Abstract
Recently, epidermal growth factor-like domain protein 6 (EGFL6) was proposed as a candidate gene for coupling angiogenesis to osteogenesis during bone repair; however, the exact role and underlying mechanism are largely unknown. Here, using immunohistochemical and Western blotting analyses, we found that EGFL6 was downregulated in the femoral head tissue of patients with steroid-induced osteonecrosis of the femoral head (SONFH) compared to patients with traumatic femoral neck fracture (FNF), accompanied by significantly downregulation of osteogenic and angiogenic marker genes. Then, bone marrow mesenchymal stem cells (BMSCs) were isolated from the FNF and the SONFH patients, respectively, and after identification by immunofluorescence staining surface markers, the effect of EGFL6 on their abilities of osteogenic differentiation and angiogenesis was evaluated. Our results of alizarin red staining and tubular formation experiment revealed that BMSCs from the SONFH patients (SONFH-BMSCs) displayed an obviously weaker ability of osteogenesis than FNF-BMSCs, and EGFL6 overexpression improved the abilities of osteogenic differentiation and angiogenesis of SONFH-BMSCs. Moreover, EGFL6 overexpression activated extracellular signal-regulated kinases 1/2 (ERK1/2). ERK1/2 inhibitor U0126 reversed the promoting effect of EGFL6 overexpression on the expression of osteogenesis and angiogenesis-related genes in the SONFH femoral head. In conclusion, EGFL6 plays a protective role in SONFH, it promotes osteogenesis and angiogenesis of BMSCs, and its effect is likely to be related to ERK1/2 activation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteonecrosis of the femoral head (ONFH), including traumatic osteonecrosis of the femoral head (TONFH) and non-traumatic osteonecrosis of the femoral head (NONFH), is a debilitating aseptic bone disease caused by ischemia of the femoral head. Up to now, ONFH remains a global problem with a relatively low recovery rate and high probabilities of surgery. In emerging developing countries in recent years, such as China, with the development of the economy and the acceleration of the pace of life, the significant increase in the abuse of glucocorticoids and hormonal drugs has led to a rapid increase and a younger trend in the morbidity of steroid-induced osteonecrosis of femoral head (SONFH), a main subtype of NONFH. Despite the continuous progress of various treatment approaches, patients with large necrotic lesion or collapsed lesion had low quality of life, and middle-aged patients exhibited relatively poor social activity and mental health (Uesugi et al. 2018). Therefore, it is essential to deeply explore the pathological mechanism of ONFH and to uncover reliable therapeutic targets.
Bone marrow mesenchymal stem cells (BMSCs) are a special type of bone marrow cells with multiple differentiation potential to differentiate into bone, cartilage, adipose, vascular endothelium, nerve, and muscle cells, and they have been applied into repair and regeneration of many tissues during the past few years. In treatment of SONFH, BMSCs is not only used in the conservative management at early stage for repair of injured bone, but also helpful to recovery after surgery (Huang et al. 2021). Hence, BMSCs are usually used to construct tissue-engineered bone or transplanted into the osteonecrotic area directly (Xu et al. 2020). The survival and differentiation efficiency of BMSCs are important to successful transplantation and bone regeneration (Zhang et al. 2021; Zhang et al. 2020). However, the stressful environment in the osteonecrotic area is disadvantageous to transplantation and functionality of BMSCs.
In long-term ischemic environment created by hormones in the femoral head tissue, massive fat decomposition products are released into the blood, increasing blood lipid concentration, causing embolism of the blood supply vessels, and contributing to accumulation of the intramedullary fat, thus resulting in disorder of lipid metabolism and intramedullary high blood pressure (Chang et al. 2020). Moreover, long-term use of hormones causes not only intravascular coagulation disorders but also secondary hyperfibrinolysis to trigger reperfusion of previously blocked small blood vessels, resulting in reperfusion injury, osteoclast activity, and osteocyte apoptosis, finally exacerbating the femoral head necrosis (Huang et al. 2021; Chang et al. 2020; Motta et al. 2022). In this unfavorable environment, BMSCs are difficult to successfully differentiate and complete their mission of osteogenesis and angiogenesis (Cui et al. 2022; Zhang et al. 2022). Taking measures to improve their differentiation into bone and blood vessels would have a significant impact on BMSCs or other stem cell-based therapies for the femoral head necrosis.
