Abstract
This study aimed to investigate whether bergapten (BG), a furanocoumarin phytohormone, holds promise for Crohn’s disease (CD)-like colitis treatment and to preliminarily explore its potential mechanisms. 2,4,6-Trinitrobenzenesufonic acid (TNBS)-treated mice were applied to establish an in vivo research model, and BG was administered with different concentrations. The status of mice in each group was evaluated by disease activity index (DAI), and the severity was evaluated by pathological sections. The intestinal barrier was assessed by measuring in vivo intestinal permeability, peripheral blood intestinal fatty acid-binding protein (I-FABP) levels, epithelial resistance values, and tight junction protein levels. Markers were then used to assess Th17/Treg levels, mitophagy, and the peroxisome proliferator–activated receptor (PPAR)γ/ nuclear factor kappa B (NF-κB) signaling pathway. BG significantly reduced colon tissue damage in a concentration-dependent manner. DAI scores showed that the loose feces, occult blood, and weight loss of mice in the BG treatment were significantly reduced, and pathological section results revealed reduced inflammatory infiltration and fibrosis. Reduced serum FITC-dextran and I-FABP and increased levels of epithelial resistance and tight junction proteins support that the intestinal barrier was protected upon BG. The proportion of Th17 in mesenteric lymph nodes increased while Treg decreased in the model group. BG treatment effectively reduced the conversion of Treg to Th17. Additionally, BG was found to enhance mitophagy and activate the PPARγ/NF-κB signaling. BG demonstrates promising effects in ameliorating intestinal barrier damage and Th17/Treg imbalance in a murine model of CD-like colitis, while also promoting intracellular mitophagy. The PPARγ/NF-κB signaling pathway may serve as a key mediator of BG’s regulatory mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic, relapsing condition that primarily affects the intestines. Historically, CD was predominantly observed in Europe, but over the past decade, its incidence has been steadily rising in Asia (Ng et al. 2017). Due to its tendency for lifelong relapse, patients require long-term drug therapy to maintain intestinal inflammation at a manageable level (Roda et al. 2020). Current treatments for CD predominantly include glucocorticoids, immunosuppressants, and intestinal mucosal protective agents. However, these medications often exhibit varying degrees of drug resistance and adverse reactions. Despite the widespread adoption of biological agents such as tumor necrosis factor-α (TNF-α) monoclonal antibodies, the surgical intervention rate for CD has not significantly decreased (Cockburn et al. 2023). Therefore, there is an urgent need for the development of new drugs with low toxicity, minimal adverse reactions, and precise efficacy (Noor et al. 2020). Although the exact pathogenesis of CD remains unclear, dysfunction of the intestinal barrier due to defects in the structure and function of intestinal epithelial cells is considered a crucial factor in initiating or perpetuating chronic intestinal inflammation (Kaminsky et al. 2021).
Bergapten (BG), primarily derived from bergamot essential oil, is a furanocoumarin phytohormone found in various herbs and fruits, exhibiting anti-inflammatory properties. Studies have also demonstrated significant antioxidant and anti-cancer effects of BG (Quetglas-Llabrés et al. 2022). For instance, in mouse models of Escherichia coli–induced sepsis and Citrobacter-induced intestinal inflammation, BG inhibited the activation of the NOD-like receptor thermal protein domain–associated protein 3 (NLRP3) inflammasome and cellular pyroptosis by maintaining mitochondrial homeostasis, thereby significantly ameliorating tissue inflammation and damage (Luo et al. 2023). Additionally, in an isolated rabbit jejunum model, BG exhibited concentration-dependent relaxation effects on both spontaneous and potassium ion-induced contractions, indicating its potential as an anti-ulcer and anti-diarrheal agent with relative safety demonstrated in acute toxicity tests (Aslam et al. 2022). These findings underscore the potential of BG in the management of gut-related diseases through the modulation of mitochondrial function.
