Abstract
Therapeutic effect of non-steroidal anti-inflammatory drugs (NSAIDs) has been related with gastrointestinal injury. Docosahexaenoic acid (DHA), an omega-3 polyunsaturated fatty acid (PUFA), can prevent gastric and small intestinal damage. Nonetheless, contribution of antioxidative action in the protective effect of DHA has not been evaluated before in the small intestine injury after indomethacin treatment. Pathogenesis of NSAID-induced small intestinal injury is multifactorial, and reactive oxidative species have been related to indomethacin’s small intestinal damage. The present work aimed to evaluate antioxidative activity in the protective action of DHA in the indomethacin-induced small intestinal damage. Female Wistar rats were gavage with DHA (3 mg/kg) or omeprazole (3 mg/kg) for 10 days. Each rat received indomethacin (3 mg/kg, orally) daily to induce small intestinal damage. The total area of intestinal ulcers and histopathological analysis were performed. In DHA-treated rats, myeloperoxidase and superoxide dismutase activity, glutathione, malondialdehyde, leukotriene, and lipopolysaccharide (LPS) levels were measured. Furthermore, the relative abundance of selective bacteria was assessed. DHA administration (3 mg/kg, p.o.) caused a significant decrease in indomethacin-induced small intestinal injury in Wistar rats after 10 days of treatment. DHA’s enteroprotection resulted from the prevention of an increase in myeloperoxidase activity, and lipoperoxidation, as well as an improvement in the antioxidant defenses, such as glutathione levels and superoxide dismutase activity in the small intestine. Furthermore, we showed that DHA’s enteroprotective effect decreased significantly LPS levels in indomethacin-induced injury in small intestine. Our data suggest that DHA’s enteroprotective might be attributed to the prevention of oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are a widely prescribed group of drugs; however, their administration has been associated with several side reactions, including gastrointestinal (GI) injury (Bindu et al. 2020). It is noteworthy that the adverse effect of NSAIDs can also extend to the lower segments of the gastrointestinal tract (GIT), mainly distal jejunum and ileum; nonetheless, most therapeutic interventions primarily target the management of upper GIT side effects, thereby leaving a significant research gap in the treatment of intestinal ulcers resulting from NSAIDs administration (Wallace 2013).
The etiology of small intestinal injury following treatment with NSAIDs is complex and challenging to treat. Prostaglandin inhibition affects upper gastrointestinal tract, such us duodenum after gastric empty. However, the most important mechanisms of NSAID-induced small intestinal injury involve pathways that are independent of prostaglandin inhibition such as enterohepatic circulation and consequently “ion trapping,” as a result of direct contact of the NSAID with intestinal mucosa. In addition, there is a theory of three important steps during NSAIDs intestinal injury; first, NSAIDs induce mucosa phospholipids lysis, resulting in cellular redox homeostasis, and consequential generation of reactive oxygen species (ROS) (Somasundaram et al. 1997; Handa et al. 2014). Therefore, the increased ROS production contributes to increase intestinal permeability, and under certain conditions, influences alterations in the composition of the microbiota (Takeuchi and Satoh 2015); then, the equilibrium of the intestinal defense mechanism becomes overwhelmed, leading to the manifestation of NSAID-induced intestinal damage and consequently neutrophil infiltration and the activation of inflammatory process. Therefore, a compound with antioxidant activity holds promise as a potential strategy for mitigating the intestinal damage induced by NSAIDs.
Our research group has previously demonstrated the potential effect of omega-3 polyunsaturated fatty acids (PUFAs), particularly docosahexaenoic acid (DHA), in mitigating gastric damage caused by NSAIDs (Pineda-Peña et al. 2018). Within this study, we identified the involvement of the antioxidative pathway in mediating DHA’s gastroprotective effect (Pineda-Peña et al. 2018). In addition to our previous findings, the protective effect and antioxidative properties of DHA have been investigated in colon (Sharma et al. 2019). In an experimental model of chronic stress-induced intestinal dysfunction, the administration of docosahexaenoic acid (DHA) resulted in a decrease in pro-inflammatory cytokines, mitigated damage to intact tight junctions, and exhibited a favorable impact on the microbiota associated with the maintenance of the intestinal epithelium (Cao et al. 2019). More recently, we demonstrated the enteroprotective effect of DHA in small intestine (Sánchez-Trigueros, et al. 2021). Nevertheless, the contribution of DHA’s antioxidative action in its protective effect against intestinal injury induced by NSAIDs treatment remains unknown.
Currently, there is a lack regarding the effects of DHA treatment on the antioxidative pathway in the context of small intestinal injury induced by indomethacin. Therefore, the objective of this study was to elucidate the role of the antioxidative pathway in modulating the protective effects of DHA against indomethacin-induced small intestinal damage.