Epidermal growth factor-like factors (EGFLs) are a modestly well characterized growth factor family belonging to the epidermal growth factor superfamily. During the past few decades, EGFLs have been widely involved in preventing cell apoptosis and blood coagulation through interacting with epidermal growth factor receptors (Wolff et al. 2007; Kurosawa 1989). EGFLs displayed a certain degree of tissue specificity but a basic regulatory role in embryonic development, tissue regeneration, and tumorigenesis. For example, EGFL7 is a quite well characterized member of EGFL family, which protected against cell death to alleviate tissue injury in response to environmental stress, such as hypoxia or hyperoxia, and enhanced activation of endothelial cell in tumor tissues to contribute to cancer progression (Xu et al. 2008; Pinte et al. 2022). EGFL6, also MAM and EGF domain-containing gene protein (MAEG), was firstly identified as a marker associated with dermatome specification and morphogenesis of its derivatives in the year 2000, which was initially activated during specification of the early lateral dermatome, and then found in dermatome derivatives, such as the trunk dermis, the hair follicles, and the mesenchyme of the cranio-facial region (Buchner et al. 2000). Thereafter, the role of EGFL6 in regulating endothelial function was gradually revealed, and it was emerging as an important regulatory factor for angiogenesis in tumors and injured non-tumor tissues (Mas et al. 2007). Recently, EGFL6 was demonstrated to facilitate osteogenic differentiation in adipose-derived SCs through activating the bone morphogenetic protein 2 (BMP-2)/Smad4 signaling pathway (Liu and Wang 2022). Moreover, a couple of recent studies indicated that EGFL6 was involved in osteogenic vascular coupling which refers to the interaction between bone cells and vascular endothelial cells and is crucial for bone repair and regeneration (Chen et al. 2021; Shen et al. 2021). However, its exact role in the progression of SONFH was largely unknown. In this study, we detected the expression pattern of EGFL6 in SONFH patients and investigated its effect on osteogenic differentiation and angiogenesis of BMSCs.
Materials and methods
Tissue specimens
Femoral head tissues were obtained from patients with SONFH and patients with the traumatic femoral neck fracture (FNF, as control) during hip joint replacement and fracture debridement. The study protocols were approved by the Ethics Committee of Xi’an Honghui Hospital, and all subjects gave informed consent. Inclusion criteria: (1) Age range from 30 to 60 years old; (2) All patients underwent artificial hip replacement at Xi’an Honghui Hospital and obtained femoral head tissue; (3) All the SONFH patients have been using glucocorticoids for more than 1 year; (4) All patients underwent preoperative X-ray, CT, and MRI examinations to confirm femoral head necrosis; (5) All patients are in stage III or IV; (6) The control group took femoral head tissue within 48 h after trauma, and preoperative imaging confirmed that there was no necrotic area of the femoral head. Exclusion criteria: Patients with pathological fractures; Patients with a history of metabolic bone disease or infectious diseases. The sample sizes of both groups of patients were obtained through power calculation. Patient information can be found in the supplementary materials.
Hematoxylin eosin (HE) staining
Femoral head tissues were fixed by 4% paraformaldehyde and decalcified for 2 days. After being fully washed by running water, they were soaked in 5% sodium sulfate solution for 12 h, and washed by running water overnight. After dehydration with different concentrations of alcohol gradient (gradually increasing), they were respectively made transparent with holly oil and xylene twice, 30 min per time, then immunohistochemistry by paraffin ding, sliced into 10 μm pieces, pasted, and dried. The dried slices were dewaxed with xylene for 3 times, 5 min per time, washed away xylene with anhydrous alcohol, and then washed with different concentration of alcohol gradient (gradually decreasing). Subsequently, hematoxylin was used to stain the slices for 10–20 min. After washed with tap water, the stained slices were washed with water to return blue and then stained with eosin semen for 2 min. The floating color (color separation) was washed with 95% alcohol 2–3 times until the nucleus and cytoplasm are clearly colored, the slices were dehydrated with anhydrous alcohol twice, 3 min per time, and xylene was used to make transparent 3 times, 3 min per time. Finally, the slices were sealed with neutral gum and cover glass, and observed under a CX43 optical microscope (Olympus, Tokyo, Japan). In order to reduce the bias in data collection and analysis, we randomly selected 10 non-experimental personnel to blind testing the experimental results.
