Abstract
Purpose: Combination therapy is a strategy aimed at the combined use of agents targeting different mechanisms in cancer treatment. This study aimed to examine the cytotoxic and apoptotic effects of the traditional chemotherapeutic agent doxorubicin (DOX) and the next-generation HSP90 inhibitor MPC-3100 on breast cancer cell lines. Methods: Firstly, molecular docking analyses were performed, and then the MTT test was conducted to evaluate the individual and combined cytotoxic effects of DOX and MPC-3100 on MCF-7 and MDA-MB-231 breast cancer cell lines. The effect of two drugs combination was assessed by the Chou and Talalay approach. To further investigate the underlying molecular mechanism responsible for this synergistic effect, the gene expression levels of apoptotic and heat shock proteins (HSP), as well as the protein expression levels, were examined using quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) and Western Blotting, respectively. Results: Based on the molecular docking results, it was observed that MPC-3100 specifically binds to the ATP binding pocket of Hsp90, exhibiting an estimated free binding energy of -7.9 kcal/mol. MTT results indicated that both DOX and MPC-3100, as well as their combination, exhibited dose-dependent cytotoxicity. The drug combination showed a synergistic effect on both MCF-7 and MDA-MB-231 cell lines. Finally, the investigated molecular mechanism demonstrated that the combination of DOX and MPC-3100 induced apoptosis in breast cancer cells more efficiently than either drug alone. Conclusions: This study showed a possible coordinated mechanism of action between DOX and MPC-3100, pointing to the possibility of a more effective therapeutic strategy for breast cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most prevalent type of cancer diagnosed worldwide. In 2020, there were about 2.3 million new instances of breast cancer and 685,000 deaths (Arnold et al. 2022). Breast cancer has a complicated origin that involves several signaling pathways. However, the biological features of these pathways are still unknown (Yersal and Barutca 2014; Arnold et al. 2022). Chemotherapy is the most vital phase in breast cancer treatment. The most common approach to cancer therapy is to utilize a combination of pharmaceuticals that increase the anticancer activity of current therapies while minimizing their negative side effects (Lainetti et al. 2020). An appropriate molecular target is one that affects many components of one or more signaling pathways simultaneously. HSP90 is a chaperone that promotes the maturation of a wide range of effectors known as client proteins (CPs) that are important in cell growth, differentiation, and survival (Ghadban et al. 2016). HSP90 is a potentially interesting therapeutic target, particularly in breast cancer, because human epidermal growth factor receptor 2 (HER2), estrogen receptor (ER), and progesterone receptor (PR) are recognized CPs of HSP90 (Wang et al. 2022). MPC-3100 was specifically chosen as a promising next-generation Hsp90 inhibitor to explore its therapeutic potential in the context of breast cancer cells. This conclusion was driven by its excellent pharmacological characteristics, which make it a desirable candidate for further research. Furthermore, the restricted scope of prior experimental and clinical investigations on breast cancer utilizing MPC-3100 adds to its relevance, emphasizing the possibility for unique insights and achievements in breast cancer treatment (Kim et al. 2012).
DOX is the most often used chemotherapy in the treatment of breast cancer. DOX has considerable dose-limiting disadvantages, mainly cardiotoxicity, despite its anticancer potency (Sohail et al. 2021; Gökşen Tosun et al. 2023).
There are two major molecular subtypes of breast cancer: hormone receptor-positive and triple-negative. Approximately 80% of breast cancers are hormone-receptor positive, whereas the remaining 20% are triple-negative (Feng et al. 2018). The purpose of this study was to see how the drug combination affected hormone-receptor-positive MCF-7 breast cancer cells and triple-negative MDA-MB-231 breast cancer cells. By utilizing these specific cell lines, which represent distinct breast cancer subtypes, the study aimed to assess the efficacy of the drug combination in a targeted manner, considering the unique characteristics and vulnerabilities of each subtype. This study focused on investigating the effects of DOX and MPC-3100 alone and in combination on breast cancer cells. A molecular docking investigation revealed that MPC-3100 specifically interacts with the ATP binding pocket of Hsp90 NTD. In this study, it was observed that the combined treatment of MPC-3100 and DOX effectively suppressed the chaperone activity of Hsp90 and induced apoptosis in breast cancer cells. This was achieved through the modulation of gene and protein expression levels associated with both the apoptotic and anti-apoptotic pathways. The findings suggest that the combination of MPC-3100 and DOX exerts a synergistic effect in promoting apoptotic cell death in breast cancer cells by targeting Hsp90 and regulating key molecular pathways involved in apoptosis. In addition, heat shock proteins related to HSP90 inhibition, such as HSP90, HSP70, and HSP27, were analyzed. These findings point to the combination of DOX and MPC-3100 as viable treatment options for triple-negative and hormone receptor-positive breast cancer. These findings suggest that the combination treatment could be an effective drug candidate in addressing the treatment needs of these specific breast cancer subtypes.
