Abstract
It has been widely documented that medicinal herbal remedies are effective, have fewer side effects than conventional medicine, and have a synergistic effect on health collaborations in the fight against complicated diseases. Traditional treatments for neurological problems in ancient times sometimes involved the use of herbal remedies and conventional methods from East Asian countries including India, Japan, China, and Korea. We collected and reviewed studies on plant-derived neuroprotective drugs and tested them in neurotoxic models. Basic research, preclinical and clinical transgene research can benefit from in silico, in vitro, and in vivo investigations. Research, summaries of the extracts, fractions, and herbal ingredients were compiled from popular scientific databases, which were then examined according to origin and bioactivity. Given the complex and varied causes of neurodegeneration, it may be beneficial to focus on multiple mechanisms of action and a neuroprotection approach. This approach aims to prevent cell death and restore function to damaged neurons, offering promising strategies for preventing and treating neurodegenerative diseases. Neurodegenerative illnesses can potentially be treated with natural compounds that have been identified as neuroprotective agents. To gain deeper insights into the neuropharmacological mechanisms underlying the neuroprotective and therapeutic properties of naturally occurring antioxidant phytochemical compounds in diverse neurodegenerative diseases, this study aims to comprehensively review such compounds, focusing on their modulation of apoptotic markers such as caspase, Bax, Bcl-2, and proinflammatory markers. In addition, we delve into a range of efficacies of antioxidant phytochemical compounds as neuroprotective agents in animal models. They reduce the oxidative stress of the brain and have been shown to have anti-apoptotic effects. Many researches have demonstrated that plant extracts or bioactive compounds can fight neurodegenerative disorders. Herbal medications may offer neurodegenerative disease patients’ new treatments. This may be a cheaper and more culturally appropriate alternative to standard drugs for millions of people with age-related NDDs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of apoptosis is a series of events that ultimately results in the death of certain cells in the body (D’Arcy 2019). These cells initiate a tightly regulated intracellular program that makes a cell perform self-destruction (Ameisen 2002). Apoptosis (a-po-toe-sis) is a unique type of cell death that was initially identified in a now-classic paper published in 1972 by Kerr, Wyllie, and Currie (Elmore 2007). It is a normal part of getting older and developing as a means of maintaining a steady cell count in the body’s tissues through a process known as homeostasis (Rando 2006). Similarly, to how a sculptor carves a statue from a block of marble by removing portions, nature utilizes a cell elimination mechanism during embryogenesis to achieve the final morphology.
When cells are damaged by things like infection or toxins, the immune system may trigger apoptosis as a protective measure (Hanisch 2002). Caenorhabditis elegans is a model organism that has been used to learn about the processes of programmed cell death (PCD) (Hengartner 1997). These worms undergo programmed cell death at key stages in their development. Cell death genes of the worm, also known as ced-3 and ced-4, are responsible for controlling this suicide of cells, whereas survival genes, such as ced-9, prevent programmed cell death from happening (Horvitz 1994). It happens when cysteine proteases called “caspases” are turned on most caspases start as procaspases, which are inactive forms that need to be cut to make the active form (Thornberry and Lazebnik 1998). In response, activated caspases break a variety of intracellular and cytoplasmic membrane substrates, which ultimately destroy the cell (Tang et al. 2019). This is a coordinated process that often needs energy and can be triggered by both normal and pathological events (Muñoz-Espín and Serrano 2014). In the body, specific hormones like corticosteroids may lead to the death of thymocytes but unaffected the rest of the cell. In response to ligand binding and protein cross-linking, apoptosis can occur in cells that express Fas or TNF receptors (Patra 2008).
There is another type of cell death known as necrosis, and it is interesting to note that apoptosis and necrosis can happen on their own, in order, or at the same time (Walczak 2011). A cell decides its type of death by the degree of stimuli like several harmful stimuli, including as heat, radiation, hypoxia, and cytotoxic anticancer medications, can trigger apoptosis at low doses, but at greater concentrations (Fulda et al. 2010), these same stimuli can cause necrosis. In mammals, apoptosis can begin either via an extrinsic pathway triggered by death receptors or by an intrinsic pathway involving the mitochondria (Burz et al. 2009).
Morphological features of apoptosis
Apoptosis is distinguished from other cell death processes by several different morphological characteristics. These characteristics include chromatin condensation, cell and nuclear shrinkage, oligonucleosomal DNA fragmentation, and cell membrane blebbing (Allen et al. 1997). By using light microscopy, one can see that the cells have shrunk and undergone pyknosis during the early stages of apoptosis (Majno and Joris 1995). This means that the cells are of a smaller size, the cytoplasm is dense, and the organelles are packed more closely together (Farquhar and Palade 1965). Condensation of chromatin causes pyknosis, which is the defining feature of apoptosis (Doonan and Cotter 2008). During a process called “budding,” there is extensive blebbing of the cell membrane, which is then preceded by karyorrhexis and the disconnection of cell fragments into apoptotic bodies (Parodi et al. 2020).
Apoptotic bodies are made up of cytosol with cellular components packed closely together, with or without a segment of the cell’s nucleus (Roach et al. 2004). The organelles are still whole, and everything is still surrounded by a healthy cell membrane (Van Den Bossche et al. 2006). After that, macrophages, parenchymal cells, or neoplastic cells eat these bodies and break them down into phagolysosomes (Ghadially 2013). There is no inflammatory response or leakage of cellular components from apoptotic cells into the surrounding interstitial tissue (Ren and Savill 1998).
Relations between caspases, Bax/Bak, Bcl-2, and TNF-α, ILs
Apoptosis, a tightly regulated process of programmed cell death, involves a complex interplay of molecular components, among which caspases, Bax, Bcl-2, TNF-α, and IL-1β play pivotal roles. Caspases, a family of protease enzymes, serve as the executioners of apoptosis. They exist in inactive forms and upon activation, particularly caspase-3, trigger the biochemical and morphological changes that lead to cell demise (Boatright and Salvesen 2003). Bax, a pro-apoptotic protein, and Bcl-2, an anti-apoptotic protein, are key regulators of mitochondrial integrity. Bax promotes mitochondrial outer membrane permeabilization, while Bcl-2 counteracts this action, thus influencing the release of apoptotic factors (Finucane et al. 1999).
TNF-α and IL-1β are cytokines that contribute to inflammation but also participate in apoptotic signaling pathways. TNF-α, when in excess or under certain conditions, can trigger apoptosis via its receptor-mediated pathways (Maianski et al. 2003). IL-1β, similarly, is implicated in apoptotic processes and inflammation. The intricate network of interactions among these molecules dictates the fate of a cell. Bcl-2 and Bax engage in a dynamic balance, determining the susceptibility of a cell to apoptotic stimuli. Caspases, particularly caspase-3, serve as downstream effectors, activated by both intrinsic and extrinsic apoptotic pathways. TNF-α and IL-1β can induce apoptosis, either directly or by modulating other apoptotic factors (Vince, et al. 2018). Cross-talk among these components amplifies or inhibits apoptotic signaling. Bcl-2 inhibits Bax, preventing mitochondrial dysfunction and subsequent caspase activation. Conversely, TNF-α can upregulate Bax or influence Bcl-2 family proteins, thereby tipping the balance towards cell death.
Understanding the intricate interactions among caspases, Bax, Bcl-2, TNF-α, and IL-1β is crucial in unraveling the complex mechanisms governing apoptosis. Their dynamic interplay orchestrates the fate of cells, highlighting their potential as targets for therapeutic interventions in diseases involving aberrant apoptosis and inflammation (Fig. 1).
Therapeutic role of phytochemicals in neurodegenerative diseases: modulating apoptotic and inflammatory markers
Neuroprotection against neurodegenerative diseases like Alzheimer’s, Parkinson’s, Huntington’s, and amyotrophic lateral sclerosis involves employing phytochemical therapy, historically utilized in crude forms. Certain herbal medications exhibit anti-inflammatory and antioxidant effects, although their specific mechanisms remain unclear. Anti-apoptotic phytochemical agents are effective neuroprotectors against various brain disorders. Developing plant-derived neuroprotective drugs involves a comprehensive approach, including in silico methods for interaction prediction, bioinformatics for target identification, and in vitro studies for assessing cellular effects. In vivo experiments, incorporating behavioral assessments, test safety and efficacy in animal models. Integration of these approaches ensures a thorough evaluation before clinical trials, optimizing the identification of effective compounds. The translation of research findings into practical applications follows a systematic progression, involving compound identification, safety assessments, understanding mechanisms of action, rigorous clinical trials, regulatory approval, and integration with conventional therapies. This approach aims to bridge the gap between laboratory discoveries and real-world applications, advancing herbal remedies as potential treatments for neurological diseases. Phytochemicals are effective and rarely cause side effects. Asian cultures treat CNS issues with 120 traditional treatments (Sharma et al. 2013). Therapeutic effects have been validated by recent clinical research. An ethnopharmacological method may provide numerous drug discovery and development resources. Plants were the source of many Western medicines. Many chemicals have been isolated from phytochemicals, although most have not been fully characterized for pharmacological application (Suk 2005). The study conducted in Thailand revealed that the primary reasons for patients in Thailand to consume herbal medications are the enhancement of their quality of life and the reduction of reliance on conventional treatments (Ming 2016). In this study, we discuss the therapeutical tools along with the molecular mechanism by which phytochemicals protect neurons, including direct modulation of the mitochondrial apoptotic machinery, change of cellular signal pathways, activation of antiapoptotic Bcl-2 protein family, PI3K, AKT, NRF2, and suppression of Bax, Bad, LPO, JNK, cytochrome-c and caspase proteins, and other apoptotic factors. Table 1 describes the upregulation and downregulation of antiapoptotic and apoptotic factors, along with inflammatory markers. It also includes details about the type of model used for the study and the phytochemical structure. Figure 2 shows representation of various targets of well reported phytochemicals as antiapoptotic and as apoptotic regulators.
Neuroinflammatory pathways
Activated microglial cells exert a substantial influence on immunological and inflammatory responses in the central nervous system and neurodegenerative diseases. Neuroinflammation releases signaling molecules that facilitate many pro-apoptotic pathways. The transcription factor NF-κB initiates and regulates the activation of several inflammatory mechanisms within glial cells, which have a role in the progression of different neurodegenerative illnesses (Shabab et al. 2017).
Microglia and neuroinflammation
Microglia are brain macrophages that live in the brain and have a crucial role in the defense and repair of tissues in an organism. These cells play a crucial role in brain inflammation and inflammatory neurodegenerative disorders (Harry and Kraft 2012). Microglia activation is the initial manifestation of neuroinflammation (Giatti et al. 2012). Microglia undergo activation in the presence of pathogens, tissue damage, aberrant stimulation, neurotoxins, infection, or injury. In this scenario, they have the ability to target and harm healthy neurons either through physical means, such as phagocytosis, or by releasing apoptotic factors (Park et al. 2011). Activated microglia have the ability to cause the death of neurons, which contributes to neurodegeneration. However, they can also exterminate and/or eliminate infections (neuro-protective function) (Carson et al. 2006). Microglial phagocytosis of deceased or deteriorating neurons prevents the discharge of harmful and/or pro-inflammatory intracellular substances. An effective and dynamic mechanism is required for the phagocytosis and removal of abnormal or surplus proteins in order to maintain a balanced protein load in the brain and prevent the onset of neurodegeneration. Neuroinflammation may directly impact neuronal death and activate microglia through the excessive production of signaling molecules in pro-apoptotic pathways (Shabab et al. 2017).
Nf-κB activation in neuroinflammation pathway
The migration of NF-κB into the nucleus and the presence of the inhibitory protein, IκBα, determine its activity. To become active, NF-κB dimers must be activated by removing the inhibitory IκB protein from the cytoplasm (Shabab et al. 2017). Dimers are transferred to the nucleus after release. Cells activate the pathway when exposed to stimuli like TNF-α and IL-1β. The IKK complex, consisting of two catalytic subunits (IKKα and IKKβ) and a regulatory/structural subunit (IKKγ or NF-κB essential modifier (NEMO), phosphorylates and ubiquitinates the inhibitory protein IκBα, leading to its proteasome degradation. Disrupting the connection between IκBα and NF-κB releases NF-κB, allowing p65/RelA to migrate from the cytoplasm to the nucleus. Attaching to promoters activates genes in the cell (Noursadeghi et al. 2008; Huang and Hung 2013). IKK activates NF-κB via conventional and non-canonical mechanisms (Diamant and Dikstein 2013). Which pathway to choose relies on whether IκB degradation is involved in the activation process. Ubiquitin plays a crucial role in three phases of the NF-κB pathway: IκB degradation, precursor molecule processing, and IKK activation. Three enzymatic steps are needed for reversible covalent modification ubiquitination (Shabab et al. 2017).
Pro-inflammatory cytokines and neuroinflammation pathway
Increased TNF-α and IL-1β levels have been observed before neuronal death. At first, inflammation is measured by the release of proinflammatory cytokines like TNF-α and IL-1β, as well as adhesion molecules. IL-1β and TNF-α play a vital role in pathological inflammation and disease progression (Lyman et al. 2014). They may disrupt the blood–brain barrier (BBB), increase adhesion-molecule expression, and promote hazardous material transport, such as nitric oxide (NO) (Blamire et al. 2000). IL-1β is critical for the course of neurodegenerative illnesses including AD and PD, as well as acute neuroinflammatory situations like stroke, ischemia, and brain injury (Swaroop et al. 2016). TNF-α, an inflammatory cytokine, induces apoptosis by activating receptors with a homologous cytoplasmic sequence indicating an intracellular death domain. TNFR1 activation triggers rapid neuronal death via caspase 3, giving a molecular basis. Membrane receptor apoptosis pathways, linked to neuronal death, involve intracellular signaling complexes such AP-1, NF-κB, and caspases (Kaushal and Schlichter 2008).
ROS and neuroinflammation pathway
The brain is constantly subjected to oxidative stress and free radicals, which can lead to many disorders. ROS are chemical species with an unpaired electron that can perform signal transduction activities in response to external stimuli. They are multi-potent and diffusible. Neuronal tissue has specific sources of oxidative stress, including excitatory amino acids and neurotransmitters. The metabolism of amino acids and neurotransmitters generates ROS (Naik and Dixit 2011). Oxidative stress can activate the NF-κB pathway due to mitochondrial malfunction. Pharmacological therapies targeting mitochondrial activity may prevent or treat metabolic and neurodegenerative disorders. Additionally, mitochondria-targeted antioxidants decrease systemic and neuroinflammation (Packer et al. 1997; Akbar et al. 2016).