Immunohistochemistry (IHC)
Femoral head tissues were fixed, dehydrated, dewaxed, and sliced similarly to HE staining. The slices were dried at 65°C for 2 h, dewaxed with xylene for 30 min, hydrated with different concentration of alcohol gradient (gradually decreasing), and then soaked in ddH2O for 2 min. Sodium citrate was applied in antigen repair under high pressure. After washed with PBS for 3 times, endogenous peroxidase was used to incubated with the slices, and the slices were incubated with specific antibodies before washed with PBS for 3 times. The slices were processed with Polink-2 plus® Polymer HRP Detection System (Golden Bridge International, Shanghai, China), and then stained with diamino Benzidine tetrahydrochloride (DAB, Sigma-Shanghai, Shanghai, China) for 3 min. The stained slices were washed with water to return blue, dehydrated with different concentration of alcohol gradient (gradually increasing), and made transparent with xylene. Finally, the slices were sealed with neutral gum and cover glass, and observed under a CX43 optical microscope (Olympus). The primary antibodies during the experiment are as follows: anti-EGFL6 (1:200, ab140079, UK), anti-VEGFA (1:100, ab52917, UK), anti-CD31 (1:50, ab28364, UK), anti-CD34 (1:2500, ab81289, UK), anti-BMP-2 (1:400, ab124715, UK), and anti-p-ERK (1:100, ab192591, UK). Goat anti-rabbit IgG H&L (HRP) (1:2000, ab6721, Abcam, UK) was used for the secondary antibody. In order to reduce the bias in data collection and analysis, we randomly selected 10 non-experimental personnel to blind testing the experimental results.
Isolation, culture, and transfection of primary BMSCs
The marrow was respectively collected from the femoral heads of FNF and SONFH patients, transferred into 1.5 mL tubes and then centrifuged at 10,000 g for 15 s at room temperature. A 25 G needle was used to pull the cell pellets up and down slowly to break up clumps, and all the samples were combined into a single 15 mL conical tube. 10 mL of BMSC Culture Media (per 500 μL of samples) were added to suspended the cells and passed through a 70 μm filter to remove possible bone fragments. The number of the cells was calculated and they were plated at 2 × 106 cells/cm2 in culture media. After 72 h of culture, at 37°C in an incubator with 5% CO2, the media were removed and replaced with fresh media. Then, the media were changed every other day.
To evaluate the effect of EGFL6 on the differentiation of BMSCs, lentiviral vector expressing the EGFL6 was constructed and packaged by the GenScript Biotechnology Co., Ltd. (Nanjing, China) and was used to infected with the cultured BMSCs. In the pre-experiments, it is determined that the multiplicity of infection (MOI) was 500. Here, GFP-labeled lentiviral overexpression vector (with a CMV promoter, the 3rd-generation lentiviral expression system, GenScript Biotechnology) of EGFL6 was used to infect the BMSCs, with the empty lentiviral vector as the negative control. Four days after infection, 2 μg/mL puromycin (GenScript Biotechnology) was used to screen the cells successfully transfected for subsequent research.
Purity identification of BMSCs
The morphology of BMSCs was observed under an inverted microscope after 3 days of culture.
BMSCs-related surface markers CD34, CD90, and CD44 were detected by flow cytometry. Firstly, 5 μL of fluorescein isothiocyanate (FITC) (BD Biosciences, Franklin Lakes, NJ, USA)–labeled anti-CD34 (1:50, ab81289, Abcam, UK), FITC-labeled anti-CD90 (1:500, ab307736, Abcam, UK), and FITC-labeled anti-CD44 (0.2 μg/ml, ab238465, Abcam, UK) were added to the FCM tube. Then 50 μL of cell suspension (2 × 107/mL) was added and the mixture was incubated in the dark at room temperature for 30 min. Then, wash 2 times with a buffer and 500 μL of staining buffer was added to re-suspend the cells. Finally, the expression levels of CD34, CD90, and CD44 were detected by FCM (Beckman Coulter Life Sciences, Brea, CA, USA). In order to reduce the bias in data collection and analysis, we randomly selected 10 non-experimental personnel to blind testing the experimental results.
Osteogenic differentiation of BMSCs
Eight hundred microliters of 1 M β-glycerol phosphate and 200 μL of 0.5 M ascorbic acid were added into 99 mL of BMSC culture media. On reaching confluent, the BMSC culture media were switched to osteoblast differentiation media. The differentiation media were replaced every other day. About 3 weeks later, the cells were visualized with the naked eye, and alizarin red staining was used to evaluate the degree of mineralization in each group. In addition, the number of calcium nodules in BMSCs was observed under an optical microscope after alizarin red staining to further evaluate the osteogenic differentiation of BMSCs cells. In order to reduce the bias in data collection and analysis, we randomly selected 10 non-experimental personnel to blind testing the experimental results.
Tube formation experiment
Fifty microliters of Matrigel (Becton, Dickinson and Company, Franklin Lake, NJ, USA) was transferred into a 96 well plate on the ice. The plate was put into the incubator for coagulation for 30 min. Then, 50 μL of cell suspension was carefully added on to Matrigel. After 24 h of cultivation, the cells were observation and photographed. In order to reduce the bias in data collection and analysis, we randomly selected 10 non-experimental personnel to blind testing the experimental results.
