Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc Diabetol 14:62
Lirussi F, Beccarello A, Zanette G, De Monte A, Donadon V, Velussi M, Crepaldi G (2002) Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr Metab 15:222–231
Liu K, Zhou S, Liu J, Wang Y, Zhu F, Liu M (2019a) Silibinin attenuates high-fat diet-induced renal fibrosis of diabetic nephropathy. Drug Des Dev Ther 13:3117–3126
Liu Y, Ye J, Cao Y, Zhang R, Wang Y, Zhang S, Dai W, Ye S (2019b) Silibinin ameliorates diabetic nephropathy via improving diabetic condition in the mice. Eur J Pharmacol 845:24–31
Liu PW, Cui LY, Liu B, Liu WW, Hayashi T, Mizuno K, Hattori S, Ushiki-Kaku Y, Onodera S, Ikejima T (2020) Silibinin ameliorates STZ-induced impairment of memory and learning by up- regulating insulin signaling pathway and attenuating apoptosis. Physiol Behav 213:112689
Mao YX, Cai WJ, Sun XY, Dai PP, Li XM, Wang Q, Huang XL, He B, Wang PP, Wu G, Ma JF, Huang SB (2018) RAGE-dependent mitochondria pathway: a novel target of silibinin against apoptosis of osteoblastic cells induced by advanced glycation end products article. Cell Death Dis 9:674
Marrazzo G, Bosco P, La Delia F, Scapagnini G, Di Giacomo C, Malaguarnera M, Galvano F, Nicolosi A, Volti GL (2011) Neuroprotective effect of silibinin in diabetic mice. Neurosci Lett 504:252–256
Mirzaei A, Mirzaei A, Najjar Khalilabad S, Askari VR, Baradaran Rahimi V (2023) Promising influences of hesperidin and hesperetin against diabetes and its complications: a systematic review of molecular, cellular, and metabolic effects. EXCLI J 22:1235–1263
Mohammadi H, Manouchehri H, Changizi R, Bootorabi F, Khorramizadeh MR (2020) Concurrent metformin and silibinin therapy in diabetes: assessments in zebrafish (Danio rerio) animal model. J Diabetes Metab Disord 19:1233–1244
Neelofar K, Arif Z, Ahmad J, Alam K (2019) Inhibitory effect of silibinin on Amadori-albumin in diabetes mellitus: a multi-spectroscopic and biochemical approach. Spectrochim Acta - Part a: Mol Biomol Spectrosc 209:217–222
Nesto RW (2004) Correlation between cardiovascular disease and diabetes mellitus: current concepts. Am J Med 116:11–22
Ong KL, Stafford LK, McLaughlin SA, Boyko EJ, Vollset SE, Smith AE, Dalton BE, Duprey J, Cruz JA, Hagins H (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402:203–234
Palomino OM, Gouveia NM, Ramos S, Martín MA, Goya L (2017) Protective effect of silybum marianum and silibinin on endothelial cells submitted to high glucose concentration. Planta Med 83:97–103
Rakelly de Oliveira D, Relison Tintino S, Morais Braga MFB, Boligon AA, Linde Athayde M, Douglas Melo Coutinho H, de Menezes IRA, Fachinetto R (2015) In vitro antimicrobial and modulatory activity of the natural products silymarin and silibinin. BioMed Res Int 2015:292797
Rezabakhsh A, Fathi F, Bagheri HS, Malekinejad H, Montaseri A, Rahbarghazi R, Garjani A (2018) Silibinin protects human endothelial cells from high glucose-induced injury by enhancing autophagic response. J Cell Biochem 119:8084–8094
Roohbakhsh Y, Baradaran Rahimi V, Silakhori S, Rajabi H, Rahmanian-Devin P, Samzadeh-Kermani A, Rakhshandeh H, Hasanpour M, Iranshahi M, Mousavi SH, Askari VR (2020) Evaluation of the effects of peritoneal lavage with Rosmarinus officinalis extract against the prevention of postsurgical-induced peritoneal adhesion. Planta Med 86:405–414
Shackelford RE, Kaufmann WK, Paules RS (2000) Oxidative stress and cell cycle checkpoint function. Free Radical Biol Med 28:1387–1404
Shi XY, Chu C, Li X, Zhang LX, Zhang XR, Chen N, Liu WW, Jiao ZX, Ikejima T, Xu FX (2023) Silibinin protects GLUTag cells from PA-induced injury via suppressing endoplasmic reticulum stress. Cyta-Journal of Food 21:442–450
Sun Y, Yang J, Liu WW, Yao GD, Xu FX, Hayashi T, Onodera S, Ikejima T (2019) Attenuating effect of silibinin on palmitic acid-induced apoptosis and mitochondrial dysfunction in pancreatic beta-cells is mediated by estrogen receptor alpha. Mol Cell Biochem 460:81–92
Tao ZS, Wang HS, Li TL, Wei S (2022) Silibinin-modified Hydroxyapatite coating promotes the osseointegration of titanium rods by activation SIRT1/SOD2 signaling pathway in diabetic rats. J Mater Sci: Mater Med 33(9):62
Toğay VA, Sevimli TS, Sevimli M, Çelik DA, Özçelik N (2018) DNA damage in rats with streptozotocin-induced diabetes; protective effect of silibinin. Mutat Res – Genet Toxicol Environ Mutagen 825:15–18