Methods
Drugs and reagents
DHA, omeprazole (OMP), indomethacin, and corn oil were purchased from Sigma-Aldrich (Toluca, México). Corn oil was used as the vehicle for DHA. Indomethacin was dissolved in a 5% NaHCO3 solution, and omeprazole was dissolved in a 0.9% saline solution. All reagents were prepared freshly (Sánchez-Trigueros et al. 2021).
Colorimetric assays
L-glutathione reduced, EDTA (Ethylenediaminetetraacetic acid), xanthine, DTPA (Diethylenetriaminepentaacetic acid), TMB (3,3′,5,5′-Tetramethylbenzidine dihydrochloride), bathocuproinedisulfonic acid disodium salt hydrate, TBA (2-Thiobarbituric acid), CTAB (Hexadecyltrimethylammonium bromide), and N,N-dimethylethylenediamine were purchased from Sigma-Aldrich (Toluca, México). TCA (Trichloroacetic acid) was obtained from specialized material company (Mexico City). Bradford kit Assay from Bio-Rad (Mexico City).
ELISA assays
For the quantification of leukotriene B4 (LTB4) (520111), the ELISA kit from Cayman Chemical Co., Ann Arbor, MI, USA, was used. The commercial kit LPS levels 96T (CEB526Ge) was purchased from Cloud-Clone.
DNA extraction
Favor-PrepTM Mini DNA Isolation Kit (FASTI001-1) for stools from Favorgen Biotech Corp, Cat was used.
Quantitative real-time PCR
The primers referenced in Table 1 were requested from Custom DNA Oligos by Sigma-Aldrich. For qPCR experiments, a SYBRTM Green PCR Master Mix reagent from Applied Biosystems was used.
Animals
Female Wistar rats (200 to 250 g) were used for the experiments. The animals were obtained from CINVESTAV-IPN and were treated according to international standards and the Norma Oficial Mexicana (NOM-062-ZOO-1999) for the production, care, and use of laboratory animals. The study was evaluated and approved by the Bioethics Committee of Escuela Nacional de Medicina y Homeopatía, Instituto Politécnico Nacional, Mexico City, México (registration number: CBE/021/2019). Rats were acclimatized for two weeks in our own facilities under 12 h light/dark cycles, air-conditioner (22 ± 2 °C), and fed ad libitum and free access to water. All experiments were conducted of six to eight animals per group. Individual rats were housed in separate cages equipped with wire net floors. Prior to the experiments, rats were fasting for a period of 12 hours, during which they received tap water. Each rat was exclusively employed for a singular experiment and subsequently euthanized at the termination of the assay (Pineda-Peña et al. 2018). Most of the laboratories at our institution use male rats; therefore, the availability of male rats is reduced. Finally, in our hands, female rats are less aggressive than male rats. Additionally, we have reported in previous work that the use of female rats is widely accepted for several animal models such as gastric injury, nociception, and inflammation (Pineda-Peña et al. 2018).
Small intestinal damage induced by indomethacin and assessment of intestinal ulcers
To induce small intestinal damage, rats were orally administered indomethacin (3 mg/kg) or vehicle (5% NaHCO3) every 24 h for 10 days through a metal cannula. Twenty-four hours later, the animals were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (7.5 mg/kg, i.p.). Blood was collected by cardiac puncture, and serum was immediately obtained by centrifugation and stored at −80°C until analysis. The small intestine (distal jejunum and ileum) was removed and opened along the antimesenteric site. The area (cm2) of all lesions in the small intestine was measured for each rat.
The percentage of enteroprotection produced by each treatment regimen in each individual was calculated as previously described (Sánchez-Trigueros et al. 2021).
where IUW is the index of ulcers for the control group (cm2), and IUT is the index of ulcers for treatment groups (cm2).
Experimental design
To evaluate the effect of DHA or OMP (reference drug) on indomethacin-induced small intestinal injury, DHA or OMP (3 mg/kg) or their respective vehicle were administered orally. After 2 h or 30 min of DHA or OMP administration, rats were gavage with indomethacin (3 mg/kg, p.o.) to induce intestinal lesions. DHA, OMP, and indomethacin were administered for ten consecutive days. The administration of doses and timing was determined based on our previous reports, and the intestinal damage was assessed in both experimental groups, as described above (Sánchez-Trigueros et al. 2021).
Histological analysis
For histological assessment, intestinal tissue was treated as it was described in Pineda-Peña et al. 2018. Briefly, tissue was excised and fixed with 10% formaldehyde and embedded in paraffin. Sections of 4–5 μm were mounted on glass slides covered with silane. Hematoxylin and eosin staining was performed on each slide, and the slides were examined under an optical microscope with a ×20 objective lens magnification (Nikon Eclipse Slog) equipped with a high-resolution digital camera (Nikon Digital Sight DS-2mv).