Western blotting
Total protein was isolated from femoral head tissues or BMSCs with RIPA lysis buffer (Sigma, USA). BCA protein assay kit (Merck, Whitehouse Station, NJ, Germany) was used to detect protein concentration. Then, 25 μg of total protein (/lane) was separated by 10% SDS-PAGE and electro-transferred onto a Polyvinylidene fluoride membrane (Roche, Basel, Switzerland). The membrane was incubated with 5% skim milk at room temperature for 2 h, and then incubated with antibodies, including anti-EGFL6 (1:500, ab167281, Abcam, UK), anti-VEGF (1:600, ab32152, Abcam, UK), anti-BMP2 (1:400, ab284387, Abcam, UK), anti-CD31 (1:300, ab28364, Abcam, UK), anti-CD34 (1:300, ab110643, Abcam, UK), anti-RUNX2 (1:500, ab236639, Abcam, UK), anti-ERK1/2 (1:300, ab17942, Abcam, UK), anti-phosphorylated ERK1/2 (1:100, ab278538, Abcam, UK), and anti-GAPDH (1:800, ab8245, Abcam, UK) antibodies at 4°C overnight. Then, the membrane was incubated with corresponding IgG (1:1500, ab6721, Abcam, UK) conjugated with horseradish peroxidase for 2 h. Protein bands were visualized with an Enhanced Chemiluminescence Kit (BioTeke, Beijing, China) in a Gel Imaging System (Thermo Fisher Scientific, USA), and quantified with the ImageJ software (Thermo Fisher Scientific, USA). In order to reduce the bias in data collection and analysis, we randomly selected 10 non-experimental personnel to blind testing the experimental results.
Statistical analyses
Data analyses were performed by the SPSS 22.0 software. All data from at least three measurements were presented as the mean ± standard deviation (SD). Student’s t-test was performed for the comparison between two groups, and analysis of variance (ANOVA) was performed for comparison among groups followed by Tukey’s test. All data were tested by Shapiro-Wilk test and homogeneity of variance test before analysis. In addition, multiple comparisons were corrected by Bonferroni. P<0.05 was considered to be statistically significant.
Results
EGFL6 was markedly downregulated in the femoral head of SONFH patients compared with FNF patients
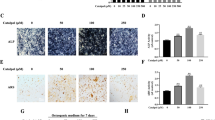
To explore the role of EGFL6 in SONFH, the femoral head tissues were sampled from SONFH patients and FNF patients; there was no significant difference in age, gender, and other potential confounding factors between these two groups of patients (Table 1). HE staining was used to observe their morphology, and the expression level of EGFL6 protein was detected with IHC and Western blotting analyses. Our results showed that, in the SONFH femoral head, there were a number of diffuse empty lacunae or pyknosis of bone nuclei in the bone trabeculae, and necrotic myelocytes and adipocytes in the bone marrow, while the FNF femoral head displayed slight lesions and few adipocytes in the bone marrow (Fig. 1A). IHC and Western blotting analyses revealed that EGFL6 protein was markedly downregulated in the SONFH femoral head compared with the FNF femoral head (Fig. 1B and C).
EGFL6 was markedly downregulated in the femoral head of SONFH patients. Femoral head tissues were sampled from the SONFH patients and the FNF patients. A HE staining was used to observe their morphology, and ratio of empty lacunae and area of adipose tissue in the femoral head were analyzed with the PRECiV Capture software (OLYMPUS). The expression level of EGFL6 protein was detected with IHC (B) and Western blotting (C) analyses. N=3, *P<0.05, **P<0.01, ***P<0.001
The SONFH femoral head displayed lower expression levels of osteogenic and angiogenic marker genes
Then, expression levels of the osteogenic marker gene BMP-2, the angiogenic marker gene VEGFA, the vascular endothelial cell surface marker CD31, and hematopoietic stem cell surface marker CD34 were detected with IHC and Western blotting analyses. The results showed that, compared with the FNF femoral head, the SONFH femoral head displayed lower levels of BMP-2 (Fig. 2A and F), VEGFA (Fig. 2B and E), CD31 (Fig. 2C and E), and CD34 (Fig. 2D and F), suggesting that the SONFH femoral head has poorer osteogenic and angiogenic abilities.