Furthermore, recent studies suggest that the development of CD may largely depend on the balance between Th17 cells and regulatory T cells (Tregs) (Gomez-Bris et al. 2023; Saez et al. 2021). Th17 cells primarily secrete pro-inflammatory factors such as interleukin (IL)-17, inducing neutrophil migration to the site of inflammation (Akhter et al. 2023). Single-cell analysis revealed a reduction in Tregs within tissues at sites of intestinal inflammation in severe CD patients, potentially exacerbating inflammation (Jaeger et al. 2021). BG has been shown to mitigate allergic rhinitis in mice exposed to PM2.5 by balancing Treg/Th17 expression (Jiang et al. 2023), suggesting its potential to modulate the course of CD by restoring Treg/Th17 balance, an area currently lacking in BG research in CD. Using the Super-PRED site, BG was found to target peroxisome proliferator-activated receptor (PPAR)γ, and previous studies have demonstrated that stimulating PPAR-γ expression alleviates inflammatory bowel disease (IBD) (Venkataraman et al. 2022).
Therefore, we hypothesize that BG may attenuate intestinal barrier damage to mitigate CD severity by regulating the PPARγ signaling pathway to promote mitophagy and balance Th17/Treg. This study utilized 2,4,6-trinitrobenzenesufonic acid (TNBS)-treated mice to establish an in vivo research model to investigate whether BG holds promise for CD treatment and to preliminarily explore its potential mechanisms.
Methods and materials
Animals
Male C57BL/6 mice (6–8 weeks, ~ 20 g) were purchased from Cavens-Biogle (Soochow, Jiangsu, China). All mice were housed in an SPF environment under controlled temperature (~ 22 °C) and humidity (~ 55%) with natural light cycles, with ad libitum access to food and water. All experimental procedures followed the National Institute of Health guidelines and were approved by the Animal Ethics Committee.
Grouping and treatment
TNBS-treated mice established preclinical models that mimic clinical CD as previously reported (Katsandegwaza et al. 2022). Mice were randomly divided into five groups (6 mice per group): Sham (saline), TNBS (model), and BG treatment (TNBS + 3, 10, and 30 mg/kg BG) groups. Briefly, after fasting overnight and anesthetizing with 2% isoflurane, saline or TNBS (50 mg/kg) dissolved in 0.5 ml 45% ethanol was carefully dripped into the colon through a flexible catheter. The mice remained inverted for 1 min to ensure contact with the intestinal mucosa. The experiment was repeated weekly for 4 weeks. BG treatment was started at the beginning of TNBS induction, and BG (dissolved in 0.5 ml of sterile water) was administered via intragastric infusion once a day for 4 weeks. The sham and model groups received sterile water via the same route. The disease activity index (DAI) based on fecal consistency, occult blood, and weight loss was scored according to a previous study (Yan et al. 2018).
Sample collection
Before the end of the handling procedure, mice were deprived of food and water for 4 h and given FITC-dextran (600 mg/kg) by gavage. Cardiac puncture and blood collection were performed under anesthesia, followed by euthanasia by cervical dislocation. FITC-dextran levels in serum were measured using a spectrophotometer (Molecular Devices, Shanghai, China). Intestinal tissue was collected, the length of the colon was measured, and then the samples were evenly divided to prepare lysates and tissue wax blocks for subsequent analysis.
Hematoxylin and eosin (H&E) staining
Colons flushed with ice-cold PBS were fixed in 4% paraformaldehyde and embedded in paraffin to prepare sections for histological evaluation. Sections cut into 4 µm thickness were treated with xylene and decreasing concentrations of alcohol. The sections were routinely stained with hematoxylin–eosin (HC0513, Fantawild Biotechnology, Beijing). Images were captured under a microscope (Olympus, Tokyo, Japan).
Masson staining
This assay was performed using a Masson staining kit (BL1538A, Biosharp, Hefei, China) according to the manufacturer’s instructions. Dewaxed and hydrated tissue sections were obtained as described above. Following the staining with Weigert’s iron hematoxylin for 5 min, samples were differentiated with acidic ethanol for 10 s, treated with the aniline blue solution for 1 min, and ponceau red magenta dyeing solution for 5 min. Subsequently, sections were washed with the phosphomolybdic acid solution for 1 min and counterstained with the aniline blue dyeing solution for 1 min. The results were observed after dehydration under a light microscope (Olympus, Tokyo, Japan).
ELISA
The blood was allowed to stand at 4 °C for 30 min, and the serum was separated by centrifugation at 1000 × g for 10 min. Serum intestinal fatty acid-binding protein (I-FABP) was determined using an ELISA kit (NBP2-82,214; Bio-Techne China) according to the manufacturer’s instructions. Absorbance was measured at 450 nm using the microplate reader (Molecular Devices, Shanghai, China).