Materials and methods
Chemicals and reagents
MPC-3100 was purchased from AdooQ® Bioscience. Doxorubicin HCI was obtained by Gold Biotechnology Incorporated, USA. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), BCA protein assay kit, and RIPA lysis buffer were supplied from Serva. Polyvinylidene difluoride (PVDF) membrane (741260) was from Macherey-Nagel. Anti-HSP27 (ab5579), anti-HSP70 (ab79852), anti-HSP90 (ab2928), Anti-Bax (ab53154), Anti-Bcl-2 (ab59348), and goat anti-rabbit IgG H&L (HRP) (ab205718) antibodies were purchased from Abcam. Anti-pro-Casp-9 (M00080-1) antibody was purchased from Boster Bio. Anti-GAPDH (10494-1-AP) antibody was purchased from Proteintech. Primers were synthesized from Metabiomics. SYBR Green master mix was from Bioline. The enhanced chemiluminescence (ECL) substrate kit (1705060) was from Bio-Rad. Dulbecco’s Modified Eagle’s medium (DMEM) High Glucose, fetal bovine serum (FBS heat-inactivated), penicillin-streptomycin solution, L-glutamine, phosphate buffer saline (PBS), and trypsin–EDTA was purchased from Biological Industries. MDA-MB-231 and MCF-7 cell lines were from ATCC (American Type Culture Collection). Other chemicals and reagents were supplied from Sigma-Aldrich.
Molecular docking
The chemical structure of MPC-3100 was retrieved from the NCBI PubChem database, while the three-dimensional (3D) structure of the Hsp90-NTD protein was obtained from the Protein Data Bank (PDB). Specifically, the HSP90 protein structure from PDB, which was in complex with ATP (PDB ID: 3T0Z) and had a resolution of 2.19 Å, was utilized. Before docking, the ATP molecule was removed from the complex, preparing the HSP90 NTD for docking analysis. Both MPC-3100 and the HSP90 protein were visualized using Chimera 1.16 software. To prepare the ligand (MPC-3100) and protein (HSP90) for docking, the Chimera 1.16 DocPrep tool was employed. The docking calculations were performed using AutoDock Vina, which involved identifying the potential interaction site between the target protein and ligand by creating a grid box. The grid box had specific x, y, and z coordinates of 9.63, -5.88, and 16.34, respectively, with a size of 22 × 22 × 22. After completing the docking analyses, Biovia Discovery Studio was utilized to visualize the interaction between the MPC-3100 ligand and the active site residues of the HSP90 protein.
Cell culture
To culture, the MCF-7 and MDA-MB-231 cancer cell lines, DMEM High Glucose medium supplemented with 0.1% penicillin-streptomycin, 1% L-glutamine, and 10% FBS was used, and the cancer cell lines were incubated at 37 °C in a humid environment containing 5% CO2. Monolayer proliferating MCF-7 and MDA-MB-231 cell lines were cultured in polystyrene, sterile T75 flasks. These breast cancer cell lines proliferated as monolayers and were passaged when they reached 90% confluency. The number of passages of breast cancer cell lines was performed not to exceed 10 times.
Cell viability assay
The MTT cell proliferation test was preferred to determine the in vitro cytotoxic effect of MPC-3100 and DOX. The cancer cell lines were grown in 96-well culture plates (5 × 104 cells per well) and treated with MPC-3100 and DOX for 48 h at concentrations ranging from 50 g/mL to 0.8 g/mL. After the incubation period, MTT solution (5 mg/mL) was added to each well and incubated at 37 °C for 3 h. The formazan product was then dissolved in 100 µL of dimethyl sulfoxide (DMSO) after MTT was removed. The absorbance was measured at 570 nm using a microplate reader, and the viability of cancer cells was calculated as a percentage of the control group.