PI3K/AKT/mTOR pathway in neuroinflammation pathway
The PI3K/AKT pathway controls inflammation, cell activation, and apoptosis. After PI3K activation, a signaling cascade occurs, leading to NF-κB translocation. The PI3K family of lipid kinases includes three classes of members. Activating AKT triggers downstream signaling via many targets and activates the PI3K pathway (Guha and Mackman 2001). AKT and mTOR are activated by P13K. The mTOR pathway phosphorylation is a key factor in microglia activation. The mTOR pathway regulates NF-κB activation and inflammation. Activated mTOR boosts NF-κB activity and enhances inflammatory molecule production, such as iNOS and COX-2 (Wang, Kou et al. 2012).
MAPK pathway in neuroinflammation
Active microglia activate MAPK family. Activation of MAPKs, such as p38 MAPK and SAPK/JNK, by stress and inflammation leads to the activation of inflammatory mediator cascades in response to LPS stimulation, which initiates various signal transduction cascades (Kyriakis and Avruch 2001; Cowan and Storey 2003; Park et al. 2011). SAPK/JNK, MAPK family members, can be triggered by environmental stress and inflammatory cytokines. Phosphorylation of c-Jun and regulation of transcription factors occur after translocation of active SAPK/JNK to the nucleus. Activating SAPK/JNK leads to binding to the c-Jun amino-terminal trans-activation domain, leading to increased AP-1-dependent gene expression. AP-1 regulates the expression of inflammatory mediators like COX-2 and Inos (Guha and Mackman 2001). In summary, p38 MAPK, AKT, and mTOR pathways have been shown to play important roles in LPS-induced microglia activation during neuroinflammation (Shabab et al. 2017).
Apoptosis and neurodegenerative disorders
Diseases of the nervous system (neurodegenerative) and apoptosis, there are many different types of neurodegenerative illnesses, all of which lead to gradual declines in mental and physical abilities over time (Wang et al. 2017). In this case, apoptotic processes stand out as likely causes of cell death due to the illness. Disorders arise when many groups of neurons die off in large numbers, as happens after trauma or illness. Alzheimer’s disease (AD) occurs when neurons in the hippocampus and cortex die; Parkinson’s disease (PD) occurs when neurons in the medulla die; Huntington’s disease (HD) occurs when neurons in the stratum spinosum die, and amyotrophic lateral sclerosis (ALS) occurs when neurons in the basal ganglia die (ALS) (Singh et al. 2019). As a putative mechanism of glucose-induced neuropathy in diabetes, we demonstrated that glucose might trigger apoptosis in PC12 cells (Tie et al. 2008). Apoptosis, also known as programmed cell death, has been proven to have a significant role in the demise of PC12 cells in our previous work.
Parkinson’s disease
PD is an age-related, chronic, progressive, neurological motion illness. In the Western world, the prevalence of PD is 315 per 100,000 people of all ages, and it is projected to double by the year 2030, raising death, morbidity, and the socioeconomic burden globally. In PD, dopamine-dependent neurons in the substantia nigra deteriorate in people with Parkinson’s disease (Association 2018). This illness appears to be driven by an increase in oxidative stress and a malfunction in the mitochondria. Bradykinesia, resting tremors, balance problems, stiffness, sadness, and anxiety are all common clinical signs of PD disease (DeMaagd and Philip 2015). Human tissues and animal models both show apoptosis in PD. Histochemical evidence and upregulation of apoptosis-related genes encoding p53, CD95, and Bax, as well as Par-4, have been identified in the brain tissue of PD patients, indicating that dopaminergic neurons die through apoptosis in PD (Fleischer et al. 2006). Levodopa (l-dopa) is the best treatment for the early motor symptoms of PD, but it is not a cure. Herbal medicine has been suggested as a way to treat PD (Yuan, et al. 2008). This includes herbal preparations and phytochemicals taken from plant foods. Cells can be protected from oxidative damage by using plant products and their bioactive phytochemicals, which can extensively scavenge oxygen free radicals and increase the antioxidative enzyme system and associated compounds. Several studies show that these antioxidant phytochemicals can regenerate lost neuronal processes and restore synaptic connections (Chandran and Abrahamse 2020).
Neuroprotective effects of phytochemicals on PD and their interactions with apoptotic markers and inflammatory markers
Phytochemicals may have therapeutic value for people with PD due to their neuroprotective mechanism and ability to reduce oxidative stress, neuroinflammation, mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis. It has been reported that phytochemicals can stimulate the Nrf2/antioxidant response element signaling pathway and Nrf2-dependent protein expression, avoiding cellular oxidative damage and PD. In our review article, we explored various experimental models utilized to evaluate the neuroprotective capabilities of antioxidant phytochemical derivatives. Our focus throughout the study was to gather specific data pertaining to apoptotic markers while measuring their capacity to diminish oxidative stress and neuroinflammation in the brain.
Vanillin interactions with IL-6, TNF-α, and Bcl-2
Vanillin is a phenolic aldehyde chemical that is widely utilized all over the world as a significant flavoring ingredient. It is a natural component of many plant species and has a wide range of applications in the food, beverage, pharmaceutical, fragrance, and beauty product manufacturing sectors (Anand et al. 2019). By increasing the activities of antioxidant enzymes and decreasing the levels of lipid peroxidation and NO generation, vanillin shows promising brain-neuroprotective potential and may readily cross the BBB (Balakrishnan et al. 2021). This polyphenolic compound is effective at scavenging O2˙ and OH˙ intermediates, which are thought to be responsible for causing damage to biological membranes. Reducing lipid peroxidation, boosting antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, glutathione), and guarding against DNA damage and histopathological alterations in man-induced mice all contribute to a reduction in renal oxidative stress (Truong et al. 2018). In an in silico study, vanillic acid exhibited a binding energy of − 62.116 kcal/mol against interleukin 6 (IL-6), − 78.0683 kcal/mol against TNF-α, and − 5.1 kcal/mol with BCL-2 (Saravanan et al. 2021; Otuechere et al. 2023). This indicates a strong binding affinity with apoptotic proteins and suggests potential inhibition of these targeted proteins according to the amino acid sequence analysis. Furthermore, vanillin’s neuroprotective effects were demonstrated in a rotenone-induced Parkinson’s disease (PD) model. When administered to SH-SY5Y cells, vanillin-suppressed rotenone–induced reactive oxygen species (ROS) production, mitigated mitochondrial dysfunction, inhibited caspase activation, and downregulated signaling molecule expression. Recent studies have also indicated that vanillin administration enhances behavioral performance by increasing dopamine levels and its metabolites in the striatum. Moreover, in rat models of PD induced by rotenone, vanillin administration was found to reduce cytochrome-c release and Bax expression while elevating Bcl-2 expression, thereby inhibiting caspase activation (Rani, et al. 2022). Vanillin also efficiently blocked NF-kB activation and MAPK phosphorylation in LPS-lesioned microglia cells. Collectively, these results point to vanillin’s neuroprotective and anti-inflammatory effect in safeguarding dopaminergic neurons and enhancing behavioral function via the inhibition of oxidative stress, inflammation, and apoptosis; consequently, vanillin may work as a natural therapeutic medication for PD (Wang et al. 2022).
Asiatic acid interactions with COX-2, IL-1β, IL-6
The natural pentacyclic triterpenoid AA has various pharmacological features that make it a promising neuroprotective therapeutic candidate. It has been discovered that some bioactive components of AA have medicinal effects (Nagoor Meeran et al. 2018). Several of the pharmacological features of asiatic acid (AA), a naturally occurring pentacyclic triterpenoid, suggest it may be useful as a neuroprotective therapeutic candidate. Intriguingly, several AA’s bioactive components were discovered to have medicinal promise in healing numerous disorders (Lv et al. 2018). In an in silico study, the interaction between asiatic acid (AA) and COX-2 was analyzed, identifying common amino acid interactions such as ARG120A, TYR 385A, and TYR348A. These interactions were found to be similar to those observed with acetosal, a drug known for inhibiting COX-2. The total binding energy observed for the asiatic acid-COX-2 complex was − 7.371 kcal/mol, suggesting evidence of AA’s inhibitory action on COX-2 (Musfiroh et al. 2023). In an MPP + -induced PD model, AA treatment directly improved the health of SH-SY5Y cells and kept the mitochondria working signaling components were shown to be affected by AA (Chen et al. 2019). Additionally, AA demonstrated interactions with IL-1B, showing a binding affinity of − 9.2626 kcal/mol. Common amino acid interactions responsible for the inhibition of IL-6 were identified, including Thr137, Asp142, Lys72, Lys77, and Gln141 (Legiawati et al. 2018).
Experimental evidence from mice subjected to MPTP-induced PD-like neurotoxicity indicated that AA treatments reduced striatal elevation of α-synuclein, apoptotic markers, and Bcl-2 expression, while increasing dopamine levels in the striatum. Furthermore, subsequent AA treatment significantly suppressed NF-kB activity (Chen et al. 2019). Curiously, subsequent studies have also discovered that AA prevented memory loss brought on by 5-fluorouracil by reducing oxidative stress and increasing antioxidant defense. This was achieved by stopping the decline in Nrf2 expression, reversing the hippocampus’s downregulation of neurogenesis, and re-establishing antioxidant defenses (Welbat et al. 2018). These findings raise the possibility that AA may one day be utilized to treat or prevent PD.
Ferulic acid interaction with Cox-2 and TNF-α/TNFR, caspase-3
As a phenolic molecule found in almost all plant tissues, ferulic acid (4-hydroxy-3-methoxy cinnamic acid) is an important bioactive component of many meals. Several commonly eaten foods, including grain bran and whole grain products, as a phenolic molecule found in almost all plant tissues, ferulic acid (4-hydroxy-3-methoxy cinnamic acid) is an important bioactive component of many meals. Bananas, grain bran, broccoli grapes juice, orange, citrus whole spinach, beetroot, cabbage, eggplant, shoots, bamboo fruits, and grains are just a few of the many vegetables and fruits that are high in FA (Gupta et al. 2021; Srinivasan et al. 2007). Individuals with a diet rich in fruits, vegetables, and drinks containing phenolic acids had an estimated daily consumption of roughly 1000 mg of total polyphenols. Clifford (1999) found that regular consumers of coffee, cereal bran, citrus fruits, and beer might be taking 500–1000 mg of caffeic acid and FA daily (Scalbert and Williamson 2000). FA’s phenolic hydroxyl group prevents lipid peroxidation and reactive oxygen species (ROS) production, allowing it to perform these roles. The BBB may be vulnerable to FA since it has been claimed that FA may readily break through it. Further, it has been demonstrated to be effective as a neuroprotective agent. In an insightful in silico study, ferulic acid (FA) exhibited a commendable docking score of − 73.41 kcal/mol with COX-2, with TYR355, Val523, and Leu352 as common amino acid interactions, mirroring those observed with the reference COX-2 inhibitor, celecoxib (Ekowati et al. 2020). Additionally, molecular docking predicted FA’s interactions with TNFR, where common amino acid interactions with apigenin-7-glucuronide (AG), an inhibitor of TNF-α, were found involving Pro16(A), Glu56(B), Cys55(B), and Glu54(B) (Ernanin Dyah 2021). Recent in vitro and in vivo findings highlighted FA’s ability to enhance protective HO-1 activity in SH-SY5Y cells, elevate levels of catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH), while reducing lipid peroxidation in MPTP-injected PD mouse models (Khurana and Gajbhiye 2013).
The effects of 6-OHDA on morphological alterations and DNA damage, as well as caspase-3 activity, were blunted after FA administration. FA also decreased Drp1 expression in mitochondria and raised PGC1 expression, effectively controlling Mfn2 expression and re-establishing mitochondrial dynamics in 6-OHDA-lesioned PD rat models.
Thymoquinone interactions with COX-2, TNF-α, and IL’s
A pharmacologically active chemical, thymoquinone (TQ) may be found in black cumin seeds and other members of the Lamiaceae family of plants. Although black cumin has been used medicinally for thousands of years, there has been a new surge of interest in this ingredient. Multiple studies have revealed that most of the principal effects of TQ are favorable, suggesting that black cumin seeds and their active ingredient, TQ, may be appropriate for clinical trials (Samarghandian, et al. 2018). The average daily dose of black cumin seed is between 250 and 1000 mg. In a recent in vitro study pre-treating MPP + -induced SH-SY5Y cells with TQ demonstrated a notable decrease in apoptosis rates compared to the control group. Additionally, TQ administration showed a suppressive effect on the expression of pro-apoptotic proteins like Bax and caspase-3 while elevating the levels of Bcl-2 in MPP + -induced SH-SY5Y cells. This suggests that TQ has a mitigating effect on apoptotic cell death triggered by MPP + . The in vivo experiments involved the use of C57/BL6 mice to induce PD using MPTP. Upon analysis, it was evident that mice receiving MPTP exhibited heightened levels of α-synuclein immunoreactivity compared to the control group. However, administration of TQ notably reduced the expression of α-synuclein in the SNc, distinctly differing from the MPTP-only group. Moreover, Western blot assays performed on SNc lysates illustrated a decrease in TH protein expression among mice exposed to MPTP. Yet, this decrease was less pronounced in the presence of TQ treatment, suggesting a mitigating effect (Radad et al. 2009). Another in vivo study where, TQ exhibited a neuroprotective impact by preventing the death of primary dopaminergic neurons in a rotenone-induced PD model in Wistar rats through the modulation of Drp-1 dependent mitochondrial fragmentation (Ebrahimi et al. 2017).
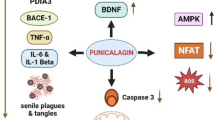
Ellagic acid interactions with Bax, Bcl-2, and caspase-3
Ellagic acid (EA) is a type of phenol that acts as a natural antioxidant and can be found in a wide variety of vegetables and fruits, including but not limited to persimmons, pomegranates, almonds, black raspberries, raspberries, peaches, strawberries. The maximal concentration of EA in the plasma was determined to be about 33 ng/mL after consuming pomegranate juice has 318 mg of punicalagin and 25 mg of EA when absorbed, 1 h later (Seeram et al. 2005). A study reveals that arsenic exposure triggers cell death via inflammation and mitochondrial dysfunction in hippocampi of Wistar rats, leading to the activation of Bax and caspase-3. Additionally, a notable reduction in the mRNA expression of the apoptotic marker Bcl-2 was observed. Notably, ROS elevation in arsenic-administered rats activated pro-inflammatory cytokines through protein kinase C and mitogen-activated protein kinase, as evidenced by increased mRNA levels of IL-1β, IFNϫ, and TNFα, as well as protein synthesis of IFNϫ and TNFα. Significantly, EA pre- and co-administration demonstrated a dose-dependent downregulation of DNA fragmentation, caspase 3 activity, and mRNA expression of IL-1β, IFNϫ, TNFα, and BAX. Concurrently, there was an upregulation of Bcl2 mRNA expression in arsenic-exposed rats (Firdaus et al. 2018). Other research found that restoring CAT activity in 6-OHDA–induced PD rats when they were treated with a combination of EA, α-lipoic acid, and myrtenal prevented the loss of DA levels and lipid peroxidation (Tancheva et al. 2020). In addition to its use in EA-mediated DA neuroprotection, EA has also been used to give protection against rotenone-induced and MPTP induced neurotoxicity through activating Nrf2 signaling. EA supplementation reduced overall ROS production in a dose-dependent manner. It also notably changed levels of apoptotic markers (Bax and Bcl-2) and inflammatory markers (IL-1β, TNFα, and INFγ) upon its administration. Furthermore, the same studies also demonstrated that EA reduced striatal MDA levels, ROS, and DNA fragmentation and improved Nrf2, HO-1, and behavioral functions (Baluchnejadmojarad et al. 2017; Ardah 2020).