Assessment of intestinal glutathione (GSH) levels and superoxide dismutase (SOD) activity
Samples of small intestine were homogenized in cold PBS solution and then centrifuged at 900 g for 5 min at 4°C. SOD activity was determined using the method described by Sun et al. (1988) with minor modifications and described in detailed by Salinas-Nolasco et al. (2023) and GSH determinations were performed with the method previously described by Pineda-Peña et al. (2018).
Assessment of lipid peroxidation
Malondialdehyde (MDA) was determined as a marker of lipid peroxidation in intestinal homogenates by quantifying MDA formation using the thiobarbituric acid method (Galicia-Moreno et al. 2016).
Measurement of intestinal myeloperoxidase (MPO) levels
Intestinal tissue concentration of MPO was determined using the method previously described by Pineda-Peña et al. (2018). MPO levels were calculated comparing them with MPO standard at 620 nm.
Determination of intestinal levels of leukotriene B4 (LTB4)
Intestinal tissue samples were treated as is described in Pineda-Peña et al. 2018 according to commercial ELISA kit instructions (Cayman Chemical Co., Ann Arbor, MI, USA).
Lipopolysaccharide (LPS) quantification
Using the manufacturer’s instructions, ELISA assay was used to quantify serum LPS levels with Cloud-Clone 96T commercial kit. Results were expressed as ng/mL (Sánchez-Tapia et al. 2020).
DNA extraction
Fecal DNA extraction was performed using fecal samples previously collected, with the Favor-PrepTM Mini DNA Isolation Kit for stools (Favorgen Biotech Corp, Cat. No: FASTI001-1), following the manufacturer’s instructions. The concentration of purified DNA was quantified using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Waltham, Ma, USA), and its quality was assessed by electrophoresis on 0.5% agarose gels, as previously reported by Cuervo-Zanatta et al. (2021).
Microbiota analysis by quantitative real-time PCR
The abundance analysis of populations of selected bacteria was estimated in fecal DNA measured by quantitative real-time polymerase chain reaction (qPCR). qPCR experiments were performed using a SYBRTM Green PCR Master Mix reagent from Applied Biosystems, universal oligonucleotide for 16S gene of the V3-V4 region F-5′ CGGTGAATACGTTCCCGG 3′ and R-5′ TACGGCTACCTTGTTACGACTT 3′ as a reference for normalization according to previously reported by López-Siles et al. (2018). The corresponding forward and reverse primers for the study are shown in Table 1.
The qPCR cycling conditions were performed according to Applied Biosystems Step One kit. The SYBR Green PCR Master Mix reagent was used to prepare the reaction mixture, and the parameters provided by SYBR® Green PCR Master Mix and SYBR® Green PCR Reagents Kit from Apply Biosystems® were followed. The abundance of each sample was calculated according the ∆Ct method (-∆∆ Ct method) where control data have been used as reference group, obtaining the fold change (FC) value (Schmittgen and Livak 2008).
Statistical analysis
The results are presented as mean ± standard error of the mean (SEM). GraphPad Prism 8.0.1 version was used for statistical analysis. Comparisons among groups were performed using a one-way analysis of variance (ANOVA) followed by the Newman-Keuls multiple comparison test. A statistically significant difference between means was considered at P ≤ 0.05.
Results
Enteroprotective effect of DHA against indomethacin-induced small intestinal damage
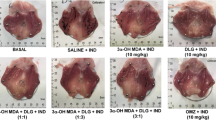
The administration of DHA (3 mg/kg, p.o.) significantly reduced macroscopic small intestinal lesions (3.42 ± 0.53 cm2) compared to the indomethacin-treated group (14.03 ± 2.13 cm2) (Figs. 1 and 2). The administration of indomethacin-induced significant morphological alterations of the villi (black arrow), thickening of the serous tunica, and loss of morphological frameworks and hyperplasia of the Lieberkühn crypts (black circle). In contrast, the histological analysis of DHA-treated rats showed villi with a continuous appearance and defined margins in the tunica, with hypertrophy limited to the tunica mucosa still observed. No alterations were observed in the small intestine of control group (Fig. 3). Interestingly, the percentage of enteroprotective action of DHA (75.61 ± 3.84%) was comparable to that of the gastric proton pump inhibitor, omeprazole (55.92 ± 6.32%) (Table 2).