Quantitative real-time RT-PCR
RT-PCR was used to investigate the expression of Hsp27, Hsp70, Hsp90, Bax, Bcl-2, Cas-9, p53, and cytC genes in MCF-7 and MDA-MB-231 cells treated with MPC-3100 and DOX. Cancer cell lines were treated with IC50 doses of MPC-3100 and DOX for 48 h. Total RNA was then extracted from these cells, and gene expression was determined using the RT-PCR technique detailed previously (Tosun et al. 2021). The total RNA isolation kit was from Favorgen Biotech Corp. RT-PCR primers were synthesized from Metabiomics Corp. SYBR Green mastermix and cDNA synthesis kits were supplied from Bio-Rad. Total RNA of treated cells with IC50 values of drugs were isolated using a commercial kit by referring to the manufacturer protocol. Synthesis of cDNA was carried out with a first-strand cDNA synthesis kit. RT-PCR (Bio-Rad CFX96TM) was set up with SYBR Green qPCR master mix in a 25 µl volume (1 µl cDNA 1 ml primer mix (10 mM stock), 11.5 µl SYBR Green mastermix, 10.5 ml RNase-free water) with following cycling condition: 1 cycle: 95 °C for 10 min, 40 cycles: 95 °C for 15 s. and 60 °C for 1 min. The primer sequences are shown in Table 1. The 2−ΔΔCt technique was used to analyze the expression levels of genes. The GAPDH gene was used as a housekeeping gene.
Western blotting
Protein expression levels of Hsp27, Hsp70, Hsp90, Bax, Bcl-2, and Cas-9 were assessed using Western blotting. After treating MCF-7 and MDA-MB-231 cells with IC50 concentrations of MPC-3100 and DOX for 48 h, the cells were trypsinized and lysed with RIPA buffer. The protein content in the cell lysates was quantified using a BCA protein assay kit. Subsequently, 30 µg of protein per well was separated by 12% SDS-PAGE and transferred to PVDF membranes using a Trans-blot turbo transfer device (Bio-Rad). The membranes were then blocked with 5% skim milk powder in TBST for 1 h and incubated overnight at four °C with primary antibodies targeting HSP27 (1:1000), HSP70 (1:500), and HSP90 (1:500). After washing with TBST, the membranes were incubated with a secondary antibody (goat anti-rabbit IgG H&L (HRP)) (1:10000) for 1 h at room temperature. Protein bands were visualized using ChemiDocTM imaging equipment (Bio-Rad) and an ECL substrate. ImageLab 6.1 software was utilized to quantify the levels of protein expression.
Statistical analysis
GraphPad Prism 8.0 software was used for statistical analysis, and p < 0.05 probability values were defined as statistical significance. To examine the efficacy of the combination of MPC-3100 and DOX, the combination index (CI) was calculated using CompuSyn software version 1.0. Chou-Talalay approach was used to determine CI. The percentage of viable cells was calculated with the GraphPad 8.0 program, and the negative control without substance is considered 100% viable by the program. Statistical analysis for cell viability: Values are expressed mean ± SEM (n = 3). p < 0.05. Statistical analysis and comparable data sets were evaluated with a two-way ANOVA with Sidak test and Dunnett’s test using GraphPad Prism 8.0 software for Real-time and Western Blot analysis.
Results and discussion
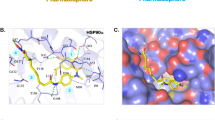
Through the implementation of molecular docking, the predicted binding conformation of MPC-3100 on Hsp90 NTD was obtained. The docking results revealed that MPC-3100 exhibits selective binding to the highly conserved ATP binding pocket, with a calculated binding energy of -7.9 kcal/mol (Fig. 1A). In the docking analysis, a conventional hydrogen bond was calculated between the Asp83, Asn41, Gly127, and Lys102 amino acid residues of the HSP90 protein and the MPC-3100 molecule. The bond distances were determined as 2.47Å, 2.32Å, 2.32Å, and 3.02Å, respectively. MPC-3100 also had a Van der Waals interaction with amino acids Phe128, Gly125, Val126, and Asn96. In addition, alkyl and pi-alkyl hydrophobic interactions were found between the amino acids Leu38, Val176, Ile81, Leu97, Met88, Tyr129, and MPC-3100 (Fig. 1B C).
The viability of MCF-7 and MDA-MB-231 cell lines was assessed using the MTT assay to evaluate the effects of DOX and the HSP90 inhibitor MPC-3100, both as single agents and in combination. As depicted in Fig. 2A and B, both DOX and MPC-3100 exhibited a dose-dependent inhibition of cell viability in MCF-7 and MDA-MB-231 cells. The half maximal inhibitory concentration (IC50) of DOX was determined to be 227.4 nM (MCF-7) and 237.0 nM (MDA-MB-231), whereas the IC50 of MPC-3100 was 598.0 nM (MCF-7) and 1185 nM (MDA-MB-231). MCF-7 cells displayed relatively higher sensitivity to both drugs. Additionally, the combined treatment of MPC-3100 and DOX demonstrated stronger inhibitory effects on cell viability in both cell lines compared to the individual agents alone (Fig. 3A and B). These findings suggest a potential synergistic effect of MPC-3100 and DOX in suppressing cell viability in breast cancer cells.