Expression levels of osteogenic and angiogenic marker genes in the FNF and the SONFH femoral head tissues. Femoral head tissues were sampled from the SONFH patients and the FNF patients. IHC assay was used to evaluate the expression of osteogenic and angiogenic marker proteins, including BMP-2 (A), VEGFA (B), CD31 (C), and CD34 (D). E The expression levels of VEGFA and CD31 proteins were detected with Western blotting. F The expression levels of BMP-2 and CD34 proteins were detected with Western blotting. N=3, *P<0.05, **P<0.01
SONFH-BMSCs displayed poorer abilities of proliferation and osteogenesis and a lower expression level of EGFL6
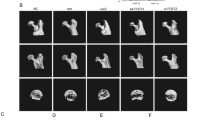
BMSCs are a special type of bone marrow cells with multiple differentiations potential to differentiate into bone, vascular endothelium, and other types of cells, which have been applied into repair and regeneration of femoral head in the SONFH patients. Here, we isolated BMSCs from the FNF and the SONFH patients, respectively, and the microscopic observation of BMSCs showed that the extracted BMSCs were arranged in a relatively uniform vortex in the medium, which was fan-shaped, with clear boundaries and good refraction (Fig. 3A). The purity of BMSCs was detected by flow cytometry. CD34 negative antigen, CD90 positive antigen, and CD44 positive antigen were detected on the surface of BMSCs, indicating that the purity of isolated BMSCs was high (Fig. 3B). Immunofluorescence staining for the cell surface markers CD73, CD90, CD45, and CD34 was used to identify the isolated cells. Our results showed that isolated BMSCs displayed classic surface protein features CD73+CD90+CD45-CD34low (Fig. 3C). Cell Counting Kit-8 was used to evaluate the proliferation of BMSCs, and the results showed that SONFH-BMSCs displayed a poorer proliferation ability than FNF-BMSCs (Fig. 3D). Moreover, SONFH-BMSCs and FNF-BMSCs were respectively induced to osteogenic differentiation, and alizarin red staining at day 21 indicated that SONFH-BMSCs displayed an obviously poorer ability of osteogenic differentiation than FNF-BMSCs (Fig. 3E). Compared with the FNF BMSCs group, the BMSCs cells in the SONFH BMSCs group showed lower osteogenic ability (less calcium nodules) (Fig. 3F). Western blotting was used to detect the expression of EGFL6 in SONFH-BMSCs and FNF-BMSCs, and the results showed that SONFH-BMSCs displayed a lower expression level of EGFL6 (Fig. 3G).
SONFH-BMSCs, expressing lower level of EGFL6, displayed poorer abilities of proliferation and osteogenesis. BMSCs from the FNF and the SONFH patients were isolated, respectively. A Microscopic observation of BMSCs morphology. B The surface markers of BMSCs were detected by flow cytometry. C Immunofluorescence staining for the cell surface markers CD73, CD90, CD45, and CD34 was used to identify the isolated BMSCs. D CCK-8 assay was used to compare the proliferation ability of FNF-BMSCs and SONFH-BMSCs. E SONFH-BMSCs and FNF-BMSCs were respectively induced to osteogenic differentiation, and alizarin red staining was used to evaluate the degree of osteogenic differentiation at day 21. F The SONFH-BMSCs and The FNF-BMSCs were induced to differentiate into osteoblasts. G The expression level of EGFL6 was detected with Western blotting. On the 21st day, alizarin red staining was used and the effect of osteogenic differentiation was observed under a microscope. N=3, *P<0.05
Overexpression of EGFL6 promoted osteogenic differentiation and angiogenesis of SONFH-BMSCs
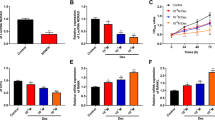
Subsequently, lentiviral vector expressing EGFL6 (OE-EGFL6) was applied to infect cultured SONFH-BMSCs, and then, their abilities of osteogenic differentiation and angiogenesis were evaluated. Our results showed that compared with control, there was no significant change in osteogenic ability of BMSCs cells in negative control for the lentiviral transduction group, and compared with negative control for the lentiviral transduction group, lentiviral vector overexpressing EGFL6 group markedly promoted SONFH-BMSC calcification (Fig. 4A, B) and tube formation (Fig. 4C). Moreover, compared with negative control for the lentiviral transduction, overexpression of EGFL6 increased SONFH-BMSC angiogenesis and the expression levels of BMP-2, VEGFA, CD31, and CD34 (Figs. 5A). These data indicated that EGFL6 overexpression was able to promote osteogenic differentiation and angiogenesis of SONFH-BMSCs.