Caffeic acid interactions with Bax, Bcl-2, and caspase-3
One of the most promising bioactive chemicals in propolis is caffeine phenethyl ester (CAPE), which has been shown to have therapeutic value in the prevention of several disorders. It has been estimated that the average human consumes up to 1000 mg of caffeic acid (CA) per day from food sources like fruits, veggies, beer, and coffee. It has also been suggested that CA may efficiently reduce certain clinical states of PD by reacting with peroxyl radicals implicated in lipid peroxidation (Clifford 1999). In a study, CAPE was found to significantly elevate Bcl-2 expression while reducing Bax expression in ARPE-19 cells subjected to oxidative stress by use of hydrogen peroxide. Moreover, the expression of genes associated with apoptosis such as apaf-1, cytochrome c, and caspase-3 was notably suppressed by CAPE (Dinc et al. 2017). Similarly, CA-phenethyl ester directly inhibited the release of cytochrome C and apoptosis-inducing factor (AIF) from mitochondria in SH-SY5Y models of Parkinson’s disease induced by MPP + , 6-OHDA exposure (Li et al. 2018; Turan et al. 2020).
Chrysin interactions with Bax, Bcl-2 and ILs, caspase-3, 9
Phytochemical chrysin is widely available in foods like propolis, blue passion flower, and honey due to its significant financial worth and medical efficacy. Due to its antioxidant, anti-inflammatory, and other pharmacological qualities (Borase et al. 2022), chrysin has been investigated for its potential neuroprotective benefits. In rats, the therapeutic potential of chrysin is limited by the concentrations that can be achieved in cells and target organs (Salehi et al. 2019). Due to quick metabolism, limited intestinal absorption, and rapid excretion, bioavailability was shown to be exceedingly low when 400 mg of chrysin was given to human volunteers (Walle et al. 2001).
Both in vitro and in vivo researches demonstrating the neuroprotective action of chrysin reveal that elevations in DA levels are negatively correlated with the death of dopaminergic neurons. Chrysin was discovered to increase DA concentrations by inhibiting monoamine oxidase B (MAO-B) in MPP +—and MPTP-treated CGN cells and mice models of PD (Guo et al. 2016). Chrysin treatment considerably suppressed the increase in ROS levels and dramatically increased antioxidant enzyme activity in aged mice (Farkhondeh et al. 2019). Lipid peroxidation levels and superoxide dismutase (SOD) activity and glutathione (GSH) content were two measures of oxidative stress that were reduced after chrysin administration in a mouse model of MPTP-induced PD (Guo et al. 2016). As shown in a mouse model of cerebral ischemia, chrysin avoided cognitive impairments, reduced IL-1β and TNF-α production, and significantly suppressed NF-kB activation to reduce neuroinflammation and apoptosis (Sarkaki et al. 2019). Inhibiting proapoptotic proteins including caspase-3, caspase-9, and Bax, and increasing antiapoptotic protein Bcl-2 expression, chrysin exerted a neuroprotective impact at the cellular level against MPP + neurotoxicity (Guo et al. 2016).
Epigallocatechin-3-gallate interactions with caspase-1, NLRP3 complex
Green tea polyphenol EGCG has been shown to have several positive physiological benefits in neurodegenerative diseases by suppressing apoptosis (Lambert and Elias 2010). Important for the advancement of treatment drugs for PD is the fact that a small number of studies have shown that EGCG may readily cross the BBB (Renaud, Nabavi et al. 2015). In the in silico study, the binding energies of EGCG with caspase-1 proteins and NLRP3 complex were found to be − 7.6 kcal/mol and − 9.6 kcal/mol, respectively. On the other hand, MCC950, a selective inhibitor of the NLRP3 complex, interacted with caspase-1 and NLRP3 with binding energies of − 6.2 kcal/mol and − 8.2 kcal/mol, respectively. The results indicate that EGCG exhibits a higher affinity toward all complex proteins compared to MCC950. Specifically, interacting amino acid residues of caspase-1 for EGCG were identified, involving van der Waals interactions with ARG383, HIS342, GLY346, ALA384, PRO380, GLN385, GLU378, HIS356, GLN379, and GLY351. These findings suggest the potential of EGCG in modulating the NLRP3 complex through its strong binding affinity with caspase-1 proteins (Jena et al. 2022). Antiapoptotic effects of EGCG were also shown in PQ-induced PC12 cell models, wherein EGCG prevented the downregulation of the proapoptotic SMAC protein in cytosol expression and prevented the elevation of caspase-3 activity (Hou et al. 2008). The reduction of dopamine (DA) levels in the middle of the brain caused by lipopolysaccharide (LPS)-induced neurotoxicity was likewise mitigated by EGCG administration (Al-Amri, et al. 2013) [Registration no:—NCT04338997 (ClinicalTrials.gov) and Registration date:—03/04/2020 (ClinicalTrials.gov).
Interactions of α- and β-asarone with ILs, Bcl-2
In particular, the antioxidant aromatic chemical components α- and β-asarone are isolated from the rhizomes of the Acorus calamus. As a result, comparable pharmacological features have been observed for both α and β asarone, suggesting they may potentially have been used in the therapeutic management of a variety of disorders. The substantial brain delivery of α and β asarone provides promising evidence for the compound’s capacity to penetrate the BBB and hence treat a wide variety of NDDs (Tronche et al. 1997). The production of IL-1, IL-6, and TNF- was inhibited in LPS-stimulated BV-2 cells, and brain inflammations were decreased in a PD model treated with α-asarone. Furthermore, α-asarone administration successfully prevented the LPS-stimulated activation in BV-2 microglial cells by regulating NF-kB and preventing the degradation of inhibitory NF-kB signaling. Furthermore, β-asarone altered the expression of Bcl-2, Beclin-1, JNK, and p-JNK in 6-OHDA-injected PD rats, as shown by in vivo experimental data. β-asarone’s neuroprotective effects arise from its suppression of JNK and p-JNK expressions and its subsequent indirect upregulation of Bcl-2 expression. Inhibition of the pERK pathway by β-asarone could enhance Bcl-2, and Bcl-2 might reduce Beclin-1 expression. β-asarone was shown to regulate autophagy and ER stress via the PERK/CHOP/Bcl-2/Beclin-1 pathway, according to the study’s findings (Johnson et al. 2010).
Theaflavin interactions with Bax, caspase-3, and Bcl-2
It is a kind of polyphenol that may be found in black tea. Due to its antioxidant qualities, theaflavin (TF) is used for a variety of medicinal purposes: the capacity to chelate metals and neutralize free radicals (Schuh and Schieberle 2006). The effects of TF on 6-OHDA-induced SH-SY5Y cell lines were analyzed by measuring changes in cell viability, nuclear morphology, apoptosis, matrix metalloproteinase (MMP) expression, and nitric oxide (NO) levels. These data showed that TF protected against 6-OHDA-induced apoptosis via decreasing NO and ROS generation (Zhang et al. 2016). By reducing Bax and caspase-3 protein expression and increasing Bcl-2 expression, TF alleviated oxidative stress in H2O2-treated PC12 cell lines. These results suggest that TF has antiapoptotic properties, which it uses to both protect cells and promote their survival (Anandhan et al. 2013). Another study found that administering MPTP increased the release of inflammatory markers like interleukin-1beta (IL-1β), IL-6, tumor necrosis factor-alpha (TNF-α), and IL-10, along with glial fibrillary acidic protein (GFAP) and the pro-apoptotic marker Bax. Additionally, it decreased levels of the anti-apoptotic marker Bcl-2. However, when mice were orally given the black tea polyphenol TF before MPTP administration, TF significantly reduced MPTP-induced neuroinflammation and apoptosis (Anandhan et al. 2013).
Alzheimer’s disease
The majority of cases of dementia are due to AD. Amyloid-beta protein plaques seen outside of the neural cell body and tau protein tangles found inside of the neural cell body are hallmarks of this widespread, progressive neurocognitive disease. In the end, these traumas destroy all mental functions and cause death (Vradenburg 2015). According to the World Alzheimer Report 2015, the number of individuals affected by dementia is expected to quadruple every 20 years, reaching 468 million by 2050. Various preclinical experiments were performed to evaluate the potential of phytochemicals in AD, and the results were found to be impressive. The target areas of phytochemicals for the treatment of AD in preclinical studies are shown in Fig. 3.
Neuroprotective effects of phytochemicals on AD and their interactions with apoptotic markers and inflammatory markers
New evidence suggests that substances found in plants, known as phytochemicals, may be a valuable resource for discovering potential treatment candidates for AD. In this review, we will introduce you to a few different types of phytochemicals and talk about how they can serve as neuroprotective agents in the future. Ginkgo biloba, Melissa officinalis, curcumin, epigallocatechin gallate catechins, and resveratrol, in particular, are engaged in anti-amyloidogenic and anti-inflammatory pathways in addition to their antioxidant activities. We will discuss the molecular targets of these carefully chosen phytochemical substances, drawing attention to the connections between their neuroprotective effects and their potential therapeutic relevance in AD.
Ginkgo biloba interactions with Bax and caspase-3
Several bioactive compounds, including diterpenes, ginkgolides A, B, C, J, and M, the sesquiterpene bilobalide, and a variety of flavonoids, may be found in G. biloba leaf extracts (Galende et al. 2021). There are evidences suggesting that Ginkgo biloba extract (GBE) inhibits neuronal loss in substantia nigral region from 6-OHDA-induced poptosis in PD rat model by modulating apoptosis, specifically by affecting Bax and caspase-3 proteins. Studies have shown that GBE possesses neuroprotective properties and can attenuate neuronal cell death by regulating apoptotic pathways (Fei et al. 2013). GBE defends against the neurotoxicity caused by beta-amyloid peptide–induced toxicity in SH-SY5Y cells, GBE was able to block Aβ (1–42)–induced cell apoptosis, mitochondrial dysfunction, and activation of c-jun N-terminal kinase (JNK) (Shi et al. 2010).
Melissa officinalis interaction with caspase-3
M. officinalis terpenes like Melissa has been used for ages to cure a variety of ailments, it is a powerful antioxidant that may directly neutralize free radicals. This plant is rich in polyphenols, which have strong antioxidant properties. Polyphenols, being polar molecules, tend to accumulate in the sour part of the mixture. It has been hypothesized that the anti-apoptotic and antioxidant properties of polyphenols in the acidic fraction shield CGNs from the damaging effects of Aβ (Shi et al. 2010). Caffeic acid analogy are the polyphenols found in M. officinalis extract. For example, rosmarinic acid is the most abundant polyphenol. Rosmarinic acid inhibits cell death or apoptosis. Rosmarinic acid’s capacity to block caspase-3 activation protected PC12 cells against Aβ-induced apoptosis (Psotova et al. 2006). Mitochondrial membrane permeability caused by Aβ aggregation can be blocked by rosmarinic acid, which also possesses mitoprotective properties. As a result of its ability to fortify mitochondrial membranes, rosmarinic acid reduces the amount of cell death caused by Aβ (Moon et al. 2010).
Ferulic acid interactions with caspases and Cox-2
Plants contain the compound 4-hydroxy-3-methoxy cinnamic acid, often known as ferulic acid (FA). Its name comes from the plant Ferula foetida, which was the source of the first isolations of the compound in 1866 (Babbar et al. 2021). The hippocampus cornus ammonis1 (CA1) area of the rat brain significantly expresses more activated p38 MAPK and IL-1 after intracerebroventricular injection of Aβ (Barone et al. 2009). A chemical that resembles an FA called sodium ferulate (SF) was able to block this action. Additionally, SF has the power to boost Akt activation and reverse the inactivation of ERK1/2 caused by Aβ (Jin et al. 2008; Chaudhary et al. 2019). The in silico analysis of ferulic acid (FA) with caspase-3 revealed interactions involving amino acids ARG179, HIS237, and CYS285 Additionally, SF reduced the caspase activation brought on by Aβ. The activation of the caspase-9, caspase-7, and caspase-3 cascade by the injection of Aβ in rats was prevented by the pre-treatment with SF, suggesting that SF helped to avoid neurotoxicity, FA has been demonstrated to improve the cell stress response (Abdulwanis Mohamed et al. 2019). These enzymes’ primary mode of activity is to mitigate the harm caused by free radicals. Contrarily, FA was found to suppress the production and/or activity of cytotoxic enzymes like cyclooxygenase-2, caspases, and inducible nitric oxide synthase (Sgarbossa et al. 2015).
Resveratrol interactions with Cox-1 and Cox-2
Researchers found that continuous resveratrol therapy decreased malondialdehyde and nitrite formation and increased glutathione (GSH) levels, suggesting that it has antioxidant capabilities (Kirimlioglu et al. 2008). Resveratrol’s antioxidant mechanisms, such as SIRT1 activation, A aggregation, and toxicity inhibition, metal chelation, and reactive oxygen species scavenging, were also reported (Gomes, et al. 2018). Trans-δ-viniferin (TVN), a stilbene dimer found in grapes, is produced in response to fungal infection. It is a major stilbene synthesized by grapevine leaves infected with Plasmopara viticola and has been shown to inhibit cox1,2 (Ficarra et al. 2016). This data demonstrates the efficacy of this chemical as a therapeutic method for the treatment of AD. In a study, the combined treatment of exercise training (ET) and resveratrol (Resv) demonstrated notable outcomes in the 3 × Tg-AD mouse model of AD. Specifically, Resv reduced neuroinflammation and Aβ oligomer buildup while elevating levels of neurotrophins and synaptic markers. It also increased silent information regulator levels and decreased markers associated with apoptosis, autophagy, endolysosomal degradation, and ubiquitination in the brains of the mice. Additionally, the treatment notably reduced caspase-3 and bax levels, indicating a potential modulation of apoptosis pathways (Broderick 2020).