Transepithelial electrical resistance (TEER) assay
Clean colon segments were placed in Krebs buffer (pH = 7.3) and cut lengthwise into flat plates. The tissue was sandwiched between two film squares and placed into the Ussing chamber system (AD Instruments, Shanghai, China). Glucose Krebs buffer (10 mM; 1.5 mL) was added to one side as an energy source, and mannitol Krebs buffer (10 mM; 1.5 mL) was added to the other side to maintain osmotic pressure balance. The voltage and current were adjusted through the system, and the data at 37 °C was recorded with the built-in acquisition software after balancing (Shao et al. 2023). TEER was calculated based on Ohm’s (Ω) law (resistance = voltage / current) / the exposed issue area (given as Ω/cm2) (Thomson et al. 2019).
Immunofluorescence (IF) assay
The mesenteric lymph node sections were dewaxed, permeabilized in 0.1% Triton X-100, and antigen retrieved. After serum blocking, the sections were incubated with primary antibodies against IL-17A or forkhead box protein P3 (FOXP3) and CD4 (Proteintech, Wuhan, China) overnight at 4 °C, followed by FITC-labeled and FRITC labeled secondary antibodies. For mitophagy, antibodies against LC3B and MitoTracker were used and slides were counterstained with DAPI. Results were examined with a fluorescence microscope (Olympus, Tokyo, Japan).
Reverse transcription-quantitative (RT-q) PCR
This assay was performed to determine the levels of T cell differentiation markers. Total RNA was gathered using TRIzol® reagent (Thermo Fisher Scientific) and subsequently reverse transcribed into cDNA using a universal reverse transcription kit (Qiagen). The mRNA expression levels were measured using QuantiTect SYBR Green PCR kit (Qiagen) on a real-time PCR system (Agilent China). Beta-actin was used as the internal reference and the relative mRNA expression levels were calculated using the 2−ΔΔCq method.
Western blot analysis
Total proteins were extracted from colon segments using RIPA lysis buffer, determined using NanoDrop equipment, separated via SDS-PAGE on a gel, and subsequently transferred onto PVDF membranes. Following blocking with 5% skimmed milk for 1.5 h, the membranes were incubated with primary antibodies (Proteintech) at 4 °C overnight. Following the membranes being washed with TBS-0.01% Tween-20 (TBST), they were incubated with a goat anti-rabbit secondary antibody (Abcam) for 2 h. Blots were visualized using an ECL kit, and the gray values were analyzed using ImageJ software (version 1.8).
Statistical analysis
Data are presented as the mean ± standard deviation, and statistical analysis was performed using GraphPad Prism (version 8.0). Comparisons were performed using one-way ANOVA followed by Tukey’s post hoc test. P < 0.05 indicates a statistically significant difference.
Results
BG reduces TNBS-induced CD-like colitis
The average weight of mice in the TNBS-induced group was significantly lower than that in the sham group (P < 0.001), and the weight of the BG-treated group was restored in a concentration-dependent manner. There was a significant difference between the body weight of mice in the highest dose treatment group and the model group (P < 0.001; Fig. 1A). The mouse DAI score is calculated by adding three observations from 0 to 4 and dividing by 3. The results showed that the DAI of the TNBS-induced group was close to 4, and the values in the BG-treated groups decreased gradually with concentration (Fig. 1B). Additionally, the length of the colon was recorded for assessment of visual intestinal damage. The colon in the TNBS-induced group was significantly shortened (P < 0.001), and BG contributed to the maintenance of colon length (P < 0.05; Fig. 1C). H&E staining exhibited that compared with the sham group, the mucosal layer structure in the model group was destroyed, inflammatory cells infiltrated, and the villus structure was disordered. The villi in the high-dose BG treatment group tended to be neat, and a clear normal mucosal layer could be observed (Fig. 1D). Masson staining displayed blue-labeled collagen fiber deposition in the model group, indicating the presence of fibrosis. In the BG treatment group, the blue visible part was significantly reduced and was very small in the high-dose group (P < 0.001; Fig. 1E).