To confirm the synergism, a fixed ratio combination of the two agents was administered to the cells, and the combination index (CI) was calculated using CompuSyn software, following the methodology described by Chou and Talalay (Chou 2014). The analysis revealed the following combination effects: CI < 1 indicates synergism, CI = 1 suggests additivity, and CI > 1 indicates antagonism (Chou 2018). The combined treatment of MPC-3100 and DOX demonstrated synergistic anticancer activity in both MCF-7 (Fig. 3C) and MDA-MB-231 (Fig. 3D) breast cancer cells. To investigate the underlying mechanism of this synergism, the impact of DOX and MPC-3100, both individually and in combination, on apoptotic and heat shock proteins in breast cancer cell lines was assessed through Real-Time PCR and Western Blot experiments.
Cytotoxic impact of DOX and MPC-3100 combination in MCF-7 (A) and MDA-MB-231 (B) cancer cell lines, as well as fraction, affected (Fa) vs. Combination index plots generated from Chou-Talalay median-effect analysis for MCF-7 (C) and MDA-MB-231 (D). *Fa, fraction affected; CI, combination index, ED30, ED50, and ED75 represent the doses required to inhibit 30%, 50%, and 75% of cells or organisms, respectively, indicating the potency of a drug or treatment in achieving the desired effect
The effect of DOX and MPC-3100 administered as a single agent and in combination on HSP genes (Hsp27, Hsp70, and Hsp90) and apoptotic genes (Bax, Bcl-2, Cas-9, p53, and Cyt C) was evaluated by RT-PCR (Fig. 4). In the MCF-7 cell line treated with MPC-3100, the Bax/Bcl-2 ratio, Cas-9, p53, and CytC gene expression levels were increased. In MDA-MB-231 cells, on the other hand, while Bax/Bcl-2 ratio and CytC increased, the p53 level decreased. There was no significant change in the Cas-9 gene level. When the changes in HSP gene expressions were examined, the level of HSP27 decreased in MCF-7 cells, while the level of HSP70 and HSP90 increased. However, the change in HSP70 and HSP27 gene expression was not statistically significant. In MDA-MB-231 cells, on the other hand, there was a decrease in the expression of three HSP genes whose expressions were examined. The decrease is not statistically significant.
Expression of apoptotic genes (Bax, Bcl-2, Cyt C, p53, Cas-9) and HSP genes (HSP27, HSP70, HSP90) at 48 h in MCF-7 (A) and MDA-MB-231 (B) cells treated with MPC-3100, DOX and DOX + MPC-3100. The results are presented as mean ± SEM (n = 3). Statistical significance was determined using *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared to the control group
Changes in the expression of apoptotic protein and HSP proteins in breast cancer cells in which MPC-3100 and DOX were treated with a single agent or in combination were analyzed by Western Blotting (Fig. 5).
The Bax/Bcl-2 ratio increased in MCF-7 cells exposed to the HSP90 inhibitor MPC-3100 for 48 h. Hsp27, Hsp70, and Hsp90 protein levels were increased. MPC-3100 treatment in MDA-MB-231 cells caused an increase in the Bax/Bcl-2 ratio, and there was no significant change in the pro-Cas-9 level. In contrast to MCF-7 cells, inhibition of Hsp90 reduced all three HSP proteins tested in MDA-MB-231 cells. DOX administration created an apoptotic profile in MCF-7 cells with increased Bax/Bcl-2 ratio, increased Hsp27, Hsp70, and Hsp90 protein levels, and decreased pro-Cas-9 levels. Similarly, in MDA-MB-231 cells, the Bax/Bcl-2 ratio increased, all the tested HSP proteins were decreased, and there was no significant change in the pro-Cas-9 level.
Analysis of apoptotic (Bax, Bcl-2, pro-Cas-9) and HSP (HSP27, HSP70, HSP90) protein expressions at 48 h in MCF-7 (A) and MDA-MB-231 (B) cells treated with MPC-3100, DOX and DOX + MPC-3100. The results are presented as mean ± SEM (n = 2). Statistical significance was determined using *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 compared to the control group
When MPC-3100 and DOX were combined, there was a large increase in Bax/Bcl-2 cells in both cells. The combination reduced the amount of pro-Cas9 protein in both cells. In general, the combination increased the quantity of HSP27 and HSP70 proteins in MCF-7 cells while decreasing the amount of these proteins in MDA-MB-231 cells. In both cell lines, the drug combination reduced the total amount of HSP90 protein.