Quercetin interactions with ILs and Bax
Quercetin, a flavonoid contained in many plant foods, has been shown to inhibit aggregation and reduce inflammation (David et al. 2016). Quercetin has been shown to diminish iNOS-mediated NO generation in lipopolysaccharide (LPS)-treated BV2 microglial cells by decreasing NF-kB activation and hence inhibiting Aβ aggregation. But in another study in human SH-SY5Y neuroblastoma cells that overexpressed APP751, the introduction of free radical toxicity and apoptosis occurred. The generation of reactive oxygen species induced by menadione significantly reduced after the cells was treated with myricetin, quercetin, or anthocyanin-rich extracts, in a dose-dependent manner. Interestingly, while these extracts effectively reduced the production of reactive oxygen species, they did not demonstrate any impact on caspase-3 activation, APP processing, or Aβ levels. In addition, quercetin has been shown to suppress the release of IL-6 and IL-8 in astrocytes and neuronal cells, hence reducing the inflammation caused by IL-1β also found to inhibit Bax activities. It has been established that quercetin can neutralize reactive oxygen species (ROS), and reactive nitrogen species (RNS), boost the production of GSH, and positively regulate the Nrf2 signaling pathway (Kang et al. 2013). Poor penetration of the blood–brain barrier, low bioavailability, and quick metabolism may explain why quercetin, despite its intriguing therapeutic potential, has been used in relatively few clinical trials (Ravishankar et al. 2016).
Luteolin interactions with Bcl-2, Bax, and caspase-3
The ability of luteolin to scavenge oxygen and nitrogen species is at the root of its many pharmacological actions and anti-oxidant characteristics. Micromolar doses of luteolin decrease cytokine production, nuclear factor kappa B (NF-kB) signaling, and toll-like receptor 4 (TLR4) signaling in immune cells which include mast cells. Additionally, luteolin has been demonstrated in vitro and in vivo to suppress microglial activation and produce BDNF-like behavior. Several genes whose mRNA expression was increased in response to exogenous 6-OHDA were downregulated by luteolin (Nabavi et al. 2015). A study investigated luteolin’s impact on triple transgenic AD (3 × Tg-AD) mice and primary neurons, revealing promising results. Luteolin supplementation notably improved memory and cognitive function in AD mice while providing neuroprotection by inhibiting Aβ generation, repairing mitochondrial damage, and reducing neuronal apoptosis. Notably, luteolin directly bound with peroxisome proliferator–activated receptor. Additionally, it led to reduced levels of caspase-3 and bax while increasing BCL-2 levels, suggesting a potential modulation of apoptotic pathways (He et al. 2023).
Epigallocatechin gallate interactions with ILs and Bcl-2
Green tea’s positive benefits are attributed in large part to epigallocatechin gallate (EGCG), the primary polyphenolic component of the tea plant Camellia sinensis (L.) Kuntze (Theaceae) (Granja, et al. 2017). EGCG and its metabolites have been shown in several in vitro and in vivo investigations to potentially have substantial neuroprotective actions. EGCG has been shown to promote APP-cleavage in experimental settings. Multiple studies have shown that EGCG has anti-inflammatory effects such as human astrocytoma U373MG cells, EGCG suppresses the activation of MAPK and NF-B, which then leads to a reduction in the production of IL-6, IL-8, and vascular endothelial growth factor along with that EGCG increases the synthesis of an antiapoptotic molecule such as Bcl-2 (Kim et al. 2007). Moreover, EGCG has been shown to improve cognitive performance by scavenging reactive oxygen species and blocking the apoptotic effects of amyloid beta (Aβ) on mitochondria (Mandel et al. 2004).
Curcumin interactions with ILs and TNF-α
Inhibition of NF-kB and mitogen-activated protein kinase (MAPK) activation by curcumin in LPS-stimulated microglia has been shown (Jung et al. 2006). Curcumin therapy reduces LPS-induced neuroinflammation and cognitive decline in an animal model of AD, supporting our findings (Millington, et al. 2014). Curcumin inhibits Erk1/2 and p38 MAPK pathways, which leads to a concentration-dependent reduction in IL-1β, IL-6, and TNF-α production in Aβ-activated microglia. Curcumin can help mitigate the damaging effects of free radicals (Shi et al. 2015). Curcumin’s β-diketone structure and phenolic groups, in particular, appear to be responsible for its antioxidant activity and its capacity to scavenge free radicals. Curcumin has a powerful protective impact on neuronal cultures by mitigating the neurotoxicity of hydrogen peroxide and ferric iron (Wright 2002).
Huntington’s disease
It is a fatal neurological disorder passed down in an autosomal dominant pattern of inheritance. Huntingtin (Htt) is a 350 kDa protein that is widely produced and typically has a repeat of 8–25 glutamines in its N-terminal portion1 (Chongtham and Agrawal 2016). The extension of the CAG tract beyond 35 repetitions in exon 1 of the IT15 gene producing the Htt protein causes the dominant neurological illness known as HD. Patients with an expanded CAG repeat of 35 to 40 are at an increased risk of developing HD compared to those whose HTT had the standard or lower to 35 CAG repeat. HD is linked to the apoptosis of striatal-specific neurons, namely GABAergic MSNs (Saudou and Humbert 2016). Recent studies have indicated that plant extracts, fractions, isolated compounds, and herbal formulations have effects on neurotoxicity and were tested on various chemically induced or transgenic HD models.
Neuroprotective effects of phytochemicals on HD and their interactions with apoptotic markers and inflammatory markers
The millions of plant species found in every corner of the globe have a wide variety of medicinal uses. It has been shown that natural substances with an antioxidant, anti-inflammatory, calcium antagonist, and anti-apoptotic, regulatory actions can prevent or treat certain forms of neurodegenerative illness. Here, we describe several plants and phytochemicals that have shown promise in combating 3-nitropropionic acid-induced neuronal dysfunction, a commonly used animal model for HD.
Centella asiatica interactions with IL-1β and TNF-α
It is a plant being used in Ayurveda medicine that is native to places like India, Sri Lanka, Madagascar, South Africa, and Malaysia. Memory improvement and improved cognitive function are two of the key outcomes identified in animal experiments with C. asiatica, which has been the subject of several recent neuropharmacological investigations (Orhan 2012). In a study CA prophylaxis significantly protected against 3-NP acid–induced GSH depletion, as demonstrated by the recovery of GSH levels in the striatum and other brain areas. The cytosolic portions of the striatum and other brain areas also showed restored GSH levels. So, some mechanism(s) connected to glutathione (GSH) is responsible for shielding different parts of the brain from 3-NPA–induced oxidative stress. Since CA consistently increased the activity of several antioxidant enzymes, this may also be a contributor to the protective effect of CA extract in the brain, apart from those protective effects of CA prophylaxis are also reported to maintain the function of the mitochondrial respiratory chain and, by extension, energy metabolism. Moreover, the protective effects of CA prophylaxis were also noted in the maintenance of mitochondrial respiratory chain function, subsequently impacting energy metabolism. Additionally, CA supplementation led to decreased levels of interleukins IL-1β and TNF-α, further highlighting its role in modulating the immune response and reducing inflammation in the brain (Shinomol 2008). The target areas of phytochemicals for the treatment of HD in preclinical studies are shown in Fig. 4 which was induced by 3-Np.
Cannabis sativa L. interactions with caspase-3
There are several blooming plants in the Cannabis genus, including the well-known Cannabis sativa, which is found across Central and South Asia. Throughout the brain and spinal cord, the cannabinoid (CB) system regulates fundamental neurophysiological processes including pain, hunger, and cognition. In addition to endogenous CB molecules, Phytocannabinoids derived from cannabis act on the G protein–coupled CB receptors CB (1) and CB (2). C. sativa contains two primary components, (9)-tetrahydrocannabinol (-9-THC), and cannabidiol (CBD) (Hashim 2011). Cannabinoids, which have anti-inflammatory, neuroprotective, and neuroregenerative effects, reduced hyperkinetic symptoms and worked as disease-modifying drugs in several animal models of HD. An HD clinical study including Sativex®, an equimolecular mix of (9)-THC and/or CBD, is imminent (Sagredo et al. 2007; Sagredo et al. 2012). Researchers found that (9)-THC and CBD had opposing impacts on human brain function and psychopathology. However, (9)-THC showed potential impacts on several brain areas, whereas CBD was discovered to engage the limbic and paralimbic systems (Fusar-Poli et al. 2009). In addition, the findings suggest that WIN55.212.2 (cannabinoid receptor agonist) has a role in limiting caspase activity in PC12 cells. This inhibition of caspase activity might be one of the mechanisms by which WIN55.212.2 exerts its protective effects against cell death induced by PSI (proteasomal synthase inhibitor) in these cells.
Garcinia kola Heckel interactions with Bax, Bcl-2, caspases, COX-2
Garcinia kola, sometimes known as bitter kola, is a plant native to the West African rain forest and is used there for the treatment of bronchitis, infections, and liver disorders in traditional medicine. The bioflavonoid kolaviron, isolated from Garcinia kola, has been credited with a variety of health benefits, including its ability to fight free radicals, prevent diabetes, inhibit cancer cell growth, and safeguard the liver (Buba et al. 2016). It was hypothesized that Garcinia kola’s analgesic or anti-inflammatory function contributed to the plant’s clinical success in treating knee osteoarthritis. The treatment with kolaviron shows notable efficacy in reversing the changes induced by atrazine in the expression of various apoptosis markers. Specifically, it leads to significant restoration in the altered expression levels of key markers associated with apoptosis. These markers include p53, Bax, Bcl2, caspase-3, caspase-9, and cyclooxygenase-2. Kolaviron treatment appears to have a regulatory effect on these markers, potentially counteracting the detrimental impact caused by atrazine on apoptosis-related pathways. In an in vivo study, when given 7 days beforehand, Garcinia kola aqueous extract (200 mg/kg) protected neurons in the hippocampus and cerebellum of 3-NP–induced starved mice, its mechanism of action is not fully reported, but it is estimated that it increases the endogenous antioxidants and prevents oxidative stress and slow down the apoptosis (Ajayi et al. 2011).
Gastrodia elata Blume interactions with Bax and caspase-3
The uncommon herb Gastrodia elata (Tianma), which is utilized in TCM, has been linked to a variety of cognitive-enhancing properties, including anticonvulsants, antihypertensive, and pro-learning and memory benefits. Tianma’s capacity to inhibit stress-related proteins and promote neuroprotective genes is responsible for the plant’s neuroprotective effect against beta-amyloid (A) caused toxicity (Kim et al. 2011). There are few evidences which indicate that Tianma has capacity to modulate Bax and caspase-3 functioning (Manavalan et al. 2012) Additionally, PC12 cells were significantly protected from H2O2-induced damage by two neuroprotective phenolics that were extracted from the rhizomes. Regulation of the adenosine A2A receptor (A(2A)-R) and proteasomal activity by G. elata inhibited mHtt aggregation in PC12 cells that had been transiently transfected with mHtt (Huang et al. 2011).
Sesamol interaction with caspase3, 9
Multiple animal studies have shown sesamol’s potent antioxidant properties. In a study, male rats given 3-NP (10 mg/kg) for 14 days exhibited impaired body weight, movement, and brain function. Pre-treatment with SML (5, 10, and 20 mg/kg) improved these factors and reduced brain damage. Additionally, SML modulated caspase-3 and Bax activity while enhancing brain enzymes, compared to the 3-NP-only group (Kumar et al. 2009a).
Schisandrin B interaction with caspase 9
Schisandrin B derived from Schisandra chinensis is a potential natural chemical with a wide range of reported pharmacokinetic properties, including antioxidant, anti-inflammatory, and neuroprotection. Schisandra chinensis contains bioactive chemicals Sch A, B, and C, which are used to prevent disease. Sch B is the most studied isoform. Sch B has been shown to have a role in neuroprotection by its ability to lower oxidative stress. Sch B protects PC12 cells from 3-NP–induced necrotic and apoptotic cell death. A mitochondria-dependent route may be involved in the process, since reduced activation of mitochondrial caspase-9 was seen in ( −) Sch B-pre-treated and 3-NP–challenged cells. Under 3-NP–challenged conditions, a rise in cellular GSH level correlated with cytoprotection. 3-NP also stimulates JNK pathway activation, which contributes to the pathophysiology of HD and cell death caused by 3-NP (–) Sch B provided cytoprotection while simultaneously decreasing the amount to which JNK was phosphorylated in response to 3-nitropropionic acid. This decreased phosphorylation of JNK was an indirect consequence of the increased glutathione redox state, which in turn lowered cellular oxidative stress (Lam and Ko 2012). Schisandrin B inhibited the signaling pathway that was dependent on toll-like receptor 4 (TLR4)/myeloid distinction primary response gene 88 (MyD88)/IB kinase (IKK)/NF-kB, and its anti-apoptotic and anti-apoptotic actions protected rat cortical neurons against A 1–42-induced neurotoxicity (Zeng et al. 2012).
Quercetin interactions with Bcl-2, bax, and caspase-3
Succinic acid dehydrogenase (SDH) staining in the striatum was recovered in 3-NP–treated rats when they were given quercetin. A possible link between 3-NP treatment and mitochondrial enlargement leads to detrimental ROS generation. Animals given 3-NP showed improved ETC enzyme performance after receiving a quercetin supplement, with ATP levels being recovered and mitochondrial swelling being prevented. Potentially attributable to quercetin’s positive effect is the flavonoid's antioxidant activity. It has been hypothesized that quercetin inhibits H2O2 generation via binding to and inactivating complex I and cyto-c. There is evidence that quercetin can inhibit mitochondrial permeability transition pore (MPT) pore opening and thereby reduce mitochondrial swelling (Sandhir and Mehrotra 2013). In a study, the administration of quercetin at a dosage of 10-mg/kg body weight per day demonstrated a reduction in aluminum-induced oxidative stress, characterized by decreased reactive oxygen species (ROS) production and increased activity of mitochondrial superoxide dismutase (MnSOD). Notably, quercetin prevented the aluminum-induced translocation of cytochrome c (cyt-c). Furthermore, quercetin exhibited a regulatory effect on key apoptotic markers, including upregulation of anti-apoptotic Bcl-2, and downregulation of pro-apoptotic Bax, p53, and caspase-3 activation (Sharma et al. 2016). The capacity of quercetin to enter cells and accumulate in mitochondria may explain why it has recently been revealed that it is most effective in guarding against indomethacin-induced mitochondrial dysfunctions (Ossola et al. 2009). It has also been established that via regulating the activities of succinate dehydrogenase and NADH oxidase, quercetin supplementation protects mitochondrial integrity, size, and functions.
Interaction of α-mangostin with Bcl-2, Bax, and caspase-3
α-Mangostin, the first xanthone isolated from Garcinia mangostana, has been characterized by its antioxidant effects. The research presents new information regarding the antioxidant molecule α-role mangostin’s in ROS scavenging and its neuroprotective impact. It has been discovered that a-mangostin can scavenge 1 O2˙ and O2˙, and we have verified that α-mangostin can scavenge ONOO. Under our test circumstances, a-mangostin did not affect OH˙ or H2O2 levels. Primary cultures of CGNs, which have been suggested as a promising in vitro model for examining mechanisms of neuroprotection due to prior research showing that this neuronal type is prone to 3-NP toxicity, were used to examine the possible neuroprotective impact of this xanthone against 3-NP (Pedraza-Chaverrí et al. 2009). In a study specifically, alpha-mangostin was effective in reducing the formation of reactive oxygen species (ROS) induced by MPP + . Additionally, the ratio of Bax/Bcl-2 expression and the expression of p53 were notably lower in cells treated with alpha-mangostin in combination with MPP + compared to cells treated solely with MPP + (Janhom and Dharmasaroja 2015).