Effect of DHA (1A) (3 mg/kg, p.o.) or omeprazole (1B) (OMP; 3 mg/kg, p.o.) in the intestinal damage induced administration (10 days) of indomethacin (3 mg/kg, p.o.). Every day the rats were pre-treated with DHA or its vehicle (corn oil) for 2 h or 30 min respectively, prior the administration of indomethacin. Control = corn oil + NaHCO3, VEH= corn oil + indomethacin for DHA. Data are expressed as the mean ± SEM (n = 6–8). ANOVA analysis, followed by a Newman-Keuls multiple comparison test. *P ≤0.05 vs. Control, #P≤0.05 vs. VEH + INDO
Representative histopathological sections of small intestine tissue after different treatments. Hematoxylin and eosin staining ×20. A Control (Corn oil + NaCOH3). B DHA (3 mg/kg, p.o.) + NaCOH3. C VEH (Corn oil) + INDO (indomethacin, 3 mg/kg, p.o.). D DHA (3 mg/kg, p.o.) + INDO (indomethacin, 3 mg/kg, p.o.). Lieberkühn crypt hyperplasia (black circle), hypertrophy of intestinal villi and necrotic remnants (black arrows)
Effect of the antioxidant activity in DHA’s protective effect in small intestine damage
As indicators of DHA’s antioxidant effect in the small intestine, the activity of SOD and GSH levels were evaluated. Indomethacin pretreatment significantly reduced SOD activity and GSH levels in intestinal tissue compared to the control group. DHA pretreatment prevented the reduction in both antioxidative markers after indomethacin injury (Table 3). MDA levels significantly increased after indomethacin administration. In contrast, pretreatment with DHA significantly reduced the MDA levels caused by the indomethacin-treated group (Table 3).
Effect of inflammatory markers in the enteroprotective effect of DHA in the indomethacin-induced small intestinal damage model
Indomethacin injury induced the increment in MPO activity and LTB4 levels in the small intestine. DHA pretreatment (3 mg/kg, p.o.) significantly reduced MPO activity in indomethacin-treated rats. However, no meaningful change was observed in LTB4 levels after DHA pretreatment compared to small intestinal damage group induced by indomethacin (Table 4).
Effects of DHA on LPS levels in the indomethacin-induced intestinal damage model
The amount of LPS in serum was evaluated to assess intestinal barrier dysfunction. The group treated with indomethacin showed increased LPS levels (641.9 ± 117 ng/mL) compared to the control group (109.5 ± 31.86 ng/mL). On the other hand, pretreatment with DHA prevented the increase induced by indomethacin administration (288.3 ± 99.3 ng/mL) (Fig. 4).
Effect of DHA (3 mg/kg, p.o.) on LPS serum levels against intestinal damage induced by the administration of indomethacin (3 mg/kg, p.o.). Data are expressed as the mean ± S.E.M (n= 6–8). ANOVA analysis, followed by a Newman-Keuls multiple comparison test. *P≤0.05 vs. Control, #P≤0.05 vs. VEH + INDO
Enteroprotective effect of DHA on the microbiota against indomethacin-induced intestinal damage
The abundance of Clostridium genus and Akkermansia muciniphila showed no significant differences among treatments. However, both quantifications showed a tendency toward an increase in abundance in the indomethacin-treated group compared with control group; moreover, DHA’s group prevented the increase induced by indomethacin-induced small intestinal damage group (Fig. 5A and B). Additionally, the abundance of the gram-positive Bacteroides genus did not differ significantly among the control and indomethacin-treated groups. Interestingly, the group treated with DHA plus indomethacin showed a significant decrease in the abundance of the Bacteroides genus compared to the other evaluated groups (Fig. 5C). Finally, our results showed that the commensal bacterium Lactobacillus spp. decreased after administration of indomethacin comparing to control group. Additionally, these alterations were prevented by DHA pretreatment, however without any significant statistical difference (Fig. 5D).
Abundance of Clostridium (A), Akkermansia muciniphila (B), Bacteroides (C), and Lactobacillus spp. (D) in the protection induced by DHA in the indomethacin-induced small bowel injury model. Data are expressed as the mean ± SEM (n= 6–8). ANOVA analysis, followed by a Newman-Keuls multiple comparison test. *P≤ 0.05 vs. Control, #P≤0.05 vs. VEH + INDO
Discussion
Traditional NSAIDs’ limited gastric and intestinal safety has encouraged the exploration for novel compounds to reduce their toxicity. Our study provides new insights into the protective effects of DHA in small intestinal injury induced by NSAIDs, highlighting the role of antioxidative activity in intestinal tissue. Our study assessed DHA’s protective action in the small intestine using a 10-day administration scheme for both DHA and indomethacin. The protective effect observed in the small intestine is consistent with our previous study, where a single dose of DHA showed a protective effect in an indomethacin-induced small intestine injury model (Sánchez-Trigueros et al. 2021). Furthermore, the present study deeply identified the antioxidant activity involved in DHA’s protective effect in the NSAID’s induced intestinal damage.