Discussion
In recent years, the combined effects of molecules targeting different mechanisms have been frequently investigated in research on breast cancer treatment. In addition to the combined use of chemotherapy and phototherapy agents, the effects of new-generation targeted agents are examined when used together with chemotherapy agents used in existing treatments. Heat shock protein inhibitors attract attention among new-generation agents (Kryeziu et al. 2019; Ye et al. 2021). In this study, the combined therapy of MPC-3100, an HSP90 inhibitor, and DOX, a chemotherapy agent, was examined on MCF-7 and MDA-MB-231 breast cancer cell lines. Within the scope of this study, MPC-3100 molecular docking analysis was first performed. MPC-3100 exhibited selective binding to the highly conserved ATP binding pocket.
In the context of the molecular docking analysis, it is crucial to emphasize the selective binding of MPC-3100 to the ATP binding pocket of Hsp90 NTD. The calculated binding energy of -7.9 kcal/mol indicates a strong affinity between MPC-3100 and the target protein. This interaction is further supported by the identification of specific amino acid residues (Asp83, Asn41, Gly127, and Lys102) involved in conventional hydrogen bonding with MPC-3100. The determined bond distances provide valuable insights into the strength and specificity of these interactions. Furthermore, the Van der Waals interactions with Phe128, Gly125, Val126, and Asn96, as well as the hydrophobic interactions with Leu38, Val176, Ile81, Leu97, Met88, and Tyr129, underscore the comprehensive binding profile of MPC-3100 within the binding pocket. These interactions collectively contribute to the stabilizing effect of MPC-3100 on Hsp90 NTD. Additionally, the comparison with other inhibitors, such as Debio-0932, highlights the superior binding affinity of MPC-3100, as evidenced by the higher calculated binding energy. the HSP90 inhibitor Debio-0932 exhibited a binding energy of -7.24 kcal/mol, whereas MPC-3100 demonstrated a superior binding energy of -7.9 kcal/mol (Özgür et al. 2021). This suggests that MPC-3100 holds significant promise as an HSP90 inhibitor in the context of next-generation cancer drug discovery.
In vitro, cytotoxic effects of MPC-3100 and DOX were tested individually and in combination on MCF-7 and MDA-MB-231 cell lines. The cytotoxic effect of MPC-3100 on MCF-7 and MDA-MB-231 breast cancer cell lines has not been previously investigated. In this study, the cytotoxic effect of MPC-3100, a new generation HSP90 inhibitor, was investigated on breast cancer cell lines, individually and in combination with DOX, and it was found that it exhibited a synergistic effect when combined with DOX.
In this study, the effects of DOX and MPC-3100 on Hsp genes and apoptotic genes were also examined both individually and in combination. In both DOX-treated breast cancer cells, the Bax/Bcl-2 ratio, Cyt C, and Cas-9 gene expression levels increased. DOX increased p53 gene expression in MCF-7 cells while decreasing it in MDA-MB-231 cells. In MCF-7 cells, the effect of DOX on HSP genes resulted in a statistically insignificant decrease in HSP27 and HSP70 gene expression and an increase in HSP90 expression. While neither HSP27 nor HSP90 levels changed significantly in MDA-MB-231 cells, HSP70 gene expression increased.
When MPC-3100 and DOX cells were combined, both showed an apoptotic profile with an increase in the Bax/Bcl-2 ratio, Cyt C, and Cas-9 expression levels. In MCF-7 cells, this combination enhanced p53 gene expression while reducing it in MDA-MB-231 cells. While the change in HSP27 gene expression was statistically insignificant in both cell lines, HSP70 expression increased in the MDA-MB-231 cell line. MCF-7 cells have more HSP90 expression than MDA-MB-231 cells. In terms of HSP gene expression, gene expression analysis revealed that both cell lines behaved differently to the drug combination.
The expression of the p53 gene increased in MCF-7 cells, whereas it decreased significantly in MDA-MB-231 cells. This might be because the two cell lines have distinct p53 statuses. The tumor suppressor protein p53 is an essential HSP90 client protein that is mutated in more than half of all human malignancies. Previous research has shown that inhibiting HSP90 activity can enhance ubiquitination and degradation of mutant p53 via the proteasome, which triggers apoptosis in a p53-dependent way (Muller et al. 2008). Our findings show that treatment of MPC-3100 resulted in a decrease in mutant p53 protein levels in MDA-MB-231 cells while at the same time increasing wild-type p53 expression in MCF-7 cells. Consequently, this treatment triggered the activation of the apoptotic pathway. On the other hand, in mutant p53 MDA-MB231 and BT-474 cells, DOX treatment has been shown to induce NF-kB translocation, DNA binding, and the upregulation of NF-kB-dependent transcription. However, this pathway did not exhibit functionality in wild-type p53 MCF-7 cancer cells. A comprehensive analysis of human breast tumors treated ex vivo with DOX revealed a significant correlation between the p53 status and the ability of DOX to induce a tumorigenic and anti-apoptotic NF-kB response (Dalmases et al. 2014).