BG reduces TNBS-induced CD-like colitis. A The average weight of mice. B DAI score based on fecal consistency, occult blood, and weight loss. C The length of the colon was recorded for assessment of visual intestinal damage. D H&E staining exhibited the mucosal layer structure. E Masson staining displayed blue-labeled collagen fiber deposition. *P < 0.05, **P < 0.01, ***P < 0.001
BG reduces TNBS-induced intestinal barrier damage
The level of FITC-dextran in the serum of mice in the TNBS induction group was significantly increased (P < 0.001), compared with that in the sham group. Compared with the model group, the FITC-dextran level in the BG treatment group showed a concentration-dependent decrease (Fig. 2A). In addition, the serum level of I-FABP, a marker of intestinal injury, was significantly increased in the model group (P < 0.001), and BG treatment could alleviate the release of I-FABP into the blood circulation (P < 0.01; Fig. 2B). The decrease in TEER also indicated that the barrier in the model group was damaged, and BG could play a role in maintaining the barrier (P < 0.001; Fig. 2C). For further validation, the levels of tight junction proteins were measured. The levels of ZO-1 and Claudin-1 protein decreased significantly in the model group, while Claudin-2 increased (P < 0.001). BG was conducive to the maintenance of high-level ZO-1 and Claudin-1 protein levels (P < 0.05; Fig. 2D). This suggested that BG antagonized the disruption of the integrity and stability of intercellular tight junction structures caused by TNBS.
BG maintains Th17/Treg balance
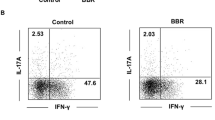
In IF analysis, IL-17A and FOXP3 were used as markers for Th17 and Treg, respectively. The results revealed that Th17 increased and Treg decreased in the mesenteric lymph nodes of the model group. BG treatment effectively alleviated the increase of Th17 cells and the decline of Treg, indicating that it might inhibit the conversion of Treg to Th17 (Fig. 3A, B). In the model group, the levels of IL-17, IFN-γ, RORγt, and T-bet elevated, and the levels of IL-10, IL-5, Foxp3, and GATA3 decreased (P < 0.001). BG treatment alleviated the fluctuations in the levels of these cytokines or transcription factors (Fig. 3C).
BG regulates mitophagy and the PPARγ/ nuclear factor kappa B (NF-κB) signaling pathway
According to IF analysis, Mito-Tracker and LC3B levels were found to be decreased in the model group, while their levels increased upon BG treatment, suggesting that BG might contribute to autophagy (Fig. 4A). By detecting mitophagy-related proteins, BG was found to promote the expression of Beclin-1, PINK1, and mito-Parkin, increase the LC3II/LC3I ratio, and reduce p62 and cyto-Parkin (P < 0.001). This proved that mitophagy was enhanced in the BG-treated groups (Fig. 4B). In addition, TNBS reduced PPARγ levels in tissues, accompanied by enhanced NFκB p65 phosphorylation (P < 0.001), indicating that the NFκB inflammatory channel was activated. BG activated PPARγ and inhibited NF-κB activation in a concentration-dependent manner, reflecting its function in regulating this pathway (Fig. 4C).
BG regulates mitophagy and the PPARγ/NF-κB signaling pathway. A Mito-Tracker and LC3B levels were determined using IF to indicate autophagy. B Mitophagy-related proteins were determined using western blotting. C PPARγ level and NFκB p65 phosphorylation were determined using western blotting. *P < 0.05, **P < 0.01, ***P < 0.001
Discussion
IBD constitutes a chronic inflammatory condition characterized by intermittent exacerbations, leading to complications and often necessitating repeated surgeries. It encompasses two major subtypes: CD and ulcerative colitis (UC) (Cheng et al. 2021). In CD, inflammation affects all layers of the intestine, whereas UC primarily involves the mucosal layer. TNBS is widely applied to establish CD models (Flacs et al. 2020; Zuo et al. 2023). Its induction mechanism is that it enters the intestinal mucosa damaged by ethanol and combines with macromolecular substances to form whole antigens and induce immune responses. TNBS induces chronic transmural colitis with severe diarrhea, mimicking the histological features of human CD (Neurath et al. 1995). This study, based on a mouse model, demonstrated that BG significantly attenuated colon tissue damage, particularly notable in CD, in a concentration-dependent manner. Assessment using DAI scores revealed significant reductions in loose feces, occult blood, and weight loss among mice in the BG treatment group. Pathological analysis indicated decreased inflammatory infiltration, restoration of crypt structure towards normalcy, and reduced fibrosis. Given that anti-inflammatory therapies often prove ineffective in patients with fibrosis in IBD (Wang et al. 2022), the observed reduction in fibrosis suggests a potentially novel mechanism of action for BG. It is speculated that beyond inflammatory stimuli, BG may target independent cellular or molecular factors contributing to fibrosis. Furthermore, the impact of BG on the intestinal barrier integrity was assessed. I-FABP, a sensitive marker for early identification of intestinal ischemia and injury (Straarup et al. 2023), exhibited a significant decrease in serum levels following BG treatment. Additionally, increased levels of TEER and tight junction proteins support the notion of BG-mediated repair or protection of the intestinal barrier.