Overexpression of EGFL6 promoted osteogenic differentiation and angiogenesis of SONFH-BMSCs. Lentiviral vector expressing EGFL6 was applied to infect cultured SONFH-BMSCs, and then, their abilities of osteogenic differentiation and angiogenesis were evaluated with alizarin red staining (A, B) and tube formation experiment (C). N=3, **P<0.01, ***P<0.001
The function of EGFL6 was related to ERK1/2. A Lentiviral vector expressing EGFL6 was applied to infect cultured SONFH-BMSCs. The expression levels of BMP-2, VEGFA, CD31, CD34, and p-ERK1/2-ERK1/2 were detected with Western blotting. B IHC assay was used to evaluate the expression of p-ERK1/2 in the FNF and the SONFH femoral head tissues. C U0126, a specific inhibitor of ERK1/2, was used to incubate EGFL6-overexpressed SONFH-BMSCs, and Western blotting was used to detect expression of osteogenic and angiogenic marker proteins, including BMP-2, RUNX2, and VEGFA. N=3, *P<0.05, **P<0.01, ***P<0.001
The promotion of EGFL6 on osteogenesis and angiogenesis was related to the activation of extracellular signal-regulated kinases 1/2 (ERK1/2)
Finally, we explored the mechanism of EGFL6 promoting osteogenesis and angiogenesis. The ERK1/2 signaling was recently reported to couple osteogenesis with angiogenesis (Jiao et al. 2021). Here, Western blotting showed that, compared with negative control for the lentiviral transduction, overexpression of EGFL6 significantly activated the ERK1/2, manifested by increased their phosphorylation (Fig. 5A). Simultaneously, we found that the SONFH femoral head displayed lower levels of p-ERK1/2 than the FNF femoral head (Fig. 5B). Furthermore, U0126, a specific inhibitor of ERK1/2 was used to incubate EGFL6-overexpressed SONFH-BMSCs, and Western blotting showed that U0126 treatment abated the promotion effect of EGFL6 overexpression on the expression of osteogenic and angiogenic marker genes (Fig. 5C). These findings indicated that promotion of EGFL6 on osteogenesis and angiogenesis of SONFH-BMSCs was related to the activation of ERK1/2.
Discussion
Bone repair is a complex biological process accompanied with the inflammatory immunity throughout and incorporated by multiple events including osteogenic differentiation, angiogenesis, and mineralization (Anani and Castillo 2022). During this process, blood vessels serve as “scaffolds” of bone development, which transported substances and regulatory factors necessary for osteogenesis into the osteogenic microenvironment, including minerals, growth factors, and osteogenic progenitor cells (Zheng et al. 2022). Therefore, factors regulating osteogenesis-angiogenesis coupling are important for bone repair. For example, melatonin was demonstrated to promote BMSC-mediated angiogenesis and to increase bone mineralization and formation around the tibia defect in rats (Zheng et al. 2022), which displayed a promising therapeutic effect on osteoporosis. Another example is that, periosteal extracellular matrix hydrogels promoted the differentiation of MSCs into endothelial cells, osteogenic differentiation of osteoblasts and MSCs, and mineralization. It can be seen that periosteal extracellular matrix hydrogel is a promising biomaterials in bone tissue engineering (Qiu et al. 2020). In this study, we found that the SONFH femoral head displayed lower expression levels of osteogenic and angiogenic marker genes, and BMSCs derived from the SONFH femoral head displayed poorer abilities of proliferation and osteogenesis, indicating the ruined repair capability of the femoral head in SONFH patients.
EGFL6 was famous as an angiogenic regulator in normal and tumor tissues, for its important regulatory role in endothelial cell proliferation and migration. A decade ago, comparative gene-expression analyses in human dental lineages revealed that EGFL6 was of the screened genes related to bone development and remodeling (Lee et al. 2013). However, its role in osteogenic differentiation was uncovered until quite recently. EGFL6 was demonstrated to facilitate osteogenic differentiation in adipose-derived SCs through activating the bone morphogenetic protein 2 (BMP-2)/Smad4 signaling pathway (Liu and Wang 2022). Almost simultaneously, a couple of study reported that EGFL6 derived from osteoblasts or BMSCs can regulate angiogenesis and osteogenesis. Overexpression of EGFL6 markedly enhanced osteogenic capacity of osteoblasts by activating the BMP/Smad and MAPK signaling, and EGFL6-deficiency leads to bone repair weaken in a bone defect model, manifested by decreased formation of type H vessels and osteoblasts (Chen et al. 2021); administration of EGFL6 in rat with tibia distraction promoted formation of type H-positive vessels and osteogenic differentiation through Wnt/β-catenin signaling (Shen et al. 2021). In our study, we also found that EGFL6 was markedly downregulated in the SONFH femoral head tissues and SONFH-BMSCs, and overexpression of EGFL6 improved osteogenic differentiation and angiogenesis of SONFH-BMSCs via the ERK1/2 signaling.