S-allyl cysteine interactions with caspase-12 and caspase-8
S-allyl cysteine (SAC), a key organosulfur compound found in garlic, has been hypothesized to have neuroprotective effects. Specifically, it protected rat brain synaptosomes against mitochondrial malfunction, lipid peroxidation, and oxidative stress caused by 3-NP. Under conditions of depleted or sequestered extracellular Ca2+, SAC is still able to significantly reduce the oxidative damage produced by a model of combined excitotoxicity and energy deficit, indicating that this agent is not only a powerful antioxidant in nerve tissue preparations involving Ca2+-induced excitotoxic events but also a potential anti-excitotoxic and energy-preserving agent (Pérez-De La Cruz et al. 2006; Colín-González, et al. 2012). The study revealed that exposure of organotypic hippocampal slice cultures to tunicamycin resulted in a notable increase in calpain activity, leading to the cleavage of a-spectrin by calpain. Additionally, this exposure led to the activation of caspase-12 (at 42 kDa) and caspase-3 (at 29 kDa), indicating the involvement of the calpain-dependent caspase-12-related apoptotic pathway in TM-induced neuronal death specifically within the hippocampus. In contrast, SAC demonstrated its efficacy by suppressing caspase-3 activity through the inhibition of calpain (Imai et al. 2014).
Naringin interactions with Bcl-2, Bax, and Bad
It has been documented that naringin, a flavanone glycoside found in citrus fruit extracts, has cardioprotective, antiapoptotic, and antioxidant properties. In the research, Bcl-2 expression was suppressed after 3-NP injection, whereas Bax and Bad expressions were elevated. As an interesting side note, naringin reduced the incidence of apoptosis in 3-NP–induced rats by restoring the expression of Bcl-2 family proteins to normal, suggesting that this protein family is involved in the protection afforded by naringin against 3-NP–induced neuronal death. Besides, cytochrome-c emission in 3-NP–induced rats is suppressed by naringin therapy (Pérez-De La Cruz et al. 2006; Cui et al. 2018).
Lycopene interactions with Bax, Bcl-2, and caspase-3
Tomato (Solanum lycopersicum L.) lycopene is a carotenoid antioxidant that has been studied for its potential to prevent heart disease, reduce inflammation, prevent genetic mutations, and fight cancer (Kumar, et al. 2021). By modulating nitric oxide, lycopene (at doses of 2.5, 5, and 10 mg/kg) enhanced the recovery of 3-NP–intoxicated rats in terms of behavior, biochemistry, and mitochondrial function. In a study conducted on primary cultured rat cortical neurons, lycopene demonstrated its efficacy by restoring the levels of proapoptotic Bax and antiapoptotic Bcl-2. Additionally, lycopene showed inhibition of caspase-3 activation (Qu et al. 2011). By protecting mitochondria, lycopene protected rat CGNs against the neurotoxicity caused by methylmercury (Kumar et al. 2009b).
Lutein interactions with Bax, Bcl-2, and caspase-3, 8, and 9
Restoration of mitochondrial complex activity by lutein prevents 3-NP–induced cell death by restoring normal ATP production. Pre-treatment with lutein (50 mg/kg) was associated with minor focal gliosis and neuronal degeneration, while treatment with lutein (100 mg/kg) was associated with mild focal gliosis alone. Therefore, lutein’s protective effect has been reaffirmed by histopathological data. The histological structure of the brain striatum was unaltered in those who were given lutein per se. Accordingly, this suggests that lutein is safe to use in typical situations. In experiments, the results of the neurobehavioral, biochemical, and histopathological studies show that lutein can reduce the neurotoxicity caused by 3-NPs by providing protection against behavioral changes, restoring antioxidant defense enzymes in the rat brain, and improving levels of mitochondrial enzymes (Binawade and Jagtap 2013). Lutein exhibited a protective effect against MPTP-induced neuronal damage and apoptosis by modulating key markers involved in the apoptotic pathway. Specifically, it inhibited the activation of pro-apoptotic markers such as Bax, caspases-3, 8, and 9, which are typically associated with promoting cell death. Additionally, lutein enhanced the expression of the anti-apoptotic marker Bcl-2 (Nataraj et al. 2016).
Ginsenoside Rg1 interactions with Bcl-2, caspase-3, 9
Panax ginseng C.A. Meyer contains ginsenosides, a wide variety of steroidal saponins. Rg1 has been shown to cross the BBB and improve several neurological conditions. The weight loss and behavioral abnormalities caused by 3-NP were dramatically reduced when the animals were pre-treated with Rg1. Additionally, Rg1 reduced 3-NP–induced neuronal loss and microscopic morphological damage in the striatum. We also discovered that Rg1 blocked 3-NP–induced apoptosis in the striatum and prevented SDH inactivity. Reduced synthesis of proinflammatory cytokines (TNF-α and IL-1) in the striatum as a result of 3-NP was likewise blocked by Rg1. Finally, study results demonstrated that Rg1 inhibited striatal 3-NP–induced activation of MAPK and NF-kB signaling (Hanna et al. 2015; Yang et al. 2021). In a study, administration of rosiglitazone was found to increase the activity of heme oxygenase-1 (HO-1). Separate research suggested that a lack of HO-1 led to a notable increase in the rate of apoptotic cells. Furthermore, additional studies highlighted that the HO-1–mediated inhibitory effect on apoptosis involved a reduction in caspase-3 cleavage in cerebral ischemia–reperfusion (IR) rats. Consequently, this reduction in apoptosis was observed in the hippocampus. Changes in the expression of related proteins, such as increased levels of bcl-2 and decreased levels of cleaved caspase-3 and cleaved caspase-9, were also observed, indicating the involvement of these proteins in the apoptosis-inhibitory effect mediated by ginsenoside (Yang et al. 2015).
Curcumin nanoparticle interactions with Bcl-2
The therapy with C-SLNs demonstrated a considerable recovery of the functioning of the brain’s mitochondrial complexes as well as the levels of cytochrome. The treatment with C-SLNs partially reversed reductions in glutathione (GSH) and superoxide dismutase (SOD) activity. It is anticipated that this treatment led to an increase in Bcl-2 levels. Treatment with C-SLNs also significantly reduced mitochondrial swelling, lipid peroxidation, protein carbonyls, and ROS (Sandhir et al. 2014).
Amyotrophic lateral sclerosis
It is a progressive and fatal neurodegenerative disorder that begins with the malfunctioning of motor neurons in the spinal cord and brain and typically manifests within months or years. This debilitating illness currently has no known therapeutic option. Between 3 and 5 years of the start of various symptoms, the majority of ALS patients die from respiratory failure (Rowland and Shneider 2001). Herbal preparations, plant extracts, fractions, isolated chemicals, and other forms of phytotherapy have been studied recently for their potential neuroprotective benefits on animal models of amyotrophic lateral sclerosis (Mir et al. 2022).
Neuroprotective effects of phytochemicals on ALS and their interactions with apoptotic markers and inflammatory markers
Clinical data reveal mixed results with promise for the use of phytochemicals as an adjuvant to the standard treatment in a variety of NDs, but findings from preclinical research show that the phytochemicals have beneficial effects in ALS. These findings demonstrate phytochemicals can affect oxidative stress, inflammation, apoptotic pathways, and gene regulation. A few species of terpenoids and flavonoids have shown their therapeutic effects.
Madecassoside interactions with caspase-3
It is a triterpenoid saponin that was first extracted from Centella asiatica. According to studies using a transgenic SOD1-G93A mouse model of ALS, madecassoside protects motor neurons against degeneration and extends the animals’ lifespan. Madecassoside has been shown to decrease malondialdehyde levels and boost SOD activity in the brain. Madecassoside increases antioxidant potential and shields neurons from an amyotrophic lateral sclerosis model that was developed using mice against apoptosis caused by free radicals. Madecassoside, it has been shown, reduces LPS-induced neurotoxicity in rats via activating the Nrf2-HO pathway (Zhang et al. 2014; Silva, et al. 2020). In a study, madecassoside at varying concentrations (10, 30, 100 μmol/L) demonstrated the ability to reverse morphological alterations, enhance cell viability, elevate glutathione levels, and reduce lactate dehydrogenase and malondialdehyde levels induced by H2O2 in a concentration-dependent manner. Moreover, it showed a capacity to attenuate apoptosis by preventing the activation of caspase-3, preserving mitochondria membrane potential, and inhibiting the phosphorylation of p38 mitogen–activated protein kinase (MAPK) in human umbilical vein endothelial cells (HUVECs) (Bian et al. 2012).
Epigallocatechin gallate interaction with Bcl-2
EGCG has been demonstrated to exhibit potent antioxidant action. Further testing of EGCG’s anti-ALS antioxidant action was conducted in transgenic SOD1 mice, where it was found to delay the onset of symptoms and increase survival. In addition, the elevation of the anti-apoptotic Bcl-2 expression was also observed with EGCG, suggesting that the antioxidant effect of EGCG in ALS is connected to the activation of the Bcl-2 gene (Koh et al. 2006).
Ampelopsin interaction with caspase-3
It is derived from the Ampelopsis grossedentata, and is a powerful antioxidant since it is a flavonoid. Against H2O2-induced apoptosis in PC-12 cells, ampelopsin was found to have therapeutic potential by decreasing ROS production, increasing HO-1 gene transcription, and decreasing caspase-3 expression. Protein HO-1 overexpression in PC-12 cells is also reliant on the 1/2 (ERK1/2) and Akt signaling pathways. Researchers concluded that ampelopsin showed promise as a potential treatment for ALS and other neurodegenerative disorders (Kou et al. 2012).
Celastrol interaction with caspase-3
Celastrol is a triterpenoid pigment that was first identified in Tripterygium wilfordii. Celastrol inhibits the production of tumor necrosis factor alpha and inducible nitric oxide synthase in the lumbar spinal cord section of transgenic mice with the SOD1-G93A model of ALS. This results in a delay in disease onset and an improvement in motor function. In addition, it was shown that celastrol, on a molecular level, prevents LPS-mediated activation of the mitogen-activated protein kinase/ERK1/2 signaling pathway and NF-kB, both of which are crucial in cellular damage and stress. Accordingly, celastrol inhibits microglial cell activation, which leads to less production of inflammatory cytokines (Sethi et al. 2007; Li, et al. 2017). In a study, celastrol demonstrated protective effects against cadmium (Cd)-induced apoptotic cell death in neuronal cells. The findings indicated that celastrol notably mitigated the reduction in cell viability, morphological alterations, nuclear fragmentation, and condensation induced by Cd. Additionally, celastrol effectively inhibited the activation of caspase-3 in neuronal cells following exposure to Cd.
Concurrently, celastrol was observed to markedly impede the Cd-induced phosphorylation of c-Jun N-terminal kinase (JNK) in neuronal cells. However, it did not significantly affect the phosphorylation status of extracellular signal-regulated kinases 1/2 (ERK1/2) and p38 in the same cells. These results suggest that celastrol’s protective mechanism against Cd-induced neuronal cell apoptosis involves the inhibition of JNK phosphorylation, contributing to its ability to prevent caspase-3 activation and subsequent cell death (Chen et al. 2014).
Paeoniflorin interactions with Bax, Bcl-2, and caspase-3
Isolated from the roots of the Paeoniae plant, paeoniflorin plays a pivotal function as a neuroprotective drug in the treatment of ALS by blocking calcium entry into the cytoplasm of PC12 cell injury models. Further, it prevents PC-12 cells from undergoing apoptosis by reducing the amount of calcium that builds up inside the cells as a result of glutamate. And further, Paeoniflorin’s neuroprotective activity is demonstrated by its ability to inhibit NMDA-induced neurotoxicity in PC-12 cells (Wang et al. 2014; Peng et al. 2022). The effects of paeoniflorin were observed to mitigate inflammation and decrease caspase-3 activity, consequently inhibiting cell death in Alzheimer’s disease (AD) mice. These protective effects were linked to specific molecular changes, including an increase in the Bcl-2/Bax ratio and elevated levels of p-Akt expression. Additionally, paeoniflorin was found to downregulate the expression of phosphorylated p38 mitogen–activated protein kinase (p-p38 MAPK), a signaling molecule associated with inflammation and cell death pathways in AD (Gu et al. 2016).
Multiple sclerosis
It is a persistent autoimmune inflammatory disorder affecting the central nervous system (CNS). It is characterized by the loss of myelin, damage to neurons, and disruption of the BBB (Qureshi et al. 2018). To make a diagnosis, it is necessary to have evidence of lesions that are both temporally and spatially distinct, and to rule out other inflammatory, structural, or genetic disorders that could present with comparable clinical symptoms (Nicholas and Rashid 2013). The susceptibility and outcome of a condition may be influenced by both genetic and non-genetic factors, such as the environment and stochastic events. However, the extent to which each of these factors contributes is still not fully understood. Every environmental influence is expected to be widespread and have an impact on a population as a whole, rather than being limited to the family’s immediate surroundings (Dyment et al. 2004). Epidemiological studies have demonstrated that the advancement and emergence of MS are influenced by immunological, genetic, and environmental variables (Qureshi et al. 2018).
Neuroprotective effects of phytochemicals on MS and their interactions with apoptotic markers and inflammatory markers
There is strong evidence indicating that some dietary or phytobioactive substances have significant antioxidant and anti-inflammatory properties. These chemicals play a role in inhibiting the oxidative and inflammatory processes linked to neurodegenerative disorders (Joseph et al. 2009; Hashimoto and Hossain 2011). Given that MS is a complex autoimmune disorder characterized by inflammation in the nervous system, it is advisable to carefully evaluate the use of different phytochemicals, particularly those that scientific research has proven to be useful in lowering inflammation, improving mood, and supporting the health of nerve cells (Crespo-Bujosa and Gonzalez 2018).