While the protective effect of DHA in the gastrointestinal tract has been previously reported, most studies have focused on its efficacy in protecting the stomach or colon. In our previous work, we reported the gastroprotective action of DHA in an indomethacin gastric injury model (Pineda-Peña et al. 2012; Pineda-Peña et al. 2018). Furthermore, the effects of DHA have been studied in experimental colitis models. For example, DHA-enriched compounds have been shown to reverse colitis in a model of dextran sodium sulfate (DSS) (Che et al. 2021), while enriched fish oil has been reported to show similar effects (Sharma et al. 2019).
Although NSAIDs treatment is prone to damage in the small intestine, the protective effect of DHA has not been extensively studied in this model. Our previous report demonstrated DHA’s enteroprotective effect after a single dose of indomethacin (Sánchez-Trigueros et al. 2021). In the actual study, we investigated the efficacy of DHA in a model of severe small intestine damage induced by 10-day administration of indomethacin as it has been done before with 10-day administration of diclofenac (Singht et al. 2017), and our results showed that DHA maintained its effectiveness. We have also described before DHA’s pharmacodynamic synergism with the antinociceptive and anti-inflammatory effects of NSAIDs, such as indomethacin, naproxen, and diclofenac, demonstrating NSAIDs maintained therapeutic actions without gastric adverse effects (Arroyo-Lira et al. 2014; Arroyo-Lira et al. 2017; Miranda-Lara et al. 2018).
Recent research has recognized that the antioxidative properties of compounds such as DHA, EPA, and ALA can help to reduce disease risk and severity (Djuricic and Calder 2021). Additionally, the role of oxidative stress in NSAID-induced small intestine injury has been well documented. For example, it has been demonstrated that mitochondrial ROS increase small intestine mucosal permeability and injury (Handa et al. 2014). This is the first report where the antioxidative action of pure DHA treatment in the indomethacin-induced small intestine injury model has been studied; previously, our group demonstrated that DHA exerts its gastroprotective action by activating gastric anti-inflammatory and antioxidative pathway (Pineda-Peña et al. 2018). Moreover, fish oil administration has been associated with redox modulatory effects in an experimental colitis model (Sharma et al. 2019).
The role of inflammation and oxidative processes has been linked to leukotriene infiltration, whereby recruited neutrophils release ROS that induce and maintain cell injury (Wallace 2013). This event was observed in our present work, where myeloperoxidase (MPO) activity was reduced in the group protected with DHA; however, leukotriene B4 levels did not change after DHA treatment. MPO catalyzes the formation of reactive oxygen intermediates, and an increase in its activity has been linked to several diseases (Aratani 2018). As a result of PUFAs supplementation, an augmented activity of antioxidant enzymes, such as SOD and glutathione peroxidase (GPx), has been observed, playing a key role in protecting tissues from oxidative damage (Saleh et al. 2022). Our data is according to this statement, DHA pretreatment-maintained control levels of GSH and SOD and decreased MDA formation, indicating its ability to prevent lipid peroxidation and improving endogenous antioxidant defense.
These findings are consistent with previous studies, Maresin 1 (MaR1), a specialized pro-resolving lipid mediator derived from DHA, reduced the infiltration of neutrophils, MPO activity, and ROS levels in a colitis model induced by DSS (Qiu et al. 2020). Similarly, treatment with enriched omega-3 fish oil restored GPx and SOD enzyme activity and prevented lipoperoxidation in the same experimental colitis model (Sharma et al. 2019). Additionally, in a murine model of brain damage, DHA’s neuroprotective effect restored SOD and GPx activity (Zhu et al. 2018). Although there is not much information on whether PUFAs directly react with ROS, it is well-established that incorporating EPA and DHA into lipoproteins and cell membranes can regulate antioxidant signaling. Furthermore, another specialized pro-resolving lipid mediators derived from DHA, resolvin D, has recently demonstrated protective effect in intestine in a mice-model of indomethacin-induced small intestinal injury. In that case, authors described the anti-inflammatory action of resolvin D in small intestine and its effect on indomethacin-induced small intestinal injury (Kuzumoto et al. 2021). In our research, the influence of DHA might be triggered through the synthesis of specialized pro-resolving lipid mediators, which recently had demonstrated beneficial effects in diverse cases of inflammatory bowel disease. Consequently, these findings suggest their potential as a viable substitute for the currently therapeutic strategies.