Extracellular signal-regulated kinases 1/2 (ERK1/2) are serine/threonine kinases that have been involved in the regulation of a large number of cellular processes including cell adhesion, progression, migration, survival, differentiation, metabolism, and proliferation. ERK1/2 catalyzed phosphorylation of nuclear transcription factors Ets, Elk, and c-Fos to play important role in many human diseases, including cancers, neurodegenerative disorders, cardiovascular diseases, and injury of muscles, skin, and bones, etc. During bone repair, ERK1/2 was a key signaling pathway coupling angiogenesis to osteogenesis. For example, activation of ERK1/2 were required for survival of BMSCs, and the peptide hormone ELABELA alleviated hypoxic/ischemic-induced apoptosis of BMSCs by activating ERK1/2 and repressing mitochondrial dysfunction (Fu et al. 2020). ERK1/2 was also reported to regulate VEGF secretion from osteoblasts to promote angiogenesis during bone healing (Rui et al. 2012). Furthermore, decreased activation of ERK1/2 was observed in the ONFH patients, and progranulin protected against osteonecrosis of the femoral head by activating the ERK1/2 pathway (Han et al. 2017). In this study, we also found that activation of ERK1/2 was decreased in the SONFH femoral head and SONFH-BMSCs, and activating ERK1/2 by EGFL6 overexpression improved osteogenesis and angiogenesis of SONFH-BMSCs. Consistent with our study, Zhao et al. also previously reported that Cx43 overexpression promoted osteogenic differentiation of BMSCs to alleviate osteonecrosis of the femoral head by activating ERK1/2 (Zhao et al. 2021).
In conclusion, EGFL6 was markedly downregulated in the SONFH femoral head of and SONFH-BMSCs that displayed lower abilities of osteogenesis and angiogenesis. Overexpression of EGFL6 promoted osteogenic differentiation and angiogenesis of SONFH-BMSCs, and the promotion of EGFL6 on osteogenesis and angiogenesis was related to the activation of ERK1/2.
References
Anani T, Castillo AB (2022) Mechanically-regulated bone repair. Bone 154:116223
Buchner G, Orfanelli U, Quaderi N, Bassi MT, Andolfi G, Ballabio A et al (2000) Identification of a new EGF-repeat-containing gene from human Xp22: a candidate for developmental disorders. Genomics 65:16–23
Chang C, Greenspan A, Gershwin ME (2020) The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun 110:102460
Chen K, Liao S, Li Y, Jiang H, Liu Y, Wang C et al (2021) Osteoblast-derived EGFL6 couples angiogenesis to osteogenesis during bone repair. Theranostics 11:9738–9751
Cui Y, Huang T, Zhang Z, Yang Z, Hao F, Yuan T et al (2022) The potential effect of BMSCs with miR-27a in improving steroid-induced osteonecrosis of the femoral head. Sci Rep 12:21051
Fu J, Chen X, Liu X, Xu D, Yang H, Zeng C et al (2020) ELABELA ameliorates hypoxic/ischemic-induced bone mesenchymal stem cell apoptosis via alleviation of mitochondrial dysfunction and activation of PI3K/AKT and ERK1/2 pathways. Stem Cell Res Ther 11:541
Han Y, Si M, Zhao Y, Liu Y, Cheng K, Zhang Y et al (2017) Progranulin protects against osteonecrosis of the femoral head by activating ERK1/2 pathway. Inflammation 40:946–955
Huang C, Wen Z, Niu J, Lin S, Wang W (2021) Steroid-induced osteonecrosis of the femoral head: novel insight into the roles of bone endothelial cells in pathogenesis and treatment. Front Cell Dev Biol 9:777697
Jiao D, Zheng A, Liu Y, Zhang X, Wang X, Wu J et al (2021) Bidirectional differentiation of BMSCs induced by a biomimetic procallus based on a gelatin-reduced graphene oxide reinforced hydrogel for rapid bone regeneration. Bioact Mater 6:2011–2028
Kurosawa S (1989) The role of complex formation and epidermal growth factor-like domains in the regulation of blood coagulation by the thrombomodulin-protein C system. Nihon Ketsueki Gakkai zasshi: J JPN Haematol Soc 52:1343–1349
Lee HS, Lee J, Kim SO, Song JS, Lee JH, Lee SI et al (2013) Comparative gene-expression analysis of the dental follicle and periodontal ligament in humans. PloS One 8:e84201
Liu H, Wang X (2022) Epidermal growth factor-like domain protein 6 recombinant protein facilitates osteogenic differentiation in adipose stem cells via bone morphogenetic protein 2/recombinant mothers against decapentaplegic homolog 4 signaling pathway. Bioengineered 13:6558–6566