Curcuma longa interaction with caspase-3
Curcumin, a hydrophobic yellow diphenolic compound found in turmeric, has the ability to interact with and regulate several cells signaling pathways. This property allows it to slow the progression of different autoimmune neurological illnesses, such as multiple sclerosis (MS). It has the ability to regulate proteins involved in cell cycle regulation, enzymes, cytokines, and transcription factors in central nervous system illnesses, such as multiple sclerosis (Qureshi et al. 2018). The primary polyphenol contents of CL are curcuminoids, which consist of three chemical components: curcumin (75–80%), demethoxycurcumin (15–20%), and bisdemethoxycurcumin (3–5%) (Wang et al. 2016). It effectively scavenges superoxide anions and has neuroprotective and anti-aging properties. Additionally, it has the capability to traverse the blood–brain barrier and access the brain. In a study, curcumin demonstrated its ability to protect neurons from oxidative damage. This protection was evidenced by a reduction in the expression of phosphorylated p38 (p-p38), decreased activation of caspase-3, and inhibition of toxic quinoprotein formation. Additionally, curcumin I facilitated the restoration of phosphorylated tyrosine hydroxylase (p-TH) levels, a key enzyme involved in dopamine synthesis and neuronal function (Meesarapee et al. 2014).
Hypericum perforatum interaction with Bax
This plant belongs to the Hypericaceae family and is commonly utilized in traditional medicine to treat mild to moderate depression. Additionally, it has been employed for its antioxidant, anti-inflammatory, and wound healing properties (Sani et al. 2016). The phytochemical composition of HP comprises several groups such as phenolic acids (specifically chlorogenic acid), flavonoids (including rutin, hyperoside, isoquercitrin, quercitrin, and quercetin), napthodianthrones (hypericin and pseudohypericin), and phloroglucinols (hyperforin and adhyperforin) (Asadian et al. 2011). Hyperforin, in addition to its neurological effects, is recognized as one of the primary active components of HP. It also has anti-inflammatory properties by suppressing the growth and triggering cell death of phagocytic cells (Naziroglu et al. 2014). A study revealed that SHP1 (extract of Hypericum perforatum) exhibited the ability to inhibit the apoptotic cascade by decreasing Bax levels. This anti-apoptotic action suggests that SHP1 may exert its neuroprotective effects, at least partially, by modulating apoptotic pathways and reducing cell death in the substantia nigra induced by rotenone (Gomez et al. 2013).
Lipoic acid interactions with ILs and caspase-3
Lipoic acid (LA) is a potent antioxidant that demonstrates efficacy in the treatment of experimental autoimmune encephalomyelitis (EAE), which serves as a model for MS (Chaudhary et al. 2011). LA exerts its influence by impeding the movement of T cells into the central nervous system (CNS), diminishing the expression of adhesion molecules in endothelial cells, and leading to a decrease in the production of MMP-9. Recently, we discovered that LA induces the generation of cAMP through the activation of prostaglandin EP2 and EP4 receptors, which are connected to G proteins, on immune cells (Schillace et al. 2007; Salinthone, et al. 2008). The therapeutic efficacy of LA has also been demonstrated in the animal model of MS; LA effectively decreased demyelination and axonal damage in mice52. Studies have indicated that the treatment of ALA to rats with EAE significantly reduces inflammation, demyelization, and axonal loss. Additionally, it decreases the amount of CD3 + T cells and CD11b + monocyte/macrophage cells in the spinal cord (Marracci et al. 2002). Neurological diseases and autoimmune disorders, such as MS, have been suggested to be caused by immunologic cascade and inflammation, according to recent research (Smith 2006; Taupin 2008). Recent studies indicate that LA can lower the IL-6 and caspase-3 levels in individuals with MS and in against hippocampal damage after pilocarpine-induced seizures by exerting an anti-inflammatory and antiapoptotic effect and stimulating protein kinase A, leading to an increase in cAMP (Salinthone et al. 2010; Gomez et al. 2013).
Omega 3-fatty acid interactions with Bax, Bcl2, and caspase-3
The key pathogenic pathways implicated in MS encompass immune-mediated inflammation, oxidative stress, and excitotoxicity (Mir, et al. 2016). Several modest investigations have shown that patients with MS had lower levels of PUFA (polyunsaturated fatty acids) in their serum, cerebral white matter, erythrocytes, and lymphocytes compared to individuals without MS (Gul et al. 1970; Fisher et al. 1987; Wilson and Tocher 1991; Koch et al. 2006). In a study, neurons treated with docosahexaenoic acid (DHA) and glutathione (GSH) showed enhanced expression levels of both Bcl-2 and Bcl-xL, which are proteins known for their anti-apoptotic properties. Additionally, these treated neurons exhibited reduced expression of cleaved caspase-3, a marker associated with apoptosis or programmed cell death. These findings suggest that the treatment with DHA and GSH contributed to a protective effect against ischemic injury in cortical neurons by promoting anti-apoptotic pathways and reducing activation of caspase-3, thus potentially preventing cell death in this context.
Herbal-conventional synergy in neurodegenerative care
The integration of herbal medicines with conventional therapies is a widespread practice. Existing evidence primarily suggests potential benefits in promoting mitochondrial health, enhancing endogenous antioxidant defenses, and reducing inflammatory markers such as TNF-α and ILs. However, it is crucial to note that there is currently no specific clinical study available on the combined use of allopathic medicine with phytochemicals. Notable examples of herbal remedies include Centella asiatica and Ashwagandha, both frequently employed in Ayurvedic practices for neurological disorders, which Ayurveda attributes to imbalances in Vata dosha. Studies indicate that Ayurvedic treatments, encompassing the use of specific herbal medicines and Panchakarma therapies such as Snehan, Swedana, Virechana, Vasti, Vamana, Shirodhara, Sirovasti, Murdha Taila, and Nadi Swedana, show synergistic effects and promise in managing neurological problems (Choudhury 2015). However, it is crucial to underscore that further research is needed to comprehensively understand the mechanisms and efficacy of these integrative approaches in neurodegenerative disease management. The World Health Organisation (WHO) reports that the global use of herbal treatments is two to three times higher than that of conventional medications (Pal and Shukla 2003). Herb use rarely has “pharmacological” effects. Knowing the effects of medicinal plants and conducting a clinical trial to determine their medical use are crucial. A proposal is to replace “side effects” with “indications” and “contraindications” when discussing herb use. Herbal medicine supports the body’s natural healing processes, while synthetic pharmaceuticals treat symptoms of specific conditions based on scientific pathology. Herbal medicines often have a minor effect by supporting weak systems or processes or removing strong factors (Karimi et al. 2015).
Challenges and future directions in herbal neuroprotective research
The research and development of herbal-based neuroprotective agents for various neurodegenerative diseases face challenges such as standardization issues, limited bioavailability, and a lack of comprehensive mechanistic understanding. Ensuring consistent product quality, enhancing bioavailability, and uncovering the precise mechanisms of action are critical challenges. Additionally, the field encounters skepticism due to a dearth of rigorous clinical trials and concerns about safety and herb–drug interactions. Future directions involve leveraging advanced analytical techniques for better compound identification, exploring combination therapies, and embracing personalized medicine approaches. Biomarker discovery, improved clinical trial design, global collaboration, robust regulatory frameworks, and enhanced public awareness are crucial for overcoming challenges and advancing herbal-based neuroprotective agents as promising interventions for neurodegenerative diseases.
Conclusion
The objective of this study was to provide a comprehensive overview of recent advancements in plant chemical research, particularly those recognized for their anti-apoptotic effects and potential applicability in treating neurodegenerative diseases. A notable revelation from this study is the discernment that targeting individual components may not suffice to impede cell death, given the intricate nature of herbal extracts and formulations containing multiple constituents. This complexity necessitates further research to evaluate the efficacy of existing botanicals in therapeutic interventions. Moreover, it underscores the need for an in-depth exploration of their therapeutic potential, unraveling the underlying mechanisms of action. Our review delved into the potential modulatory roles of caspases, Bcl-2, and Bax—key regulators of apoptosis—and elucidated the interactions of phytochemicals with these variables. Additionally, we examined the impact of plant-derived bioactive compounds on proinflammatory indicators, shedding light on their potential in mitigating inflammation, a pivotal factor in neurodegenerative diseases. Further investigations are imperative, considering the ongoing clinical trials involving promising compounds like Centella asiatica (phase 1), quercetin (phase 4 for mental disorders, phase 2 for Alzheimer’s), palmitoylethanolamide combined with luteolin (phase 2 for frontotemporal dementia), EGCG (epigallocatechin gallate) (phase 2 for Huntington’s disease), sunphenon EGCg (epigallocatechin-gallate) (phase 2 for early-stage Alzheimer’s disease), and CBD (cannabidiol) in phase 4 for amyotrophic lateral sclerosis. The promising outcomes of these trials underscore significant potential for utilizing phytochemicals in the future treatment of neurodegenerative disorders, suggesting a potential breakthrough in developing effective interventions for neurodegenerative segments.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PCD:
-
Programmed cell death
- Fas:
-
Fatty acid synthetase
- mFas:
-
Membrane fatty acid synthetase
- sFas:
-
Soluble fatty acid synthetase
- FasL:
-
Fatty acid synthetase ligand
- FasR:
-
Fatty acid synthetase receptor
- TNF:
-
Tumor necrosis factor
- TNFα:
-
Tumor necrosis factor-alpha
- TNFR1:
-
Tumor necrosis factor receptor 1
- TNFR2:
-
Tumor necrosis factor receptor 2
- APO-1:
-
Apoptosis antigen 1
- DISC:
-
Death-inducing signaling complex
- CD95:
-
Cluster of differentiation 95
- DD:
-
Death domain
- CD4:
-
Cluster of differentiation 4
- Bcl-2:
-
B cell lymphoma protein 2
- TRADD:
-
TNF receptor–associated death domain
- NF-kB:
-
NF-kappa B
- JNK:
-
C-Jun N-terminal kinase
- MOMP:
-
Mitochondrial outer membrane permeabilization
- SMAC:
-
Second mitochondrial activator of caspases
- DIABLO:
-
Direct IAP–binding protein with low PI
- HtrA2:
-
High-temperature requirement
- AIF:
-
Apoptosis-inducing factor
- CAD:
-
Caspase-activated DNase
- Bcl-10:
-
B cell lymphoma protein 10
- BAX:
-
BCL2-associated X protein
- BAK:
-
BCL2 antagonist killer 1
- BID:
-
BH3-interacting domain death agonist
- BAD:
-
BCL2 antagonist of cell death
- BIM:
-
BCL2-interacting protein BIM
- BIK:
-
BCL2-interacting killer
- BLK:
-
Bik-like killer protein
- Bcl-X:
-
BCL2 like 1
- Bcl-XL:
-
BCL2-related protein, long isoform
- Bcl-XS:
-
BCL2-related protein, short isoform
- Bcl-W:
-
BCL2 like 2 proteins
- BAG:
-
BCL2-associated athanogene
- Puma:
-
BCL2-binding component 3
- Noxa:
-
Phorbol-12-myristate-13-acetate–induced protein 1
- Caspase-9:
-
Cysteinyl aspartic acid-protease-9
- UPR:
-
Unfolded protein response
- ER:
-
Endoplasmic reticulum
- IP3R:
-
1,4,5-Triphosphate receptor
- RyR:
-
Ryanodine receptor
- LMP:
-
Lysosomal membrane permeabilization
- AD:
-
Alzheimer’s disease
- PD:
-
Parkinson’s disease
- HD:
-
Huntington’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- Cyto-C:
-
Cytochrome C
- LPS:
-
Lipopolysaccharide
- COX-2:
-
Cycloxygenase-2
- IL-1:
-
Interluekin-1
- IL-6:
-
Interluekin-6
- MAPK:
-
Mitogen-activated protein kinase
- AA:
-
Asiatic acid
- MMP:
-
Mitochondrial matrix metalloproteinase
- NLRP3:
-
NLR family pyrin domain containing 3
- GSH:
-
Glutathione
- SOD:
-
Superoxide dismutase
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- CA1:
-
Cornu ammonis 1
- CA3:
-
Cornu ammonis 3
- DA:
-
Dopamine
- FA:
-
Ferulic acid
- CAT:
-
Catalase
- GPx:
-
Glutathione peroxidase
- 6-OHDA:
-
6-Hydroxydopamine hydrobromide
- DRP-1:
-
Dynamin-related protein 1
- PGC1:
-
Peroxisome proliferator–activated receptor-gamma coactivator
- MFN-2:
-
Mutofusin-2
- HSP-70:
-
70 Kilodalton heat shock proteins
- TH:
-
Thyroxine
- TQ:
-
Thymoquinone
- IFN:
-
Interferon
- EA:
-
Ellagic acid
- MDA:
-
Malondialdehyde
- NRF-2:
-
Nuclear factor erythroid 2–related factor 2
- CA:
-
Caffeic acid
- CAPE:
-
Caffeine phenethyl ester
- RNS:
-
Reactive nitrogen species
- AKT:
-
Protein kinase B
- AIF:
-
Apoptosis-inducing factor
- MAO-B:
-
Monoamine oxidase B
- EGCG:
-
Epigallocatechin-3-gallate
- PQ:
-
Paraquat
- Caspase-3:
-
Cysteinyl aspartic acid-protease-3
- NDD:
-
Neurodegenerative disorders
- PERK:
-
Protein kinase RNA-like endoplasmic reticulum kinase
- CHOP:
-
C/EBP homologous protein
- TF:
-
Theaflavin
- GBE:
-
Gingko biloba leaf extract
- ERK1/2:
-
Extracellular signal–regulated protein kinase
- SF:
-
Sodium ferulate
- Aβ:
-
Amyloid beta
- SIRT1:
-
Sirtuin 1
- STZ:
-
Streptozocin
- TLR4:
-
Toll-like receptor 4
- BIM:
-
Bcl-2-like protein 11
- GADD45:
-
Growth arrest and DNA damage–inducible 45 proteins
- CA:
-
Centella asiatica
- CB:
-
Cannabinoid
- 9-THC:
-
(9)-Tetrahydrocannabinol
- CBD:
-
Cannabidiol
- GABA:
-
Gamma-aminobutyric acid
References
Abdulwanis Mohamed Z et al (2019) Neuroprotective potential of secondary metabolites from Melicope lunu-ankenda (Rutaceae). Molecules 24(17):3109
Ajayi SA et al (2011) The microstructural effects of aqueous extract of Garcinia kola (Linn) on the hippocampus and cerebellum of malnourished mice. Asian Pac J Trop Biomed 1(4):261–265
Akbar M et al (2016) Mitochondrial dysfunction and cell death in neurodegenerative diseases through nitroxidative stress. Brain Res 1637:34–55
Al-Amri JS et al (2013) Effect of epigallocatechin-3-gallate on inflammatory mediators release in LPS-induced Parkinson’s disease in rats. Indian J Exp Biol 51(5):357–62
Allen RT et al (1997) Morphological and biochemical characterization and analysis of apoptosis. J Pharmacol Toxicol Methods 37(4):215–228