Th17 cells, a subset of CD4+ T cells, play a crucial role in IBD pathogenesis. It has been confirmed that cytokines and transcription factors, such as IL-6 and RORγt, are required for the early differentiation of Th17 cells (Kumar et al. 2021). This study revealed a significant increase in Th17 cells alongside a decrease in Tregs in mesenteric lymph nodes of the model group. BG treatment effectively mitigated this imbalance by reducing the conversion of Tregs to Th17 cells. Elevated local and serum IL-17 levels in CD patients underscored the significance of IL-17+ T cells in disease severity (Pedersen et al. 2022). BG treatment increased anti-inflammatory factors such as IL-10, IL-5, Foxp3, and GATA3 in the colon, indicative of enhanced anti-inflammatory responses. Notably, GATA3 expression in Tregs residing in barrier sites, including the intestine (Choi et al. 2023), highlights its potential role in BG-mediated immunomodulation.
Given BG has previously been demonstrated to have a protective effect on mitochondrial function as aforementioned, markers of mitophagy were evaluated in colon tissue. BG was found to enhance mitophagy, suggesting a potential mechanism by which BG regulates immune responses. Increasing evidence suggests that the elimination of dysfunctional mitochondria is a powerful means used by autophagy to control the immune system, and that the mitophagy process may limit inflammatory cytokine secretion (Xu et al. 2020). Moreover, the regulation of BG on the PPARγ/NF-κB signaling pathway was elucidated. Activation of PPARγ has been associated with mitigating experimental CD through regulation of mitochondrial function (Yao et al. 2017), while inhibition of NF-κB has been linked to reduced inflammation (Huang et al. 2020). Activating PPARγ has also been found to have positive effects in other disease models. For example, regulating PPARγ-dependent mitophagy enhances brain glucose metabolism and improves cognitive impairment in APP/PS1 mice (Li et al. 2022), and inhibiting NF-κB reduces brain ischemia–reperfusion injury in rats (Yao et al. 2022). Therefore, PPARγ is considered a strong candidate target.
In summary, BG demonstrates promising effects in ameliorating intestinal barrier damage and Th17/Treg imbalance in a murine model of CD-like colitis, while also promoting intracellular mitophagy. The PPARγ/NF-κB signaling pathway may serve as a key mediator of BG’s regulatory mechanisms. These findings provide a valuable experimental foundation for the potential therapeutic use of BG in CD treatment. Nevertheless, in addition to the colon, the ileum is also the primary segment affected by CD (Alrubia et al. 2022; Narula et al. 2022). Therefore, the impact of Bergapten on ileal lesions deserves future investigation, and the lack of research on other intestinal segments is a limitation of the present study.
Data availability
No datasets were generated or analysed during the current study.