Differences in HSP27 expression levels can be due to a variety of reasons. To begin, it is critical to recognize the underlying biological heterogeneity of two breast cancer cell lines, MCF-7 and MDA-MB-231. These cell lines may respond differently to applied treatments due to differences in their genetic structure and signaling pathways. In addition, the response to MPC-3100 and DOX, both individually and in combination, may be affected by the presence or absence of specific regulatory elements or mutations in the HSP27 gene in these cell lines. These genetic variations can lead to differential transcriptional activity and subsequent protein expression levels. It is also worth noting that although there are observable changes in HSP27 expression, some of these changes did not reach statistical significance. This may be attributed to the limited sample size in our study. Larger sample sizes or additional replicates could potentially provide more robust statistical support for these observations. Finally, it is crucial to consider the dynamic nature of cellular responses to drugs. The complex network of cellular pathways and feedback mechanisms can lead to complex and sometimes contradictory outcomes. These responses may be affected by various factors, such as administration time, dosage, and interaction between different cellular processes. In summary, the observed differences in HSP27 expression levels highlight the complexity of cellular responses to MPC-3100 and DOX treatments. Further research, including molecular studies and functional analyses, may be necessary to elucidate the underlying mechanisms driving these observed differences. This could potentially reveal new information regarding the interaction between HSP27 and apoptotic pathways in these breast cancer cell lines.
The findings clearly indicate that the combination of drugs activates caspases, resulting in programmed cell death known as apoptosis. Apoptosis is an important process for assessing cell responses to cytotoxic and tumor-related damage. The expression levels of pro-apoptotic proteins (such as Bax) and anti-apoptotic proteins (such as Bcl-2) might provide vital information about a cell’s apoptotic profile. Both pro-apoptotic and anti-apoptotic proteins carefully regulate cytochrome-c release during the apoptotic process. In this mechanism, cytochrome-c, pro-cas-9, and APAF-1 create a complicated apoptosome complex, which eventually leads to Cas-9 activation by proteolytic cleavage. Cas-9, once triggered, activates caspase-3 and caspase-7, activating the intrinsic processes that cause apoptotic cell death within the cells. Consequently, the increase in the Bax/Bcl-2 expression ratio and the reduction in pro-Cas-9 protein levels emerge as vital biomarkers capable of stimulating apoptosis in cancer cells and indicating the initiation of apoptotic processes (Ramalingam et al. 2021).
HSP90 inhibitors that specifically target the N-terminus of the protein have been observed to induce the translocation of the transcription factor HSF-1 into the nucleus. This translocation event triggers a series of cellular responses, leading to the upregulation of various heat shock proteins, including HSP70 and HSP90. Activation of the heat shock response survival cascade plays a vital role in cellular stress adaptation. Notably, HSP70, a prominent member of the heat shock protein family, acts as a pro-survival factor with implications in the regulation of cell survival and apoptosis. Its upregulation in response to HSP90 inhibition contributes to the suppression of apoptosis induction and can confer resistance to therapeutic interventions. This phenomenon highlights the complex interplay between heat shock proteins, such as HSP70, and cellular survival mechanisms (Kumar et al. 2016). Selective inhibition of HSP90 in tumor cells is a compelling strategy in oncology due to the significantly higher activity of HSP90 in tumor cells compared to normal cells (Kamal et al. 2003). While the clinical use of geldanamycin, an HSP90 inhibitor, is limited by hepatotoxicity, its derivative 17-AAG has shown promise in phase II/III clinical trials for breast cancer and other malignancies (Gorska et al. 2012) and in the context of HER2-positive metastatic breast cancer, tanspimycin, another derivative of 17-AAG, exhibited encouraging antitumor activity when combined with trastuzumab in phase II trials (Modi et al. 2011). Low-dose ganetespib, yet another HSP90 inhibitor, demonstrated the ability to inhibit oncogenic signaling and suppress tumor growth in MDA-MB-231 cells by upregulating apoptotic markers such as Parp and Bim (Friedland et al. 2014). HSP90 inhibitors, either alone or in combination, hold significant potential in inducing apoptotic cell death pathways in breast cancer. Additionally, the relevance of HSP90 inhibition to angiogenesis has been demonstrated, with HSP90 inhibition shown to modulate angiogenesis. High expression levels of HSP90 have been associated with poor outcomes in breast cancer (Pick et al. 2007). In a previous study, our research focused on Debio-0932, an HSP90 inhibitor that effectively inhibited the ATPase activity of human Hsp90 and reduced the proliferation of MCF-7 and MDA-MB-231 breast cancer cell lines through the induction of apoptotic pathways. Debio-0932 also upregulated the mRNA and protein expression of the Bax/Bcl-2 ratio and stimulated Casp-9 cleavage. Furthermore, Debio-0932 displayed potential antimetastatic activity by reducing the migration of HUVEC cells (Özgür et al. 2021).