Ameisen JC (2002) On the origin, evolution, and nature of programmed cell death: a timeline of four billion years. Cell Death Differ 9(4):367–393
Anand A et al (2019) Vanillin: a comprehensive review of pharmacological activities. Plant Arch 19(2):1000–1004
Anandhan A et al (2013a) Therapeutic attenuation of neuroinflammation and apoptosis by black tea theaflavin in chronic MPTP/probenecid model of Parkinson’s disease. Neurotox Res 23(2):166–173
Ardah MT et al (2020) Ellagic acid prevents dopamine neuron degeneration from oxidative stress and neuroinflammation in MPTP model of Parkinson’s disease. Biomolecules 10(11):1519
Asadian G et al (2011) Study of variation of biochemical components inhypericum perforatum l. grown in north of Iran. J Med Plants Res 6(3)
Association A (2018) 2018 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia 14(3):367–429
Babbar R et al (2021) A comprehensive review on therapeutic applications of ferulic acid and its novel analogues: a brief literature. Mini Rev Med Chem 21(12):1578–1593
Balakrishnan R et al (2021) Natural phytochemicals as novel therapeutic strategies to prevent and treat Parkinson’s disease: current knowledge and future perspectives. Oxid Med Cell Longev 25:2021:6680935
Baluchnejadmojarad T et al (2017) Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson’s disease: possible involvement of ERβ/Nrf2/HO-1 signaling. Brain Res 1662:23–30
Barone E et al (2009) Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 10(2):97–108
Bian D et al (2012) Madecassoside, a triterpenoid saponin isolated from Centella asiatica herbs, protects endothelial cells against oxidative stress. J Biochem Mol Toxicol 26(10):399–406
Binawade Y, Jagtap A (2013) Neuroprotective effect of lutein against 3-nitropropionic acid–induced Huntington’s disease–like symptoms: possible behavioral, biochemical, and cellular alterations. J Med Food 16(10):934–943
Blamire A et al (2000) Interleukin-1β-induced changes in blood–brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci 20(21):8153–8159
Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15(6):725–731
Borase HP et al (2022) Design and evaluation of natural deep eutectic solvents system for chrysin to elicit its solubility, stability, and bioactivity. J Mol Liq 345:118205
Broderick TL et al (2020) Neuroprotective effects of chronic resveratrol treatment and exercise training in the 3xTg-AD mouse model of Alzheimer’s disease. Int J Mol Sci 21(19)
Buba CI et al (2016) Garcinia kola: the phytochemistry, pharmacology and therapeutic applications. Int J Pharmacognosy 3(2):67–81
Burz C et al (2009) Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol 48(6):811–821
Carson MJ et al (2006) CNS immune privilege: hiding in plain sight. Immunol Rev 213(1):48–65
Chandran R, Abrahamse H (2020) Identifying plant-based natural medicine against oxidative stress and neurodegenerative disorders. Oxid Med Cell Longev 2020:8648742
Chaudhary P et al (2011) Lipoic acid decreases inflammation and confers neuroprotection in experimental autoimmune optic neuritis. J Neuroimmunol 233(1–2):90–96
Chaudhary A et al (2019) Ferulic acid: a promising therapeutic phytochemical and recent patents advances. Recent Pat Inflamm Allergy Drug Discov 13(2):115–123
Chen S et al (2014) Celastrol prevents cadmium-induced neuronal cell death via targeting JNK and PTEN-Akt/mTOR network. J Neurochem 128(2):256–266
Chen D et al (2019) Asiatic acid protects dopaminergic neurons from neuroinflammation by suppressing mitochondrial ROS production. Biomol Ther 27(5):442
Chongtham A, Agrawal N (2016) Curcumin modulates cell death and is protective in Huntington’s disease model. Sci Rep 6(1):1–10
Choudhury B (2015) Approach to neurological disorder in Ayurveda. Ind J Med Res and Pharm Sci 2:69–73
Clifford MN (1999) Chlorogenic acids and other cinnamates–nature, occurrence and dietary burden. J Sci Food Agric 79(3):362–372
Colín-González AL et al (2012) The antioxidant mechanisms underlying the aged garlic extract-and S-allylcysteine-induced protection. Oxid Med Cell Longev 2012:907162
Cowan KJ, Storey KB (2003) Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J Exp Biol 206(7):1107–1115
Crespo-Bujosa HB, Gonzalez MJ (2018) Phytochemicals for the treatment of multiple sclerosis? A review of scientific evidence. J Orthomol Med 33(1):1–8
Cui J et al (2018) Neuroprotective effect of naringin, a flavone glycoside in quinolinic acid-induced neurotoxicity: possible role of PPAR-γ, Bax/Bcl-2, and caspase-3. Food Chem Toxicol 121:95–108
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592
David AVA et al (2016) Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev 10(20):84
DeMaagd G, Philip A (2015) Parkinson’s disease and its management: part 1: disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. Pharmacy and Therapeutics 40(8):504
Diamant G (1829) Dikstein R (2013) Transcriptional control by NF-κB: elongation in focus. Biochim Biophys Acta 9:937–945
Dinc E et al (2017) Protective effect of combined caffeic acid phenethyl ester and bevacizumab against hydrogen peroxide-induced oxidative stress in human RPE cells. Curr Eye Res 42(12):1659–1666
Doonan F, Cotter TG (2008) Morphological assessment of apoptosis. Methods 44(3):200–204
Dyment DA et al (2004) Genetics of multiple sclerosis. The Lancet Neurology 3(2):104–110
Ebrahimi SS et al (2017) Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol Lett 276:108–114
Ekowati J et al (2020) Ferulic acid prevents angiogenesis through cyclooxygenase-2 and vascular endothelial growth factor in the chick embryo chorioallantoic membrane model. Turk J Pharm Sci 17(4):424–431
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Ernanin Dyah W et al (2021) Virtual prediction of purple rice ferulic acid as anti-inflammatory of TNF-α signaling. Berkala Penelitian Hayati 27(2):59–66
Farkhondeh T et al (2019) Chrysin attenuates inflammatory and metabolic disorder indices in aged male rat. Biomed Pharmacother 109:1120–1125
Farquhar MG, Palade GE (1965) Cell junctions in amphibian skin. J Cell Biol 26(1):263–291
Fei C et al (2013) Ginkgo biloba extract protects substantia nigral neurons from apoptosis in PD rat model. J Neurol Disord 1(118):2
Ficarra S et al (2016) Insights into the properties of the two enantiomers of trans-δ-viniferin, a resveratrol derivative: antioxidant activity, biochemical and molecular modeling studies of its interactions with hemoglobin. Mol BioSyst 12(4):1276–1286
Finucane DM et al (1999) Bax-induced caspase activation and apoptosis via cytochromec release from mitochondria is inhibitable by Bcl-xL. J Biol Chem 274(4):2225–2233
Firdaus F et al (2018) Ellagic acid attenuates arsenic induced neuro-inflammation and mitochondrial dysfunction associated apoptosis. Toxicol Rep 5:411–417
Fisher M et al (1987) Linoleic acid levels in white blood cells, platelets, and serum of multiple sclerosis patients. Acta Neurol Scand 76(4):241–245
Fleischer A et al (2006) Modulating apoptosis as a target for effective therapy. Mol Immunol 43(8):1065–1079
Fulda S et al (2010) Targeting mitochondria for cancer therapy. Nat Rev Drug Discovery 9(6):447–464
Fusar-Poli P et al (2009) Distinct effects of Δ9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry 66(1):95–105
Galende AV et al (2021) Report by the Spanish Foundation of the Brain on the social impact of Alzheimer disease and other types of dementia. Neurología (english Edition) 36(1):39–49
Ghadially FN (2013) Ultrastructural pathology of the cell and matrix: a text and atlas of physiological and pathological alterations in the fine structure of cellular and extracellular components. Butterworth-Heinemann
Giatti S et al (2012) Neuroactive steroids, their metabolites, and neuroinflammation. J Mol Endocrinol 49(3):R125–R134
Gomes BAQ et al (2018) Neuroprotective mechanisms of resveratrol in Alzheimer’s disease: role of SIRT1. Oxid Med Cell Longev 2018:8152373
Gomez DR et al (2013) Neuroprotective properties of standardized extracts of Hypericum perforatum on rotenone model of Parkinson’s disease. CNS Neurol Disord Drug Targets 12(5):665–679
Granja A et al (2017) Therapeutic potential of epigallocatechin gallate nanodelivery systems. Biomed Res Int 2017:5813793
Gu X et al (2016) Protective effect of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic mouse model of Alzheimer’s disease. Mol Med Rep 13(3):2247–2252
Guha M, Mackman N (2001) LPS induction of gene expression in human monocytes. Cell Signal 13(2):85–94
Gul S et al (1970) Fatty acid composition of phospholipids from platelets and erythrocytes in multiple sclerosis. J Neurol Neurosurg Psychiatry 33(4):506–510
Guo B et al (2016) Multifunction of chrysin in Parkinson’s model: anti-neuronal apoptosis, neuroprotection via activation of MEF2D, and inhibition of monoamine oxidase-B. J Agric Food Chem 64(26):5324–5333
Gupta A et al (2021) Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. Adv Protein Chem Struct Biol 125:215–257
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155
Hanna DM et al (2015) ADIOL protects against 3-NP-induced neurotoxicity in rats: possible impact of its anti-oxidant, anti-inflammatory and anti-apoptotic actions. Prog Neuropsychopharmacol Biol Psychiatry 60:36–51
Harry GJ, Kraft AD (2012) Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology 33(2):191–206
Hashim P (2011) Centella asiatica in food and beverage applications and its potential antioxidant and neuroprotective effect. Int Food Res J 18(4):44056033
Hashimoto M, Hossain S (2011) Neuroprotective and ameliorative actions of polyunsaturated fatty acids against neuronal diseases: beneficial effect of docosahexaenoic acid on cognitive decline in Alzheimer’s disease. J Pharmacol Sci 116(2):150–162
He Z et al (2023) Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer’s disease. Redox Biol 66:102848
Hengartner MO (1997) Genetic control of programmed cell death and aging in the nematode Caenorhabditis elegans. Exp Gerontol 32(4–5):363–374
Horvitz HR (1994) Genetic control of programmed cell death in the nematode Caenorhabditis elegans. Springer, Apoptosis, pp 1–13
Hou RR et al (2008) Neuroprotective effects of (−)-epigallocatechin-3-gallate (EGCG) on paraquat-induced apoptosis in PC12 cells. Cell Biol Int 32(1):22–30
Huang W-C, Hung M-C (2013) Beyond NF-κB activation: nuclear functions of IκB kinase α. J Biomed Sci 20:1–13
Huang C-L et al (2011) Gastrodia elata prevents huntingtin aggregations through activation of the adenosine A2A receptor and ubiquitin proteasome system. J Ethnopharmacol 138(1):162–168
Imai T et al (2014) Protective effect of S-allyl-L-cysteine against endoplasmic reticulum stress-induced neuronal death is mediated by inhibition of calpain. Amino Acids 46:385–393
Janhom P, Dharmasaroja P (2015) Neuroprotective effects of alpha-mangostin on MPP+-induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J Toxicol 2015:919058
Jena AB et al (2022) An in silico investigation on the interactions of curcumin and epigallocatechin-3-gallate with NLRP3 inflammasome complex. Biomed Pharmacother 156:113890
Jin Y et al (2008) Neuroprotective effect of sodium ferulate and signal transduction mechanisms in the aged rat hippocampus 1. Acta Pharmacol Sin 29(12):1399–1408
Johnson TV et al (2010) Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol vis Sci 51(4):2051–2059
Joseph J et al (2009) Nutrition, brain aging, and neurodegeneration. J Neurosci 29(41):12795–12801
Jung KK et al (2006) Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci 79(21):2022–2031
Kang C-H et al (2013) Quercetin inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-dependent HO-1 pathway. Int Immunopharmacol 17(3):808–813
Karimi A et al (2015) Herbal versus synthetic drugs; beliefs and facts. J Nephropharmacol 4(1):27
Kaushal V, Schlichter LC (2008) Mechanisms of microglia-mediated neurotoxicity in a new model of the stroke penumbra. J Neurosci 28(9):2221–2230
Khurana N, Gajbhiye A (2013) Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson’s disease. Neurotoxicology 39:57–64
Kim S-J et al (2007) Epigallocatechin-3-gallate suppresses NF-κB activation and phosphorylation of p38 MAPK and JNK in human astrocytoma U373MG cells. J Nutr Biochem 18(9):587–596
Kim IS et al (2011) Neuroprotective effects of vanillyl alcohol in Gastrodia elata Blume through suppression of oxidative stress and anti-apoptotic activity in toxin-induced dopaminergic MN9D cells. Molecules 16(7):5349–5361
Kirimlioglu V et al (2008) Effect of resveratrol on oxidative stress enzymes in rats subjected to 70% partial hepatectomy. Elsevier, Transplantation proceedings
Koch M et al (2006) Erythrocyte membrane fatty acids in benign and progressive forms of multiple sclerosis. J Neurol Sci 244(1–2):123–126
Koh S-H et al (2006) The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci Lett 395(2):103–107
Kou X et al (2012) Ampelopsin inhibits H2O2-induced apoptosis by ERK and Akt signaling pathways and up-regulation of heme oxygenase-1. Phytother Res 26(7):988–994
Kumar P et al (2009a) Lycopene modulates nitric oxide pathways against 3-nitropropionic acid-induced neurotoxicity. Life Sci 85(19–20):711–718
Kumar P et al (2009b) Sesamol attenuate 3-nitropropionic acid-induced Huntington-like behavioral, biochemical, and cellular alterations in rats. J Asian Nat Prod Res 11(5):439–450
Kumar M et al (2021) Tomato (Solanum lycopersicum L.) seed: a review on bioactives and biomedical activities. Biomed Pharmacother 142:112018
Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81(2):807–869
Lam PY, Ko KM (2012) Beneficial effect of (–) Schisandrin B against 3-nitropropionic acid-induced cell death in PC12 cells. BioFactors 38(3):219–225
Lambert JD, Elias RJ (2010) The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys 501(1):65–72
Legiawati L et al (2018) In silico study of Centella asiatica active compounds as anti-inflammatory agent by decreasing Il-1 and Il-6 activity, promoting Il-4 activity. J Pharm Sci Res 10(9):2142–2147
Li Y-H et al (2017) Antioxidant effects of celastrol against hydrogen peroxide-induced oxidative stress in the cell model of amyotrophic lateral sclerosis. Sheng Li Xue Bao 69(6):751–758
Li C et al (2018) Pinostrobin exerts neuroprotective actions in neurotoxin-induced Parkinson’s disease models through Nrf2 induction. J Agric Food Chem 66(31):8307–8318
Lv J et al (2018) Pharmacological review on asiatic acid and its derivatives: a potential compound. SLAS Technology 23(2):111–127
Lyman M et al (2014) Neuroinflammation: the role and consequences. Neurosci Res 79:1–12
Maianski NA et al (2003) Tumor necrosis factor α induces a caspase-independent death pathway in human neutrophils. Blood, J Am Soc Hematol 101(5):1987–1995
Majno G, Joris I (1995) Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146(1):3