von Schönfeld J, Weisbrod B, Müller MK (1997) Silibinin, a plant extract with antioxidant and membrane stabilizing properties, protects exocrine pancreas from cyclosporin A toxicity. Cell Mol Life Sci 53:917–920
Wang Q, Liu M, Liu WW, Hao WB, Tashiro S, Onodera S, Ikejima T (2012) In vivo recovery effect of silibinin treatment on streptozotocin-induced diabetic mice is associated with the modulations of sirt-1 expression and autophagy in pancreatic beta-cell. J Asian Nat Prod Res 14:413–423
Wang T, Cai L, Wang Y, Wang Q, Lu D, Chen H, Ying X (2017) The protective effects of silibinin in the treatment of streptozotocin-induced diabetic osteoporosis in rats. Biomed Pharmacother 89:681–688
Wang H, Zhu J, Jia Z, Lu S (2022a) Pretreatment of diabetic aged rats with combination of ginsenoside-Mc1 and silibinin protects liver from ischemia-reperfusion injury through an AMPK-dependent mechanism. Turkish J Biochem 47:23–32
Wang J, Zhang L, Cao H, Shi X, Zhang X, Gao Z, Ikeda K, Yan T, Jia Y, Xu F (2022b) Silibinin improves L-cell mass and function through an estrogen receptor-mediated antioxidative mechanism. Phytomed 99:154022
Wing Ying Cheung C, Gibbons N, Wayne Johnson D, Lawrence Nicol D (2010) Silibinin-a promising new treatment for cancer. Anti-Cancer Agents Med Chem (Form Curr Med Chem-Anti-Cancer Agents) 10:186–195
Xu F, Yang J, Negishi H, Sun Y, Li D, Zhang X, Hayashi T, Gao M, Ikeda K, Ikejima T (2018) Silibinin decreases hepatic glucose production through the activation of gut-brain-liver axis in diabetic rats. Food Funct 9:4926–4935
Yang J, Sun Y, Xu FX, Liu WW, Hayashi T, Onodera S, Tashiro S, Ikejima T (2018) Involvement of estrogen receptors in silibinin protection of pancreatic beta-cells from TNF alpha- or IL-1 beta-induced cytotoxicity. Biomed Pharmacother 102:344–353
Yang J, Sun Y, Liu XL, Xu FX, Liu WW, Hayashi T, Imamura Y, Mizuno K, Hattori S, Tanaka K, Fujisaki H, Tashiro SI, Onodera S, Ikejima T (2019) Silibinin’s regulation of proliferation and collagen gene expressions of rat pancreatic beta-cells cultured on types I and V collagen involves beta-catenin nuclear translocation. Connect Tissue Res 60:463–476
Ying XZ, Chen XW, Liu HX, Nie PF, Shui XL, Shen Y, Yu KH, Cheng SW (2015) Silibinin alleviates high glucose-suppressed osteogenic differentiation of human bone marrow stromal cells via antioxidant effect and PI3K/Akt signaling. Eur J Pharmacol 765:394–401
Ying X, Chen X, Wang T, Zheng W, Chen L, Xu Y (2020) Possible osteoprotective effects of myricetin in STZ induced diabetic osteoporosis in rats. Eur J Pharmacol 866:172805
Zhan T, Digel M, Küch EM, Stremmel W, Füllekrug J (2011) Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J Cell Biochem 112:849–859
Zhang JQ, Xm Mao, Zhou YP (1995) Silybin decreases erythrocytic sorbitol level and improves peripheral nerve conduction velocity in patients with non-insulin dependent diabetes mellitus. Chin J Integr Tradition West Med 1:84–86
Zhang HT, Shi K, Baskota A, Zhou FL, Chen YX, Tian HM (2014) Silybin reduces obliterated retinal capillaries in experimental diabetic retinopathy in rats. Eur J Pharmacol 740:233–239
Acknowledgements
This study was supported by Mashhad University of Medical Sciences, Mashhad, Iran.
Author information
Authors and Affiliations
Contributions
P. Z. M.: investigation, resources, and writing—original draft; S. A.: investigation, resources, and writing—original draft; E. E.: investigation and writing—original draft; V. R. A.: conceptualization, supervision, and writing—review and editing; V. B. R.: conceptualization, project administration, supervision, and writing—review and editing. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethics approval
This is a review article. Ethical approval is not required for the study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Parisa Zare Mehrjerdi, Sara Asadi, and Elham Ehsani share the co-first authorships.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zare Mehrjerdi, P., Asadi, S., Ehsani, E. et al. Silibinin as a major component of milk thistle seed provides promising influences against diabetes and its complications: a systematic review. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03172-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03172-x