Interestingly, in addition to the antioxidant activity of DHA, in the present investigation, we observed that augmented LPS levels were linked to small intestinal injury induced by indomethacin; nonetheless, this increase was prevented in the group protected with DHA. It appears that NSAIDs induce disruption in intestinal epithelium and facilitate the translocation of commensal bacteria and their constituents, such as LPS. It has been established before that LPS enters the bloodstream after intestinal permeability and triggers an inflammatory response. Participation of LPS/TLR4 signaling pathway has been related in NSAIDs-small intestine injury (Watanabe et al. 2008), and some studies have shown that PUFA omega-3 treatment have been shown to prevent LPS increase (Zhu et al. 2021). Additionally, NSAIDs may cause changes in the microbiota, resulting in an unfavorable environment for the growth of beneficial bacteria and an overgrowth of potentially harmful bacteria, decreasing mucosal defense, possibly by releasing endotoxin or microbial metabolites (Maseda and Ricciotti 2020).
Moreover, intake of protective compounds such as PUFAs has been associated to beneficial gut microbiota, while dysbiosis in gut microbiota diversity has been linked in the development of enteropathy. In our study, we observed a tendency to increase the abundance of Akkermansia muciniphila following indomethacin treatment in the small intestinal injury model. Consistent with our findings, Maseda et al. (2019) previously reported that Akkermansia and Bacteroides genera were affected by indomethacin administration. Furthermore, our study demonstrated that DHA pretreatment prevented the tendency in the augmentation of Akkermansia muciniphila.
Our results also showed a significant decrement on Bacteroides abundance after DHA pretreatment in the group of intestinal damage with indomethacin. Bacteroides are considered a beneficial bacterium that protects against intestinal inflammation and injury. Furthermore, there is a report where it was found that in a model of indomethacin-induced enteritis injury the relative abundance of Bacteroides augmented (Terán-Ventura et al. 2014). Taken together, our results suggest that DHA administration might regulate at some point Bacteroides abundance, in the indomethacin-induced small intestine injury model. However, more studies need to be conducted to investigate the relationship between specific strains of bacteria and DHA’s protective effect in the intestine. The composition of the gut microbiota is complex and dynamic. Our study also examined other genus and species in microbiota (Lactobacillus and Clostridium); however, we were not able to draw a conclusion related to microbiota modification after DHA protective effect in the intestine and further investigation is needed to elucidate the tendency that we observed.
Conclusions
Our findings highlight the potential of PUFAs, specifically DHA, to prevent oxidative stress, thereby reducing the adverse effects of NSAIDs, such as small intestinal injury. However, more research is needed to fully understand the complex interplay among oxidative stress and the treatment of intestinal injury with omega-3 fatty acid’s supplementation.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Aratani Y (2018) Myeloperoxidase: its role for host defense, inflammation, and neutrophil function. Arch Biochem Biophys 640:47–52. https://doi.org/10.1016/j.abb.2018.01.004
Arroyo-Lira AG, Rodríguez-Ramos F, Chávez-Piña AE (2014) Synergistic antinociceptive effect and gastric safety of the combination of docosahexaenoic acid and indomethacin in rats. Pharmacol Biochem Behav 122:74–81. https://doi.org/10.1016/j.pbb.2014.03.015
Arroyo-Lira AG, Rodríguez-Ramos F, Ortiz MI, Castañeda-Hernández G, Chávez-Piña AE (2017) Supra-additive interaction of docosahexaenoic acid and naproxen and gastric safety on the formalin test in rats. Drug Dev Res 78:332–339. https://doi.org/10.1002/DDR.21396
Bindu S, Mazumder S, Bandyopadhyay U (2020) Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol 180:114147. https://doi.org/10.1016/j.bcp.2020.114147
Cao W, Wang C, Chin Y, Chen X, Gao Y, Yuan S, Xue C, Wang Y, Tang Q (2019) DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL) prevent intestinal dysfunction induced by chronic stress. Food Funct 10:277–288. https://doi.org/10.1039/C8FO01404C