Manavalan A et al (2012) Gastrodia elata Blume (Tianma) mobilizes neuro-protective capacities. Int J Biochem Mol Biol 3(2):219
Mandel S et al (2004) Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. J Neurochem 88(6):1555–1569
Marracci GH et al (2002) Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J Neuroimmunol 131(1–2):104–114
Meesarapee B et al (2014) Curcumin I mediates neuroprotective effect through attenuation of quinoprotein formation, p-p38 MAPK expression, and caspase-3 activation in 6-hydroxydopamine treated SH-SY5Y cells. Phytother Res 28(4):611–616
Millington C et al (2014) Chronic neuroinflammation in Alzheimer’s disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int 2014:309129
Ming LC (2016) Use of herbal products in Southeast Asian countries. Arch Pharm Pract 7(5):S1
Mir RH et al (2022) Plant-derived natural compounds for the treatment of amyotrophic lateral sclerosis: an update. Curr Neuropharmacol 20(1):179
Mir SM et al (2016) Omega-3 fatty acids in inflammatory diseases. Omega-3 Fatty Acids: Keys to Nutritional Health 141–155. https://doi.org/10.1007/978-3-319-40458-5
Moon D-O et al (2010) Rosmarinic acid sensitizes cell death through suppression of TNF-α-induced NF-κB activation and ROS generation in human leukemia U937 cells. Cancer Lett 288(2):183–191
Muñoz-Espín D, Serrano M (2014) Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15(7):482–496
Musfiroh I et al (2023) Stability analysis of the asiatic acid-COX-2 complex using 100 ns molecular dynamic simulations and its selectivity against COX-2 as a potential anti-inflammatory candidate. Molecules 28(9):3762
Nabavi SF et al (2015) Luteolin as an anti-inflammatory and neuroprotective agent: a brief review. Brain Res Bull 119:1–11
Nagoor Meeran MF et al (2018) Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: a pentacyclic triterpenoid of therapeutic promise. Front Pharmacol 9:892
Naik E, Dixit VM (2011) Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med 208(3):417–420
Nataraj J et al (2016) Lutein protects dopaminergic neurons against MPTP-induced apoptotic death and motor dysfunction by ameliorating mitochondrial disruption and oxidative stress. Nutr Neurosci 19(6):237–246
Naziroglu M et al (2014) Modulation of oxidative stress, apoptosis, and calcium entry in leukocytes of patients with multiple sclerosis by Hypericum perforatum. Nutr Neurosci 17(5):214–221
Nicholas R, Rashid W (2013) Multiple sclerosis. Am Fam Physician 87(10):712
Noursadeghi M et al (2008) Quantitative imaging assay for NF-κB nuclear translocation in primary human macrophages. J Immunol Methods 329(1–2):194–200
Orhan IE (2012) Centella asiatica (L.) Urban: from traditional medicine to modern medicine with neuroprotective potential. Evid Based Complement Alternat Med 2012:946259
Ossola B et al (2009) The multiple faces of quercetin in neuroprotection. Expert Opin Drug Saf 8(4):397–409
Otuechere CA et al (2023) Action of vanillin-spiked zinc ferrite nanoparticles against cadmium-induced liver damage: computational insights with AKT 1, BCl-2 and TLR 8 proteins. J Drug Deliv Sci Technol 80:104139
Packer L et al (1997) Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radical Biol Med 22(1–2):359–378
Pal SK, Shukla Y (2003) Herbal medicine: current status and the future. Asian Pac J Cancer Prev 4(4):281–288
Park S et al (2011) Kaempferol acts through mitogen-activated protein kinases and protein kinase B/AKT to elicit protection in a model of neuroinflammation in BV2 microglial cells. Br J Pharmacol 164(3):1008–1025
Parodi V, Jacchetti E, Osellame R, Cerullo G, Polli D, Raimondi MT (2020) Nonlinear Optical Microscopy: From Fundamentals to Applications in Live Bioimaging. Front Bioeng Biotechnol 8:585363
Patra SK (2008) Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta 1785(2):182–206
Pedraza-Chaverrí J et al (2009) ROS scavenging capacity and neuroprotective effect of α-mangostin against 3-nitropropionic acid in cerebellar granule neurons. Exp Toxicol Pathol 61(5):491–501
Peng W et al (2022) Paeoniflorin is a promising natural monomer for neurodegenerative diseases via modulation of Ca2+ and ROS homeostasis. Curr Opin Pharmacol 62:97–102
Pérez-De La Cruz V et al (2006) Protective effect of S-allylcysteine on 3-nitropropionic acid-induced lipid peroxidation and mitochondrial dysfunction in rat brain synaptosomes. Brain Res Bull 68(5):379–383
Psotova J et al (2006) Photoprotective properties of Prunella vulgaris and rosmarinic acid on human keratinocytes. J Photochem Photobiol, B 84(3):167–174
Qu M et al (2011) Protective effects of lycopene against amyloid β-induced neurotoxicity in cultured rat cortical neurons. Neurosci Lett 505(3):286–290
Qureshi M et al (2018) Therapeutic potential of curcumin for multiple sclerosis. Neurol Sci 39:207–214
Radad K et al (2009) Thymoquinone protects dopaminergic neurons against MPP+ and rotenone. Phytother Res 23(5):696–700
Rando TA (2006) Stem cells, ageing and the quest for immortality. Nature 441(7097):1080–1086
Rani L et al (2022) Evaluation of potential neuroprotective effects of Vanillin against MPP+/MPTP-induced dysregulation of dopaminergic regulatory mechanisms in SH-SY5Y cells and a mouse model of Parkinson’s disease. Mol Neurobiol 60(8):4693–4715
Ravishankar D et al (2016) Thioflavones as novel neuroprotective agents. Bioorg Med Chem 24(21):5513–5520
Ren Y, Savill J (1998) Apoptosis: the importance of being eaten. Cell Death Differ 5(7):563–568
Renaud J et al (2015) Epigallocatechin-3-gallate, a promising molecule for Parkinson’s disease? Rejuvenation Res 18(3):257–269
Roach H et al (2004) Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis 9(3):265–277
Rowland LP, Shneider NA (2001) Amyotrophic lateral sclerosis. N Engl J Med 344(22):1688–1700
Sagredo O et al (2007) Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur J Neurosci 26(4):843–851
Sagredo O et al (2012) Cannabinoids: novel medicines for the treatment of Huntington’s disease. Recent Pat CNS Drug Discov 7(1):41–48
Salehi B et al (2019) The therapeutic potential of apigenin. Int J Mol Sci 20(6):1305
Salinthone S et al (2008) Lipoic acid: a novel therapeutic approach for multiple sclerosis and other chronic inflammatory diseases of the CNS. Endocr Metab Immune Disord Drug Targets 8(2):132–142
Salinthone S et al (2010) Lipoic acid attenuates inflammation via cAMP and protein kinase A signaling. PLoS ONE 5(9):e13058
Samarghandian S et al (2018) A review on possible therapeutic effect of Nigella sativa and thymoquinone in neurodegenerative diseases. CNS Neurol Disord Drug Targets 17(6):412–420
Sandhir R, Mehrotra A (2013) Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington’s disease. Biochim Biophys Acta 1832(3):421–430
Sandhir R et al (2014) Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. NeuroMol Med 16(1):106–118
Sani MM et al (2016) Phytopharmacology and phytotherapy of regulatory T cells: a new approach to treat multiple sclerosis. Pharm Lett 8(3):215–220
Saravanan V et al (2021) Molecular docking of active compounds from Phoenix pusilla root extract against antidiabetic and anti-inflammatory drug targets. J Appl Biol Biotechnol 9(1):26–30
Sarkaki A et al (2019) Chrysin prevents cognitive and hippocampal long-term potentiation deficits and inflammation in rat with cerebral hypoperfusion and reperfusion injury. Life Sci 226:202–209
Saudou F, Humbert S (2016) The biology of huntingtin. Neuron 89(5):910–926
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130(8):2073S-2085S
Schillace R et al (2007) Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem Biophys Res Commun 354(1):259–264
Schuh C, Schieberle P (2006) Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: quantitative differences between tea leaves and infusion. J Agric Food Chem 54(3):916–924
Seeram NP et al (2005) In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem 16(6):360–367
Sethi G et al (2007) Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κB–regulated gene products and TAK1-mediated NF-κB activation. Blood 109(7):2727–2735
Sgarbossa A et al (2015) Ferulic acid: a hope for Alzheimer’s disease therapy from plants. Nutrients 7(7):5764–5782
Shabab T et al (2017) Neuroinflammation pathways: a general review. Int J Neurosci 127(7):624–633
Sharma J et al (2013) Ethnomedicinal plants used for treating epilepsy by indigenous communities of sub-Himalayan region of Uttarakhand, India. J Ethnopharmacol 150(1):353–370
Sharma D et al (2016) Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience 324:163–176
Shi C et al (2010) Ginkgo biloba extract in Alzheimer’s disease: from action mechanisms to medical practice. Int J Mol Sci 11(1):107–123
Shi X et al (2015) Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: involvement of ERK1/2 and p38 signaling pathways. Neurosci Lett 594:105–110
Shinomol GK (2008) Effect of Centella asiatica leaf powder on oxidative markers in brain regions of prepubertal mice in vivo and its in vitro efficacy to ameliorate 3-NPA-induced oxidative stress in mitochondria. Phytomedicine 15(11):971–984
Silva JM et al (2020) Secondary metabolites with antioxidant activities for the putative treatment of amyotrophic lateral sclerosis (ALS): “Experimental evidences”. Oxid Med Cell Longev 2020:5642029
Singh R et al (2019) Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat Rev Mol Cell Biol 20(3):175–193
Smith KJ (2006) Pathophysiology of multiple sclerosis. Rev Prat 56(12):1299–1303
Srinivasan M et al (2007) Ferulic acid: therapeutic potential through its antioxidant property. Journal of Clinical Biochemistry and Nutrition 40(2):92–100
Suk K (2005) Regulation of neuroinflammation by herbal medicine and its implications for neurodegenerative diseases. Neurosignals 14(1–2):23–33
Swaroop S et al (2016) HSP60 plays a regulatory role in IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J Neuroinflammation 13:1–19
Tancheva LP et al (2020) Neuroprotective mechanisms of three natural antioxidants on a rat model of parkinson’s disease: a comparative study. Antioxidants 9(1):49
Tang D et al (2019) The molecular machinery of regulated cell death. Cell Res 29(5):347–364
Taupin P (2008) Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int J Med Sci 5(3):127
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281(5381):1312–1316
Tie L et al (2008) Down-regulation of brain-pancreas relative protein in diabetic rats and by high glucose in PC12 cells: prevention by calpain inhibitors. J Pharmacol Sci 106(1):28–37
Tronche F et al (1997) Analysis of the distribution of binding sites for a tissue-specific transcription factor in the vertebrate genome. J Mol Biol 266(2):231–245
Truong VL et al (2018) Role of resveratrol in regulation of cellular defense systems against oxidative stress. BioFactors 44(1):36–49
Turan D et al (2020) Evaluation of the neuroprotective potential of caffeic acid phenethyl ester in a cellular model of Parkinson’s disease. Eur J Pharmacol 883:173342
Van Den Bossche K et al (2006) The quest for the mechanism of melanin transfer. Traffic 7(7):769–778
Vince JE et al (2018) The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and-7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep 25(9):2339-2353. e2334
Vradenburg G (2015) A pivotal moment in Alzheimer’s disease and dementia: how global unity of purpose and action can beat the disease by 2025. Expert Rev Neurother 15(1):73–82
Walczak H (2011) TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev 244(1):9–28
Walle T et al (2001) Disposition and metabolism of the flavonoid chrysin in normal volunteers. Br J Clin Pharmacol 51(2):143–146
Wang L et al (2012) Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-κB, and MAPK pathways. Arch Toxicol 86:879–896
Wang K et al (2014) Protective effect of paeoniflorin on Aβ25–35-induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 34(2):227–234
Wang J et al (2016) Neuroprotective effect of several phytochemicals and its potential application in the prevention of neurodegenerative diseases. Geriatrics 1(4):29
Wang Y et al (2017) Toxicity of inhaled particulate matter on the central nervous system: neuroinflammation, neuropsychological effects and neurodegenerative disease. J Appl Toxicol 37(6):644–667
Wang T-F et al (2022) Inhibition of nigral microglial activation reduces age-related loss of dopaminergic neurons and motor deficits. Cells 11(3):481
Welbat JU et al (2018) Neuroprotective properties of asiatic acid against 5-fluorouracil chemotherapy in the hippocampus in an adult rat model. Nutrients 10(8):1053
Wilson R, Tocher DR (1991) Lipid and fatty acid composition is altered in plaque tissue from multiple sclerosis brain compared with normal brain white matter. Lipids 26(1):9–15
Wright JS (2002) Predicting the antioxidant activity of curcumin and curcuminoids. J Mol Struct (thoechem) 591(1–3):207–217
Yang Y et al (2015) Ginsenoside Rg1 suppressed inflammation and neuron apoptosis by activating PPARγ/HO-1 in hippocampus in rat model of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol 8(3):2484
Yang X et al (2021) Ginsenoside Rg1 exerts neuroprotective effects in 3-nitropronpionic acid-induced mouse model of Huntington’s disease via suppressing MAPKs and NF-κB pathways in the striatum. Acta Pharmacol Sin 42(9):1409–1421
Yuan C-X et al (2008) Effects of traditional Chinese herbal medicine on the neurobehavioral manifestations and the activity of dopamine D2 receptor in corpora striatum of rats with levodopa-induced dyskinesias. Zhong Xi Yi Jie He Xue Bao 6(10):1024–1028
Zeng K-W et al (2012) Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-κB signaling pathway in lipopolysaccharide-induced microglia. Eur J Pharmacol 692(1–3):29–37
Zhang X et al (2014) A review of experimental research on herbal compounds in amyotrophic lateral sclerosis. Phytother Res 28(1):9–21
Zhang J et al (2016) Neuroprotective effects of theaflavins against oxidative stress-induced apoptosis in PC12 cells. Neurochem Res 41(12):3364–3372
Acknowledgements
The authors are thankful to the Institute for providing excellent support to complete this work.
Author information
Authors and Affiliations
Contributions
BS: writing original draft and preparation of figures; NR—helped in writing manuscript and referencing; CS—critically review and editing; AS: conceptualization, supervision, critical review, and editing. The authors confirm that no paper mill and artificial intelligence was used.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors give full consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarkar, B., Rana, N., Singh, C. et al. Medicinal herbal remedies in neurodegenerative diseases: an update on antioxidant potential. Naunyn-Schmiedeberg's Arch Pharmacol 397, 5483–5511 (2024). https://doi.org/10.1007/s00210-024-03027-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-024-03027-5