In investigations focusing on triple-negative breast cancer models, the apoptotic potential of ganetespib in combination with docetaxel, paclitaxel, and DOX was evaluated. The combination of ganetespib and DOX resulted in increased expression of Parp in MDA-MB-231 cells. Similarly, In BT-20 cells, treatment with ganetespib led to the upregulation of Parp and Caspase-7 (Proia et al. 2014). These findings collectively highlight the promising therapeutic implications of HSP90 inhibitors in breast cancer treatment, specifically in terms of inducing apoptosis and inhibiting metastasis. Mohammadian et al. showed that the HSP 90 inhibitor NVP-AUY922, in combination with DOX, has a potent effect on the MCF-7 cell line. The possible mechanism of this cytotoxic effect was identified as induction of apoptosis, inhibition of cell growth, and downregulation of VEGF as the angiogenic factor (Mohammadian et al. 2020).
In our research, DOX and MPC-3100 alone caused cellular death in breast cancer cell lines by inducing apoptosis, and the combination of the drugs dramatically increased apoptotic cell death compared to their alone usage.
Conclusion
In this study, we proved the synergistic effect of DOX and MPC-3100 on MCF-7 and MDA-MB-231 breast cancer cell lines. It has been demonstrated that DOX and MPC-3100 agents may be more effective when used in combination than when used alone. The findings in our study are the first for the use of DOX and MPC-3100 in breast cancer cells and may form the basis for future combination cancer therapy research. The results of the synergistic effect offer a possible path for more effective anticancer treatments in future treatment techniques.
Data Availability
Data and materials are available from the author upon request.
References
Arnold M, Morgan E, Rumgay H et al (2022) Current and future burden of Breast cancer: global statistics for 2020 and 2040. The Breast 66:15–23. https://doi.org/10.1016/j.breast.2022.08.010
Chou T-C (2014) Frequently asked questions in drug combinations and the mass-action law-based answers. Synergy 1:3–21. https://doi.org/10.1016/j.synres.2014.07.003
Chou T-C (2018) The combination index (CI < 1) as the definition of synergism and of synergy claims. Synergy 7:49–50. https://doi.org/10.1016/j.synres.2018.04.001
Dalmases A et al (2014) Deficiency in P53 is required for doxorubicin induced transcriptional activation of NF-КB target genes in human breast cancer. Oncotarget 5(1):196–210.
Feng Y, Spezia M, Huang S et al (2018) Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 5:77–106. https://doi.org/10.1016/j.gendis.2018.05.001
Friedland JC et al (2014) Targeted inhibition of Hsp90 by ganetespib is effective across a broad spectrum of breast cancer subtypes. Invest New Drugs 32(1):14–24. https://doi.org/10.1007/s10637-013-9971-6
Ghadban T, Jessen A, Reeh M et al (2016) In vitro study comparing the efficacy of the water-soluble HSP90 inhibitors, 17-AEPGA and 17-DMAG, with that of the non–water-soluble HSP90 inhibitor, 17-AAG, in Breast cancer cell lines. Int J Mol Med 38:1296–1302. https://doi.org/10.3892/ijmm.2016.2696
Gökşen Tosun N, Erden Tayhan S, Gökçe İ, Alkan C (2023) Doxorubicin-loaded mPEG-pPAd-mPEG triblock polymeric nanoparticles for drug delivery systems: Preparation and in vitro evaluation. J Mol Struct 1291:135959. https://doi.org/10.1016/j.molstruc.2023.135959
Gorska M et al (2012) Geldanamycin and its derivatives as Hsp90 inhibitors. Front Biosci (Landmark edn) 17(6):2269–2277.