References
Akhter S, Tasnim FM, Islam MN et al (2023) Role of Th17 and IL-17 cytokines on inflammatory and auto-immune diseases. Curr Pharm Des 29:2078–2090. https://doi.org/10.2174/1381612829666230904150808
Alrubia S, Al-Majdoub ZM, Achour B, Rostami-Hodjegan A, Barber J (2022) Quantitative assessment of the impact of Crohn’s disease on protein abundance of human intestinal drug-metabolising enzymes and transporters. J Pharm Sci 111:2917–2929. https://doi.org/10.1016/j.xphs.2022.07.012
Aslam H, Khan AU, Qazi NG, Ali F, Hassan SSU, Bungau S (2022) Pharmacological basis of bergapten in gastrointestinal diseases focusing on H(+)/K(+) ATPase and voltage-gated calcium channel inhibition: a toxicological evaluation on vital organs. Front Pharmacol 13:1005154. https://doi.org/10.3389/fphar.2022.1005154
Cheng WX, Ren Y, Lu MM et al (2021) Palmitoylation in Crohn’s disease: current status and future directions. World J Gastroenterol 27:8201–8215. https://doi.org/10.3748/wjg.v27.i48.8201
Choi SI, Shin YC, Lee JS, Yoon YC, Kim JM, Sung MK (2023) N-Acetylglucosamine and its dimer ameliorate inflammation in murine colitis by strengthening the gut barrier function. Food Funct 14:8533–8544. https://doi.org/10.1039/d3fo00282a
Cockburn E, Kamal S, Chan A, Rao V, Liu T, Huang JY, Segal JP (2023) Crohn’s Disease: an Update. Clin Med (lond) 23:549–557. https://doi.org/10.7861/clinmed.2023-0493
Flacs M, Collard M, Doblas S et al (2020) Preclinical model of perianal fistulizing Crohn’s disease. Inflamm Bowel Dis 26:687–696. https://doi.org/10.1093/ibd/izz288
Gomez-Bris R, Saez A, Herrero-Fernandez B, Rius C, Sanchez-Martinez H, Gonzalez-Granado JM (2023) CD4 T-cell subsets and the pathophysiology of inflammatory bowel disease. Int J Mol Sci 24. https://doi.org/10.3390/ijms24032696
Huang T, Pu Q, Zhou C et al (2020) MicroRNA-302/367 cluster impacts host antimicrobial defense via regulation of mitophagic response against Pseudomonas aeruginosa infection. Front Immunol 11:569173. https://doi.org/10.3389/fimmu.2020.569173
Jaeger N, Gamini R, Cella M et al (2021) Single-cell analyses of Crohn’s disease tissues reveal intestinal intraepithelial T cells heterogeneity and altered subset distributions. Nat Commun 12:1921. https://doi.org/10.1038/s41467-021-22164-6
Jiang Y, Nguyen TV, Jin J, Yu ZN, Song CH, Chai OH (2023) Bergapten ameliorates combined allergic rhinitis and asthma syndrome after PM2.5 exposure by balancing Treg/Th17 expression and suppressing STAT3 and MAPK activation in a mouse model. Biomed Pharmacother 164:114959. https://doi.org/10.1016/j.biopha.2023.114959
Kaminsky LW, Al-Sadi R, Ma TY (2021) IL-1β and the intestinal epithelial tight junction barrier. Front Immunol 12:767456. https://doi.org/10.3389/fimmu.2021.767456
Katsandegwaza B, Horsnell W, Smith K (2022) Inflammatory bowel disease: a review of pre-clinical murine models of human disease. Int J Mol Sci 23. https://doi.org/10.3390/ijms23169344
Kumar R, Theiss AL, Venuprasad K (2021) RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol 42:1037–1050. https://doi.org/10.1016/j.it.2021.09.005
Li Z, Meng X, Ma G et al (2022) Increasing brain glucose metabolism by ligustrazine piperazine ameliorates cognitive deficits through PPARγ-dependent enhancement of mitophagy in APP/PS1 mice. Alzheimers Res Ther 14:150. https://doi.org/10.1186/s13195-022-01092-7
Luo T, Jia X, Feng WD et al (2023) Bergapten inhibits NLRP3 inflammasome activation and pyroptosis via promoting mitophagy. Acta Pharmacol Sin 44:1867–1878. https://doi.org/10.1038/s41401-023-01094-7
Narula N, Wong ECL, Dulai PS, Marshall JK, Jairath V, Reinisch W (2022) Comparative effectiveness of biologics for endoscopic healing of the ileum and colon in Crohn’s disease. Am J Gastroenterol 117:1106–1117. https://doi.org/10.14309/ajg.0000000000001795
Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W (1995) Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 182:1281–1290. https://doi.org/10.1084/jem.182.5.1281
Ng SC, Shi HY, Hamidi N et al (2017) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390:2769–2778. https://doi.org/10.1016/s0140-6736(17)32448-0