Mas VR, Maluf DG, Archer KJ, Yanek KC, Fisher RA (2007) Angiogenesis soluble factors as hepatocellular carcinoma noninvasive markers for monitoring hepatitis C virus cirrhotic patients awaiting liver transplantation. Transplantation 84:1262–1271
Motta F, Timilsina S, Gershwin ME, Selmi C (2022) Steroid-induced osteonecrosis. J Transl Autoimmun 5:100168
Pinte S, Delfortrie S, Havet C, Villain G, Mattot V, Soncin F (2022) EGF repeats of epidermal growth factor-like domain 7 promote endothelial cell activation and tumor escape from the immune system. Oncol Rep 47:8
Qiu P, Li M, Chen K, Fang B, Chen P, Tang Z et al (2020) Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials 227:119552
Rui Z, Li X, Fan J, Ren Y, Yuan Y, Hua Z et al (2012) GIT1Y321 phosphorylation is required for ERK1/2- and PDGF-dependent VEGF secretion from osteoblasts to promote angiogenesis and bone healing. Int J Mol Med 30:819–825
Shen J, Sun Y, Liu X, Zhu Y, Bao B, Gao T et al (2021) EGFL6 regulates angiogenesis and osteogenesis in distraction osteogenesis via Wnt/β-catenin signaling. Stem Cell Res Ther 12:415
Uesugi Y, Sakai T, Seki T, Hayashi S, Nakamura J, Inaba Y et al (2018) Quality of life of patients with osteonecrosis of the femoral head: a multicentre study. Int Orthop 42:1517–1525
Wolff GS, Chiang PJ, Smith SM, Romero R, Armant DR (2007) Epidermal growth factor-like growth factors prevent apoptosis of alcohol-exposed human placental cytotrophoblast cells. Biol Reprod 77:53–60
Xu D, Perez RE, Ekekezie II, Navarro A, Truog WE (2008) Epidermal growth factor-like domain 7 protects endothelial cells from hyperoxia-induced cell death. Am J Physiol Lung Cell Mol Physiol 294:L17–L23
Xu Y, Jiang Y, Xia C, Wang Y, Zhao Z, Li T (2020) Stem cell therapy for osteonecrosis of femoral head: opportunities and challenges. Regen Ther 15:295–304
Zhang F, Peng W, Wang T, Zhang J, Dong W, Wang C et al (2022) Lnc Tmem235 promotes repair of early steroid-induced osteonecrosis of the femoral head by inhibiting hypoxia-induced apoptosis of BMSCs. Exp Mol Med 54:1991–2006
Zhang F, Peng W, Zhang J, Dong W, Wu J, Wang T et al (2020) P53 and Parkin co-regulate mitophagy in bone marrow mesenchymal stem cells to promote the repair of early steroid-induced osteonecrosis of the femoral head. Cell Death Dis 11:42
Zhang F, Yan Y, Peng W, Wang L, Wang T, Xie Z et al (2021) PARK7 promotes repair in early steroid-induced osteonecrosis of the femoral head by enhancing resistance to stress-induced apoptosis in bone marrow mesenchymal stem cells via regulation of the Nrf2 signaling pathway. Cell Death Dis 12:940
Zhao X, Alqwbani M, Luo Y, Chen C, Ge A, Wei Y et al (2021) Glucocorticoids decreased Cx43 expression in osteonecrosis of femoral head: the effect on proliferation and osteogenic differentiation of rat BMSCs. J Cell Mol Med 25:484–498
Zheng S, Zhou C, Yang H, Li J, Feng Z, Liao L et al (2022) Melatonin accelerates osteoporotic bone defect repair by promoting osteogenesis-angiogenesis coupling. Front Endocrinol 13:826660
Data availability
The data that support the findings of this study are available on request from the corresponding author upon reasonable request.
Funding
This study was funded by the General Project of Shaanxi Provincial Department of Science and Technology (2021SF-250) and Xi’an Science and Technology Bureau’s “Science and Technology+” Action Plan-Medical Research Project (20YXYJ0004).
Author information
Authors and Affiliations
Contributions
Penghui Bu prepared the manuscript; Penghui Bu, Weipeng Xie, and Sicheng Wang designed the study; Zhi Yang, Kan Peng, and Shouye Hu performed the experiments and analyzed data; Weisong Zhang prepared the figures. All authors reviewed and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethics Committee of Xi’an Hong Hui Hospital, Xi’an Jiaotong University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(XLSX 21 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bu, P., Xie, W., Wang, S. et al. EGFL6 activates the ERK signaling to improve angiogenesis and osteogenesis of BMSCs in patients with steroid-induced osteonecrosis of the femoral head. Naunyn-Schmiedeberg's Arch Pharmacol 397, 4287–4298 (2024). https://doi.org/10.1007/s00210-023-02880-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02880-0