Kamal A et al (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425(6956): 407–410. https://doi.org/10.1038/nature01913
Kim S-H, Bajji A, Tangallapally R et al (2012) Discovery of (2S)-1-[4-(2-{6-Amino-8-[(6-bromo-1,3-benzodioxol-5-yl)sulfanyl]-9H-purin-9-yl}ethyl)piperidin-1-yl]-2-hydroxypropan-1-one (MPC-3100), a purine-based Hsp90 inhibitor. J Med Chem 55:7480–7501. https://doi.org/10.1021/jm3004619
Kryeziu K, Bruun J, Guren TK et al (2019) Combination therapies with HSP90 inhibitors against Colorectal cancer. Biochim Biophys Acta - Rev Cancer 1871:240–247. https://doi.org/10.1016/j.bbcan.2019.01.002
Kumar S, Stokes J, Singh UP et al (2016) Targeting Hsp70: a possible therapy for cancer. Cancer Lett 374:156–166. https://doi.org/10.1016/j.canlet.2016.01.056
Lainetti PD, Leis-Filho AF, Laufer-Amorim R et al (2020) Mechanisms of resistance to chemotherapy in Breast Cancer and possible targets in Drug Delivery systems. Pharmaceutics 12
Modi S et al (2011) HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res 17(15):5132–5139. https://doi.org/10.1158/1078-0432.CCR-11-0072
Mohammadian M et al (2020) Hsp90 inhibitor; NVP-AUY922 in combination with doxorubicin induces apoptosis and downregulates VEGF in MCF-7 breast cancer cell line. Asian Pac J Cancer Prev: APJCP 21(6):1773–1778.
Muller P et al (2008) Chaperone-dependent stabilization and degradation of P53 mutants. Oncogene 27(24):3371–3383. https://doi.org/10.1038/sj.onc.1211010
Özgür A et al (2021) Debio-0932, a second generation oral Hsp90 inhibitor, induces apoptosis in MCF-7 and MDA-MB-231 cell lines. Mol Biol Rep 48(4):3439–349.
Pick E et al (2007) High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res 67(7):2932–2937. https://doi.org/10.1158/0008-5472.CAN-06-4511
Ramalingam M, Jang S, Jeong H-S (2021) Therapeutic effects of conditioned medium of neural differentiated human bone marrow-derived stem cells on rotenone-induced alpha-synuclein aggregation and apoptosis. Stem Cells Int 2021:6658271. https://doi.org/10.1155/2021/6658271
Sohail M, Sun Z, Li Y et al (2021) Research progress in strategies to improve the efficacy and safety of doxorubicin for cancer chemotherapy. Expert Rev Anticancer Ther 21:1385–1398. https://doi.org/10.1080/14737140.2021.1991316
Tosun NG, Kaplan Ö, Özgür A (2021) Apoptosis Induced by Tarantula Cubensis Crude Venom (Theranekron® D6) in Cancer cells. Rev Bras Farmacogn 31:824–831. https://doi.org/10.1007/s43450-021-00221-x
Wang F, Zhang H, Wang H et al (2022) Combination of AURKA inhibitor and HSP90 inhibitor to treat Breast cancer with AURKA overexpression and TP53 mutations. Med Oncol 39:180. https://doi.org/10.1007/s12032-022-01777-x
Ye M, Huang W, Liu R et al (2021) Synergistic activity of the HSP90 inhibitor Ganetespib with Lapatinib reverses acquired Lapatinib Resistance in HER2-Positive Breast Cancer cells. Front Pharmacol 12. https://doi.org/10.3389/fphar.2021.651516
Yersal O, Barutca S (2014) Biological subtypes of Breast cancer: prognostic and therapeutic implications. World J Clin Oncol 5:412–424. https://doi.org/10.5306/wjco.v5.i3.412
Acknowledgements
The author would like to thank Prof. Dr. Ìsa Gök?e and Dr. Özlem Kaplan for their contributions
Funding
No support was received in this study. However, the facilities of Tokat Gaziosmanpasa University, Faculty of Engineering and Architecture, Department of Bioengineering laboratories were used.
Author information
Authors and Affiliations
Contributions
Nazan Gökşen Tosun: investigation, methodology, formal analysis, writing-review & editing, visualization, methodology, resources.N.G.T: Investigation, Methodology, Formal analysis, Writing-review & editing, Visualization, Methodology, Resources. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethical approval
Ethical approval is not required for this study.
Competing interests
The authors confirm that this article content has no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gökşen Tosun, N. Enhancing therapeutic efficacy in breast cancer: a study on the combined cytotoxic effects of doxorubicin and MPC-3100. Naunyn-Schmiedeberg's Arch Pharmacol 397, 3249–3259 (2024). https://doi.org/10.1007/s00210-023-02807-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02807-9