Noor NM, Verstockt B, Parkes M, Lee JC (2020) Personalised medicine in Crohn’s disease. Lancet Gastroenterol Hepatol 5:80–92. https://doi.org/10.1016/s2468-1253(19)30340-1
Pedersen TK, Brown EM, Plichta DR et al (2022) The CD4(+) T cell response to a commensal-derived epitope transitions from a tolerant to an inflammatory state in Crohn’s disease. Immunity 55:1909-1923.e1906. https://doi.org/10.1016/j.immuni.2022.08.016
Quetglas-Llabrés MM, Quispe C, Herrera-Bravo J et al (2022) Pharmacological properties of bergapten: mechanistic and therapeutic aspects. Oxid Med Cell Longev 2022:8615242. https://doi.org/10.1155/2022/8615242
Roda G, Chien Ng S, Kotze PG et al (2020) Crohn’s Disease. Nat Rev Dis Primers 6:22. https://doi.org/10.1038/s41572-020-0156-2
Saez A, Gomez-Bris R, Herrero-Fernandez B, Mingorance C, Rius C, Gonzalez-Granado JM (2021) Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int J Mol Sci 22. https://doi.org/10.3390/ijms22147618
Shao R, Yang Z, Zhang W et al (2023) Pachymic acid protects against Crohn’s disease-like intestinal barrier injury and colitis in miceby suppressingintestinal epithelial cell apoptosis via inhibiting PI3K/AKT signaling. Nan Fang Yi Ke Da Xue Xue Bao 43:935–942. https://doi.org/10.12122/j.issn.1673-4254.2023.06.08
Straarup D, Gotschalck KA, Christensen PA, Krarup H, Lundbye-Christensen S, Handberg A, Thorlacius-Ussing O (2023) Exploring I-FABP, endothelin-1 and L-lactate as biomarkers of acute intestinal necrosis: a case-control study. Scand J Gastroenterol 58:1359–1365. https://doi.org/10.1080/00365521.2023.2229930
Thomson A, Smart K, Somerville MS et al (2019) The Ussing chamber system for measuring intestinal permeability in health and disease. BMC Gastroenterol 19:98. https://doi.org/10.1186/s12876-019-1002-4
Venkataraman B, Almarzooqi S, Raj V et al (2022) α-Bisabolol mitigates colon inflammation by stimulating colon PPAR-γ transcription factor: in vivo and in vitro study. PPAR Res 2022:5498115. https://doi.org/10.1155/2022/5498115
Wang Y, Huang B, Jin T, Ocansey DKW, Jiang J, Mao F (2022) Intestinal fibrosis in inflammatory bowel disease and the prospects of mesenchymal stem cell therapy. Front Immunol 13:835005. https://doi.org/10.3389/fimmu.2022.835005
Xu Y, Shen J, Ran Z (2020) Emerging views of mitophagy in immunity and autoimmune diseases. Autophagy 16:3–17. https://doi.org/10.1080/15548627.2019.1603547
Yan YX, Shao MJ, Qi Q et al (2018) Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol Sin 39:1633–1644. https://doi.org/10.1038/aps.2017.185
Yao J, Lu Y, Zhi M, Hu P, Wu W, Gao X (2017) Dietary n-3 polyunsaturated fatty acids ameliorate Crohn’s disease in rats by modulating the expression of PPAR-γ/NFAT. Mol Med Rep 16:8315–8322. https://doi.org/10.3892/mmr.2017.7673
Yao H, Zhao J, Song X (2022) Protective effects of fraxin on cerebral ischemia-reperfusion injury by mediating neuroinflammation and oxidative stress through PPAR-γ/NF-κB pathway. Brain Res Bull 187:49–62. https://doi.org/10.1016/j.brainresbull.2022.06.010
Zuo L, Geng Z, Song X et al (2023) Browning of mesenteric white adipose tissue in Crohn’s disease: a new pathological change and therapeutic target. J Crohns Colitis 17:1179–1192. https://doi.org/10.1093/ecco-jcc/jjad046
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. B.Z., H.C, and G.L. contributed to material preparation, data collection, and analysis. L.X. contributed to the draft and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. This study was approved by the Ethics Review Committee of Zhangjiagang Hospital affiliated with Soochow University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Zhao, B., Cheng, H. et al. Bergapten enhances mitophagy to regulate intestinal barrier and Th17/Treg balance in mice with Crohn’s disease-like colitis via PPARγ/NF-κB signaling pathway. Naunyn-Schmiedeberg's Arch Pharmacol (2024). https://doi.org/10.1007/s00210-024-03113-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00210-024-03113-8