Che H, Li H, Song L, Dong X, Yang X, Zhang T, Wang Y, Xie W (2021) Orally administered DHA-enriched phospholipids and DHA-enriched triglyceride relieve oxidative stress, improve intestinal barrier, modulate inflammatory cytokine and gut microbiota, and meliorate inflammatory responses in the brain in dextran sodium sulfate induced colitis in mice. Mol Nutr Food Res 65:2000986. https://doi.org/10.1002/mnfr.202000986
Cuervo-Zanatta D, Garcia-Mena J, Perez-Cruz C (2021) Gut microbiota alterations and cognitive impairment are sexually dissociated in a transgenic mice model of Alzheimer’s disease. J Alzheimers Dis 82(s1):S195–S214. https://doi.org/10.3233/JAD-201367
Djuricic I, Calder PC (2021) Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: an update for 2021. Nutrients 13:2421. https://doi.org/10.3390/nu13072421
Galicia-Moreno M, Rosique-Oramas D, Medina-Avila Z, Álvarez-Torres T, Falcón D, Higuera-de la tijera F, Béjar YL, Cordero-Pérez P, Muñoz-Espinosa L, Pérez-Hernández JL, Kershenobich D, Gutierrez-Reyes G (2016) Behavior of oxidative stress markers in alcoholic liver cirrhosis patients. Oxid Med Cell Longev 2016:1–10. https://doi.org/10.1155/2016/9370565
Handa O, Majima A, Onozawa Y, Horie H, Uehara Y, Fukui A, Omatsu T, Naito Y, Yoshikawa T (2014) The role of mitochondria-derived reactive oxygen species in the pathogenesis of non-steroidal anti-inflammatory drug-induced small intestinal injury. Free Radic Res 48:1095–1099. https://doi.org/10.3109/10715762.2014.928411
Kuzumoto T, Tanigawa T, Higashimori A, Kitamura H, Nadatani Y, Otani K, Fukunaga S, Hosomi S, Tanaka F, Kamata N, Nagami Y, Taira K, Watanabe T, Fujiwara Y (2021) Protective role of resolvin D1, a pro-resolving lipid mediator, in nonsteroidal anti-inflammatory drug-induced small intestinal damage. PLoS One. 16(5):e0250862. https://doi.org/10.1371/journal.pone.0250862
Lopez-Siles M, Enrich-Capó N, Aldeguer X, Sabat-Mir M, Duncan SH, Garcia-Gil LJ, Martinez-Medina M (2018) Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front Cell Infect Microbiol. 8:281. https://doi.org/10.3389/fcimb.2018.00281
Maseda D, Ricciotti E (2020) NSAID–gut microbiota interactions. Front Pharmacol 11. https://doi.org/10.3389/fphar.2020.01153
Maseda D, Zackular JP, Trindade B, Kirk L, Roxas JL, Rogers LM, Washington MK, Du L, Koyama T, Viswanathan VK, Vedantam G, Schloss PD, Crofford LJ, Skaar EP, Aronoff DM (2019) Nonsteroidal anti-inflammatory drugs alter the microbiota and exacerbate Clostridium difficile colitis while dysregulating the inflammatory response. mBio 10. https://doi.org/10.1128/mBio.02282-18
Miranda-Lara CA, Ortiz MI, Rodríguez-Ramos F, Chávez-Piña AE (2018) Synergistic interaction between docosahexaenoic acid and diclofenac on inflammation, nociception, and gastric security models in rats. Drug Dev Res 79:239–246. https://doi.org/10.1002/ddr.21438
Pineda-Peña EA, Jiménez-Andrade JM, Castañeda-Hernández G, Chávez-Piña AE (2012) Docosahexaenoic acid, an omega-3 polyunsaturated acid protects against indomethacin-induced gastric injury. Eur J Pharmacol 697:139–143. https://doi.org/10.1016/j.ejphar.2012.09.049
Pineda-Peña EA, Martínez-Pérez Y, Galicia-Moreno M, Navarrete A, Segovia J, Muriel P, Favari L, Castañeda-Hernández G, Chávez-Piña AE (2018) Participation of the anti-inflammatory and antioxidative activity of docosahexaenoic acid on indomethacin-induced gastric injury model. Eur J Pharmacol 818:585–592. https://doi.org/10.1016/j.ejphar.2017.11.015
Qiu S, Li P, Zhao H, Li X (2020) Maresin 1 alleviates dextran sulfate sodium-induced ulcerative colitis by regulating NRF2 and TLR4/NF-kB signaling pathway. Int Immunopharmacol 78:106018. https://doi.org/10.1016/j.intimp.2019.106018
Saleh A, Noguchi Y, Aramayo R, Ivanova ME, Stevens KM, Montoya A, Sunidhi S, Carranza NL, Skwark MJ, Speck C (2022) The structural basis of Cdc7-Dbf4 kinase dependent targeting and phosphorylation of the MCM2-7 double hexamer. Nat Commun 13:2915. https://doi.org/10.1038/s41467-022-30576-1
Salinas-Nolasco C, Barragán-Zarate GS, Lagunez-Rivera L, Solano R, Favari L, Jiménez-Andrade JM, Chávez-Piña AE (2023) Role of LTB4 and nitric oxide in the gastroprotective effect of Prosthechea karwinskii leaves extract in the indomethacin-induced gastric injury in the rat. Nat Prod Res. 37(5):819–822. https://doi.org/10.1080/14786419.2022.2089880
Sánchez-Tapia M, Miller AW, Granados-Portillo O, Tovar AR, Torres N (2020) The development of metabolic endotoxemia is dependent on the type of sweetener and the presence of saturated fat in the diet. Gut Microbes 12:1801301. https://doi.org/10.1080/19490976.2020.1801301
Sánchez-Trigueros MI, Méndez-Cruz F, Pineda-Peña EA, Rivera-Espinoza Y, Castañeda-Hernández G, Chávez-Piña AE (2021) Synergistic protective effects between docosahexaenoic acid and omeprazole on the gastrointestinal tract in the indomethacin-induced injury model. Drug Dev Res 82:543–552. https://doi.org/10.1002/DDR.21772
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 3(6):1101–8. https://doi.org/10.1038/nprot.2008.73
Sharma M, Kaur R, Kaushik K, Kaushal N (2019) Redox modulatory protective effects of ω-3 fatty acids rich fish oil against experimental colitis. Toxicol Mech Methods 29:244–254. https://doi.org/10.1080/15376516.2018.1553220
Singh DP, Borse SP, Nivsarkar M (2017) Overcoming the exacerbating effects of ranitidine on NSAID-induced small intestinal toxicity with quercetin: providing a complete GI solution. Chem Biol Interact. 272:53–64. https://doi.org/10.1016/j.cbi.2017.04.006
Somasundaram S, Rafi S, Hayllar J, Sigthorsson G, Jacob M, Price AB, Macpherson A, Mahmod T, Scott D, Wrigglesworth JM, Bjarnason I (1997) Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 41:344–353. https://doi.org/10.1136/gut.41.3.344
Sun Y, Oberley LW, Li Y (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500. https://doi.org/10.1074/jbc.M113.471342
Takeuchi K, Satoh H (2015) NSAID-induced small intestinal damage - roles of various pathogenic factors. Digestion 91:218–232. https://doi.org/10.1159/000374106
Terán-Ventura E, Aguilera M, Vergara P, Martínez V (2014) Specific changes of gut commensal microbiota and TLRs during indomethacin-induced acute intestinal inflammation in rats. J Crohns Colitis 8:1043–1054. https://doi.org/10.1016/j.crohns.2014.02.001
Wallace JL (2013) Mechanisms, prevention and clinical implications of nonsteroidal anti-inflammatory drug-enteropathy. World J Gastroenterol 19:1861. https://doi.org/10.3748/wjg.v19.i12.1861
Watanabe T, Higuchi K, Kobata A, Nishio H, Tanigawa T, Shiba M, Tominaga K, Fujiwara Y, Oshitani N, Asahara T, Nomoto K, Takeuchi K, Arakawa T (2008) Non-steroidal anti-inflammatory drug-induced small intestinal damage is Toll-like receptor 4 dependent. Gut 57:181–187. https://doi.org/10.1136/gut.2007.125963
Zhu W, Ding Y, Kong W, Li T, Chen H (2018) Docosahexaenoic Acid (DHA) provides neuroprotection in traumatic brain injury models via activating Nrf2-ARE signaling. Inflammation 41:1182–1193. https://doi.org/10.1007/s10753-018-0765-z
Zhu X, Bi Z, Yang C, Guo Y, Yuan J, Li L, Guo Y, 2021. Effects of different doses of omega-3 polyunsaturated fatty acids on gut microbiota and immunity. Food Nutr Res 65. https://doi.org/10.29219/fnr.v65.6263
Acknowledgements
Martha Ivonne Sánchez Trigueros is a CONACyT, Mexico fellow (Grant Number 781715). Authors acknowledge to Q.F.B. Martha Patricia González García from Pharmacology Deparment-Cinvestav, M.V.Z. Ricardo Gaxiola Centeno, M.V.Z. Benjamín Chávez Álvarez, M.V.Z. Carlos Giovanni Sam Miranda from UPEAL-Cinvestav for their technical assistance in this project.
Funding
Financial support was provided by Consejo Nacional de Ciencia y Tecnología (CONACyT Project 285416) and SIP, Mexico 20220896.
Author information
Authors and Affiliations
Contributions
MIST conducted all experiments and wrote the draft. EAPP, GC, and AECP analyzed the data. EAP contributed to the experiments presented in Tables 1 and 2. CPC contributed to the design of the experiments performed in Figs. 4 and 5. IMV and DC contributed to the experiments conducted for Fig. 5. AECP wrote the final draft, supervised the research, and edited the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
The study was evaluated and approved by the Bioethics Committee of Escuela Nacional de Medicina y Homeopatía, Instituto Politécnico Nacional, Mexico City, México (registration number: CBE/021/2019).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sánchez-Trigueros, M.I., Martínez-Vieyra, I.A., Pineda-Peña, E.A. et al. Role of antioxidative activity in the docosahexaenoic acid’s enteroprotective effect in the indomethacin-induced small intestinal injury model. Naunyn-Schmiedeberg's Arch Pharmacol 397, 4275–4285 (2024). https://doi.org/10.1007/s00210-023-02881-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02881-z